UNIVERSIDADE DE LISBOA
FACULDADE DE CIÊNCIAS
DEPARTAMENTO DE BIOLOGIA ANIMAL
Modelling the distribution of São Tomé bird species: Ecological
determinants and conservation prioritization
Filipa Macedo Coutinho de Oliveira Soares
Mestrado em Biologia da Conservação
Dissertação orientada por:
Doutor Ricardo Faustino de Lima
Professor Doutor Jorge Palmeirim
AGRADECIMENTOS
Quero começar por agradecer aos meus orientadores por todo o apoio incansável ao longo deste ano. Este trabalho não seria possível sem todos os “brainstormings” durante as extensas reuniões ao longo de várias semanas. Obrigada por me terem sempre incentivado a dar o meu melhor. Ricardo quero agradecer-te toda a ajuda, logo desde o início quando esta tese era nada mais do que uma pequena ideia. Não poderia ter pedido mais ou melhor orientação, obrigada pela tua infinita disponibilidade (eu sei o quão “chata” eu consigo ser!). O meu obrigado também ao Professor Jorge Palmeirim, a sua ajuda foi indispensável. Este trabalho não seria possível sem a incrível ajuda de ambos, o meu mais sincero obrigado!
Este trabalho não teria sido possível sem os dados recolhidos no âmbito da tese de doutoramento “Land-use management and the conservation of endemic species in the island of São Tomé” de Ricardo Faustino de Lima, e da “BirdLife International São Tomé and Príncipe Initiative”. A tese de doutoramento foi financiada pela FCT - Fundação para a Ciência e Tecnologia, através de uma bolsa de doutoramento cedida pelo Governo Português (Ref.: SFRH/BD/36812/2007), e pela “Rufford Small Grant for Nature Conservation”, que forneceu financiamento adicional para o trabalho de campo (“The impact of changing agricultural practices on the endemic birds of Sao Tome” - Ref.: 50.04.09). A “BirdLife International São Tomé and Príncipe Initiative” foi financiada pela “BirdLife’s Preventing Extinctions Programme”, através da família Prentice no âmbito da “BirdLife’s Species Champion Programme”, pela “Royal Society for the Protection of Birds”, pela “Disney Worldwide Conservation Fund”, pela “U.S. Fish and Wildlife Service Critically Endangered Animals Conservation Fund” (AFR-1411 - F14AP00529), pela “Mohammed bin Zayed Species Conservation Fund” (Project number 13256311) e pela “Waterbird Society Kushlan Research Grant”.
Quero ainda agradecer a toda a equipa de trabalho de campo da Associação Monte Pico que esteve envolvida na recolha de dados, nomeadamente Gabriel Cabinda, Ricardo Fonseca, Gabriel Oquiongo, Joel Oquiongo, Sedney Samba, Aristides Santana, Estevão Soares, Nelson Solé e Leonel Viegas. Este trabalho não teria sido possível sem a coordenação do Hugo Sampaio, da Sociedade Portuguesa para o Estudo das Aves (SPEA), ou sem o apoio institucional e empenho pessoal de Luís Costa (SPEA) e de Alice Ward-Francis (“Royal Society for the Protection of Birds” - RSPB), a quem agradecemos igualmente a disponibilização de dados. Finalmente, um agradecimento especial a Graeme M. Buchanan, pelas orientações e pelo apoio no planeamento experimental deste trabalho.
Agradeço também à Associação Monte Pico, pelo alojamento durante a minha estadia em São Tomé. Gostaria também de agradecer a todos os que contribuíram para o “Plano de acção internacional para a conservação das espécies de aves Criticamente em Perigo de São Tomé”, especialmente à Direção Geral do Ambiente, ao Parque Natural do Obô de São Tomé, à Direção das Florestas, à Associação dos Biólogos Santomenses e à associação MARAPA. Queria ainda agradecer em especial ao Eng. Arlindo Carvalho, Diretor Geral do Ambiente por apoiar as nossas atividades em São Tomé. O trabalho de campo não teria sido possível sem a ajuda de Silvino Dias, José Malé, Filipe Santiago, Lidiney e inúmeros outros santomenses. Uma dedicação especial para "Dakubala". Agradecemos a António Alberto, Nuno Barros, Mariana Carvalho, Martin Dallimer, Hugulay Maia, Stuart Marsden, Martim Melo, Fábio Olmos e Longtong Turshak por partilharem todas as suas observações.
Quero agradecer a Teotónio Soares pela disponibilidade e ajuda na construção dos loops para o script dos modelos lineares generalizados.
Não posso deixar de agradecer a todas as pessoas que conheci em São Tomé. Obrigada Nity e Estevão por terem sido os melhores ajudantes de campo. Aos dois, obrigada por terem respondido às minhas perguntas, por terem sempre confiado em mim atrás do volante do nosso táxi (nem eu confiaria!), por terem esperado sempre por mim em todas as nossas escaladas intermináveis. Obrigada por me terem dado a conhecer todas as paisagens incríveis de São Tomé. Obrigada Lucy por nos teres recebido em tua casa, por nos tratares praticamente como filhas quando não era tua obrigação, por teres sido para mim a minha família longe de casa. Nunca conseguirei agradecer-te o suficiente tudo o que fizeste. Obrigada Gégé por todas as conversas, por todos os risos e gargalhadas, por todos os cafés e bolachas, por todas as caminhadas e passeios pela cidade. Obrigada por teres sido um grande amigo quando eu mais precisava. Obrigada Adilécio por toda a ajuda com o carro, por vires sempre ao meu auxílio, ou porque o carro não andava, ou porque andava pouco, ou porque a mala não fechava (acho que praticamente tudo aconteceu àquele carro!). Obrigado Octávio por nos teres recebido em tua casa, ainda hoje consigo lembrar-me dos teus famosos cozinhados. Obrigado Filipe e Fica por me terem recebido de braços abertos e terem sempre mil e uma histórias para contar. Obrigada Mito e Sá também por me terem acolhido, por me mostrarem Emolve e por todos os jantares à luz das velas cheios de gargalhadas e boa disposição. Obrigada Juary, Gabi, Leonel, Catoninho, Lito, Lau, e todos os outros que me ajudaram e tornaram a minha estadia em São Tomé uma das melhores experiências que até hoje vivi. Quero agradecer aos meus pais, à minha irmã Rita e, também, às minhas duas avós por o apoio e companhia ao longo deste ano (particularmente difícil!). Também, quero agradecer ao Afonso por ter estado sempre lá, por ter aturado todas as minhas longas conversas sobre “bichos” (mesmo quando já não conseguia ouvir mais!). Obrigada por seres quem és e por acreditares sempre em mim, mesmo quando já nem eu acredito.
Obrigada a todos os meus companheiros e amigos pertencentes à “team cócós”. Obrigada Rita (e Zeus, o melhor cão do mundo!), Manel, Catarina Vegy, Cátia, Catarina Vet, Marvel por toda as aventuras ao longo deste ano (e que aventuras…desde atolar carros a perseguir assassinos em série!). Em especial, quero agradecer ao Professor Francisco Petrucci-Fonseca, protagonista de grande parte das nossas aventuras, por me ter dado a oportunidade de conhecer o que são talvez as serras mais bonitas de Portugal!
Obrigada a todos os meus amigos e colegas que me ajudaram e apoiaram ao longo deste ano. Em especial, um grande obrigado à Martina e à Bárbara por toda a companhia durante este longo ano e, principalmente, durante a nossa aventura de dois meses em São Tomé. Foi difícil mas não a trocava por nada, ou escolhia outras pessoas para irem comigo!
RESUMO ALARGADO
O Homem tem vindo a alterar a ecologia do planeta, influenciando a distribuição das espécies e o funcionamento dos ecossistemas. A comunidade científica tem dedicado muita atenção ao estudo do impacto das atividades humanas na biodiversidade, uma vez que estas são largamente tidas como responsáveis pela atual crise da perda de biodiversidade. Apesar da dificuldade em determinar com exatidão os processos envolvidos, sabe-se que o aumento da população humana tem tido diversos impactos negativos sobre os ecossistemas naturais. Há então necessidade de definir prioridades globais de conservação, começando pela identificação das principais ameaças, como a alteração antropogénica dos usos do solo. As florestas estão entre os ecossistemas terrestres mais ricos e também mais ameaçados, sendo que nas últimas décadas a pressão humana tem vindo a aumentar sobretudo nas florestas tropicais, estando muitas das suas espécies entre as mais ameaçadas do mundo.
A ocupação pelo Homem é sinónimo de fortes alterações na paisagem, tanto nos continentes como em ilhas. No entanto, as ilhas tendem a possuir ecossistemas mais sensíveis, ricos em espécies endémicas, que são particularmente vulneráveis à extinção. Posto isto, assumem uma elevada importância na preservação da biodiversidade, principalmente dada a taxa de alteração do uso do solo ser mais elevada nas ilhas do que nos continentes.
São Tomé é uma pequena ilha oceânica situada no Golfo da Guiné, África Central, a cerca de 255 km do continente. De origem vulcânica, possui uma topografia acidentada constituída por encostas de declive acentuado e vales encaixados, com rios pontuados por grandes cascatas. Nas zonas costeiras ocorrem estuários e mangais. Esta topografia explica o gradiente climático, caracterizado por elevados níveis de humidade e chuvas frequentes trazidas pelos ventos fortes do sudoeste da ilha, que contrastam com o nordeste semiárido. O forte gradiente climático tem vindo a moldar a distribuição dos ecossistemas da ilha, mas a paisagem originalmente dominada por floresta tem sofrido alterações desde a colonização humana, que teve início no final do século XV pelos Portugueses. As zonas planas de baixa altitude são as mais intervencionadas, sendo constituídas maioritariamente por áreas não florestadas, tais como savanas e áreas cultivadas. As florestas de baixa altitude foram substituídas por plantações de sombra com árvores exóticas, como cafeeiro, cacaueiro e palmeiras. A floresta nativa mais bem preservada está hoje restrita às áreas montanhosas no centro e sudoeste da ilha, rodeada por floresta secundária, que resultou sobretudo da regeneração de plantações de sombra abandonadas. Apesar da paisagem humanizada, São Tomé mantem uma flora e fauna muito diversas com um número muito elevado de endemismos. As suas florestas têm um enorme interesse para a conservação, tendo sido identificadas como as terceiras mais importantes no mundo para a conservação de espécies de aves florestais.
Esta tese está dividida em dois capítulos com objetivos distintos, ambos relacionados com a diversidade das aves de São Tomé. No primeiro capítulo, o objetivo principal é compreender como se distribuem as aves ao longo da ilha, tendo como objetivos específicos: (1) identificar os principais determinantes da distribuição das espécies de aves de São Tomé; (2) compreender como se relaciona o endemismo com as respostas das espécies às variáveis ambientais; (3) analisar a relação entre as guildes tróficas e a resposta das espécies às variáveis ambientais. No final, explorámos a relação entre as respostas das espécies e os fatores determinantes da sua distribuição, dando um foco especial às espécies endémicas e ameaçadas. Neste estudo foram realizados pontos de contagem de aves com duração de 10 minutos, onde foram registadas todas as espécies de aves. O período de amostragem foi de Janeiro a Março de 2017, tendo sido a amostragem direcionada para as zonas não florestadas e de plantação de sombra, bem como algumas zonas de floresta secundária. Estas observações foram agrupadas com observações ocasionais e sistemáticas de estudos anteriores, que se tinham focado sobretudo nas áreas
florestais, atingindo um total de 3056 pontos amostrados em toda a ilha, onde foram registadas de forma inequívoca 34 espécies de aves terrestres. Algumas variáveis ambientais, tais como o tipo de uso do solo, a topografia, a precipitação, o declive, a altitude, a acessibilidade e a distância à costa, foram mapeadas e utilizadas na construção dos modelos lineares generalizados para cada espécie. A ordenação dos melhores modelos de distribuição potencial de cada espécie permitiu explorar a resposta de cada espécie às variáveis ambientais. Uma análise de correspondência detrended foi realizada para avaliar a relação entre endemismo, guildes tróficas e variáveis ambientais. O tipo de uso do solo foi identificado como a variável mais importante para explicar a presença das espécies: as espécies endémicas tendem a ocorrer preferencialmente na floresta, em zonas mais remotas, de elevada altitude e precipitação, por sua vez as não endémicas preferem zonas não florestadas e mais humanizadas. A paisagem altamente florestada de São Tomé permite, de uma forma geral, que haja uma dominância das espécies endémicas na ilha. Muitas espécies endémicas estão ameaçadas, o que salienta a necessidade de proteger os habitats florestais. Como tal, propomos um incremento da matriz florestal na paisagem, através da proteção da floresta nativa remanescente e da expansão da floresta secundária, para a conservação das aves de São Tomé.
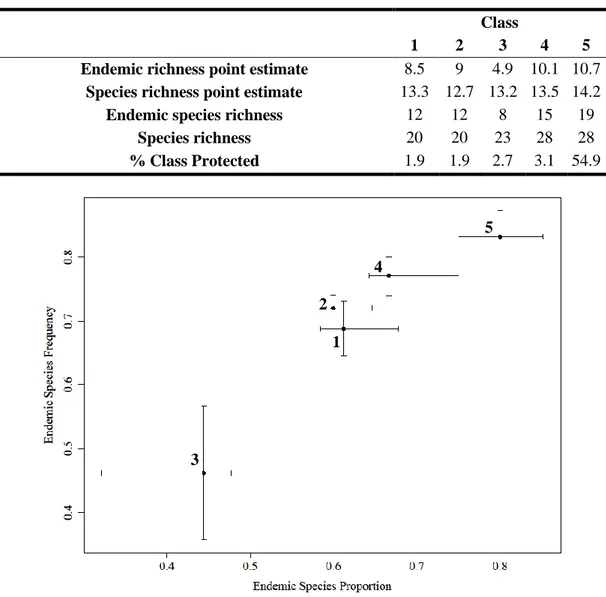
No segundo capítulo, o objetivo principal é avaliar se o Parque Natural do Obô (PNO) inclui uma representação adequada da diversidade de aves da ilha. Como tal, foi modelada a riqueza específica e a composição das aves, dando especial atenção à distribuição de espécies endémicas e não endémicas. A distribuição da diversidade de aves foi comparada com os limites da área protegida. Foi construída uma base de dados com os pontos de contagem de aves de estudos anteriores, que foi complementada por pontos adicionais realizados entre Janeiro e Março de 2017. Os pontos de contagem pertencentes à mesma quadrícula de 1x1 km foram agrupados, criando conjuntos de cinco pontos de contagem por quadrícula num total de 187 quadrículas, onde 36 espécies de aves terrestres foram registadas. Foram utilizadas seis variáveis ambientais, tendo sido excluídas a rugosidade e a acessibilidade, para modelar e mapear a riqueza específica total, das espécies endémicas e não endémicas, bem como a composição da comunidade. Os resultados mostram que o número de espécies endémicas diminui nos habitats mais humanizados, onde aumenta o de espécies não endémicas. O PNO não está a proteger as comunidades mais ricas em aves, mas aquelas que têm mais aves endémicas, que ocorrem nas florestas mais bem preservadas. Definidos com base na distribuição dos habitats e da população humana, os limites do parque permitem a proteção das espécies endémicas ameaçadas, indiscutivelmente as de maior interesse conservacionista. As florestas secundárias atuam como zona de transição para as zonas mais humanizadas, protegendo as espécies endémicas das diversas ameaças antropogénicas. Deve ser realizada uma revisão do zonamento do parque, de modo a integrar o atual conhecimento da distribuição das espécies.
Este estudo permitiu aumentar o conhecimento atual sobre a distribuição das aves de São Tomé, salientando a importância do tipo de uso do solo para a ocorrência das espécies e dando, pela primeira vez, uma perspetiva sobre a distribuição da riqueza e da composição das comunidades de aves ao longo da ilha. Esta informação deve ser utilizada na definição de estratégias de conservação e monitorização. No entanto, é necessário aprofundar o conhecimento sobre a distribuição de cada espécie, ao longo do ano e a escalas espaciais mais pormenorizadas, por forma a compreender melhor a resposta de cada espécie à degradação florestal. Destacamos ainda a importância de quantificar o impacto de outras ameaças, como a caça e as espécies introduzidas. Toda esta informação irá permitir definir ações prioritárias de conservação para espécies-alvo, adequadas às necessidades ecológicas de cada espécie, o que é especialmente importante no caso das espécies mais ameaçadas como a galinhola Bostrychia
bocagei, o picanço Lanius newtoni e o anjoló Neospiza concolor.
Palavras-chave: endemismo; guilde trófica; Parque Natural do Obô; espécies ameaçadas; riqueza específica
ABSTRACT
Human actions are rapidly changing ecosystems all over the world. Anthropogenic land use change affects the structure and functioning of ecosystems, leading to irreversible biodiversity losses. Understanding how human actions influence biodiversity is therefore key to prevent further biodiversity loss. Tropical forests are among the most diverse and threatened ecosystems, and the increasing human pressure, high number of threatened species and major habitat loss calls for conservation actions.
São Tomé is a small oceanic island located in the Gulf of Guinea, Central Africa. Despite the human-dominated landscape, this island maintains a high biodiversity, rich in endemic species, and its forests are of great conservation value. This study has the main goals of:
- Understanding how bird species are distributed throughout the island. Occasional and systematic observations were gathered from previous studies and complemented by additional 10-minute point counts. A total of 3056 sampling locations were used to understand the distribution of 34 terrestrial bird species. Species-specific generalized linear models and detrended correspondence analysis based on presence-absence, were used to explore the links between endemism, feeding guilds and environmental variables. Land use was the most important variable to explain bird species occurrence. The endemics tended to prefer forests located in remote, wetter areas, on rugged terrain and at higher altitudes, while the non-endemics favoured the drier flat lowlands, in more accessible locations and devoid of forest. The change in bird species assemblage from forest endemics to open habitat non-endemic granivores is clearly a result of the land use intensification gradient. The current overall dominance of endemic species across the island is maintained by São Tomé’s forest-dominated landscape. The dependency of endemics on forest highlights the urgent need for their protection. Based on these results, we suggest that protecting remaining native forests and expanding secondary forests will improve landscape matrix and contribute to the survival of the endemic-rich island avifauna worldwide.
- Assessing how the São Tomé Obô Natural Park (STONP) represents the avifauna of the island. The boundaries of the STONP were defined in 2006, based on the distribution of native ecosystems and of the human population. We compared them to the distribution of bird diversity, by modelling species richness and composition. We used systematic observations from previous studies supplemented by additional bird counts. A total of 187 1x1 km quadrats were sampled by five 10-minute point counts each. Thirty-six terrestrial bird species were identified unambiguously and considered for analyses. The proportion of endemic species decreased along the land use intensification gradient. The STONP did not protect the most species-rich bird assemblages, but included those that were richest in endemics, the best-preserved forests. Thus, the STONP is focusing on the protection of endemic threatened birds, which arguably have the highest global conservation interest. The secondary forests surrounding the park act as a transition zone to areas with more intensive land use types, hence protecting it from pervasive threats. We suggest the zonation of STONP is revised, using the same factors considered for the delimitation of the protected area and the current knowledge on species distribution. This study reveals that protecting well-preserved natural areas with low human density might be a good proxy to identify areas of high conservation interest, when there is little information on the distribution of the multiple components of biodiversity.
Keyword: community ecology; species distribution modelling; endemism; feeding guild; threatened species
TABLE OF CONTENTS
GENERAL INTRODUCTION ... 1
CHAPTER 1: The role of natural gradients and ecosystem humanization in determining the distribution of bird species in São Tomé ... 5
INTRODUCTION ... 5 METHODS ... 6 Study Area ... 6 Data Compilation ... 7 Field Methods ... 7 Sampling design ... 7 Bird sampling ... 7
Characterizing environmental variables ... 8
Data Analysis ... 9
Exploratory analysis ... 9
Generalized linear models ... 9
Relative variable importance ... 10
Response to environmental variables ... 10
RESULTS ... 10
Relative variable importance ... 10
Response of endemic and non-endemic species to environmental variables ... 11
Feeding guilds response to environmental variables ... 14
Species land use type preferences ... 16
DISCUSSION ... 17
Determinants of bird species distribution ... 17
Differential response of endemic and non-endemic bird species ... 17
Differential response of bird species based on feeding guilds ... 18
Consequences of land use intensification to the endemic-rich avifauna of São Tomé ... 18
CHAPTER 2: Is the existing protected network adequate for the conservation of the endemic-rich avifauna of São Tomé Island? ... 20
INTRODUCTION ... 20
METHODS ... 21
Study Area ... 21
Data Compilation ... 22
Sampling design ... 22
Bird sampling ... 22
Characterizing environmental variables ... 23
Data Analysis ... 24
Exploratory analysis ... 24
Generalized linear models ... 24
Generalized dissimilarity modelling... 25
Generalized dissimilarity model categorization ... 25
Assessing the adequacy of the Obô Natural Park to represent São Tomé bird diversity ... 26
RESULTS ... 26
Modelling bird species richness ... 26
Bird species compositional dissimilarity ... 28
Is the São Tomé Obô Natural Park adequate to protect the island’s avifauna? ... 29
DISCUSSION ... 31
Contrasting responses of endemic and non-endemic species to the environment ... 31
Species assemblages vary mostly in response to habitat humanization ... 32
Is the São Tomé Obô Natural Park adequate to protect the island’s bird diversity? ... 33
Final remarks ... 33
FINAL CONSIDERATIONS ... 35
REFERENCES ... 36
SUPPLEMENTARY MATERIALS ... 45
SECTION I: Environmental Variables ... 45
SECTION II: São Tomé Bird Species ... 60
SECTION III: Binomial Generalized Linear Models ... 61
SECTION IV: Proportion of species occurrence per land use type ... 67
SECTION V: Exploratory analysis for species richness and composition modelling... 68
SECTION VI: Poisson Generalized Linear Models ... 70
SECTION VII: Generalized Dissimilarity Modelling ... 73
LIST OF TABLES
Table 1.1. Response of endemic (E) and non-endemic (N), and of distinct feeding guilds (omnivores - O, granivores
- G, frugivores – F, and carnivores – C) to environmental variables………12
Table 2.1. Species richness and endemic species richness estimated for each average point inside 1x1 quadrats, called, respectively, species and endemic richness point estimate……….30
Table S1. (Section I – Supp. Materials) Environmental variables description……….45
Table S2. (Section I – Supp. Materials) Environmental raster’s characteristics………...46
Table S3. (Section II – Supp. Materials) Bird species’ characteristics………60
Table S4. (Section III – Supp. Materials) Validation of the best multivariable model………..61
Table S5. (Section III – Supp. Materials) Relative variable importance (RVI)………...…62
Table S6. (Section III – Supp. Materials) Single-variable model coefficients. ………...63
Table S7. (Section III – Supp. Materials) Kruskal-Wallis rank test to analyse the difference in environmental variables between endemic and non-endemic species, as well as among feeding guilds. ……….64
Table S8. (Section IV – Supp. Materials) Proportion of species occurrence per land use type and topography class………...67
Table S9. (Section V – Supp. Materials) Bird species’ characteristics………...68
Table S10. (Section VI – Supp. Materials) Validation of the best model………70
Table S11. (Section VI – Supp. Materials) Species richness and environmental variables………...72
Table S12. (Section VII – Supp. Materials) Significance test of GDM model………73
Table S13. (Section VII – Supp. Materials) Significance test for each variable in GDM model………....74
Table S14. (Section VII – Supp. Materials) Importance of each predictor variable……….75
LIST OF FIGURES
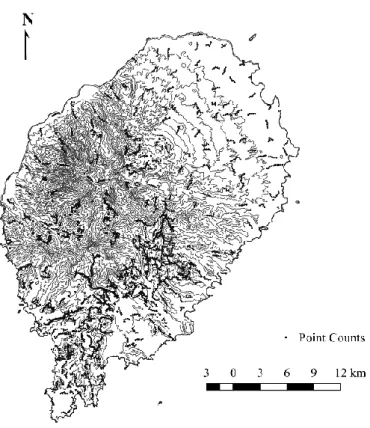
Figure 1.1. Location of sampling point counts and occasional observations (n = 3056) in São Tomé Island………...8Figure 1.2. Relative variable importance (RVI) of each environmental variable for each bird species generalized linear model………..11
Figure 1.3. Response of endemic (E) and non-endemic (N) species to environmental variables………13
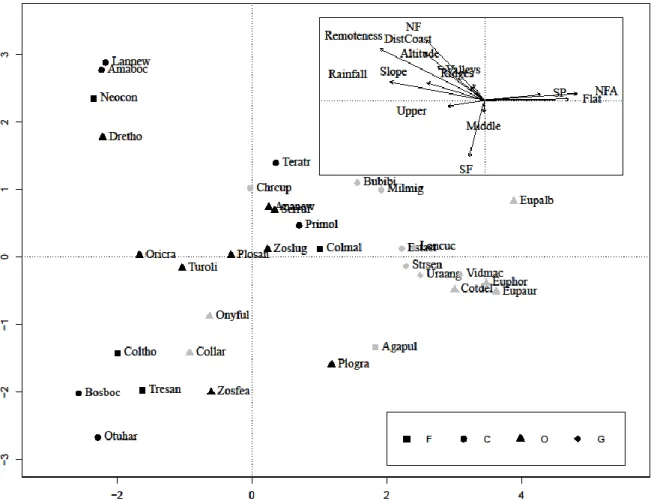
Figure 1.4. Detrended Correspondence Analysis (DCA) showing the relationship between endemism, feeding guilds and environmental variables………...14
Figure 1.5. Feeding guild (omnivores - O, granivores - G, frugivores – F, and carnivores - C) response to environmental variables……….15
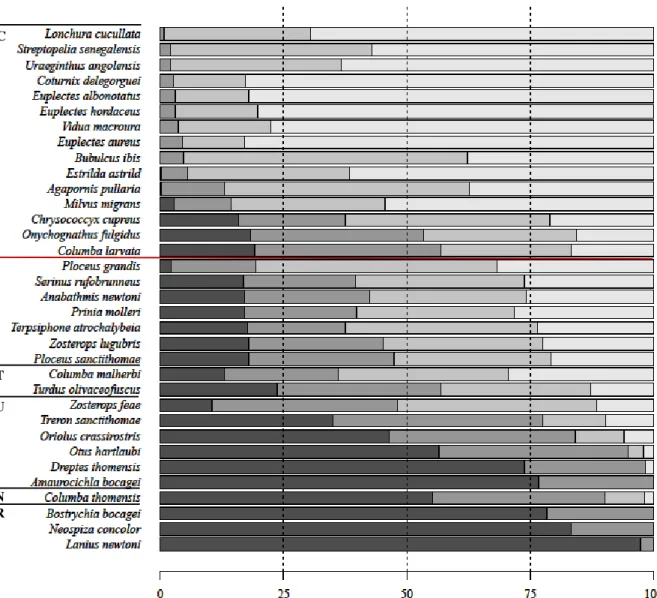
Figure 1.6. Proportion of occurrence of each species by land use types………..16
Figure 2.1. São Tomé Island sampling locations………..23
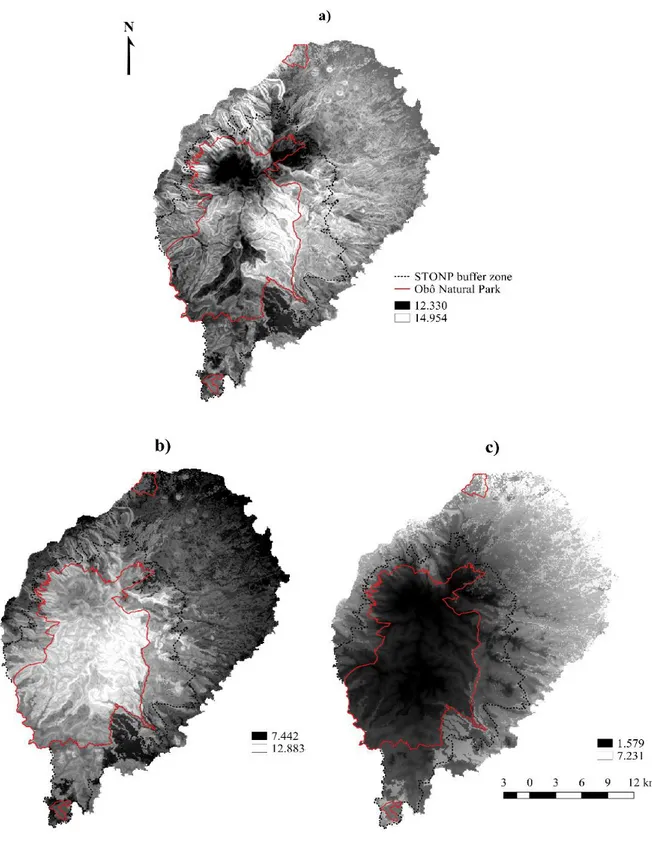
Figure 2.2. Predictive maps of (a) total species richness, (b) endemic species richness and (c) non-endemic species richness, shown in contrast to the boundaries of the Obô Natural Park and buffer zone………27
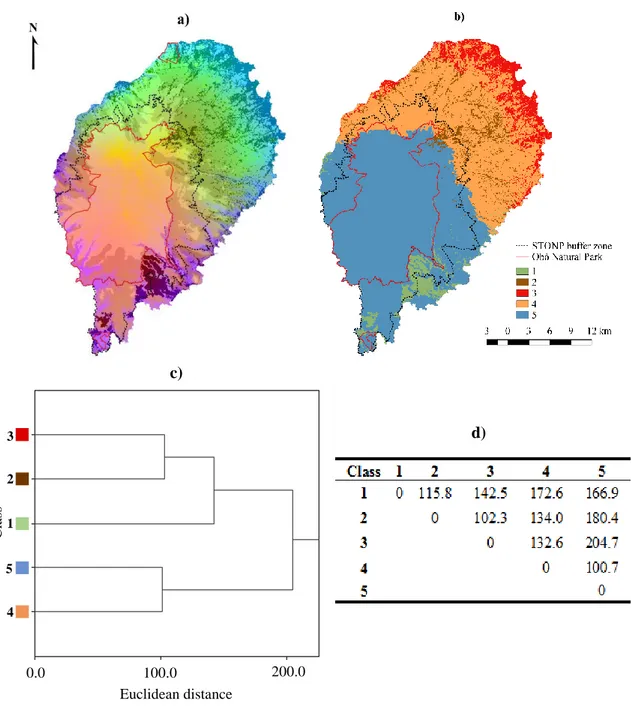
Figure 2.3. (a) Continuous and (b) categorical composition dissimilarity maps, as obtained from generalized dissimilarity modelling (GDM)………..28
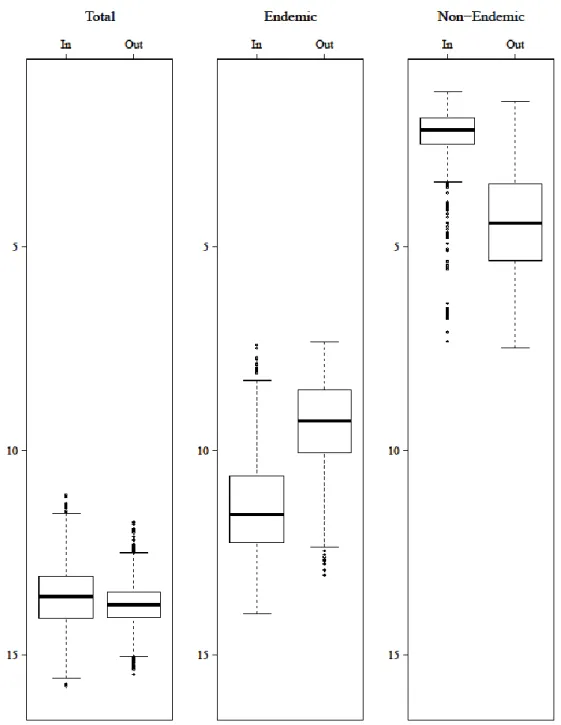
Figure 2.4. Total, endemic and non-endemic species richness inside (In) and outside (Out) Obô Natural Park…….29
Figure 2.5. Proportion of endemic species and frequency of endemic species for each GDM class (1 to 5)………...30
Figure S1. (Section I – Supp. Materials) Altitude in meters………...47
Figure S2. (Section I – Supp. Materials) Ruggedness………...48
Figure S3. (Section I – Supp. Materials) Slope in degrees……….49
Figure S4. (Section I – Supp. Materials) Distance to coast line in degrees………..50
Figure S5. (Section I – Supp. Materials) Separation of flat plain areas and middle slope areas……….51
Figure S6. (Section I – Supp. Materials) Transforming continuous Topographic Position Index in a categorical variable……….52
Figure S8. (Section I – Supp. Materials) Building remoteness index……….54 Figure S9. (Section I – Supp. Materials) Remoteness Index……….55 Figure S10. (Section I – Supp. Materials) Rainfall in millimetres………..56 Figure S11. (Section I – Supp. Materials) Land use map created by S. Mikulane (resolution of 10x10 meters)…….57 Figure S12. (Section I – Supp. Materials) Land use………..58 Figure S13. (Section I – Supp. Materials) Correlogram between environmental variables ...59 Figure S14. (Section III – Supp. Materials) Relative variable importance (RVI) of each continuous environmental variable……….65 Figure S15. (Section III – Supp. Materials) Relative variable importance (RVI) of each continuous environmental variable in endemic and non-endemic species………..65 Figure S16. (Section III – Supp. Materials) Relative variable importance (RVI) of each continuous environmental variable in every feeding guild species group………...66 Figure S17. (Section V – Supp. Materials) Correlogram between environmental variables and response variables...69 Figure S18. (Section VI – Supp. Materials) Pearson and Deviance Residuals………71 Figure S19. (Section VII – Supp. Materials) Overall model fit in explaining the observed dissimilarities………….73 Figure S20. (Section VII – Supp. Materials) K-fold cross-validation of GDM………...74 Figure S21. (Section VII – Supp. Materials) Response curves of each predictor variable………76
LIST OF ABBREVIATIONS AND ACRONYMS
E Endemics N Non-endemics O Omnivores G Granivores F Frugivores C Carnivores NF Native forest SF Secondary forest SP Shade plantation
NFA Non-forested areas
F Flat areas
V Valleys and deep valleys M, Middle Middle slope areas U, Upper Upper slope areas
R Ridges
Amaboc Amaurocichla bocagei, São Tomé Short-tail
Ananew Anabathmis newtonii, São Tomé Sunbird
Bosboc Bostrychia bocagei, Dwarf Ibis
Colmal Columba malherbii, São Tomé Bronze-napped Pigeon
Coltho Columba thomensis, São Tomé Maroon Pigeon
Dretho Dreptes thomensis, Giant Sunbird
Lannew Lanius newtoni, São Tomé Fiscal
Neocon Neospiza concolor, São Tomé Grosbeak
Oricra Oriolus crassirostris, São Tomé Oriole
Otuhar Otus hartlaubi, São Tomé Scops Owl
Plosan Ploceus sanctithomae, São Tomé Weaver
Primol Prinia molleri, São Tomé Prinia
Serruf Serinus rufobrunneus, (São Tomé) Príncipe Seed-eater
Teratr Terpsiphone atrochalybeia, São Tomé Paradise Flycatcher
Tresan Treron sanctithomae, São Tomé Green Pigeon
Turoli Turdus olivaceofuscus, São Tomé Thrush
Zosfea Zosterops feae, São Tomé White-eye
Zoslug Zosterops lugubris, São Tomé Speirops
Agapul Agapornis pullaria, Red-headed Lovebird
Bubibi Bubulcus ibis, Cattle Egret
Chrcup Chrysococcyx cupreus, Emerald Cuckoo
Collar Columba larvata, São Tomé Cinnamon Dove
Cotdel Coturnix delegorguei, Harlequin Quail
Estast Estrilda astrild, Common Waxbill
Eupalb Euplectes albonotatus, White-winged Widowbird
Eupaur Euplectes aureus, Golden-backed Bishop
Euphor Euplectes hordeaceus, Fire-crowned Bishop
Loncuc Lonchura cucullata, Bronze Mannikin
Milmig Milvus migrans, Yellow-billed Kite
Onyful Onychognathus fulgidus, São Tomé Chestnut-winged Starling
Strsen Streptopelia senegalensis, Laughing Dove
Uraang Uraeginthus angolensis, Southern Cordon-bleu
Vidmac Vidua macroura, Pin-tailed Whydah
DistCoast Distance to coast
SR Species richness
ESR Endemic species richness NSR Non-endemic species richness STONP São Tomé Obô Natural Park PNO Parque Natural do Obô
GENERAL INTRODUCTION
Human population is a major threat to biodiversity
Humans have been shaping the environment all over the planet, influencing the distribution of species and functioning of ecosystems. Many studies have associated human activities to the current crisis of biodiversity loss (Balmford & Bond 2005). Defining and measuring biodiversity is a complex and difficult task, therefore studying how human actions affect biodiversity is a major challenge. Additionally, biodiversity threats are unevenly distributed throughout the world, making it difficult to allocate conservation efforts. The urgent need to establish global conservation priorities has been a hot topic between conservationists (Brooks et al. 2006). Myers et al. (2000) identified 25 “biodiversity hotspots”, characterized by having a high concentration of endemic species and also great levels of habitat loss. Anthropogenic land use change is considered a main threat to species across all taxonomic groups (Luck 2007). Tropical forests, known to have both high species diversity and human pressure, are rapidly being converted for agriculture, timber production and other uses, generating human-dominated landscapes and leading to forest degradation and destruction (Gardner et al. 2009). Habitat loss is considered to be one of the main reasons for the extinction of many species in the past decades (Sodhi et al. 2004; Stork 2010; Szabo et al. 2012). Many extinct species were island-endemics and because the projected rate for land-cover changes in islands is expected to increase, these fragile ecosystems are a growing global concern for conservationists (Manne et al. 1999). Many believe that given their conservation risks, smaller areas and high endemic species richness, islands could offer high returns for species conservation efforts, and therefore should be a high priority in global biodiversity conservation (Johnson & Stattersfield 1990).
São Tomé Island as a study case
São Tomé is an oceanic island, which is an excellent model to study the factors influencing species distribution, as well the adequacy of protected areas to represent biodiversity. It is an 857 km2 island,
holding a remarkable biodiversity with many endemic species and a wide gradient of land use intensification.
Together with Príncipe, it constitutes the Democratic Republic of São Tomé and Príncipe, which is in the Gulf of Guinea, Central Africa. At about 255 km from mainland Africa, São Tomé is of volcanic origin, which explains its rugged topography composed of steep slopes, deep valleys and high ridges, up to 2024 meters above sea level at the São Tomé Peak (Salgueiro & Carvalho 2001). Rivers are intrinsically associated with these narrow valleys, creating multiple waterfalls. The water slows down near the ocean creating small estuaries occasionally with mangroves. In the north-east, the terrain is flatter, especially if compared to the centre and west of the island. This diverse topography explains the incredibly varied climate found in São Tomé. The high mountains are a barrier to the strong winds, bringing heavy rains and coming from the south-west of the island. Thus, the south-west is characterized by high levels of humidity, having an almost permanent cloud cover, frequent rains and an annual rainfall of over 6000 mm, while the north-east is much drier, some areas receiving less than 600 mm of rain each year (Tenreiro 1961). São Tomé’s climate is characterized by a wet season, which occurs for most of the year, and two drier seasons. The longer dry season, called “gravana”, starts in May and ends in September, being more evident in the north of the island, and corresponding to the coldest months of the year. The shorter dry season, the “gravanito”, lasts for a few weeks in January and February. The
strong altitudinal gradient influences the mean annual temperature; coastal areas can reach maximum mean annual temperatures of 25.5º C, while at higher altitudes it might be as low as 9º C (Silva 1958).
The strong climatic gradient has shaped the distribution of ecosystems throughout São Tomé. Having highly diverse landscapes with many different ecosystems, four land use types are usually recognized: non-forested areas, shade plantations, secondary forests and native forests (Jones & Tye 2006). Native forests are characterized by having a high density of native flora and few exotic species (e.g. Elaeis guineensis). Mangroves established along the lowest parts of the rivers and coastal lagoons can also be considered native forests. Exell (1944) defined three distinct rainforest types following the altitudinal gradient: lowland forests (up to 800 meters a.s.l), montane forests (between 800 and 1400 meters a.s.l.) and mist forests (above the 1400 meters a.s.l., along ridges of the central mountain range). Secondary forests appeared with the regeneration of abandoned shade plantations and with the intensive exploitation of timber, holding an assemblage poorer in forest species and with shade and fruit trees (e.g. breadfruit Artocarpus altilis, African nutmeg Pycnanthus angolensis). Shade plantations initially created as intensive monocultures by the Portuguese replaced most of the lower altitude forests. It is an agroforestry system composed mostly of exotic trees, such as cocoa Theobroma cacao, coffee Coffea sp. and coral trees Erythrina sp. (Salgueiro & Carvalho 2001). Nowadays, shade plantations have become more varied and produce many other crops, mostly for the internal market (banana Musa sp., cocoyam Colocasia sculenta and Xanthosoma sp., oil palm Elaeis guineensis, avocado Persea
americana, papaya Carica papaya). Non-forested land uses include active and resting agricultural areas
with different systems, such as monocultures of sugar cane Saccharum sp, coconut Cocos nucifera or oil palm, and artificial savannahs and smallholder horticultures (Diniz et al. 2002).
The human occupation of São Tomé started in the late 15th century, after the Portuguese discovered
the island, allegedly uninhabited and entirely covered by forest. Since then, the dried coastal lowland forests have suffered the most, being first cleared for sugar cane (Tenreiro 1961). During the 19th and
20th century, extensive cocoa and coffee plantations were grown in shade plantations, in large
agricultural plantation systems, known as “roças”, further decreasing the area covered by native forests (Oliveira 1993; Frynas 2003). Nowadays, many shade plantations rely on medium and smallholdings that produce many subsistence products besides the main export crops. Swidden agriculture appeared to meet the demand for horticultural foods, expanding in forest borders and replacing abandoned shade plantations, being therefore included in non-forested land uses (Eyzaguirre 1986; Albuquerque et al. 2008). In the centre and south-west of the island a large patch of well-preserved native forest remains, nowadays enclosed by secondary forest, which in turn is surrounded by active shade plantations mixed with several non-forested land uses (Jones et al. 1991; Diniz et al. 2002).
São Tomé has an incredible diverse flora and fauna. The right amount of isolation allowed many species to evolve in environments distinct from those found in the mainland (Miller et al. 2012). São Tomé and Príncipe hold 28 endemic bird species in an area little over 1000 km2 (Melo 2006). Out of 45
resident terrestrial species, São Tomé alone has 17 single-island endemics, 3 endemics to the Gulf of the Guinea oceanic islands (Annobón, São Tomé and Príncipe) and 8 widespread species represented in the island by an endemic subspecies (Jones & Tye 2006). As is often the case in other islands, some species are larger than their mainland relatives. That is the case of the Giant Sunbird Dreptes thomensis, the Giant Weaver Ploceus grandis, the São Tomé Grosbeak Neospiza concolor, the São Tomé Speirops
Zosterops lugubris and the São Tomé Thrush Turdus olivaceofuscus. However, a few species, like the
Dwarf Ibis Bostrychia bocagei, become smaller (Melo 2006; Melo et al. 2017). The lack of natural predators also made some species tame, such as the São Tomé Green Pigeon Treron sanctithomae, the São Tomé Maroon Pigeon Columba thomensis and the Dwarf Ibis.
São Tomé is in a “biodiversity hotspot” and about 23.3% of its territory is included in Important Bird Areas (Myers et al. 2000; Fishpool & Evans 2001). Its forests are of great conservation interest, belonging to one of Earth’s biological ecoregions, named Gulf of Guinea Islands (Olson & Dinerstein
1998). Also, the forests were identified as the third most important in the world for forest bird species conservation (Buchanan et al. 2011). The long history of human occupation has led to habitat destruction and degradation, especially in the lower altitude forests, which were mostly converted to shade plantations. Endemic species have a long relationship with native forest, and many are dependent on these habitats (Rocha 2008; de Lima 2012). This way, the destruction or transformation of these forests might make them into unsuitable habitats. Apart from land use change, the introduction of species and direct exploitation are the main threats to São Tomé avifauna (Jones et al. 1991; de Lima 2012). Like in many oceanic islands, free of native predators of birds, introduced land mammals like rats, mice, dogs, cats, pigs, among others, become a serious threat to native bird species (Johnson & Stattersfield 1990; Dutton 1994; Blackburn et al. 2004). Three endemic bird species are considered Critically Endangered, the Dwarf Ibis, the São Tomé Fiscal Lanius newtoni and the São Tomé Grosbeak, the São Tomé Maroon Pigeon is Endangered, while six other endemic bird species are Vulnerable, two are Near Threatened and eight are Low Concern (IUCN 2017).
To protect both native fauna and flora species, as well as their natural habitats, from human activities, the São Tomé Obô Natural Park (STONP) was created in 2006, covering 295 km2 (Direcção
Geral do Ambiente 2006). This protected area was born under the umbrella of the “Ecosystemes Forestiers en Afrique Centrale” (ECOFAC) program, which started in 1992, funded by the European Commission, to encourage the conservation and sustainable use of forests in Central Africa. A buffer zone was also envisaged, but never official. The STONP action and management plan were first created in 2008, and revised in 2014 (Albuquerque et al. 2008), but implementation remains weak (de Lima et al. 2015).
Thesis scope
This thesis has two main goals, both related to understanding the bird diversity in São Tomé. In the first chapter, we explore bird species distribution and their responses to several environmental variables, using generalized linear models (GLMs) and paying close attention to the differences between endemic and non-endemic species, as well as between feeding guilds. Predictive distribution models are used to understand where species occur, which is essential to understand ecological requirements, as well as for conservation and population management (Guisan & Zimmermann 2000; Rushton et al. 2004). Logistic regressions are frequently used by ecologists to model species distribution, having gained a certain appeal because presence-absence data is easy to collect in the field. We considered vegetation, topographic, climatic and anthropogenic variables as potential predictors in logistic models, improving our understanding of which factors condition species occurrence (Seoane et al. 2003; Thuiller et al. 2004).
In the second chapter, we model bird species richness and composition patterns to assess if the STONP adequately covers the island’s diverse avifauna. Three generalized linear models with poisson distribution were created to explain total, endemic and non-endemic species richness (Guisan & Zimmermann 2000), while generalized dissimilarity modelling (GDM) was used to map composition patterns (Ferrier et al. 2007). GDM is a novel statistical technique that analyzes and predicts spatial patterns of turnover in community composition (beta diversity). Being an extension of matrix regression, it is designed specifically to accommodate two types of nonlinearity commonly encountered in large-scaled ecological data sets: (1) the curvilinear relationship between increasing ecological distance, and observed compositional dissimilarity, between sites; and (2) the variation in the rate of compositional turnover at different positions along environmental gradients (Ferrier et al. 2007; Arponen et al. 2008). In short, this approach compares community composition and environmental variables at pairs of sites to predict compositional difference as a function of environmental difference, extrapolating the prediction beyond surveyed sites. The resulting models give a spatially continuous prediction of
turnover, and thus of the spatial structure of diversity (Fitzpatrick et al. 2013; Brown et al. 2014). Predictive distribution maps are used nowadays to design protected areas, evaluate human impacts on biodiversity and test biogeographical hypotheses (Seoane et al. 2004). In this study, maps describing species richness and composition patterns were built to evaluate if the STONP is covering relevant components of the bird assemblage in São Tomé.
CHAPTER 1:
The role of natural gradients and ecosystem humanization in
determining the distribution of bird species in São Tomé
Abstract: Anthropogenic land use change is the main driver of the ongoing biodiversity crisis. Understanding how species respond to land use changes is thus key to minimize the current species extinction rate. São Tomé is a small oceanic island, where forest degradation is a main threat to the endemic-rich avifauna. To preserve this invaluable avifauna, we tried to understand how bird species are distributed throughout the island. We gathered occasional and systematic observations from previous studies, which were later combined with additional 10-minute point counts, adding to a total of 2398 bird point counts and 658 occasional observations. Thirty-four terrestrial bird species were unambiguously identified and considered in subsequent analyses. Species-specific generalized linear models and detrended correspondence analysis based on presence-absence, were used to explore the links between endemism, feeding guilds and environmental variables. Land use was the most important variable to explain bird species occurrence. The endemics tended to prefer forests in wetter, rugged, higher altitude, and remote areas, while the non-endemics favoured flat lowland non-forested areas and shade plantations. São Tomé’s forest-dominated landscape ensures an overall dominance of endemic species, but a change in bird species assemblage from forest endemic species to open habitat non-endemic granivore species was found to be a result of the land use intensification gradient. Many of the forest endemics are threatened, highlighting the urgent need to protected forested habitats. We suggest landscape matrix improvement, through the protection of the remaining native forest and the expansion of secondary forest, as the most important conservation measure to ensure the future of the endemic-rich avifauna of the islands.
Keyword: endemism; feeding guild; generalized linear model; land use types; threatened species
INTRODUCTION
Understanding how animals and plants are distributed on Earth, in both space and time, is a challenging task, especially in our constantly changing planet. A wide range of factors, such as food availability, shelter, environmental abiotic factors (e.g. temperature, humidity), biotic interactions (e.g. competition, predation, mutualism, host-parasite interactions, facilitation), physical barriers (e.g. rivers, mountains), climate (e.g. global climate change), disturbances (e.g. fires, floods, pathogens), among many others, are listed to influence species distribution (Brown 1984; Lawton 1999; Mackey & Lindenmayer 2001; Thomas et al. 2004). All these factors interact at different spatial and temporal scales, imposing limits on species distribution which are expressed from local to global spatial scales.
Our understanding of species distribution started with qualitative analyses: observing and recording the relationship between species distributions and the physical environment. Today, numerical techniques are widely used for describing species distribution patterns and making predictions (Elith & Leathwick 2009). For example, species distribution models (SDMs), that combine observations of species occurrence or abundance with environmental variables, allow the prediction of species distributions across the landscape (Guisan & Zimmermann 2000; Rushton et al. 2004).
Human activities have been shaping ecosystems across the globe, especially by land use change that is known to alter ecosystem patterns and processes, as well as species distributions (Blair 1996; Cincotta et al. 2000). Anthropogenic land use changes have been considered a major driver of the
ongoing biodiversity crisis (Myers et al. 2000). Therefore, understanding species response to human-induced land use change is essential to guide conservation actions (Maestas et al. 2003; Benton et al. 2003; Chacea & Walsh 2006). Agricultural demand is by far the main cause for land use change (Phalan et al. 2011). Urban sprawling is also promoting the conversion of natural and even agricultural land, further reducing the availability of habitats for wildlife (Assandri et al. 2017). Both are predicted to continue growing in the nearby future. Land use change has consistently reduced overall habitat quality, increased ecosystems fragmentation, isolation and degradation, and promoted the introduction of exotic species (Cadenasso & Pickett 2001; Foley 2005; McKinney 2006; Stork 2010). A study conducted in the north-eastern Brazilian Amazonia showed plantations had a relatively impoverished amphibian and lizard communities, a frequently discussed consequence of land use change (Gardner et al. 2007). In tropical forests, where the species diversity and human pressure is higher, land use change is expected to cause great habitat loss (Sodhi et al. 2004; Walter et al. 2007; Gardner et al. 2009; Stork 2010; Szabo et al. 2012).
Local extinction of birds and mammals have been described as a consequence of anthropogenic land use change (Brooks et al. 1999; Sodhi et al. 2004; IUCN 2017). Extinctions have been far more frequent on islands than on continents (Manne et al. 1999). The unique flora and fauna found on insular ecosystems are extremely vulnerable to human actions, and with the increasing rate of land use change, these fragile ecosystems are becoming a growing global concern among conservationists.
This main goal of this study is to understand how bird species are distributed in response to natural and anthropogenic factors, using São Tomé, an endemic-rich oceanic island with a known land use intensification gradient, as an example (Melo 2006; Miller et al. 2012; de Lima et al. 2015). We focus on three specific goals: (1) identifying the key determinants of the distribution of bird species; (2) understanding how endemism relates to the response of bird species to environmental variables; and (3) analyse the relationship between feeding guilds and bird species response to environmental variables. We will also explore the relationship between key determinants and species response, paying special attention to endemic and threatened species.
METHODS Study Area
São Tomé, together with the neighbouring island of Príncipe, form the Democratic Republic of São Tomé and Príncipe, located in the Gulf of Guinea, Central Africa. This oceanic island is just north of the Equator and about 255 km west of the African Continent. For an 857 km2 island, it has a remarkably
unique avifauna (Stattersfield et al. 1990; Peet & Atkinson 1994; Leventis & Olmos 2009). Out of 45 resident terrestrial species, 17 are single-island endemics, 3 are endemic to the Gulf of the Guinea oceanic islands (Annobón, São Tomé and Príncipe) and 8 are widespread species represented in the island by an endemic subspecies (Jones & Tye 2006). The high endemism rate is associated with its location in relation to the African continent: close enough to allow migration, and far enough to allow speciation by isolation (Melo 2006). This island is considered a “biodiversity hotspot” and, recently, its lowland forest belong to one of Earth’s biological ecoregions, the Gulf of Guinea Islands (Olson & Dinerstein 1998; Myers et al. 2000). Also, these forests were identified as the third most important in the world for forest bird species conservation (Buchanan et al. 2011).
As in many other oceanic islands, human occupation in São Tomé led to the introduction of several species, namely several bird species, most of which native from the African Continent. Before human intervention, the island was almost entirely covered by forest and the topography was responsible for
the strong climatic gradients that shaped the distribution of ecosystems. Human colonization, resulted in much of the lowland forests and some montane forests being replaced by plantations (Jones et al. 1991). Only the inaccessible rugged wet areas in the south-west and centre of the island remain covered by native forest, which is currently surrounded by secondary forest, resulting from logging and plantation abandonment. Enclosing this land use type are extensive areas of active shade coffee and cocoa plantations, a type of agroforestry, which is mixed with non-forested areas, such as oil palm monocultures, horticultures and open savannahs (Exell 1944; Tenreiro 1961; Jones et al. 1991).
Despite the long history of intensive conversion to anthropogenic land use, São Tomé’s landscape is still dominated by forested ecosystems. The native forest is almost entirely classified as São Tomé Obô Natural Park (STONP), which covers almost one third of the island (Albuquerque et al. 2008). Unfortunately, the protection and conservation efforts have not been effective and in the last decades human pressure on natural resources has been increasing fast, and the area covered by native forest and shade plantations has decreased, while secondary forest and non-forested areas have been expanding (Salgueiro & Carvalho 2001).
Data Compilation
In this study, we gathered all records from a single observer, obtained in 2009 and in 2010, for a total of 300 point counts (de Lima 2012), plus 1653 point counts and 677 occasional from BirdLife International São Tomé and Príncipe Initiative (BISTPI), collected between 2013 and 2015 (de Lima et al. 2017). In both studies, point counts were separated by at least 200 meters, to ensure independence, and all birds detected during 10 minutes were registered, regardless of the distance. This information was compiled in a single bird species occurrence database, which had a GIS component.
Field Methods
Sampling design
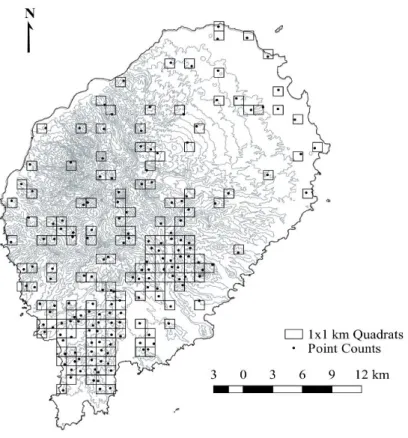
To identify under-sampled areas in previous studies from which we compiled data, we over-imposed the bird occurrence database on the map of São Tomé. The island was then divided in 1x1 km quadrats, grouped in groups of four to form 2x2 km quadrats (de Lima et al. 2017). All 2x2 km quadrats that had more than half of their area occupied by the ocean were excluded. We considered sampled all the 2x2 km quadrats that had at least one 1x1 km quadrat with five 10-minute point counts sampled. Between January and March 2017, we sampled 91 out of the remaining unsampled 96 2x2 km quadrats, located mostly in non-forested low-altitude areas across the island.
Bird sampling
Each of the 2x2 quadrats were sampled by performing five bird point counts in a randomly selected 1x1 km quadrat (Fig. 1.1), largely following the BISTPI methodology (de Lima et al. 2017). The location of the point counts was chosen to ensure a distance of at least 200 meters between point counts, thereby ensuring independence and that the environmental variability inside each quadrat was sampled in the approximate proportion in which they occurred in the quadrat.
In each point count, all bird species detected visually and aurally were registered by an experienced observer, during a 10 minute period, and regardless of the distance. To maximize the number of sampled points during our short sampling period, counts were made throughout the day, from approximately 6 am until 5 pm.
Characterizing environmental variables
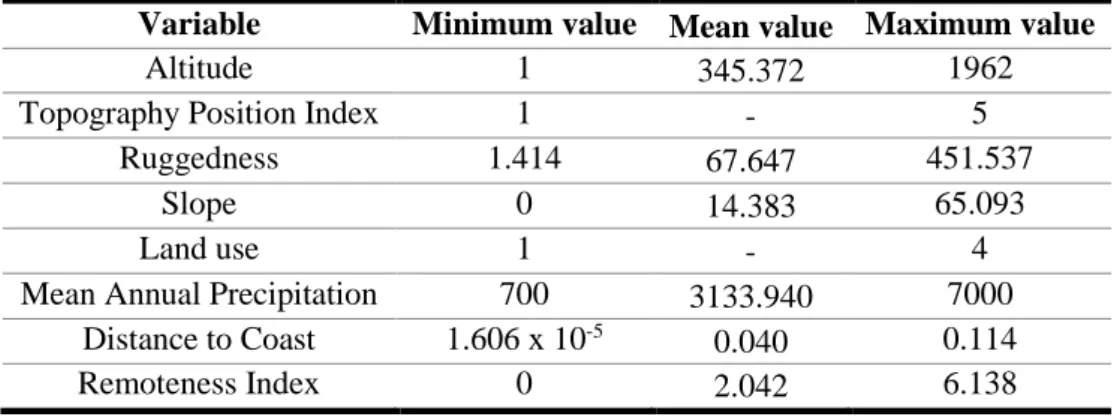
To model the distribution of bird species, we obtained geographically explicit information on altitude, ruggedness, slope, distance to the coast, topography, remoteness, rainfall and land use across São Tomé, using Quantum GIS v. 2.8.3 and v. 2.14.8 (Quantum GIS Development Team 2009a; Table S1 & S2).
The variable altitude was derived in meters from a 90 meters resolution Digital Elevation Model (DEM) (Silva 1958; Salgueiro & Carvalho 2001;NASA Jet Propulsion Laboratory 2016; Fig. S1). The ruggedness and slope were also calculated from the DEM raster, using the “raster terrain analysis” QGIS plugin (Quantum GIS Development Team 2009b; Fig. S2 & S3). Slope was primarily calculated in decimal degrees and then transformed to percentage. Distance to the coast was calculated as the minimum linear distance in decimal degrees between each pixel and the nearest point on the coast line, using the DEM and the QGIS “distance matrix” tool (Quantum GIS Development Team 2009a; Fig. S4). The topography was represented using a Topography Position Index (TPI) which allows comparing of each cell’s elevation to the mean elevation of a specified neighbourhood (Jenness 2007). The TPI was calculated using the DEM and the “topography position index” tool in the QGIS GDAL algorithm provider (Quantum GIS Development Team 2009c) and a 0.05º radius neighbourhood, which allows for a good representation of terrain ruggedness and elevation in São Tomé. TPI was later transformed in a five-category discrete variable: flat areas, valleys, middle slopes, upper slopes and ridges (Fig. S5, S6 & S7). Remoteness is expressed as an index that translates the difficulty of movement through the landscape, and it was created using the “accumulated cost” QGIS GDAL algorithm provider (Quantum
Figure 1.1. Location of sampling point counts and occasional observations (n = 3056)
GIS Development Team 2009d). This index is a cost accumulated surface based on a friction surface derived from slope and weighted by the human population density (Tobler 1993; Instituto Nacional de Estatística 2016;Fig. S8 & S9). Rainfall was obtained by digitizing a map with the island’s mean annual precipitation in millimetres (Silva 1958;Fig. S10). The land use map (Fig. S12) was created mostly by visual interpretation of 2014 satellite images (Google Earth 2017), supplemented by 2009-2017 field land cover information (de Lima 2012; de Lima et al. 2012), 1970 land use map (de Carvalho Rodrigues 1974), military maps (Missão Hidrográfica de Angola e S. Tomé 1958), a 2011-13 preliminary land use map (S. Mikulane, unpublished data - see Fig. S11) and expert knowledge.
All variables were standardised to a common in raster grid, using the nearest neighbour sampling method and the TPI raster as a geometric reference. This standardization was made using QGIS “align rasters” tool (Quantum GIS Development Team 2009a), and resulted in a pixel’s size of 0.000833º x 0.000833º and a raster with 359 x 471 cells. Each point count was characterized for each environmental variable using the “point sampling tool” QGIS plugin (Quantum GIS Development Team 2009e). Data Analysis
All statistical analyses were made in R v. 3.3.2 using RStudio v. 1.0.143 (R Development Core Team 2017).
Exploratory analysis
All bird data was compiled in a single database of 2408 point counts and 677 occasional observations. We excluded all species that are aquatic, difficult to identify or had less than 20 presences (Table S3), obtaining a total of 34 species that was considered for subsequent analyses. Point counts with no record of these species or that had inconsistencies between the field land cover classification and the 2014 land use map were also removed, leading to a final of 2398 point counts, plus 658 occasional observations.
Multicollinearity was tested using Spearman’s rank correlation coefficient, and visualized in a correlogram built using the “corrgram” package (Wright 2016; Part I, Section VIII). Ruggedness was excluded, since its correlation coefficient with slope was higher than 0.8 (Fig. S13).
Variance homogeneity and no outliers were identified by the boxplots drawn for each environmental variable using the “vegan” package (Oksanen 2015).
Generalized linear models
The data were divided in training and testing sets, using the “caTools” package: 70% of the points were used to create binomial generalized linear model (GLM) to explain species presence (Rushton et al. 2004), while the remaining 30% were used to validate the models (Tuszynski 2014; Part II, Section VIII).
We used Variance Inflation Factors (VIF) to double-check multicollinearity, and, once again, ruggedness was chosen to be excluded from all species models for being the only predictor variable having VIFs larger than 10. For each species, we generated all possible models based on the different combinations of explanatory variables, and ranked based on the Akaike Information Criterion corrected for small sample sizes (AICc), using the “dredge” function from the “MuMIn” package (Barton 2016). The goodness of fit was analysed with the McFadden’s index in the “pscl” package (Jackman et al. 2015). We validated the predicted values and calculated the receiving operating characteristic (ROC) curve. The area under the curve (AUC) was calculated to examine the model’s performance with the “ROCR” package (Sing et al. 2015; Table S4).
Relative variable importance
To identify which variables best explain the presence of each species, we ran the “model averaging” function of the “MuMIn” package to obtain relative variable importance (RVI). Bird species were separated in endemic and non-endemic species, and by feeding guild: carnivore (including insectivore), frugivore, granivore and omnivore (Jones & Tye 2006; HBW Alive 2017; Table S3). For each explanatory variable, we used Kruskal-Wallis rank tests to evaluate the difference in RVI values between endemic and non-endemic, and between feeding guilds (Table S5). To perform post hoc pairwise comparisons between feeding guilds we used Dunn-tests with Benjamini-Hochberg corrections (Thissen et al. 2002). These analyses were done using the “stats” and “FSA” packages (Ogle 2017; Part V, Section VIII).
Response to environmental variables
To analyse the response of each species to continuous variables, single-variable logistic regression models were created to explain species’ presence and obtain coefficient values (Table S6).
The proportion of occurrence in each land use type and in each TPI class was calculated for every species, correcting for sampling effort. Then, it was calculated for each group of species: endemics, non-endemics, carnivores, frugivores, granivores and omnivores. To evaluate the differences between endemic and non-endemic species, and between feeding guilds, among each land use type and topography class, Kruskal-Wallis rank tests were performed using the “stats” package. As previously, Dunn-tests with the Benjamini-Hochberg correction for multiple comparisons were run to analyse differences between feeding guilds (Part V, Section VIII). Both these tests were also used to evaluate the differences in coefficient values between endemic and non-endemic species, and between feeding guilds.
To visualize the links between endemism, feeding guilds and environmental variables, a detrended correspondence analysis (DCA) was made. The proportion of occurrence of each species in each land use type was also explored graphically, to gain a better understanding of how endemism and threat status relate to land use types.
RESULTS
Only 658 out of the 3056 final data points referred to occasional observations. On average, each species appeared in 24.4% of the systematic point counts, ranging from 88.4% for the São Tomé Sunbird
Anabathmis newtonii to 0.6% for the São Tomé Grosbeak Neospiza concolor (Table S3).
Relative variable importance
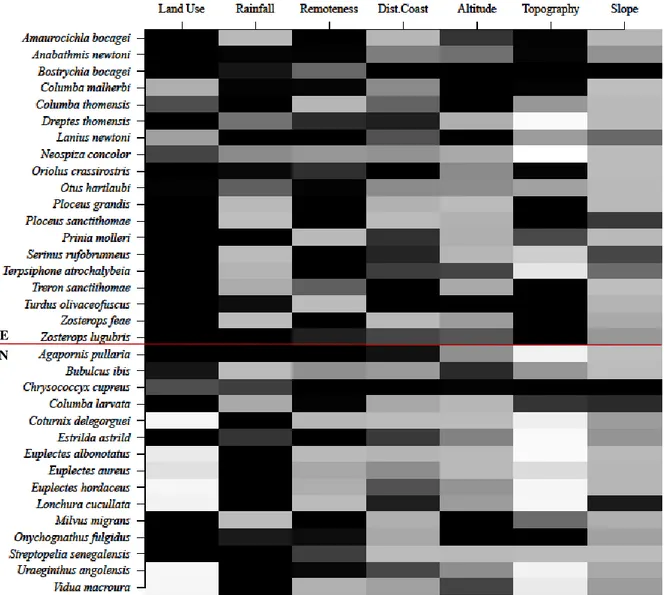
The most important variable to explain the occurrence of bird species in São Tomé was land use, followed by rainfall and remoteness (Fig. 1.2 & S14). Distance to coast, altitude and topography had intermediate importance, while slope was the least important.
When looking at the species individual responses to environmental variables, it is clear that land use was more important to the endemic species than to the non-endemic. On the other hand, rainfall was more important to non-endemic species. Topography seemed relevant to endemic species distribution, but was the least important variable to non-endemic species.
When comparing the RVI of endemic and non-endemic species, only land use (H = 6.19, df = 1, p = 0.013) and topography (H = 5.674, df = 1, p = 0.017) had significant differences, and both were more important to the endemics (Table S7 & Fig. S15).
Among feeding guilds, altitude (H = 8.3603, df = 3, p = 0.039) and slope (H = 10.373, df = 3, p = 0.016) were the only variables having significantly different RVI values. Altitude was more important to explain the presence of carnivores than that of omnivores, while slope was less important to frugivores than to any other feeding guild (Table S7 & Fig. S16).
Response of endemic and non-endemic species to environmental variables
The endemic species tended to have significantly higher values for all continuous environmental variables, when compared to the non-endemic (rainfall: H = 14.295, df = 1, p = 0.0002; remoteness: H = 12.765, df = 1, p = 0.0004; distance to coast: H = 11.555, df = 1, p = 0.0007; altitude: H = 12.032, df = 1, p = 0.0005; slope: H = 13.519, df = 1, p = 0.0002; Table 1.1, Fig. 1.3 & 1.4).
The proportion of occurrence in almost all land use types was significantly different between endemic and non-endemic species. Endemics tended to occur preferentially in native (H = 17.794, df =
Figure 1.2. Relative variable importance (RVI) of each environmental variable for each bird species generalized linear
model. The RVI is represented by a colour gradient, in which: darker cells indicate higher values. The RVI values range from 0 to 1. Endemic (E) and non-endemic (N) species are grouped together and separated by a black line.
E N
1, p = 2.461 x 10-5; Table 1.1 & S8, Fig. 1.3 & 1.4) and secondary forest (H = 11.672, df = 1, p = 0.0006),
while non-endemic species preferred non-forested areas (H = 17.206, df = 1, p = 3.355 x 10-5).
Endemic and non-endemic species occurrence among each topography class was also almost significantly different for all classes (Table 1.1, Fig. 1.3 & 1.4). Endemics tended to occur mostly in valleys, middle and upper slope areas, and also ridges (valleys: H = 13.911, df = 1, p = 0.0002; middle slope: H = 16.328, df = 1, p = 5.328 x 10-5; upper slope: H = 16.609, df = 1, p = 4.593 x 10-5; ridges: H
= 18.115, df = 1, p = 2.08 x 10-5), while the non-endemic species occur in a bigger proportion in flat
areas (H = 17.468, df = 1, p = 2.922 x 10-5).
Variables Endemism (KW test) Feeding Guilds (Dunn-test) Rainfall 0.0002 E >>> N 0.0219 C > G Remoteness 0.0004 E >>> N 0.0048 C >> G Distance to Coast 0.0007 E >>> N 0.0052 C >> G Altitude 0.0005 E >>> N 0.0185 C > G Slope 0.0002 E >>> N 0.0240 0.0420 C > G O > G Land Use Native Forest 2.461 x 10-5 E >>> N 0.020 0.023 C > G F > G Secondary Forest 0.0006 E >>> N 0.021 0.015 F > G O > G Shade Plantation - - - - Non-Forested Areas 3.355 x 10-5 E <<< N 0.038 0.038 C < G F < G Topography
Flat Plain Areas 2.922 x 10-5 E <<< N
0.024 0.019 0.047 C < G F < G O < G Valleys 0.0002 E >>> N - - Middle Slope 5.328 x 10-5 E >>> N 0.033 0.020 0.024 C > G F > G O > G Upper Slope 4.593 x 10-5 E >>> N 0.036 F > G Ridges 2.08 x 10-5 E >>> N 0.021 0.028 C > G F > G
Table 1.1. Response of endemic (E) and non-endemic (N), and of distinct feeding guilds (omnivores - O, granivores
- G, frugivores – F, and carnivores – C) to environmental variables. For continuous variables, the differences between E and N coefficients were assessed using Kruskal-Wallis rank tests (KW), while between feeding guild coefficients were assessed using Dunn-tests with Benjamini-Hochberg correction. For categorical variables, land use and TPI, Kruskal-Wallis rank tests were used to analyse differences between endemic and non-endemic species, while between feeding guilds Dunn-tests with Benjamini-Hochberg correction were used. Only p-value < 0.05 are shown.
Fi g u re 1. 3 . Re sp o n se o f en d em ic (E) a n d n o n -e n d em ic (N) sp ec ies to e n v iro n m en tal v ariab les . T h e b o x p lo ts re p re se n t th e co n ti n u o u s v ariab les c o e ff icie n ts o b tain ed f ro m sin g le -v ariab le m o d els : th e th ick li n e sh o w s th e m ed ian , th e b o x th e first an d th ir d q u arti les , th e w h isk er s th e ex tre m e s, an d th e d o ts th e o u tl iers . T h e b ar -p lo ts re p re se n t th e sta n d ard ize d p ro p o rti o n o f o cc u rre n ce i n e v er y lan d u se ty p e (n ati v e fo re st - NF, se co n d ary f o re st - S F , sh ad e p la n tatio n – SP a n d n o n -f o re st are as - NFA ) an d t o p o g ra p h y c las s (f la t are as – F, v all e y s - V , m id d le slo p e are as - M , u p p er sl o p e are as - U, ri d g es – R) .