www.jped.com.br
ORIGINAL
ARTICLE
Bone
densitometry
by
dual-energy
X-ray
absorptiometry
(DXA)
in
preterm
newborns
compared
with
full-term
peers
in
the
first
six
months
of
life
夽
Virginia
S.
Quintal
a,∗,
Edna
M.A.
Diniz
b,
Valeria
de
F.
Caparbo
c,
Rosa
M.R.
Pereira
caHumanMilkBank,HospitalUniversitário,FaculdadedeMedicina,UniversidadedeSãoPaulo(USP),SãoPaulo,SP,Brazil bDepartmentofPediatrics,HospitalUniversitário,FaculdadedeMedicina,UniversidadedeSãoPaulo(USP),SãoPaulo,SP,Brazil cDivisionofRheumatology,FaculdadedeMedicina,UniversidadedeSãoPaulo(USP),SãoPaulo,SP,Brazil
Received23January2014;accepted21March2014
Availableonline17June2014
KEYWORDS
Prematurity; Newborn;
Bonemineralization; Densitometry; Humanmilk
Abstract
Objectives: Tolongitudinallyassessbonemineralcontent(BMC),bonemineraldensity(BMD), andwhole-bodyleanmassobtainedthroughbonedensitometrybydual-energyX-ray absorp-tiometry(DXA)inpretermnewborns(PTNs)andcomparethemwithfull-termnewborns(FTNs) frombirthto6monthsofcorrectedpostnatalage.
Methods: Atotalof28adequateforgestationalage(AGA)newbornswerestudied:14preterm and14 full-term newborns.DXA was usedto determineBMC, BMD,andlean massinthree moments:40weekscorrected post-conceptualage,aswellas3and6monthsofcorrected postnatalage.PTNshadgestationalage≤32weeksatbirthandwerefedtheirmother’sown milkormilkfromthehumanmilkbank.
Results: AllinfantshadanincreaseinBMC,BMD,andleanbodymassvaluesduringthestudy. PTNshadlowerBMC,BMD,andleanmassat40weeksofcorrectedpost-conceptualagein rela-tiontoFTNs(p<0.001,p<0.001,p=0.047,respectively).However,therewasanacceleration inthemineralizationprocessofPTNs,whichwassufficienttoachievethenormalvaluesofFTNs at6monthsofcorrectedage.
Conclusions: Thisstudysuggeststhatbonedensitometrybydual-energyX-rayabsorptiometry isagoodmethodfortheassessmentofbodycompositionparametersatbaseline,andatthe follow-upofthesePTNs.
©2014SociedadeBrasileiradePediatria.PublishedbyElsevierEditoraLtda.Allrightsreserved.
夽
Pleasecitethisarticleas:QuintalVS,DinizEM,CaparboVF,PereiraRM.Bonedensitometrybydual-energyX-rayabsorptiometry(DXA) inpretermnewbornscomparedwithfull-termpeersinthefirstsixmonthsoflife.JPediatr(RioJ).2014;90:556---62.
∗Correspondingauthor.
E-mail:virginia@hu.usp.br,quintal.md@gmail.com(V.S.Quintal).
http://dx.doi.org/10.1016/j.jped.2014.03.001
PALAVRAS-CHAVE
Prematuridade; Recém-nascido; Mineralizac¸ãoóssea; Densitometria; Leitehumano
Densitometriaósseadeduplaabsorc¸ãoderaio-X(DXA)emcrianc¸asnascidas pré-termocomparadacomseusparesatermonosprimeiros6meses
Resumo
Objetivos: Avaliarlongitudinalmenteoconteúdo mineral ósseo(CMO), adensidade mineral óssea(DMO)eamassamagradocorpointeiroobtidosatravésdadensitometriaósseadedupla absorc¸ãodeRaios-X(DXA)emrecém-nascidospré-termo(RNPT)ecompararcomseusparesa termo(RNT)desdeonascimentoaté6mesesdeidadepós-natalcorrigida.
Métodos: Foramestudados28recém-nascidosadequadosparaaidadegestacional:14 recém-nascidospré-termoe14recém-nascidosatermo.Utilizando-seaDXA,foramdeterminadosCMO, DMOemassamagraemtrêsmomentos:40semanasdeidadepós-concepcionalcorrigida,3e6 mesesdeidadepós-natalcorrigida.Osrecém-nascidospré-termoapresentavamaonascimento umaidadegestacionaligualouinferiora32semanasereceberamleitedaprópriamãeouleite humanodebanco.
Resultados: Todososrecém-nascidosapresentaramumaumentonosvaloresdeCMO,DMOe massamagraduranteoestudo.Osrecém-nascidospré-termoapresentarammenorCMO,DMO emassamagra,com40semanasdeidadepós-concepcionalcorrigida,emrelac¸ãoaos recém-nascidosatermo(p<0,001,p<0,001,ep=0,047,respectivamente).Entretanto,houveuma acelerac¸ãonoprocessodemineralizac¸ãonospré-termos,suficienteparaatingiremosvalores normaisdorecém-nascidosatermoaos6mesesdeidadecorrigida.
Conclusões: Esteestudosugerequeadensitometriaósseadeduplaabsorc¸ãodeRaios-X cons-tituiumbommétodoparaaavaliac¸ãodosparâmetrosdecomposic¸ãocorporalnoinícioeno seguimentodestesrecém-nascidospré-termo.
©2014SociedadeBrasileiradePediatria.PublicadoporElsevierEditoraLtda.Todososdireitos reservados.
Introduction
Metabolicbonediseaseischaracterizedbychangesin skele-talmineralizationduetopoorbonemineralcontent(BMC) accrual.Inpretermnewborns(PTNs), theBMC isinversely proportionaltobirthweightandgestationalage;decreased BMC is also related to inadequate intake of calcium and phosphorusinextra-uterinelife.1---3
Risk factors related to inadequate mineralization are: very-lowbirthweight,intrauterinegrowthrestriction, pro-longed use of parenteral nutrition, use of diuretics and glucocorticoids,bronchopulmonarydysplasia,delayin intro-duction of food,low mineral supply in thediet, andlong immobilizationperiods.4---6Theuseofsupplementedhuman milkisabletoprovidepropergrowthandbone mineraliza-tionintheshortterm.7
Metabolic bone disease of PTNs has no characteristic clinical presentation. It can be observed with longitudi-nal growth arrest, maintenance of head circumference, andevenradiologicalsignssimilartorickets,with sponta-neousfracturesdescribedin10%ofPTNswithvery-lowbirth weight.8
InPTNswithmineraldeficiency,somebiochemical mark-ers may be altered. Hypophosphaturia and hypercalciuria that precedeserum alterations (reductionin calcium and phosphorus,andelevatedalkalinephosphatase)and radio-logicalalterations canbeobserved.5,9Othermorespecific serum or urinary markers, such as bone-specific alkaline phosphatase(BAP),deoxypyridinoline(DPD),osteocalcin, C-terminal telopeptideof type Icollagen (CTX),and C-type natriureticpeptide(CNP)maybeusedforgrowthandbone remodelingassessment.10---12
Additionally, bone densitometry by dual-energy X-ray absorptiometry(DXA)hasbeenconsideredthegoldstandard methodtoassessbonemineralizationinnewborns,showing highprecisionandaccuracy.4,10,11,13---15
Theaimof thisstudy wastoevaluatebone mineraliza-tionbyDXAinthefirst6monthsofcorrected ageinPTNs comparedwithFTNs.
Methods
Thiswasa longitudinalstudyinvolving newbornsadmitted totheNeonatalUnitoftheHospitalUniversitárioda Univer-sidadedeSãoPaulo(USP),withgestationalage≤32weeks, followedfromJuly of2006toSeptemberof2008. As con-trolgroup,agroupoffull-termnewborns(FTNs)borninthe sameperiodwereselected.
Newbornswithcongenitalmalformations,chromosomal disorders,or geneticdisorders were excluded, aswell as newbornsofdiabetic mothers.Newbornsmallorlarge for gestationalagewerealsoexcluded.Informedconsentwas obtained fromall parents of the assessed newborns. The study was approved by the Ethics Committee of Hospital UniversitáriodaUSP.
weeks(mean40.1).Birthweightof thePTNsranged from 1,115gto2,130g(mean1,540g),andinFTNs,from2,900g to3,700g(mean3,260g).Allhadweightbetweenthe10th and90th percentiles of thereference curve of Alexander etal.16
Samplesizecalculation
AccordingtothereferencevaluesofBMCforPTNsandFTNs, theestimatedvariabilityisapproximately6.5g(SD=6.5g) at40weeksofcorrectedage.2AssumingadifferenceinBMC betweenPTNsandFTNsisfoundat6-monthsoffollow-upof atleast7g(withtheinitialdifferencebeing10gbetween thetwogroups), an improvement of at least30% in PTNs shouldbeexpected,withan80%powerand95%confidence. Basedonthiscalculation, thesample requiredtoperform thestudywouldbe14patientsineachgroup.
Risk factorsfor inadequate mineralization(pathologies andmedications)foundinpre-terminfantsweresepsiswith positivebloodcultures,whichwasobservedin28.5%; necro-tizing enterocolitis (Bell’s criteria) with clinical therapy, whichwasseen in14.3%;andbronchopulmonarydysplasia (requiring oxygentherapy for 28 days or more),observed in35.7%. Oftotal PTNswithbronchopulmonary dysplasia, three received hydrochlorothiazide and two, furosemide. Elevenreceivedparenteralnutrition;twoforlessthanone week,andnineforbetweenoneweekandonemonth,with 12daysasthemeandurationofparenteralnutrition.
Enteral feedingwasintroducedonthefirstday oflife. Duringhospitalizationintheneonatalunit,allPTNsreceived human milk,both their ownmother’s milk andmilk from theUniversityHospitalbank.Onlyfourofthesevenpreterm infantswithbirthweight<1,500greceivedhumanmilk sup-plementedwithanadditive(FM85®)inpumpedbreastmilk orpasteurizedhuman milkandadministeredbyorogastric tubeor cup. The remainingthreePTNs had hypophospha-turia (urinary phosphorus< 1mg kg−1 d−1) and therefore
receivedanadditionalsupplyofcalciumandphosphorusto achieveasupplyof200mgofcalcium/kgand110mg phos-phorus/kgper day.9 All newborns (preterm andfull-term) receivedenteralsupplementationofvitaminDatadoseof 400IU/day,whichwasmaintainedduringthefirsttwoyears ofage.
Afterdischarge,thechildrenwereassessedmonthly. Dur-ingthestudyperiod,allinfantswerefedexclusivelyhuman milk.Childrenwithindication for additive use receiveda combinedsolutionofcalciumgluconateanddibasiccalcium phosphatebetweenbreast-feedings,whichwasmaintained forthefirstsixmonthsofage(agecorrectedforthePTNs and chronological age for the FTNs), and complementary feedingwasnotintroduced.
Allnewbornswereweighedonanelectronicscale(Baby Model;Filizola-SãoPaulo,Brazil)andheightwasobtained usingananthropometricrulergraduatedincentimeters.
In the groupof PTNs, serum calcium,phosphorus,and alkalinephosphatasemeasurementswereperformedatthe agesof40post-conceptualweeksand6monthsofcorrected postnatalage. Furthermore,the concentrationof calcium and phosphorus was determined in 6-hour urine samples between the third and fourth weeks of life (uncorrected age).
InthegroupofFTNs,measurementsof serumcalcium, phosphorus,andalkalinephosphatasewereperformedonly at 40weekspost-conceptualage, asbloodcollectionat 6 monthswasnotapprovedbytheEthicsCommittee.
BonedensitometrywasperformedattheLaboratoryfor BoneMetabolismof Rheumatology,Faculdade deMedicina da USP. The following parameters were evaluated: BMC, bonemineraldensity(BMD),andleanmassinthreeperiods: 40weeksofcorrectedpost-conceptualage,aswellas3and 6monthsofcorrectedpostnatalage.BMCreflectsthetotal amount of material (mineral bone) measured by absorp-tiometry,ingrams;BMDisdefinedasbonemineralcontent dividedby boneareaingramspersquarecentimeter,and leanbodymassisfat-freemass.
Adual X-ray absorptiometry (DXA)apparatuswasused (DXA:DiscoveryA;HologicInc.-Bedford,MA,USA)withthe InfantWhole-Bodyscanningmode(softwareversion12.3.3; HologicInc.).
The software used is considered superior to pediatric softwarefor the analysisofbone mineral, accurately val-idatedforbothPTNsandFTNs.17Inaddition,thefan-beam technique,usedinthestudy,makestheDiscoveryA scan-nermoreaccuratewhencomparedtothepriorpencil-beam technique.18 The study byBlake etal.demonstrated that the Discovery A scanner has additional advantages, as it requiresalowerradiationdosewhencomparedtothe pre-viouslyusedDiscoveryW(HologicInc.-Bedford,MA,USA) andQDR4500HologicInc.-Bedford,MA,USA)models.For anewborn,theeffectiveradiationdoseis 8.9mSv forthe wholebody,anditis7.5mSvforachildaged1year.19
Examinations were performed without sedation after breastfeeding.The coefficientof variationfor wholebody BMD was0.004g/cm2(0.4%), andtheminimumsignificant
difference for newborns evaluatedin the study was 1.2% (95%confidenceinterval).Thesevaluesareappropriate,as theliteraturedescribescoefficientvaluesrangingfrom0.8 to2.2%.20
Statisticalanalysis
StatisticalanalysiswasperformedusingtheStatistical Anal-ysis System, release 9.1.3 (SAS Institute, Cary, NC, USA), and analysisof variance (ANOVA)with repeated measure-mentswasperformedinthePROCMIXEDmoduleofSAS(SAS Institute,Cary,NC,USA).Tukeymultiplecomparisonswere performedaftertheANOVA,whichallowedfordetectionof thedifferences.Toassesstheassociationofvariableswith thegroup,Fisher’sexacttestwasused.Asignificancelevel of5%wasadoptedinallanalyses.
Results
Table1 Comparisonofbirthweight,gestationalage,andgenderofchildrenexcludedfromandincludedinthestudy.
NB Preterm Full-Term p
Mean SD n Mean SD n
Birthweight(kg)
Excluded 1.65 0.24 3 3.24 0.27 11
0.658
Included 1.54 0.30 14 3.26 0.24 14
GA(weeks)
Excluded 30.76 0.22 3 39.71 0.99 11
0.366
Included 31.15 1.41 14 40.10 0.99 14
Gender(male)n(%)
Excluded 2(66.7) 3 6 (54.5) 11
0.495a
Included 9(64.3) 14 10 (71.4) 14
ANOVAresult.
a chi-squaredtestresult.
ANOVA,analysisofvariance;SD,standarddeviation;GA,gestationalage.
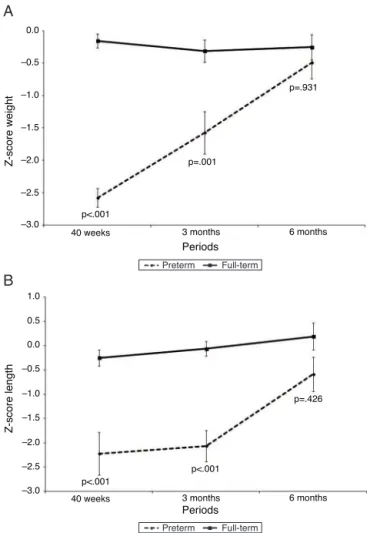
Fig.1showsthez-scoreforweight(kg)andheight(cm), andFig.2showsBMC(g),BMD(g/cm2),andleanmass(g)of
PTNsandFTNsthroughoutthefollow-upperiod,at40weeks ofpost-conceptualage,andat3and6monthsofcorrected gestational age. At all times, differences were observed between thePTNs and FTNs,in both Z-scoremeasures of weight and height, aswell asBMC, BMD, and lean mass. The comparisonbetween the PTNs andFTNs showed that attheinitialassessment(40weeksofpost-conceptualage), PTNs had lowervalues whencompared toFTNs regarding weight,Z-scoresforweightandheight,BMC,BMD,andlean mass(p<0.05)(Table2).However,thesedifferences disap-pearedat the 6-month evaluationof postnatal age, when allparametersshowedsimilarmeansbetweenthePTNsand FTNs(p>0.05)(Table2).
Serum biochemical parameters didnot differ between PTNsandFTNs,exceptalkalinephosphatase,whichwas sta-tisticallyhigher inPTNs in relation totheFTNs(Table 2). Only two patients, both preterm, had alkaline phos-phataselevels>1,200IU/L,whichisconsideredsuggestive ofmetabolicbonedisease.
AmongthePTNs,three(21.4%)hadresultsofurinetests suggestiveofphosphorusdeficiencysyndrome(urinary cal-cium>4mg/kg perday, and urinaryphosphorus< 1mg/kg perday).Forthisreason,theuseoftheoralsolutionof cal-ciumandphosphoruswasindicated,whichwasmaintained untilthecorrectedageof6months.
Inadditiontothissolutionwithcalciumandphosphorus oralsupplementation,humanmilkadditivewasusedinfour PTNs,totaling50%ofpreterminfantsrequiringhumanmilk supplementation.
Discussion
InBrazil, thiswasthefirststudy conductedonthe evolu-tionofbodycompositionofPTNsandFTNsfedhumanmilk, assessedbyDXAafterdischarge.
Bonemineralization ofPTNs is frequently addressedin theliterature,andDXAhasbecomethemethodof choice forassessingbodycompositionofnewborns.21---24
0.0
–0.5
–1.0
–1.5
–2.0
p<.001
p<.001
p<.001
p=.426 40 weeks
Periods
Periods
Z-score weight
Z-score length
3 months 6 months
40 weeks 3 months 6 months p=.001
p=.931
–2.5
–3.0
–3.0 –2.5 –2.0 –1.5 –1.0 –0.5 0.0 0.5 1.0
A
B
Preterm Full-term
Preterm Full-term
Figure1 Evolution ofgrowthwithZ-scores for weightand lengthofpreterminfantsinrelationtotheirfull-termpeersat 40weeks,3months,and6monthsofcorrectedage.
Table2 Measurementsofweight,z-scoreforweightandlength,bonemineralcontent(BMC),bonemineraldensity(BMD), leanmass,calcium,phosphorus,andalkalinephosphataseinpretermnewbornsandfull-termnewbornsat40weekscorrected post-conceptualage,aswellas3and6monthsofcorrectedpostnatalage.
Preterm n=14
Full-Term n=14
p
Weight(kg)
Initial 2.25±0.21 3.26±0.24 <0.001
3months 5.03±0.60 6.08±0.59 0.001
6months 7.29±0.80 7.55±0.81 0.701
Z-score
Initial -2.58±0.54 -0.16±0.40 <0.001
Weight
3months -1.58±1.14 -0.32±0.60 0.001
6months -0.49±0.90 -0.25±0.70 0.931
Z-score
Initial -2.22±1.63 -0.25±0.61 <0.001
Length
3months -2.06±1.12 -0.06±0.51 <0.001
6months -0.59±1.27 0.19±1.01 0.426
BMC(g)
Initial 31.80±6.10 60.76±7.32 <0.001
3months 84.57±14.23 117.66±14.10 <0.001
6months 137.14±22.46 152.86±20.92 0.054
BMD(g/cm2)
Initial 0.13±0.02 0.19±0.01 <0.001
3months 0.19±0.02 0.21±0.01 0.008
6months 0.22±0.02 0.23±0.02 0.618
Leanmass(g)
Initial 2,377.52±267.75 3,084.82±227.47 0.047
3months 4,084.72±1,030.99 4,863.21±587.74 0.035
6months 5,750.13±765.11 5,745.88±726.52 1.000
Calcium(mg/dL)
Initial 9.86±0.53 10.25±0.49 0.076
6months 10.54±0.41 NA
Phosphorus(mg/dL)
Initial 6.50±0.78 5.85±1.12 0.085
6months 6.13±0.54 NA
Alkalinephosphatase(IU/L)
Initial 959.50±561.64 392.85±101.47 <0.001
6months 743.42±327.80 NA
Datashownasmean±SD.
IU/L,internationalunits/liter;Initial,40weeksofcorrectedgestationalage;NA,notassessed.
Thepresentstudydemonstrated,throughevaluationby DXA,thatPTNsreachBMCandBMDsimilartothoseofFTNs after6monthsofcorrectedage.
Thestudysampleconsistedof14PTNs,ofwhom50%had very-lowbirthweight.Nutritionalsupportwasrequiredfor propergrowthoftheseinfantsweighing lessthan1,500g, withahighsupplyofcalcium,phosphorus,andprotein,as these infants show an accelerated bone-remodeling rate. Atbirth,thesePTNshadlowerBMCandBMDinrelationto FTNs,whichpersisteduntil6monthsofcorrectedage.This observationisinagreementwiththeliterature,wherethere
arereportsofPTNswho,althoughreceivinghumanmilkand supplementation,didnotsignificantlyimprovebone miner-alizationuntiltheyreachedfullterm.4
Thisstudydemonstrated,throughanalysisbyDXA,that the process of bone mineralization showed a significant accelerationinPTNs,butwasstillfarfromthatobservedin FTNsupto6monthsofcorrectedage,suggestingthat min-eralsupplementationshouldbecarriedoutforaprolonged periodinvery-lowbirthweightnewborns.
p<.001
p<.001
p=.008
p=.047
p=.035
p=1 p=.618 p<.001
p=.054
40 weeks 3 months 6 months
40 weeks 3 months 6 months
40 weeks 3 months 6 months 0
20 40 60 80 100 120 140 160 180
0.0
0 1000 2000 3000 4000 5000 6000 7000 0.05 0.10 0.15 0.20 0.25
Periods
Periods
Periods
Whole-body bone mineral
content (g)
Whole-body bone mineral
density (g/cm2)
Whole-body lean mass (g)
Preterm Full-term
Preterm Full-term
Preterm Full-term
A
B
C
Figure2 Evolution ofbonemineral content(g), bone min-eraldensity(g/cm2),andleanmass(g),inpretermnewborns inrelationtotheirfull-termpeersevaluatedbyDXA.
No.ofsample:40weeks(pretermandfull-term)=14;3months (preterm and full-term)=12; 6 months (preterm and full-term)=13.
absorption, and renal function in these infants. Some authorsrecommendaurinalysisoftheseionsasamethod to determine the need for supplementation, aiming to improveBMCand reducetheincidence ofmetabolicbone disease.However,theseanalyses donotappearto substi-tute thedirectmeasurementof BMCand BMD.9,25 In fact, thepresentstudydemonstratedthattheBMCandBMDwere significantlylowerinPTNswhencomparedtoFTNs,evenin infantswithnormalurinaryandserummeasurements.
Among serum markers of metabolic bone disease, the most widely used is alkaline phosphatase. However, the cutoffvalue forosteopeniadefinitionvaries widely in the literature, between 300 and 1,200 IU/L. In this sense, Figueras-Aloyetal.evaluatedalkalinephosphataseandBMD
in336 PTNs andconsidered metabolic bone diseasewhen bothvariableswerealtered(alkalinephosphatase>500IU/L andBMD<0.068g/cm2)athospitaldischarge.26
Although metabolic bone disease of prematurity is a self-limitingprocess,therapidrecoveryofBMC(catchup) has many advantages: better growth in height and head circumference, prevention of fractures, and reduction of osteopeniainadulthood.27
LeanmassalsonormalizedinPTNs at6 monthsof cor-rectedpostnatalage,afindingsimilartothatreportedby Cookeet al., albeit in children assessed at 12 months of corrected age.28 These authors found that lean mass was lowerinPTNswhencorrectedforage.However,when cor-rectedforweight,PTNshadleanmassvaluessimilartothe referencevaluesfortheFTNs.
In conclusion, the present study showed that PTNs recoverBMD,BMC,andleanbodymassat6monthsof cor-rectedage,andsuggeststhatbonedensitometryisagood methodfortheassessmentoftheseparametersattheinitial assessmentandespeciallyatfollow-upoftheseinfants.
Funding
ConselhoNacionaldeCiênciaeTecnologia(CNPQ),Process No.305691/2006-6.
Conflicts
of
interest
Theauthorsdeclarenoconflictsofinterest.
References
1.SteichenJJ,GrattonTL,TsangRC.Osteopeniaofprematurity: thecauseandpossibletreatment.JPediatr.1980;96:528---34.
2.RigoJ,NyamugaboK,PicaudJC,GerardP,PieltainC,De Cur-tisM.Referencevaluesofbodycompositionobtainedbydual energyX-rayabsorptiometryinpretermandtermneonates.J PediatrGastroenterolNutr.1998;27:184---90.
3.RigoJ,SenterreJ.Nutritionalneedsofprematureinfants: cur-rentissues.JPediatr.2006;149:S80---8.
4.FaerkJ,PetersenS,PeitersenB,MichaelsenKF.Dietandbone mineral content at term in premature infants. Pediatr Res. 2000;47:148---56.
5.CatacheM,LeoneCR.Análisecríticadosaspectos fisiopatológi-cos,diagnósticos eterapêuticos dadoenc¸ametabólicaóssea em recém-nascidos de muito baixo peso. J Pediatr (Rio J). 2001;77:S53---62.
6.HarrisonCM,JohnsonK,McKechnieE.Osteopeniaof prematu-rity:anationalsurveyandreviewofpractice.ActaPaediatr. 2008;97:407---13.
7.AlkalayAL, MoghimiA,VanderhalA,SimmonsCF.Osteopenia ofprematurity:practiceguidelinesfortheclinician.Neonatal IntensiveCare.2003;16:40---5.
8.RigoJ,MohamedMW,DeCurtisM.Disordersofcalcium, phos-phorusandmagnesiummetabolism.In:MartinRJ,FanaroffAA, Walsh MC, editors. Fanaroff and Martin’s neonatal-perinatal medicinediseases ofthefetusand infant. 8th ed.St. Louis:
Mosby;2006.p.1491---523.
10.VignochiCM,MiuraE,CananiLH.Effectsofmotorphysical ther-apyonbonemineralizationinprematureinfants:arandomized controlledstudy.JPerinatol.2008;28:624---31.
11.Vignochi CM, Silveira RC, Miura E, Canani LH, Procianoy RS. Physical therapy reduces bone resorption and increases bone formationinpreterminfants.AmJPerinatol.2012;29: 573---8.
12.Kilpeläinen L, Ivaska KK, Kuiri-Hänninen T, Väänänen HK, Rehfeld JF, Goetze JP, et al. Urinary osteocalcinand serum pro-C-typenatriureticpeptidepredictlinearcatch-upgrowth ininfants.JBoneMinerRes.2012;27:1528---35.
13.BishopNJ,DahlenburgSL,FewtrellMS,MorleyR,LucasA.Early diet of preterminfants and bone mineralization at age five years.ActaPaediatr.1996;85:230---6.
14.Wauben IP, AtkinsonSA, Shah JK,Paes B. Growth and body compositionofpreterminfants:influenceofnutrient fortifica-tionofmother’smilkinhospitalandbreastfeedingpost-hospital discharge.ActaPaediatr.1998;87:780---5.
15.De Schepper J, Cools F, Vandenplas Y, Louis O. Whole body bonemineralcontentissimilaratdischargefromthehospital inprematureinfantsreceivingfortifiedbreastmilkorpreterm formula.JPediatrGastroenterolNutr.2005;41:230---4.
16.AlexanderGR,HimesJH,KaufmanRB,MorJ,KoganM.AUnited States national reference for fetal growth.Obstet Gynecol. 1996;87:163---8.
17.Picaud JC, Lapillonne A, Pieltain C, Reygrobellet B, Claris O, SalleBL, et al. Softwareand scanacquisition technique-related discrepancies in bone mineral assessment using dual-energy X-rayabsorptiometryinneonates.ActaPaediatr. 2002;91:1189---93.
18.Hammami M, Koo WW, Hockman EM. Body composition of neonates from fan beam dual energy X-ray absorptiome-try measurement. JPEN J Parenter Enteral Nutr. 2003;27: 423---6.
19.BlakeGM,NaeemM,BoutrosM.Comparisonofeffectivedose tochildrenandadultsfromdualX-rayabsorptiometry exami-nations.Bone.2006;38:935---42.
20.BaimS,WilsonCR,LewieckiEM,LuckeyMM,DownsJrRW,Lentle BC.Precisionassessmentandradiationsafetyfordual-energy X-rayabsorptiometry:positionpaperoftheInternationalSociety forClinicalDensitometry.JClinDensitom.2005;8:371---8.
21.LapillonneA,BraillonP,ClarisO,ChatelainPG,DelmasPD,Salle BL.Bodycompositioninappropriateandinsmallforgestational ageinfants.ActaPaediatr.1997;86:196---200.
22.AkcakusM,KokluE,BudakN,KulaM,KurtogluS,KokluS.The relationshipbetweenbirthweight,25-hydroxyvitaminD concen-trationsandbonemineralstatusinneonates.AnnTropPaediatr. 2006;26:267---75.
23.BeltrandJ,AlisonM,NicolescuR,VerkauskieneR,DeghmounS, SibonyO, etal.Bonemineralcontentatbirthisdetermined both bybirth weightand fetal growthpattern. Pediatr Res. 2008;64:86---90.
24.Avila-DíazM,Flores-HuertaS,Martínez-Mu˜nizI,AmatoD. Incre-mentsin whole bodybone mineral content associated with weightandlengthinpre-termandfull-terminfantsduringthe first6monthsoflife.ArchMedRes.2001;32:288---92.
25.PohlandtF,MihatschWA.Referencevaluesforurinarycalcium andphosphorustopreventosteopeniaofprematurity.Pediatr Nephrol.2004;19:1192---3.
26.Figueras-Aloy J, Álvarez-Domínguez E, Pérez-Fernández JM, Moretones-Su˜nol G,Vidal-SicartS,Botet-MussonsF.Metabolic bonediseaseandbonemineraldensityinverypreterminfants. JPediatr.2014;164:499---504.
27.FewtrellMS.Doesearlynutritionprogramlaterbonehealthin preterminfants?AmJClinNutr.2011;94:1870S---3S.