w ww.e l s e v i e r . c o m / l o c a t e / b j p
Original
Article
Anti-inflammatory,
and
antinociceptive
effects
of
Campomanesia
adamantium
microencapsulated
pulp
Danieli
Z.
Viscardi
a,∗,
Vinícius
S.
de
Oliveira
b,
Jucicléia
da
S.
Arrigo
a,
Ana
C.
Piccinelli
c,
Claudia
A.L.
Cardoso
d,
Iriani
R.
Maldonade
e,
Cândida
A.L.
Kassuya
c,
Eliana
J.
Sanjinez-Argando˜na
faProgramadePós-graduac¸ãoemBiotecnologiaeBiodiversidade,FaculdadedeCiênciasExataseTecnologia,UniversidadeFederaldaGrandeDourados,Dourados,MS,Brazil bFaculdadedeCiênciasExataseTecnologia,UniversidadeFederaldaGrandeDourados,Dourados,MS,Brazil
cFaculdadedeCiênciasdaSaúde,UniversidadeFederaldaGrandeDourados,Dourados,MS,Brazil dCursodeQuímica,UniversidadeEstadualdeMatoGrossodoSul,Dourados,MS,Brazil eEmbrapaHortalic¸as,Brasília,DF,Brazil
fFaculdadedeEngenharia,UniversidadeFederaldaGrandeDourados,Dourados,MS,Brazil
a
r
t
i
c
l
e
i
n
f
o
Articlehistory:
Received1March2016 Accepted7September2016 Availableonline11November2016
Keywords:
Campomanesiaadamantium
Inflammation Hyperalgesia Microencapsulation Guavira
a
b
s
t
r
a
c
t
Guavirafruitshaveantimicrobial,antioxidant,antinociceptive,andanti-inflammatoryactivities.Spray dryinghasbeenwidelyusedinthefoodindustrypresentinggoodretentioninbioactivecompoundsused totransformthepulp/fruitjuiceintopowderform.Therefore,thepresentstudyhasevaluatedthe anti-inflammatoryandantinociceptiveactivitiesofthemicroencapsulatedpulpofCampomanesiaadamantium (Cambess.)O.Berg,Myrtaceae,byspraydrying.Differentgroupsofmiceweretreatedwiththedosesof 100and300mg/kgofmicroencapsulated“guavira”pulpandinflammatoryparameterswereassessedin acarrageenanpawedema-modelandleukocytemigrationwithpleurisymodel,whilethe antinocicep-tiveactivitywasassessedusingtheformalinmethodandCFA-inducedhyperalgesiamodel.Asignificant reductioninleukocytemigrationandinpawedemawasobservedinrodentsinalltimeaftercarrageenan injectionforbothdosesofmicroencapsulatedpulpofC.adamantiumwhencomparedwithcontrolgroup. MicroencapsulatedpulpofC.adamantiumalsoreducedlickingtimeatthefirst(nociceptive)and sec-ond(inflammatory)phasesintheformalinmodel.InCFA-inducedcoldandmechanicalhyperalgesia, depressivebehavior,andkneeedema,allparametersanalyzedweresignificantlyinhibitedby microen-capsulatedpulpofC.adamantium.Microencapsulationbyspraydryingprovedtobeatechniquethat promotesbioavailabilityandthepreservationofbioactivecomponentsinguavirapulp.
©2016SociedadeBrasileiradeFarmacognosia.PublishedbyElsevierEditoraLtda.Thisisanopen accessarticleundertheCCBY-NC-NDlicense(http://creativecommons.org/licenses/by-nc-nd/4.0/).
Introduction
AmongtheBrazilian Cerrado,there is anative plantspecies known as Campomanesia adamantium (Cambess.) O.Berg, Myr-taceae,(guavira)(Lorenzi,2000)thatiswidelyfoundinisolated fieldsinMidwesternandsoutheasternBrazil.Thefruitsgrowon smallbushesandhavespecificcharacteristics,suchasbright col-orsfromgreentoyellow,withastrongandcitricaroma(Fernandes etal.,2014).Itsfruithasthepotentialtobeusedinnaturainthe foodindustryandasflavoringinthedrinkindustryduetoits juici-ness,mineralcontent,fiber,andinterestingbioactivesubstances fromthenutritionalandfunctionalpointsofviewasphenolic com-pounds.Inadditiontotheirpleasanttaste,thefruitsareconsidered asourceofvitaminC(Bredaetal.,2012),whichisanimportant
∗ Correspondingauthor.
E-mail:danieliviscardi@ufgd.edu.br(D.Z.Viscardi).
micronutrientinvolvedinseveralbiologicalfunctionsinthehuman body(Pascoaletal.,2014).
In folk medicine “guavira” fruits are used as antirheumatic, antidiarrheal, hypocholesterolemic, anti-inflammatory (Ramos etal.,2007),andtothetreatmentofcystitisandurethritis(Pascoal etal.,2014).Previousstudieswiththefruitshaveobserved antimi-crobial (Pavan et al., 2009; Cardoso et al., 2010), antioxidant (Coutinho et al., 2010), antinociceptive, and anti-inflammatory activities(Ferreiraetal.,2013),aswellasapoptoticand antiprolifer-ativeactivitiesinPC-3humanprostatecarcinomacells(Fernandes etal.,2014).Phytochemical investigationshavefoundthe pres-enceofflavanonesandchalconesintheethylacetateextractfrom itsfruits(Pavanetal.,2009)andphenolic contentsintheethyl acetate, ethanol, and hexane extracts fromthe leaves, particu-larlyflavonoids(Coutinhoetal.,2010).Ferreiraetal.(2013)has demonstratedthepresenceofmyricitrin,quercetin,andmyricetin in ethyl acetate in the extract from the leaves of C. adaman-tium.Thehydro-alcoholicextractofC.adamantiumfruitpeelshas
http://dx.doi.org/10.1016/j.bjp.2016.09.007
anti-inflammatory,antihyperalgesic,andantidepressantactivities inrodents(Souzaetal.,2014).
The fruits of C. adamantium are restricted to the period of harvesting (Coutinho et al., 2008).Conservation alternatives to improve the availability of pulp, such as spray drying, has been widely used in the food industry (Tonon et al., 2009, 2013)presenting good retention in bioactive compounds used to transform the pulp/fruit juice into powder form (Chen et al., 2014), allowing prolonged storage and greater sta-bility of the product, giving it a longer shelf life (Souza et al., 2014; Mahdavi et al., 2014). Together with this idea, recent technological advances,such as the microencapsulation technique, have helped to solve these issues and renewed interest in natural products in drug discovery (Lescano et al., 2014).
Phenoliccompounds,particularlyflavonoids,havefree-radical scavengingpropertiesandalsoinhibitlipidperoxidation.The pres-enceofflavonoidsandchalconesinthehumandietcanreducethe riskofcancersandtumorsandalsoexhibitseveralactivities,suchas antibacterial,antifungal,anti-inflammatory,antileishmanial, anti-malarial,andanti-HIVprotease(Hodeketal.,2012;Tewtrakuletal., 2003;Djeridaneetal.,2006;Cabrera etal.,2007;Nowakowska, 2007).
Therefore,thestudyofmicrocapsuledfruitsofC.adamantium
enablesscientific knowledge of thepharmacological properties of the plant, considering that there may be loss of bioac-tivecompoundsandthemicroencapsulationtechniquepromotes its preservation. Thus, this study aims to evaluate the anti-inflammatory parameters and antinociceptive effects of the microencapsulatedpulpofC.adamantium(MPCA)inrodents.
Materialsandmethods
Plantmaterial
Fruitsof Campomanesiaadamantium(Cambess.)O.Berg, Myr-taceae,werecollectedat“Cerrado,”Brazil,onNovember2014.A voucherspecimenwasdepositedintheherbariumoftheFaculty ofBiologicalSciencesofUFGD(DDMS4602).Fruitsweresanitized andthepulp,peel,andseedswereseparated.Thepulpwaspacked inrigidpolypropylenecontainersandstoredat−18◦Cuntiluse.
High-performanceliquidchromatography(HPLC)
The solvents employed were methanol (HPLC grade, Tedia Company,Fairfield,OH,USA)andacetonitrile(HPLCgrade,Tedia Company,Fairfield,OH,USA).SamplesofthepulpofC. adaman-tiumwerepreparedwith1gofpulpextractdissolvedin5mlof methanolinanultrasoundfor20min.SamplesofMPCAwere pre-paredwith5gofmicrocapsulesthatwereextractedwith25mlof methanolinanultrasoundfor20minandthedriedmaterialintoa chapelwasreconstructedin5mlofmethanol.
Thesamplesandstandardswereanalyzedusingananalytical HPLC(Varian210)system,withaternarysolventdeliverysystem andanautosampler.Aphotodiodearraydetectorwasmonitored at=200–800nm.TheHPLCcolumnwasC-18(25cm×4.6mm;
particlesize,5m;Luna,Phenomenex,Torrance,CA,USA),with
a small pre-column (2.5cm×3mm) containingthe same
pack-ingtoprotect theanalytical column.Theflowrateandinjected volumewere1.0mlmin−1and20l,respectively.All
chromato-graphicanalyseswereperformedat22◦C.
The elutionwas conducted using in 0min, acetonitrile12%, water50%,andmethanol38%;in40min,acetonitrile10%,water 10%,andmethanol80%;andin45min,returningtotheinitial con-dition.
ThesubstancesusedinHPLCanalysiswereisolatedfromthe leavesofC.adamantium.CompoundswerepurifiedbyHPLC, result-inginpuritybetween86%and96%.Thesubstancesweredissolved separatelyinmethanoltoaconcentrationof10g/mldegree
chro-matographicpreparationofstocksolutionsusedintheanalysisby HPLC.ThestandardswereeasilyidentifiedbytheirUVabsorption spectraandretentiontimes.Thesubstancesfoundintheextracts wereunambiguouslyidentifiedbyperformingcoinjection experi-mentsinwhichaliquotsofsamplesandstandardsweremixedand dilutedtoaknownvolumeandanalyzedbyHPLC.
MicrocapsulationofCampomanesiaadamantiumfruits
Afterpreliminarytestsandthroughotherstudiesdescribedin theliterature,microcapsuleswereproducedusingmaltodextrin8% (DE20Maltogill,Cargill,Uberlândia,Brazil),gumArabic8%(Synth, Brazil),andchitosan8%(Purifarma,SãoPaulo,Brazil)purchased fromJKLAB(QuímicaDiagnósticaeSeguranc¸aLtda)(Oliveiraetal., 2014).Sampleswerepreparedusing24%encapsulatingagent,16% distilledwater,and60%pulp.Themixtureofeachformulationwas homogenizedinanUltra-Turraxataspeedof18,000rpmuntil com-pletedissolutionofthecarrieragent,obtainingsamplescomprising 30%solids(encapsulatingagentandpulp).Theatomization pro-cesswasconductedinaconcurrentflowpatternusingaminispray dryer–LM(modelMSD1.0LABMAQ).Sampleswerefedintothe atomizerataflowrateof0.5lh−1witha1.2mmnozzlediameter,
airflowof35lmin−1,andadryingairtemperatureof180◦C.The
determinationofmoisturecontentandascorbicacid(AOAC,2000) wereperformedonfreshandmicroencapsulatedpulpofguavira.
Experimentalanimals
MaleandfemaleSwissmice(20–25g)wereobtainedfrom Uni-versidade Federal daGrandeDourados (UFGD)biotherium. The animalswerekeptincollectivecagesunder controlled temper-ature(23±1◦C)andlightconditions(12-hlight/darkcycle),and
hadaccesstofoodandwateradlibitum.The23/2014protocolwas approvedbytheEthicsCommitteeonAnimalUse(CEUA/UFGD).
Pleurisy
Different groups of femaleSwiss mice (n=5 animals/group) wereorallytreatedwithMPCAatdosesof100and300mg/kgor vehicle(0.9%salinesolution,alsocalledthecontrolgroup).The positivecontrolgroupreceiveddexamethasonesubcutaneouslyat adoseof1mg/kg.Pleurisywasinducedinexperimentalgroupsby intrapleuralinjectionof100lof1%carrageenandilutedinsaline,
after1hoftreatment,aspreviouslydescribed(Veloetal.,1973).The naivegroupreceived100lofsterilesalinebyintrapleural
injec-tion.After4h,animalswereeuthanizedandthepleuralcavitywas washedwith1mlphosphate-bufferedsaline.Analiquotof20lof
lavage(exudate)wascollectedfromthepleuralcavity,anddiluted withTurcksolution(1:20)andusedfortotalleukocytecountina Neubauerchamber(Kassuyaetal.,2009).
Formalin-inducednociception
Sixtyminbeforeformalininjection,maleSwissmice(n=6 ani-mals/group)weredividedintogroups:dexamethasone(1mg/kg, subcutaneousroute),MPCA(100and300mg/kg,oralroute),and vehicle(salinesolution,0.9%,oralroute).Afterrespective treat-ment,20lofsalinecontaining2.5%offormalinwasinjectedin
formalininthepaw(Kassuyaetal.,2009).Afterthat,animalswere submittedtocoldsensitivity,pawedemameasurement.
Openfieldtest
Fifty minutes after oral treatment with MPCA (100 and 300mg/kg)orvehicle(salinesolution,0.9%,oralroute),themice wereplacedindividuallyinthecenterofthearena,andthe behav-iorwasquantified for 5min.The number of “squares”invaded (ambulation)inthecenterandtheperipheryofthearenawere ana-lyzed.Ambulationwasusedtoevaluatethehorizontalmovement (Piccinellietal.,2015).
Carrageenan-inducedpawedema
DifferentgroupsofmaleSwissmice(n=5animals/group)were orallytreatedwithMPCA(100and300mg/kg),orvehicle(control group).Anothergroupwastreatedsubcutaneouslywith dexameth-asone(1mg/kg). After1h, animals receiveda solutionof 50l
carrageenaninjection(300g/paw)inthelefthindpaw.Theother
pawreceivedthesamevolumeofsterilesaline0.9%.Thepaw vol-umewasmeasuredat1,2,3,and4haftercarrageenaninjection withaplethysmometer.Theresultswereexpressedasthe differ-encebetweentheleftandrightpawsateachtime(Kassuyaetal., 2009).
CFA-inducedpawinflammation,coldandmechanical hyperalgesia,anddepression
Animals were subjected to induction of peripheral inflam-mation by administration of 20l of CFA (Freund’s Complete
Adjuvant) into the right paw. For 10 days after CFA, different groupsofmaleSwissmice(n=6/group)wereorallytreatedwith MPCA (100mg/kg).Basal values of mechanical sensitivity were determinedbeforeCFAinjection.Pawedemawasperformedas pre-viouslydescribedat1,2and4honthefirstdayandonceperday for10days.Inaddition,responsetoacetone(sensitivitytocold), tailsuspension(toanalyzedepression),andkneeedemawerealso performed.
Cold hyperalgesia was measured by the acetone test as describedpreviously(25).Aneedleconnectedtoasyringewasused todrop30lofacetoneonthepawandtheduration(inseconds)of
thepawwithdrawalwasrecorded.Minimalandmaximalcut-offs wereassignedat0.5sand20s,respectively.Pawwithdrawaldue tolocomotionorweightshiftingwerenotcountedandsuchtrials wererepeated(Ferreiraetal.,2013).
MechanicalsensitivitywasmeasuredwithElectronic analges-imeter(Aquinoetal.,2015)at0(basalvalues),1,2,and4hafter theinjectiononthefirstdayandonceperdayfor10days.Oral treatmentwasadministereddailyfor10daysafterCFAinjection.
Thicknesspawedemawasassessedusinga plethysmometer 30minbeforeanytreatmentand after1hofformalin injection. Theresultswereexpressedinmicrolitersasthedifferencebetween thebaselineandpost-injectionedemavalues,withmodifications ofKassuyaetal.(2009).Onthe10thdayafterCFAinjection, assess-mentofkneeedemawasperformedinmice,usingamicrometer.
Tailsuspensiontestisadepressionmodelthatassesses immo-bilityofmice.Micewereindividuallysuspendedonanacrylicbox withtheirtailsattachedwithtape.Thebehavior wasfilmedfor 6minandthedurationofimmobilitywasmeasuredinseconds.
Statisticalanalyses
Theresultsareexpressedasmean±standarderrorofthemean.
Forcomparisonofresultsbetweenexperimentalgroups,analysis ofvariance(one-wayandtwo-way ANOVA)wasused,followed
mv
10 40
10 20 30
1
2 3 5
6 7
4
40 (min)
mv
10 40
10 20 30
1
2 3 5
6 7
4
40 (min)
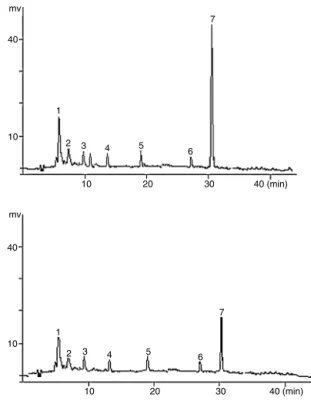
Fig.1.ChromatogramofthepulpandmicrocapsulesofCampomanesiaadamantium
obtained by HPLC. Identified substances: 3,5,7,3′,4′,5′-hexahydroxy-flavonol
(peak 1), 3,5,7,3′,4′,5′-hexahydroxy-flavonol-3-O-␣-l-arabinofuranoside (Peak 2), 3,5,7,3′,4′,5′-hexahydroxy-flavonol-3-O-␣-l-raminopyranoside (peak 3), 7-dihydroxy-5-metoxiflavanone(peak4), 6-methyl-7-hydroxy-5-metoxiflavanone (peak 5), 2′,4′-dihydroxy-6′-metoxichalcone (peak 6), and 2′,4′-dihydroxy-5′
-methyl-6′-metoxichalcone(peak7).
bytheNewman–KeulsorBonferronitest.Thenumberofanimals pergroupisindicatedinthelegends.Statisticaldifferenceswere consideredsignificantatp<0.05.Asterisk(*)or(#)denotesa sig-nificantdifferencebetweengroups.
Results
Analyses by high-performance liquid chromatography in the pulp and the C. adamantium microcapsules identified the
0.0 0.5 1.0 1.5 2.0 2.5
Vehicle
Microcapsules Dexa
1 300
100 mg/kg
4 hours after carrageenan injection
Naive
* *
* *
Le
uk
oc
y
te
(
x 1
0
7)
Fig.2.EffectoforaladministrationofthepulpandmicrocapsulesofCampomanesia adamantiumontheinhibitionoftheleukocytemigrationatbothdosestestedon pleurisytest.Miceweretreated1hbeforeanintrapleuralinjectionofcarrageenan, withmicrocapsules(100or300mg/kg),dexamethasone(DEXA,1mg/kg,s.c.),or salinesolution(vehicle).Naivegroup,alsotreatedwithsaline,receivedan intrapleu-ralinjectionofsterilesaline.Eachbarrepresentsthemean±SEMoffiveanimals.
0 50 100 150
Vehicle
Microcapsules Dexa
1 300
100 mg/kg
Formalin phase I
A
*
* *
Se
c
ond
s
0 100 200 300 400
Vehicle
Microcapsules Dexa 1 300
100 mg/kg
Formalin phase II
B
*
* *
Second
s
0.0 0.2 0.4 0.6 0.8
Vehicle
Microcapsules Dexa
1 300
100 mg/kg
C
**
** **
Paw oedema
Pa
w o
e
de
ma
(m
l)
0 5 10 15
20 Cold sensitivity
Vehicle
Microcapsules Dexa
1 300
100 mg/kg
***
*** ***
D
S
econd
s
Fig.3. EffectofthepulpandmicrocapsulesofCampomanesiaadamantiumonformalin-inducedpawlickingorbitinginmiceandonformalin-inducedcoldsensitivityand edemainducedbyformalin.(A,B)Theessentialoilofmicrocapsulesatdosesof100and300mg/kgpresentedantinociceptiveeffectsinphaseIandIItest.(C)Edemainduced byformalin.(D)EffectoftheMPCAoncoldsensitivityaftertreatmentwithmicrocapsulescoldsensitivitywithacetoneinmice.Eachbarrepresentsthemean±SEMofsix
animals.*p<0.001whencomparedtothecontrolgroup.Differencesbetweengroupswereanalyzedbyanalysisofvariance(one-wayANOVA)followedbytheNewman–Keuls test.
0.0 0.2 0.4 0.6 0.8 1.0
Vehicle
Microcapsules Dexa
1 300
100 mg/kg
* *
A
1.0 hour after carrageenan
**
Pa
w o
e
de
ma
(
ml)
0.0 0.2 0.4 0.6 0.8 1.0
Vehicle
Microcapsules Dexa
1 300
100 mg/kg
2.0 hour after carrageenan
B
**
** **
Pa
w o
e
de
ma
(
ml)
0.0 0.2 0.4 0.6 0.8
Vehicle
Microcapsules Dexa
1 300
100 mg/kg
____________________________ 3.0 hour after carrageenan
C
**
** **
Pa
w o
e
de
ma
(
ml)
0.0 0.2 0.4 0.6 0.8
Vehicle
Microcapsules Dexa
1 300
100 mg/kg
____________________________
4.0 hour after carrageenan
D
**
** **
Pa
w
oe
de
ma
(
ml)
Fig.4. EffectofthepulpandmicrocapsulesofCampomanesiaadamantiumincarrageenan-inducedpawedemainmice.Animalsreceivedmicrocapsules(100or300mg/kg,
p.o.)orcontrol(vehicle)ordexamethasone(DEXA,1mg/kg,s.c.)andafter1h,anintraplantarinjectionofcarrageenan(300g/paw).Graphics(A),(B),(C),and(D)represent
substances:3,5,7,3′,4′,5′-hexahydroxy-flavonol(peak1),3,5,7,3′,4′,
5′-hexahydroxy-flavonol-3-O-␣-l-arabinofuranoside(peak2),3,5,
7,3′,4′,5′-hexahydroxy-flavonol-3-O-␣-l-raminopyranoside (peak
3),7-dihydroxy-5-metoxiflavanone(peak4), 6-methyl-7-hydroxy-5-metoxiflavanone (peak 5), 2′,4′-dihydroxy-6′-metoxichalcone
(peak6),and2′,4′-dihydroxy-5′-methyl-6′-metoxichalcone(peak
7)(Fig.1).
MPCApreventedmigrationofleukocytestothepleuralcavity inducedbycarrageenan
TheoraladministrationofC.adamantiummicroencapsulated pulpsignificantlyinhibitedtheleukocytemigration atalldoses tested(100and300mg/kg),beinggreateratdoseof100mg/kgwith maximalinhibitionof50±4%comparedtocontrols.The
microen-capsulatedalsoshowedsignificantactivity,reducingtheprotein extravasationatbothdosestested,butwithbetterresponseatdose of300mg/kgwithmaximuminhibitionof67±3%.Forthepositive
controltheinhibitionwas91±2%(Fig.2).
MPCApreventednociception,coldsensitivity,andneurogenic edemainducedbyformalininrats
MPCA(Fig.3A, Phase I) produced significantantinociceptive effectsinthefirstphasewhencomparedwiththecontrolgroup. Atthedoseof100mg/kg,maximuminhibitionwas59±2%,while
atthedoseof300mg/kg,itwas63±3%,andfordexamethasone,it
was73±2%.
MPCAatdosesof100and300mg/kgsignificantlyreduced lick-ingtimeinthesecondphaseoftheformalintestinrats(Fig.3B, PhaseII).Atadoseof100mg/kg,maximuminhibitionwas72±1%,
atadoseof300mg/kg,itwas70±2%,andfordexamethasone,it
was85±3%.
MPCAcausedareductioninpawedemainducedbyformalin.
Fig.3Cshowstheresultsofpawedemawithmaximalinhibitionof 69±1%forMPCAatthedoseof300mg/kg,64±3%atthedoseof
100mg/kg,and87±2%fordexamethasone.
MPCA(100and300mg/kg)significantlyattenuatedtheduration ofcoldhypersensitivityafterformalininjection.Animalsalmostdid notmoveandraisedtheirpawsafewtimesafteracetone applica-tion.Atadoseof100mg/kg,maximuminhibitionwas55±2%,ata
doseof300mg/kg,itwas60±2%,andfordexamethasone,86±3%.
Furthermore,hypersensitiveresponsetocoldwas<10s(Fig.3D). Openfieldresultsdemonstrated thattheorallyadministered microcapsuleswere not capableof reducing locomotor activity whencomparedwiththecontrolgroup(resultsnotshown).
MPCApreventedcarrageenan-inducedpawedema
Anhourafterthecarrageenan-inducedinflammation,the con-trolgroupcontinuedtoshowedema,whereasthegroupstreated withmicrocapsulesatdosesof100and300mg/kgshoweda signifi-cantdecreaseinedemacomparedtothecontrolgroup(Fig.4A)and thisreductioncontinuedafterthesecond,thirdandfourthhourof observation(Fig.4B–D).Fig.4showspawedemawasalsoinhibited atalltimes,andmaximalinhibitionatthedoseof100mg/kgwas 52±2%,63±3%,86±3%,and77±2%,after1,2,3,and4h,
respec-tively.Theinhibitions,doseof300mg/kg,were53±2%,68±3%,
86±2%,and80±3%after1,2,3,and4h,respectively.Theanimals
treatedwithdexamethasone,thepositivecontrol,showeda signif-icantreductionatalltimepoints,withinhibitionsof93±7%after
2hand85±5%after4h.
0 1 2 3 4 5
0.0 0.5 1.0 1.5
*
*
A
Hours post-injection CFA
Mechanical threshold 50 %
von Frey (g)
0 1 2 3 4 5 6 7 8 9 10 11 12 0
1 2 3 4
CFA (Vehicle) CFA + microcapsule 100 mg/kg Basal
B
*
* * * * * * * * *
Days post-injection CFA
Mechanical threshold 50 %
von Frey (g)
0 1 2 3 4 5 6 7 8 9 10 11 12 0.0
0.5 1.0 1.5
C
*
* * * * * * * * *
Days post-injection CFA
Paw oedema (mL)
Fig.5.EffectoftheoraladministrationofmicroencapsulatedpulpofCampomanesia adamantium(MPCA)(100mg/kg)onmechanicalhyperalgesiaandpawedemain mice.(A)AnimalsreceivedtheMPCA(10mand300mg/kg,p.o.)orvehicle,and after1h,20lofCFA(Freund’sCompleteAdjuvant)injectionintherighthindpaw.
MechanicalsensitivitywasmeasuredwithvonFreyanalgesimeterat1,2,and4 aftertheinjectiononthefirstdayand(B)onceadayfor10days.(C)Effectoforal administrationofMPCAonCFA-inducedpawedema.Pawedemawasperformedas previouslydescribedonceadayduring10days.Eachbarrepresentsthemean±SEM
ofsixanimals.*p<0.001whencomparedtothecontrolgroup.Differencesbetween groupswereanalyzedbyanalysisofvariance(two-wayANOVA)followedbythe Bonferronitests.
MPCAfruitspreventedCFA-inducedmechanicalandcold hyperalgesiaandkneeedema
Theresults ofmechanical hyperalgesia assessedby analges-imeter are shown in Fig. 5. Treatment with CFA and MPCA demonstratedasignificantincreaseinpawwithdrawalthreshold after10daysoftreatmentwhencomparedwiththecontrolgroup. MicrocapsulesofC.adamantiumpulppresentedaneffectagainst mechanicalhyperalgesiaatalldaysanalyzed(Fig.5A).Onthefirst day,significantresultswereobservedafter2and3hofCFA injec-tionatadoseof100mg/kgwithmaximalinhibitionof61±1%after
2hand55±2%after3h.On day9,theydemonstratedmaximal
inhibitionof86±2%andreductionofedemaonday6withmaximal
inhibitionof87±3%(Fig.5B).Significantdifferencesin
mechani-calthresholdswereobservedinthegrouptreatedwithCFAand microencapsulatedpulpofC.adamantium.Theseresultsarevery similartothoseofthecontrolgroup.
0 2 4 6 8 10
*
CFA (Vehicle) CFA +Microcapsule 100 mg/kg
Day 1 after CFA-injection
A
Cold sensitivity (s)
0 2 4 6 8 10
CFA (Vehicle) CFA +Microcapsule 100 mg/kg
***
Day 10 after CFA-injection
B
Cold sensitivity (s)
0.0 0.1 0.2 0.3 0.4 0.5
CFA (Vehicle) CFA +Microcapsule 100 mg/kg
Knee edema - 10th day
**
C
Knee edema (
µ
m)
0 50 100 150 200
CFA (Vehicle) CFA + Microcapsule 100 mg/kg
Tail suspension - 10th day
***
D
Seconds
Fig.6.(A)EffectofmicroencapsulatedpulpofCampomanesiaadamantium(MPCA)oncoldsensitivityaftertreatmentwithmicrocapsulesatthedoseof100mg/kgandan injectionofCFA(Freund’sCompleteAdjuvant)intherighthindpaw.(B)EffectofMPCAoncoldsensitivityaftertreatmentwithmicrocapsulesonthe10thdaysafterCFA injection.(C)EffectofMPCAonkneeedemaatthe10thdayafterCFAinjection.Thetestwasperformedinmiceusingamicrometer.(D)EffectofMPCAontailsuspensiontest. Micewereindividuallysuspendedonanacrylicboxwiththeirtailsattachedwithtape.Behaviorhasbeenfilmedfor6minandthedurationofimmobilitywasmeasuredin seconds.Eachbarrepresentsthemean±SEMofsixanimals.*p<0.05,**p<0.01,***p<0.001whencomparedtothecontrolgroup.Differencesbetweengroupswereanalyzed byanalysisofvariance(one-wayANOVA)followedbytheNewman–Keulstest.
movementswhencomparedwiththecontrol(Fig.6A),andoneday afterCFAinjection,themaximuminhibitionwas44±2%.Therewas
adecreaseinlickingtimeafter10days.Onthefirstday,lickingtime was6sandonthelastday,itwas4s,whileonthe10thdayafter applyingtheCFAthemaximuminhibitionwas62±3%(Fig.6B).
Forkneeedematestafter10dayswecouldseemaximum inhi-bitionof63±1%(Fig.6C)andfortailsuspensiontestmaximum
inhibitionwas67±2%(Fig.6D),whencomparedwiththecontrol
group.
Discussion
DespiteitseconomicandculturalimportanceintheBrazilian Cerrado,therearefewclinicaltrialswiththespeciesC.adamantium. Manyethnopharmacologicalstudieshavereported pharmacolog-icalefficiency (Souzaet al.,2014).Myrtaceae family plants are distributed throughout the Cerrado region of Brazil and some species have been used to treat pain, inflammation and other diseases. These anti-inflammatory properties are attributed to flavonoidsandchalcones,presentasthemainconstituentsofthe extractofthisplant(Pavanetal.,2009;Ramosetal.,2007;Coutinho et al., 2009) and fruit pulp. Flavonoids (Coutinho et al., 2008) arewidelydistributedamongplantsandexhibitpharmacological effectsontheinflammatoryprocess.
The present study demonstrated the anti-inflammatory and antinociceptive analyses of C. adamantium microencapsulated pulp.Experimental datademonstrated C. adamantium microen-capsulated pulp inhibited leukocyte migration, inflammatory, neurogenicpainandedema,CFA-inducedpawinflammationand mechanichypersensitivitymeasurementsuggestingtheiruseasa nutraceuticalorpharmacologicalagent.
Previous results from our group have demonstrated that the hydroalcoholic extract of the fruit peel of C. adamantium
exhibitedanti-inflammatoryand antihypernociceptiveeffectsin pleurisyand mechanicalnociception(Souzaetal.,2014).In the present study,theresultshave indicated theanti-inflammatory and antinociceptive actions of the microencapsulated pulp C. adamantium. Experimental data have demonstrated that MPCA inhibitedleukocytemigration,inflammatoryandneurogenicpain, andedema,suggestingtheiruseasanutraceuticalor pharmaco-logicalagent.Thosefindingscorroboratewiththepopularuseof
C.adamantiumfruitsasanti-inflammatory(Ramos etal.,2007). The anti-inflammatory activity of MPCA in acute inflammation was evaluated by induction of a carragenan-induced pleurisy model.Thisisaclassictesttoevaluatethistypeofinflammation and theformation ofa pleural exudate inthecavity is charac-terizedbyinfiltrationof polymorphonuclearleukocytesand the releaseofseveralimportantchemicalmediatorsinthe inflamma-toryprocess(Oliveiraetal.,2014).Anti-inflammatorydrugs,such asindomethacinanddexamethasone,inhibitleukocytemigration between3and6haftercarrageenanadministration.
TreatmentwithMPCA1hbeforecarrageenaninjectionwasable to significantlydecrease the total leukocyterecruitment in the pleural cavity. Thisresult wasalsoconfirmed byFerreira etal. (2013)andSouzaetal.(2014).
In thepaw edema test, there was a significant decrease in swellingafter3hfromthetimeofadministrationwhencompared with thecontrol group, as previously observed (Ferreiraet al., 2013).AccordingtoSouzaetal.,thisprobablyhappens because ofareductioninlocalvascularpermeability.Thus,weconcluded thatC.adamantiumpromotesareductionincellleakage,leadingto theconsequentreductionofproinflammatorymediators.
HPLC analyses of C. adamantium microcapsules have iden-tified 2′,4′-dihydroxy-5′-methyl-6′-metoxichalcone as a major
theisolationandidentificationofthechalcone(2′,4′
-dihydroxy-6′-metoxichalcona), which showed promising antiproliferative
activityagainstsomeofthehumantumorcelllinesstudied. Someofthesecompoundsandsubstancesoftheclassof chal-cones have been described as natural analgesic agents in the literature(Raygudeetal.,2012)andpresentedchemopreventive andantitumoreffects(Rahman,2012).Ferreiraetal.hasreported myricetinas apotentialcompound responsiblefor antinocicep-tiveactionofC.adamantiumextract.Maybemyricetinandother compoundsfoundinthisspeciesexhibitantihyperalgesicand anti-inflammatoryeffects.
Phytochemicalevaluationhasidentifiedthepresenceofthree typesofflavonols,twoofflavonones,andtwoofchalcones,which presentedthehighestchromatographicprofileofbothpulp micro-capsules.AccordingtoDewick (2005),theincrease in chalcone content may be related to a biosynthetic strategy for the for-mation of flavonones that could interferein theproduction of secondarymetabolitesofplantsasadefensestrategyoftheseplants (Harbone,1994).Furthermore,accordingtoCoutinhoetal.(2010)
thisincreasemaybeduetotheaccumulationofflavonoidsonthe surfaceoftheleafasaformofprotectionfromsunlightorasaplant defensemechanismagainstinsects.
Itisknownthatflavonoidsareplantpolyphenolcompounds thatpresentanalgesicandanti-inflammatorypropertiesby inhibi-tingenzymesinvolvedininflammationandpaininseveralparts of thenervous system (Coutinho et al., 2010).In the inflamed tissue, flavonoids are able to inhibit cyclooxygenase, prevent prostaglandinformationandTNFsecretion,whichareresponsible instimulatingpainreceptorsinthebrain.Flavonoidsalsodecrease analgesicactivitybyinhibitingnitricoxidesynthesisandNO pro-duction.
Weknowthatthechemistryofnaturalproductsappearstobea promisingalternativeandhasattractedtheincreasingattentionof scientiststosearchfornewpharmacologicallyactiveagents,mainly toimprovethetreatmentofpain.Therefore,thepresent investiga-tionoftheadministrationofMPCA(100mg/kgperdayfor10days) isanewapproachandhasabeneficialeffectonthetreatmentof neuropathybecauseofitsmultipleeffects:anti-inflammatory,as indicatedbytheCFA-inducedpawedematest,and antinocicep-tive,asindicatedbytheCFA-inducedcoldsensitivity,inducedknee edema,andtailsuspensiontests.
Inthisstudy,wehave observedthatthemicroencapsulation methodofspray dryinghasgreat applicability,being character-izedasaneffectivemethodandoneofextremeimportanceinthe preservationofseveralnutritionalcomponents,protectingagainst themostaggressivemethodsofprocessing.Themaintenanceof bioactive compounds, such as flavonoids in microencapsulated pulpfromC.adamantium,canalsobeobservedinthiswork.
MPCA has demonstrated anti-inflammatory and antihyper-algesic activities, probably due to the presence of bioactive compounds that interfere with inflammatory parameters, sup-portingtheuseof this plantpartin folkmedicine.In addition, the microcapsules retained the stability of the bioactive com-pounds,enhancingthe developmentofnew productsbasedon naturalproducts.Additionalstudiesareneededtoelucidatethe exactmechanismofactionandthecompoundsresponsibleforthis activity.
Ethicaldisclosures
Protectionofhumanandanimalsubjects. Theauthorsdeclare
thattheproceduresfollowedwereinaccordancewiththe regula-tionsoftherelevantclinicalresearchethicscommitteeandwith thoseoftheCodeofEthicsoftheWorldMedicalAssociation (Dec-larationofHelsinki).
Confidentialityofdata. Theauthorsdeclarethatnopatientdata
appearinthisarticle.
Righttoprivacyandinformedconsent. Theauthorsdeclarethat
nopatientdataappearinthisarticle.
Authors’contributions
DZV(PhDstudent):contributedtothecollectionand identi-ficationof plantsamples,performingthelaboratorywork,data analysis, and drafting of the article. VSO: contributed to the developmentof C. adamantiummicrocapsules. JSA: contributed tocarrying outtheinvivolaboratorywork.ACP:contributedto dataanalysisanddraftedthearticle.CALC:Professorresponsible for high-performanceliquid chromatography.CALK: design and supervisionoftheinvivoexperiments.IRM:contributedto criti-calreadingofthemanuscript.EJSA:designedthestudy,supervised the laboratorywork, and contributed to critical reading of the manuscript.Alloftheauthorshavereadthefinalmanuscriptand approvedthesubmission.
Conflictsofinterest
Theauthorsdeclarenoconflictsofinterest.
Acknowledgements
The authors alsowish to thank theFundac¸ão de Amparo à PesquisadoEstadodoMatoGrossodoSul(FUNDECT)forthe finan-cialsupport,throughtheprojectsFUNDECT/CAPESn◦ 45/2014–
PAPOSREDEPRÓ-CENTROOESTE–FASEII–N◦23/200.622/2014
andtheCNPq.
References
Aquino,D.F.S.,Piccinelli,A.C.,Soares,F.L.,Arena,A.C.,Salvador,M.J.,Kassuya,C.A., 2015.Anti-hyperalgesicandanti-inflammatoryactivityofalternanthera mar-itimaextractand2′′-O-␣-l-rhamnopyranosylvitexininmice.Inflammation1, 1–10.
AOAC,2000.OfficialMethodsofAnalysisofAOACInternational.Associationof Offi-cialAnalyticalChemists.AOAC,Maryland.
Breda,C.A.,Sanjinez-Argando˜na,E.J.,Correia,C.A.C.,2012.Shelflifeofpowdered Campomanesiaadamantiumpulpincontrolledenvironments.FoodChem.135, 2960–2964.
Cabrera,M.,Simoens,M.,Falchi,G.,Lavaggi,M.L.,Piro,O.E.,Castellano,E.E.,2007.
Syntheticchalcones,flavanones,andflavonesasantitumoralagents: biolog-icalevaluationandstructure–activityrelationships.Bioorg.Med.Chem.15, 3356–3367.
Cardoso,C.A.,Salmazzo,G.R.,Honda,N.K.,Prates,C.B.,Vieira,M.C.,Coelho,R.G., 2010.Antimicrobialactivityoftheextractsandfractionsofhexanicfruitsof Campomanesiaspecies(Myrtaceae).J.Med.Food13,1273–1276.
Chen,Q.,Zhou,Y.,Liu,X.,Wu,X.,Chen,R.,2014.Multi-objectiveoptimizationof spraydryingofjujube(ZizyphusjujubaMiller)powderusingresponsesurface methodology.FoodBioprocessTechnol.7,1807–1818.
Coutinho, I.D., Cardoso, C.A.L., Ré-Poppi, N., Melo, A.M., Vieira, M.C., Honda, N.K.,Coelho,R.G.,2009.GasChromatography–MassSpectrometry(GC–MS) evaluationofantioxidantandantimicrobialactivitiesofessentialoilof Cam-pomanesiaadamantium(Cambess.)OBerr.(Guavira).Braz.J.Pharm.Sci.45, 767–776.
Coutinho, I.D., Coelho, R.G., Kataoka, V.M.G., Honda, N.K., Silva, J.R.M., Vile-gas, W., 2008. Determination of phenolic compounds and evaluation of antioxidantcapacityofCampomanesiaadamantium leaves.Eclet.Quim.33, 53–60.
Coutinho,I.D.,Kataoka,V.M.G.,Honda,N.K.,Coelho,R.G.,Vieira,M.C.,Cardoso,C.A.L., 2010.Influênciadavariac¸ãosazonalnosteoresdeflavonóideseatividade antiox-idantedasfolhasdeCampomanesiaadamantium(Cambess.)O.Berg,Myrtaceae. Rev.Bras.Farmacogn.20,322–327.
Dewick,P.M.,2005.MedicinalNaturalProducts:ABiosyntheticApproach.John Wiley&Sons,England.
Djeridane,A.,Yousfi,M.,Nadjemi,B.,Boutassouna,D.,Stocker,P.,Vidal,N.,2006.
AntioxidantactivityofsomeAlgerianmedicinalplantsextractscontaining phe-noliccompounds.FoodChem.97,654–660.
Ferreira,L.C.,Grabe-Guimarães,A.,dePaula,C.A.,Michel,M.C.P.,Guimarães,R.G., Rezende,S.A.,Filho,J.D.S.,Saúde-Guimarães,D.A.,2013.Anti-inflammatoryand antinociceptiveactivitiesofCampomanesiaadamantium. J.Ethnopharmacol. 145,100–108.
Harbone,J.B.,1994.TheFlavonoids-advancesinResearchSince1986.Chapman& Hall,London.
Hodek,P.,Trefil,P.,Stiborová,M.,2012.Flavonoids–potentandversatilebiologically activecompoundsinteractingwithcytochromesP450.Chem.Biol.Interact.139, 1–21.
Kassuya,C.A.,Cremoneze,A.,Barros,L.F.,Simas,A.S.,Lapa,F.daR.,Mello-Silva,R., Stefanello,M.E.,Zampronio,A.R.,2009.Antipyreticandanti-inflammatory prop-ertiesoftheethanolicextract,dichloromethanefractionandcostunolidefrom Magnoliaovate(Magnoliaceae).J.Ethnopharmacol.124,369–376.
Lescano, C.H., Iwamoto, R.D., Sanjinez-Argandon, E.J., 2014. Diuretic and anti-inflammatoryactivitiesofthemicroencapsulatedacrocomiaaculeata (Are-caceae)oilonWistarrats.J.Med.Food18,656–662.
Lorenzi,H.,2000.ÁrvoresBrasileiras:ManualdeIdentificacãoeCultivodePlantas ArbóreasNativasdoBrasil.InstitutoPlantarum,NovaOdessa.
Mahdavi,S.A.,Jafari,S.M.,Ghorbani,M.,Assadpoor,E.,2014.Spray-drying microen-capsulationofanthocyaninsbynaturalbiopolymers:areview.DryTechnol.32, 509–518.
Nowakowska,Z.,2007.Areviewofanti-infectiveandanti-inflammatorychalcones. Eur.J.Med.Chem.42,125–137.
Oliveira,V.S.,Sanjinez-Argandona,E.J.,Alves,A.V.,2014.RetentionofvitaminCin microencapsulatedGuavirapulpbyspraydrying.RevistaFacultadNacionalde Agro67,202–204.
Pascoal,A.C.R.F.,Ehrenfried,C.A.,Lopez,B.G.C.,Arauj,T.M.,Pascoal,V.,Gilioli,R., Pavan,F.R.,Leite,C.Q.F.,Coelho,R.G.,Coutinho,I.D.,Honda,N.K.,Cardoso,C.A.L., 2014.AntiproliferativeactivityandinductionofapoptosisinPC-3cellsby thechalconecardamoninfromCampomanesiaadamantium(Myrtaceae)ina bioactivity-guidedstudy.Molecules19,1843–1855.
Pavan,F.R.,Leite,C.Q.F.,Coelho,R.G.,Coutinho,I.D.,Honda,N.K.,Cardoso,C.A.L.,etal., 2009.Evaluationofanti-mycobacteriumtuberculosisactivityofCampomanesia adamantium(Myrtaceae).QuimNova32,1222–1226.
Piccinelli,A.C.,Santos,J.A.,Konkiewitz,E.C.,Oesterreich,S.A.,Formagio,A.S.N.,Croda JulioZiff,E.B.,Kassuya,C.A.L.,2015.Antihyperalgesicandantidepressiveactions of(R)-(+)-limonene,␣-phellandrene,andessentialoilfromSchinus
terebinthi-foliusfruitsinaneuropathicpainmodel.Nutr.Neurosci.18,217–224.
Rahman,M.A.,2012.Chalcone:avaluableinsightintotherecentadvancesand potentialpharmacologicalactivities.Chem.Sci.J.29,1–16.
Ramos,D.D.,Cardoso,C.A.L.,Yamamoto,N.T.,2007.Avaliac¸ãodopotencial citotóx-icoeatividadeantioxidanteemCampomanesiaadamantium(Cambess.)O.Berg (Myrtaceae).Rev.Bras.Biocienc.5,774–776.
Raygude,K.S.,Kandhare,A.D.,Ghosh,P.,Ghule,A.E.,Bodhankar,S.L.,2012. Eval-uationofameliorativeeffectofquercetininexperimentalmodelofalcoholic neuropathyinrats.Inflammopharmacology20,331–341.
Souza, J.C., Piccinelli, A.C.,Aquino, D.F.S., Souza, V.V., Schmitz, W.O., Traesel, G.K., Cardoso, C.A.L.,Kassuya, C.A.L.,Arena, A.C.,2014. Toxicological anal-ysisand antihyperalgesic,antidepressant,andanti-inflammatoryeffectsof
Campomanesiaadamantiumfruitbarks.Nutr.Neurosci.,http://dx.doi.org/10. 1179/1476830514Y.0000000145.
Tewtrakul,S.,Subhadhirasakul,S.,Puripattanavong,J.,Panphadung,T.,2003. HIV-1proteaseinhibitorysubstancesfromBoesenbergiapandurateHoltt.Anim.Sci. Technol.25,503–508.
Tonon,R.V.,Brabet,C.,Hubinger,M.D.,2009.Influênciadatemperaturadoarde secagemedaconcentrac¸ãodeagentecarreadorsobreaspropriedades físico-químicasdosucodeac¸aíempó.Cien.Tec.Aliment.9,444–450.
Tonon,R.V.,Brabet,C.,Hubinger,M.D.,2013.Aplicac¸ãodasecagemporatomizac¸ão paraaobtenc¸ãodeprodutosfuncionaiscomaltovaloragregadoapartirdoac¸aí. InclusãoSocial6,70–71.