www.jped.com.br
ORIGINAL
ARTICLE
Pneumococcal
meningitis:
epidemiological
profile
pre-and
post-introduction
of
the
pneumococcal
10-valent
conjugate
vaccine
夽
,
夽夽
Tatiane
E.
Hirose
a,∗,
Eliane
M.C.P.
Maluf
b,c,
Cristina
O.
Rodrigues
aaDepartmentofPediatrics,HealthSciencesSection,UniversidadeFederaldoParaná(UFPR),Curitiba,PR,Brazil bDepartmentofInternalMedicine,HealthSciencesSection,UniversidadeFederaldoParaná(UFPR),Curitiba,PR,Brazil cDisciplineofFamilyHealth,Maternal-ChildSection,UniversidadePositivo,Curitiba,PR,Brazil
Received25February2014;accepted10July2014 Availableonline16October2014
KEYWORDS Streptococcus pneumoniae; Pneumococcal meningitis; Invasive pneumococcal disease; Vaccines
Abstract
Objectives: Toevaluatethepossibleeffectsoftheintroductionofthepneumococcalconjugate 10-valentvaccinescheduleinthestateofParana onpneumococcalmeningitiscasesandto assessthedistributionofserotypesamongcases.
Method: Cross-sectional study with retrospectivedata collection of casesof pneumococcal meningitisinthestateofParanáreportedtoSistemadeInformac¸ãodeAgravosdeNotificac¸ão (SINAN),from1998to2011.Atotalof1,339casesofpneumococcalmeningitiswereanalyzed; 1,205 casesfromthepre-vaccineperiod (1998-2009)were comparedto134cases fromthe post-vaccineperiod(2010-2011).Descriptiveandcomparativestatisticalanalyses(chi-squared testandprevalenceratio)wereperformedusingJMP5.1.2statisticalsoftware(JMPStatistical Discovery,NorthCarolina,USA) andEPIINFO6(Centersfor DiseaseControlandPrevention, Georgia,EUA).
Results: Therewasasignificantreductioninthemeanratesofincidenceandmortalityinthe generalpopulation.Theanalysisofcasesinthepre-andpost-vaccinationperiodsintheage groupscoveredbyvaccination(youngerthan2years)showedsignificantreductionsinincidence rates(6.01cases/100,000to2.49cases/100,000individuals)andmortality(1.85cases/100,000 populationto0.47cases/100,000 population),whilethemeanlethalityratedidnotchange significantly.Therewas asignificantreductionincaseswhose serotypesareincludedinthe vaccine(80.7%to53.3%).
Conclusion: Evenafter ashort timeofuse, the10-valentpneumococcal conjugatevaccine has already hada significantimpact inreducing the incidenceandmortality ofmeningitis
夽 Pleasecitethisarticleas:HiroseTE,MalufEM,RodriguesCO.Pneumococcalmeningitis:epidemiologicalprofilepre-andpost-introduction
ofthepneumococcal10-valentconjugatevaccine.JPediatr(RioJ).2015;91:130---5.
夽夽
StudyconductedatUniversidadeFederaldoParaná(UFPR),Curitiba,PR,Brazil.
∗Correspondingauthor.
E-mail:tatianehirose@hotmail.com(T.E.Hirose).
http://dx.doi.org/10.1016/j.jped.2014.07.002
cases amonginfants,aswellasthereductionofcaseswhose serotypesareincludedinthe vaccine.
©2014SociedadeBrasileiradePediatria.PublishedbyElsevierEditoraLtda.Allrightsreserved.
PALAVRAS-CHAVE Streptococcus pneumoniae; Meningite pneumocócica; Doenc¸a pneumocócica invasiva; Vacinas
MeningitePneumocócica:perfilepidemiológicopréepósaintroduc¸ãodavacina pneumocócicaconjugada10valente
Resumo
Objetivos: Avaliarospossíveisefeitos daintroduc¸ãodavacina pneumocócica conjugada 10 valentenocalendáriovacinalnoParanásobreoscasosdemeningitepneumocócica;avaliara distribuic¸ãodossorotiposdentreoscasos.
Método: Estudoobservacional,transversal, comcoletade dadosretrospectivadoscasosde meningite pneumocócica noEstado doParaná, notificadosao SINAN,no período de1998 a 2011.Foramanalisados1339casosdemeningitepneumocócicaecomparadosos1205casosdo períodopré-vacina(1998a2009)comos134doperíodopós-vacina(2010a2011).A análise estatísticadescritivaecomparativa(testequi-quadradoerazãodeprevalência)foirealizada nosoftwaredeestatísticaJMP5.1.2(JMPStatisticalDiscovery,CarolinadoNorte,EUA)eno ProgramaEPIINFO6.
Resultados: Observou-sereduc¸ãosignificativadastaxasmédiasdeincidênciaemortalidade na populac¸ão geral. A análise dos casos nos períodos pré e pós-vacina nas faixas etárias contempladaspela vacinac¸ão(menoresde2anos)mostrou reduc¸ões significativasdastaxas de incidência (6,01 casos/100.000 para 2,49casos/100.000 habitantes), mortalidade (1,85 casos/100.000 habitantes para 0,47 casos/100.000 habitantes), enquanto que a letalidade médianãoapresentouvariac¸ãosignificativa.Houvereduc¸ãosignificativadoscasoscujos soroti-posestãoincluídosnavacina(80,7%para53,3%).
Conclusão: Mesmo com um tempo reduzido de uso, a vacina pneumocócica conjugada 10 valentejáapresentouumimpactorelevantenadiminuic¸ãodoscoeficientesdeincidênciae mor-talidadedoscasosdemeningiteentreoslactentes,alémdereduc¸ãodecasoscujossorotipos estãoincluídosnavacina.
©2014SociedadeBrasileiradePediatria.PublicadoporElsevierEditoraLtda.Todososdireitos reservados.
Introduction
Streptococcus pneumoniae (pneumococcus) can be found in the nasopharynx and oropharynx mucosa of healthy humans,andisimportantduetoitsmorbidityandmortality relatedtodiseasessuchasmeningitis,pneumonia,and sep-ticemia,amongothers.1 InBrazil,it isthesecond-leading
causativeagentofbacterialmeningitis,followingNeisseria meningitidis.2
Pneumococcal diseasepreventionis primarily basedon activeimmunization.Atotalof93pneumococcusserotypes3
have been identified, according to the antigenicity and immunogenicity of the polysaccharide capsule, the main bacterial virulence factor. The polysaccharide antigens induce a serotype-specific immunologicalresponse,which is very useful for the composition of pneumococcal vac-cines. The 23-valent vaccine consistsof purifiedcapsular polysaccharidesof23 serotypesof Streptococcus pneumo-niae,producesthymus-independentimmuneresponse,and isthereforeindicatedonlyforchildrenolderthan2yearsof age.Whenthepolysaccharidesareindividuallyconjugated toproteincarriers,thereisimmunogenicityimprovement, as they are capable of triggering the immune memory response(thymus-dependent) andcan be administeredto childrenyounger than2yearsof age,themain age group affectedbyinvasivepneumococcaldisease.1,4,5
The pneumococcalconjugatevaccinesreleased by reg-ulatoryagenciesand currentlymarketed inBrazil arethe 10-valentand13-valent,whichprotectagainsttenand13 pneumococcalserotypes,respectively.The10-valent pneu-mococcalconjugate vaccine became part of the National Immunization Program(NIP) schedule from 2010 for chil-drenyoungerthan24months.The23-valentpneumococcal polysaccharidevaccineisavailableattheSpecial Immuno-biological Reference Centers (Centros de Referências de Imunobiológicos Especiais---CRIE) to patients older than 2 yearsofage consideredat risk for invasivepneumococcal disease,andinstitutionalizedelderlypatientsolderthan60 years.6
Theobjectiveofthisstudywastoevaluatetheeffectof theintroduction ofthe10-valentpneumococcalconjugate vaccineintotheNIPvaccinationscheduleonthe epidemio-logicalindicatorsandserotypesofpneumococcalmeningitis inthestateofParaná,Brazil.
Methods
(SistemadeInformac¸ãodeAgravosdeNotificac¸ão---SINAN), fromJanuaryof1998toDecemberof2011.
The analysis of cases of pneumococcal meningitis was performedbycomparingtheperiodsaccordingtothe intro-ductionof10-valentpneumococcalconjugatevaccineinthe NIP.Thepre-vaccineperiodincludedtheyears1998to2009, andthepost-vaccineperiod,theyears2010and2011.
The indicators used for the analysis of cases over the years, or in the pre- and post-vaccine periods, based on population data from the Brazilian Institute of Geog-raphy and Statistics (Instituto Brasileiro de Geografia e Estatística---IBGE)wereasfollows:
• Meanincidencerate:(meannumberofmeningitiscases
intheperiod/meanpopulationoftheperiod)×100,000 • Mean mortality rate: (mean number of deaths due to
pneumococcalmeningitisintheperiod/meanpopulation oftheperiod)×100,000.
• Meanlethality:(meannumberofdeathsby
pneumococ-cal meningitis in the period/mean number of cases of pneumococcalmeningitisintheperiod)×100.
Theterm‘‘vaccinationcoverage’’wasusedforthe fre-quencyoftheserotypesavailableintheconjugatevaccines ofthestudiedsample.
DatawererecordedinaMicrosoftExcel®2010(Microsoft, NewYork,USA)spreadsheet,verified,andthenexportedto JMPStatisticalDiscoverySoftware5.1.2(JMPStatistical Dis-covery, NorthCarolina, USA) and EPI INFO 6 (Centers for Disease Control and Prevention, Georgia, USA). The esti-mateofthe differencebetween categoricalvariables was performedbychi-squaredtest,withaminimumsignificance levelof5%.Theprevalenceratiowasusedinthecomparison ofcasesin childrenyoungerthan1yearbetweenthe pre-andpost-vaccineperiods,withaconfidenceintervalof95%. The study was approved by the Research Ethics Com-mittee of the Department of Health of the State of Paraná/HospitaldoTrabalhador,onAugust26, 2010,Case No.218/2010.
Results
BetweenJanuaryof1998andDecemberof2011,1,354cases ofpneumococcalmeningitiswerereportedtoSINANinthe stateofParaná.Ofthispopulation,15caseswereexcluded duetoinconsistencyofinformationandduplicate notifica-tions, totaling1,339 cases, with1,205 in the pre-vaccine and134inthepost-vaccineperiod.
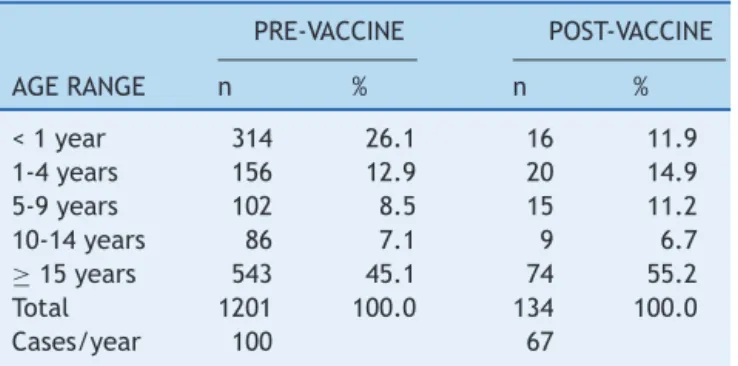
Thedistributionofcasesaccordingtoagerangeinboth periodsisshowninTable1.Whenanalyzingthepre-vaccine andpost-vaccineperiods,a54%reductioncanbeobserved inthefrequency ofcases intheage group<1 year(26.1% to11.9%,respectively).The prevalenceratioamongcases ofpneumococcalmeningitisinchildrenyoungerthan1year betweenthe pre-and post-vaccineperiods was2.68(95% CI:1.62to4.44,p<0.01).
1.Evaluationofepidemiologicalindicatorsinthepreand post-vaccineperiods:
Whencomparingtheepidemiologicalindicatorsbetween the two studied periods in the general population, there was a significant decrease. The mean incidence rate
Table1 Distributionofcasesofpneumococcalmeningitis intheperiodspre-(1998-2009)andpost-vaccination (2010-2011),accordingtoagerange.Paraná,1998-2011.
PRE-VACCINE POST-VACCINE
AGERANGE n % n %
<1year 314 26.1 16 11.9
1-4years 156 12.9 20 14.9
5-9years 102 8.5 15 11.2
10-14years 86 7.1 9 6.7
≥15years 543 45.1 74 55.2
Total 1201 100.0 134 100.0
Cases/year 100 67
Obs:Fourcaseshadnodataonageinthepre-vaccineperiod.
decreasedby36.0%(1.00cases/100,000inhabitantsto0.64 cases/100,000 inhabitants; p<0.001) and the mean mor-tality rate decreased by 65.5%, from 0.29 cases/100,000 inhabitantsto0.10cases/100,000inhabitants(p<0.001).
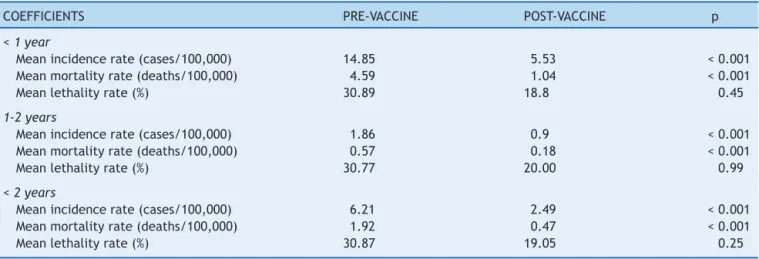
Whenanalyzing the profileindicators between pre-and post-vaccineperiods,specificallyinthepopulationof chil-drenyoungerthan2years,thereweresignificantreductions inmeanincidenceandmortalityrates(Table2).Themean incidenceratedecreased by59.9% (6.21cases/100,000to 2.49 cases/100,000 inhabitants; p<0.01), and the mean mortality rate decreased by 75.5% (1.92 deaths/100,000 to 0.47 deaths/100,000; p<0.01). Similar results were obtained in the analysis of the age rangeyounger than 1 year and between 1and 2 years.Although lethalityrates also decreased between the two periods, the differences werenotstatisticallysignificant.
2. Distribution of serotypes identified in the pre- and post-vaccineperiods:
Inthepre-vaccineperiod,46.3%(558/1205)ofthecases wereserotyped. Ofthese,58.1% correspondedtocasesof whichpneumococcusserotypewasincludedinthe10-valent conjugatevaccine(1,4,5,6B,7F,9V,14,18C,19F,23F). In the post-vaccine period, 45.5% (61/134) of the cases wereserotyped,of which47.5%werecausedbyserotypes includedinthevaccine.Whenthepossiblecross-protection between serotypes 6A and 6B is considered, in the pre-and post-vaccine periods, the identification of 62.2% and 52.5%oftheserotypesincludedinthe10-valentvaccineare observed,respectively.
When analyzing the most frequent serotypes between the periods in the general population, an increase in the frequencyofcaseswithserotype3,10A,and4inthe post-vaccinationperiodisobserved,withthefirsttwoserotypes notincludedinthe10-valentconjugatevaccine.
3.Distributionofserotypesintheagerangeyoungerthan 2years:
Table2 Epidemiologicalindicatorsforpneumococcalmeningitisinchildrenyoungerthan2years.Paraná,1998-2011.
COEFFICIENTS PRE-VACCINE POST-VACCINE p
<1year
Meanincidencerate(cases/100,000) 14.85 5.53 <0.001
Meanmortalityrate(deaths/100,000) 4.59 1.04 <0.001
Meanlethalityrate(%) 30.89 18.8 0.45
1-2years
Meanincidencerate(cases/100,000) 1.86 0.9 <0.001
Meanmortalityrate(deaths/100,000) 0.57 0.18 <0.001
Meanlethalityrate(%) 30.77 20.00 0.99
<2years
Meanincidencerate(cases/100,000) 6.21 2.49 <0.001
Meanmortalityrate(deaths/100,000) 1.92 0.47 <0.001
Meanlethalityrate(%) 30.87 19.05 0.25
Table3 Coverageofthe10-valentpneumococcal conju-gatevaccineinthepre-andpost-vaccineperiodsaccording toage.
PRE-VACCINE POST-VACCINE p
<12MONTHS
10-valent 76.6% 60.0% 0.42
Cross-protection 80.90% 60.0% 0.14
12-23MONTHS
10-valent 73.9% 20.0% 0.05
Cross-protection 80.4% 40.0% 0.24
<24MONTHS
10-valent 75.9% 46.7% 0.03
Cross-protection 80.7% 53.3% 0.03
Obs:Cross-protectionwithserotype6A.
pre- and post-vaccine periods in children younger than 2 years,with the expansion of cross-protectionof serotype 6A,showsasignificantdecreaseincasesofwhichserotypes areincludedinthevaccine(from80.7%to53.3%,p=0.030;
Table3).
Discussion
The analysis of theeffects of the introduction of the 10-valentconjugate vaccine inthe NIPin theBrazil must be carefully considered. The 10-valent pneumococcal conju-gatevaccinewasintroducedintotheroutineimmunization programinBrazilfromMarchtoSeptemberof2010.7
In the present study, when comparing the pre-vaccine (1998-2009)withthepost-vaccineperiod(2010and2011), and evenincludingthe year 2010, which wasa transition period when municipalities were progressively implemen-ting pneumococcal vaccine at the basic health units, a reductionof54%incasesofmeningitisinchildrenyounger than1yearwasfound,withthehighestprevalenceofcases inthisagegroupinthepre-vaccineperiod.Whenanalyzing thegeneralpopulation, theincidenceratesand themean mortalityratesdecreasedby36.0%and65.5%,respectively. Fortheagegroupcoveredbythevaccine,i.e.,youngerthan
2years, significant reductions in the mean incidenceand mortalityrates(reductionof59.9%and75.5%,respectively) werealsoobserved.
Since the Kaiser Permanent Study Center,8 studies
haveshownthe benefitsofusingconjugatepneumococcal vaccines.9,10
Theuseofthe7-valentpneumococcalconjugatevaccine (nolongermarketed)showed97%efficacyagainstinvasive pneumococcal diseases caused by vaccine serotypes and 89%againstinvasivepneumococcaldiseasesingeneral.The impactofthisvaccinewasdemonstratedbythereductionof invasivediseases,from79%to100%,andincasesofmedical consultationsforacuteotitismedia,withanimpactof13% to43%.11Regardingthe10-valentpneumococcalconjugate
vaccine,Afonsoetal.foundsignificantreductionsin hospi-talizationsforpneumoniain BeloHorizonte,Curitiba,and Recife,afteritsintroductionintheBrazilianimmunization schedule.12Thisvaccinealsoshowedtobeeffectivein
stud-iesperformedinFinland13andQuebec,14withreductionsin
theincidenceratesofinvasivepneumococcaldiseases. Althoughthepost-vaccineperiodinBrazilstillcomprises a short time period, with a three-dose vaccine coverage inthefirstyear oflifeof35.36%in2010,reaching94.19% in2011,15areductioninepidemiologicalindicators canbe
observed,whichisinagreementwithstudiesthatshowed benefitswiththeuseofpneumococcalconjugatevaccines within15monthsafteritsintroduction.14Theimpactofthe
vaccineuse andindirect benefitsof theherd effect,i.e., decreased cases in unvaccinated individuals by reducing nasopharyngeal colonization and transmission of vaccine serotypes by vaccinated children16 would be tooearly at
thisstageofthe analysis.Itis expectedthatdatawillbe compatiblewiththosefoundineffectivenessstudiesafter the introduction of the 7-valent pneumococcal conjugate vaccine,inwhichtheindirecteffectswereresponsiblefor a19%to62%reductionintheoverallincidenceofinvasive pneumococcaldiseases in individuals aged>18 years,and 81%to92%ofcasesrelatedtovaccineserotypesinthesame agegroup.11
other reportedstudies. However,considering the possible cross-protectionbetweenserotypes6Aand6B,therewould beanidentificationof62.2%inthepre-vaccineperiod.
RatesfoundinBrazilwere77.6%forthe10-valent conju-gatevaccine and 86%for the13-valentconjugate vaccine for invasive pneumococcal diseases;17,18 data from Africa
showratesof70%to84%forthe10-valentconjugatevaccine andfrom79%to88%forthe13-valentconjugatevaccine.19
Formeningitis,thecoverageofthe10-valentand13-valent conjugatevaccineswouldbe82.8%and94.2%,respectively.2
In this study, when comparing the periods before and afterthe introduction of the 10-valentconjugate vaccine intheBrazilianNIP,asignificantreductionwasobservedin ratesofserotypesincludedinthevaccineasthecauseof dis-easesintheagerange<2years(from80.7%to53.3%),which couldbeexplainedbytheroutineuseofthis immunobiolog-icalagent.
When making a projection,comparingthe coverage of pneumococcalconjugatevaccinesavailableinBrazil,there isbettercoverage(serotypesincludedinthevaccines coin-cident with the isolates in the state) with the 13-valent vaccine, when compared to the 10-valent, both in the pre-vaccine (69.2% and 58.1%, respectively, p<0.01) and the post-vaccine period (68.9% and 47.5%, respectively, p=0.017).Ifthe10-valentvaccinecoveragewereextended with the cross-protection of serotype 6A, this difference would not be maintained in the post-vaccine period (13-valent:68.9% and10-valent:52.5%,p=0.064),but rather, only in the pre-vaccine period (13-valent: 69.2% and 10-valent:62.2%,p=0.014).
The effect of serotype replacement, i.e., increase in casesbyserotypes notincluded intheconjugatevaccines throughareductioninthecirculationofvaccineserotypes inavaccinated population,20 appears tobepremature, in
additiontothefactthatfluctuationsinthefrequencyofthe serotypescanoccurwithouttheneedforselectivepressure ofvaccines.21Inthepresentstudy,anincreaseinthe
num-berofcasesinthepost-vaccineperiodwasobserveddueto serotypes3and10A,notincludedinthe10-valentconjugate vaccine. InEurope andthe United States,after usingthe 7-valentpneumococcal conjugate vaccine, the main non-vaccineserotypesfoundininvasivepneumococcaldiseases were1,3,7F,15,19A,27F,and33F.22
Theanalysisofcasesofpneumococcalmeningitisinthe pre-andpost-vaccineperiodsshowedareductionofcases in the age range<1 year, significant reductions in inci-denceand overallmortality coefficients,andof thesame coefficientsintheagegroupscoveredbythevaccine.The coverageofpneumococcalvaccinesformeningitiscaseswas lowerthanthatdescribedintheliterature,butwitha reduc-tionofthevaccineserotypesinthepost-vaccineperiod.
Onelimitationof thisstudywasthe sourceof datafor analysis,whichisbasedonnotificationdata.Although noti-fication of meningitis is compulsory in Brazil, the lack of some information, the divergence of data, and the lack ofpropercompletionof formsat timesprevented amore complete analysis of the available data, informationthat couldnot be included in this research.Nevertheless, the development of new studies that follow the scenario of invasive pneumococcal diseases in the state (not only of meningitis),theeffectsoftheintroductionof pneumococ-calconjugatevaccineintotheNIP,andthemaintenanceof
surveillanceonserotypescirculating inthepopulationare encouraged.
Permanentsurveillanceofcasesofpneumococcal menin-gitisandthemostprevalentserotypesallowsthechoiceof conjugatevaccinesthatarebestadaptedtothe geographi-calregionanditspopulation.
Conflicts
of
interest
Theauthorsdeclarenoconflictsofinterest.
Acknowledgments
The authors would like to thank the staff of the Depart-mentofEpidemiologicalSurveillance(DEVE),theDivisionof CommunicableDiseasesSurveillance(DVVTR),andtheState SecretariatofParaná,particularlyMs.MarleneSera Wille, fortheircollaborationwithdatacollection.
References
1.Vieira AC, Gomes MC, Rolo Filho M, Eudes Filho J, Bello EJ, deFigueiredo RB.Streptococcuspneumoniae:a studyof strains isolated from cerebrospinal fluid. J Pediatr (RioJ). 2007;83:71---8.
2.Alvares JR, Mantese OC, Paula AdWolkers PC, Almeida VV, AlmeidaSC,etal.Prevalenceofpneumococcalserotypesand resistancetoantimicrobialagentsinpatientswithmeningitis: ten-yearanalysis.BrazJInfectDis.2011;15:22---7.
3.dosSantosSR,PassadoreLF,TakagiEH,FujiiCM,YoshiokaCR, GilioAE,etal.SerotypedistributionofStreptococcus pneumo-niaeisolatedfrompatientswithinvasivepneumococcaldisease inBrazilbeforeandafterten-pneumococcalconjugatevaccine implementation.Vaccine.2013;31:6150---4.
4.Mantese OC, Paula A, Moraes AB, Moreira TA, Guerra ML, BrandileoneMC.Prevalênciadesorotiposeresistência antimi-crobianade cepasinvasivas doStreptococcuspneumoniae. J Pediatr(RioJ).2003;79:537---42.
5.Mantese OC, Paula Ad Almeida VV, Aguiar PA, Wolkers PC, AlvaresJR, et al.Prevalence of serotypes and antimicrobial resistanceofinvasivestrainsofpneumococcusinchildren: anal-ysisof9years.JPediatr(RioJ).2009;85:495---502.
6.Brasil.MinistériodaSaúdeSecretariadeVigilânciaemSaúde. DepartamentodeVigilânciaEpidemiológica.Manualdoscentros de referência para imunobiológicos especiais. Brasília: Min-istériodaSaúde;2006.
7.SartoriAM, de Soárez PC, NovaesHM. Cost-effectivenessof introducingthe10-valentpneumococcalconjugatevaccineinto the universal immunisation of infantsin Brazil. J Epidemiol CommunityHealth.2012;66:210---7.
8.BlackS1,ShinefieldH,FiremanB,LewisE,RayP,HansenJR, etal.Efficacy,safetyandimmunogenicityofheptavalent pneu-mococcal conjugate vaccine in children. Northern California KaiserPermanenteVaccineStudyCenterGroup.PediatrInfect DisJ.2000;19:187---95.
9.Bruce MG, Deeks SL, Zulz T, Bruden D, Navarro C, Lovgren M, et al. International Circumpolar Surveillance System for invasivepneumococcaldisease, 1999-2005.EmergInfect Dis. 2008;14:25---33.
11.Fitzwater SP, Chandran A, Santosham M, Johnson HL. The worldwideimpactoftheseven-valentpneumococcalconjugate vaccine.PediatrInfectDisJ.2012;31:501---8.
12.AfonsoET,MinamisavaR,BierrenbachAL,EscalanteJJ, Alen-carAP,DominguesCM,etal.Effectof10-valentpneumococcal vaccineonpneumoniaamongchildren,Brazil.EmergInfectDis. 2013;19:589---97.
13.Palmu AA, Jokinen J, Borys D, Nieminen H, Ruokokoski E, Siira L, et al. Effectiveness of the ten-valent pneumo-coccal Haemophilus influenzae protein D conjugate vaccine (PHiD-CV10)againstinvasivepneumococcaldisease:acluster randomisedtrial.Lancet.2013;381:214---22.
14.DeWalsP, LefebvreB, Defay F,Deceuninck G, Boulianne N. Invasivepneumococcaldiseasesinbirthcohortsvaccinatedwith PCV-7and/orPHiD-CVintheprovinceofQuebec,Canada. Vac-cine.2012;30:6416---20.
15.Departamento de Informática do SUS (DATASUS). [cited 15 Aug 2013]. Available from: http://www2.datasus.gov.br/ DATASUS/index.php
16.deMenezesOAP,CamposLC,dosSantosMS,AzevedoJ,Dos SantosRC,CarvalhoM daG, etal.Serotypedistributionand antimicrobialresistanceofStreptococcuspneumoniaepriorto introductionofthe10-valentpneumococcalconjugatevaccine inBrazil,2000-2007.Vaccine.2011;29:1139---44.
17.Casta˜neda E, Agudelo CI, Regueira M, Corso A, Brandileone MC, Brandão AP, et al. Laboratory-based surveillance of
Streptococcuspneumoniaeinvasive diseasein childrenin 10 LatinAmericancountries:aSIREVAIIproject,2000-2005. Pedi-atrInfectDisJ.2009;28:e265---70.
18.AndradeAL,OliveiraR,VieiraMA,MinamisavaR,PessoaVJr, BrandileoneMC,etal.Population-basedsurveillancefor inva-sivepneumococcaldiseaseandpneumoniaininfantsandyoung childreninGoiânia,Brazil.Vaccine.2012;30:1901---9.
19.MudhuneS, WamaeM. NetworkSurveillancefor Pneumococ-cal Disease in the East African Region. Report on invasive disease and meningitis due to Haemophilus influenzae and
StreptococcuspneumoniafromtheNetworkforSurveillanceof PneumococcalDiseaseintheEast AfricanRegion.ClinInfect Dis.2009;48:S147---52.
20.Brandileone MC, Kfouri RA. Doenc¸as pneumocócicas. In: Neto VA, editor.Atualizac¸ões,orientac¸õesesugestões sobre imunizac¸ões.SãoPaulo:SegmentoFarma;2011.p.340---58. 21.Pírez MC, Algorta G, Cedrés A, Sobrero H, Varela A,
Gia-chettoG,etal.Impactofuniversalpneumococcalvaccination on hospitalizations for pneumonia and meningitis in chil-dren in Montevideo, Uruguay. Pediatr Infect Dis J. 2011;30: 669---74.