Temporally unstructured electrical stimulation to the amygdala
suppresses behavioral chronic seizures of the pilocarpine animal model
Jasiara Carla de Oliveira
a, Daniel de Castro Medeiros
b, Gustavo Henrique de Souza e Rezende
b,
Márcio Flávio Dutra Moraes
b, Vinícius Rosa Cota
a,⁎
aLaboratório Interdiciplinar de Neuroengenharia e Neurociências, Departamento de Engenharia de Biossistemas (DEPEB), Universidade Federal de São João Del-Rei, Pça. Dom Helvécio, 74, 36301-160 São João Del-Rei, MG, Brazil
bNúcleo de Neurociências, Instituto de Ciências Biológicas (ICB), Universidade Federal de Minas Gerais, Av. Antônio Carlos 6627, CEP 31270-901 Belo Horizonte, MG, Brazil
a b s t r a c t
a r t i c l e
i n f o
Article history:
Received 17 January 2014 Revised 30 April 2014 Accepted 6 May 2014 Available online 13 June 2014
Keywords:
Temporal lobe epilepsy Pilocarpine
Electrical stimulation Temporal coding Basolateral amygdala Desynchronization Chronic seizures
Electrical stimulation applied to the basolateral amygdala in the pentylenetetrazole animal model of seizures may result in either a proconvulsant or an anticonvulsant effect depending on the interpulse intervals used: pe-riodic or nonpepe-riodic, respectively. We tested the effect of this electrical stimulation temporal coding on the spontaneous and recurrent behavioral seizures produced in the chronic phase of the pilocarpine animal model of temporal lobe epilepsy, an experimental protocol that better mimics the human condition. After 45 days of the pilocarpine-inducedstatus epilepticus, male Wistar rats were submitted to a surgical procedure for the im-plantation of a bipolar electrical stimulation electrode in the right basolateral amygdala and were allowed to re-cover for seven days. The animals were then placed in a glass box, and their behaviors were recorded daily on DVD for 6 h for 4 consecutive days (control period). Spontaneous recurrent behavioral seizures when showed in animals were further recorded for an extra 4-day period (treatment period), under periodic or nonperiodic electrical stimulation. The number, duration, and severity of seizures (according to the modified Racine's scale) during treatment were compared with those during the control period. The nonperiodically stimulated group displayed a significantly reduced total number and duration of seizures. There was no difference between control and treatment periods for the periodically stimulated group. Results corroborate previousfindings from our group showing that nonperiodic electrical stimulation has a robust anticonvulsant property. In addition, results from the pilocarpine animal model further strengthen nonperiodic electrical stimulation as a valid therapeutic approach in current medical practice. Our working hypothesis is that temporally unstructured electrical stimula-tion may wield its effect by desynchronizing neural networks involved in the ictogenic process.
© 2014 Elsevier Inc. All rights reserved.
1. Introduction
Epilepsy is a chronic neurological disorder characterized by recur-rent and spontaneous seizures caused by hypersynchronous and exces-sive neural activity[1]. It has high prevalence, affecting aboutfifty million people worldwide[2]. Temporal lobe epilepsy (TLE) is the most common type of partial epilepsy[3], which accounts for about 60% of all patients[4]. It has a focal onset, and it is the most common type of drug-resistant epilepsy[5].
Intracranial electrical stimulation has long been considered a poten-tially viable therapy for patients with drug-resistant epilepsy who are not eligible for ablative surgery[6]. Currently, electrical stimulation (i.e., current or voltage pulses) may be applied to the peripheral nervous system, in structures such as the vagus nerve (vagus nerve stimulation)
[7]and the trigeminal nerve (trigeminal nerve stimulation)[8], or di-rectly to the central nervous system, in substrates such as the anterior thalamic nucleus[9], subthalamic nuclei[10], and epileptogenic focus itself[11].
Although the literature reports an overwhelming amount of data showing the effect of electrical stimulation on seizure suppression (for a review, see[6]), its mechanisms of action on neural network modula-tion need further investigamodula-tion. While debatable, the most widely accepted framework for its therapeutic effectiveness posits that electri-cal stimulation would recruit substrates and/or neural networks capable of modulating seizure-like activity in areas involved in ictogenesis or rather by impairing the coupling of neural oscillators necessary to prop-agate and sustain aberrant activity[12,13]. Among others, in silico stud-ies have shown that neural circuits thatfire synchronously are coupled in a positive feedback fashion[14], that synaptic weights increase in proportion to the coincidence in neuronalfiring[15], corroborating
Hebbian postulates[16], and that this coincidence may be increased or decreased by a synchronizing or desynchronizing electrical stimulation, respectively[15]. Finally, Medeiros et al. have shown that electrical
⁎ Corresponding author at: DEPEB, Pça. Dom Helvécio, 74, B. Fábricas, 36301-160 São João Del-Rei, MG, Brazil. Tel.: +55 32 3379 2541 (office), +55 32 8861 8074 (mobile).
E-mail addresses:vrcota@ufsj.edu.br,vrcota@pq.cnpq.br,vrcota@gmail.com
(V.R. Cota).
http://dx.doi.org/10.1016/j.yebeh.2014.05.005
1525-5050/© 2014 Elsevier Inc. All rights reserved.
Contents lists available atScienceDirect
Epilepsy & Behavior
stimulation may drive the temporal occurrence of preictal oscillatory neural networks; that is, cortical preictal discharges gradually synchro-nize with electrical stimulation minutes before seizure onset[17].
In recent investigations, we approached this issue by testing the hy-pothesis that afixed four-stimuli-per-second electrical stimulation in the amygdaloid complex would modulate convulsive behavior of rats with acute pentylenetetrazole (PTZ)-induced seizures according to the temporal pattern used: structured (constant interpulse intervals or periodic — PS) or nonstructured (random interpulse intervals or
nonperiodic—NPS). It is important to highlight that the average fre-quency used (4 pulses per second) is substantially lower than what is usually considered to be anticonvulsant[6]. It was shown that PS is proconvulsant and NPS is anticonvulsant, suggesting that a putative synchronization/desynchronization effect of structured/nonstructured electrical stimulation may be an underlying mechanism of action[18]. In addition, these results corroborate the notion that reverberation of neural networks has an important role in ictogenesis.
Based on the promising results obtained with the use of a nonstruc-tured temporal pattern in the suppression of acutely induced seizures and on the urge to develop safe and efficient therapeutic alternatives to treatment-resistant epilepsy such as TLE, in this study, we set out to investigate the hypothesis that NPS may also have a positive effect on animal models that best mimic this clinical scenario. For this, we tested NPS applied to the amygdaloid complex of the pilocarpine animal model during chronic seizures. Pilocarpine is a cholinergic agonist that binds to muscarinic receptors to increase cholinergic excitatory neurotransmis-sion. When administered systemically in high doses, it induces limbic seizures that become generalized and are associated withstatus epilep-ticus(SE) in rodents[19–22]. Such a state of enduring seizures induces epileptogenesis due to large cellular reorganization that culminates in permanent late neural tissue hyperexcitability[23–35]. Due not only to the similarities between pathophysiological mechanisms but also to the behavioral manifestation of its spontaneous and recurrent chronic seizures, the pilocarpine animal model is considered an experimental protocol that mimics human TLE. The amygdala was used as a target for electrical stimulation because it has an important role in the cou-pling of neural oscillators within the limbic system during seizures [18,23,24,26,27]and also in the activation of modulatory circuits such as the nucleus accumbens–temporal lobe[36,37].
2. Materials and methods
2.1. Animals and groups
All experiments were done in accordance with the Ethical Commit-tee for Animal Experimentation (Comitê de Ética em Experimentação Animal — CETEA) of the Federal University of Minas Gerais
(Universidade Federal de Minas Gerais—UFMG) and with the Ethical Committee on Research Involving Animals (Comitê de Ética em Pesquisa Envolvendo Animais—CEPEA) of the Federal University of
São João del Rei (Universidade Federal de São João del Rei—UFSJ). The procedures for animal care were previously approved by these organizations under protocols 150/2006/UFMG and 01/2011/UFSJ. A total of 14 male Wistar rats, weighing 250–300 g, supplied by UFMG (n = 8) and UFSJ (n = 6) vivariums, were kept in a light–dark cycle of 12 h (lights on at 7 am and off at 7 pm) with free access to food and water.
The animals were randomly divided into two groups: NPS (n = 7) received nonperiodic stimulation and PS (n = 7) received periodic stimulation. After a recovery period of 5 to 7 days, the animals were placed in a glass box with free access to food and water, and their behav-iors were recorded on DVD for 6 h (10 am to 4 pm) daily for 4 consecu-tive days (control period—CRTL). Animals that showed spontaneous
recurrent seizures underwent electrical stimulation (pattern according to group) and video recording for an extra 4 days (treatment period).
2.2. Status epilepticus induction
Status epilepticuswas induced in the pilocarpine animal model by
first applying methylscopolamine (1 mg/kg; Sigma Aldrich, St. Louis, MO, USA) by means of an intraperitoneal (i.p.) injection, followed 30 min later by pilocarpine hydrochloride (320 mg/kg; Sigma Aldrich, St. Louis, MO, USA) i.p. injection. Methylscopolamine is a cholinergic antagonist that does not cross the blood–brain barrier and was used to avoid undesirable systemic effects of the cholinergic stimulation in-duced by pilocarpine (i.e., excessive defecation, urination, sweating, and bronchial secretion). Animals that did not develop SE during the
first 30 min after pilocarpine injection received an overdose of 40% of
the initial dose. Animals that did not develop SE even with the overdose were excluded from the study. At the 90-minute mark, SE was interrupted by i.p. injection of diazepam (20 mg/kg; Laboratório Teuto Brasileiro, Anápolis, GO, Brazil). The animals received intensive care, including rehydration with dextrose (2 mL) i.p., during 48 h after the SE induction to ensure survival.
2.3. Stereotaxic procedures
Between 45 and 50 days after the SE induction, the animals underwent a surgical procedure for implantation of a bipolar stimula-tion electrode in the right basolateral amygdala. Electrodes were made of a twisted pair of stainless-steel teflon-coated wires (model 791400, A-M Systems Inc., California, USA) and were surgically implanted at coordinates derived from the Paxinos and Watson's atlas for rats[38]: AP = 2.8 mm and ML = 5.0 mm referenced from the bregma suture and DV = 7.2 mm from dura mater.
Briefly, the animals were anesthetized by means of an i.p. injection
containing the mixture of ketamine (100 mg/kg—König do Brasil,
Santana do Paraíba, SP, Brazil), xylazine (5 mg/kg—Syntec do Brasil, Cotia, SP, Brazil), and fentanyl (0.025 mg/kg—Union Chemical do Brasil, Londrina, PR, Brazil). After hair shaving and proper asepsis proce-dures, the animals were positioned in a stereotaxic frame (Insight Equipamentos Ltda, Ribeirão Preto, SP, Brazil). The electrode wasfixed to the skull with zinc cement and soldered to a telephone jack (model RJ-11), which wasfixed onto the skull with dental acrylic. After surgery, the animals received a prophylactic pentabiotic (2.5 mg/kg) treatment and were allowed to recover for 5–7 days before the experimental
procedure.
2.4. Electrical stimulation
For stimulation delivery, we designed and built an electrical stimula-tor composed of a constant-voltage isolation unit driven by the output of an MP3 player (model NWZ-B152 26B, Sony). Control signals for both periodic electrical stimulation and nonperiodic electrical stimula-tion were digitally designed using Adobe Audistimula-tion 1.0 and transformed into a 44.1 KHz, 16-bit, mono-waveform, MP3 format compatible with the D/A hardware output. Pulses were always square, biphasic waves of 100μs duration. Current amplitudes, measured by a built-in shunt resistor, varied from 400 to 600μA due to differences in the impedance of electrode–brain sets among the animals. Yet, there was no difference in average currents between periodic and nonperiodic groups. Pulses were biphasic to prevent electrodeposition along the extended period of stimulation and subsequent tissue damage, as reported in the litera-ture[39,40].
2.5. Behavioral analysis
The number, duration, and severity–according to the modified Racine's scale[41]–of seizures, during control and treatment periods of both groups, were assessed by two separate and experienced researchers. Their independent results were compared, and reproduc-ibility of data was assessed in order to guarantee minimum subjectivity or bias. Between researchers, there was absolutely no difference in measures of the severity and number of seizures, while the duration of seizure measurements differed no more than 2 s. In this last case, we used the average between measures. Only animals displaying sei-zures with Racine's index greater than or equal to 3 during the control period were used to computefinal results.
2.6. Histology
After the end of stimulation, the animals were deeply anesthetized with ketamine (100 mg/kg) and xylazine (5.0 mg/kg) and were transcardially perfused with formaldehyde (4%) before brain removal. Coronal sections of 50μm thickness were cut on a vibratome (Leica), mounted on glass slides, and stained with cresyl violet. Animals with in-correct positioning of electrodes were not included in our analysis.Fig. 2 shows a representative location of the electrode tip.
2.7. Statistical analysis
The Kolmogorov–Smirnov normality test and Student t test were used to evaluate parametric data (number and duration of seizures)
and Wilcoxon test to evaluate nonparametric data (severity of seizures). Statistical significance was set at pb0.05. Values in the text are
displayed as means ± standard error.
3. Results
All animals had histological confirmation of the positioning of
elec-trode in the BLA. Only one animal was excluded from the study because it had no spontaneous and recurrent behavioral seizures during the control period (first 4 days of experimental protocol).
Seizures were observed during control and stimulation periods. The animals developed the typical behaviors of the pilocarpine animal model: myoclonic jerks, forelimbs and head clonus, rearing, and gener-alized tonic–clonic seizures (GTCS).
During treatment when compared to the control period, NPS was able to significantly reduce the total number (CTRL: 3.8 ± 1.3; NPS: 1.3 ± 0.6; pb0.05) (Fig. 3A) and duration (CTRL: 26.6 ± 2.1; NPS:
8.3 ± 3.9; pb0.05) (Fig. 3B) of seizures. Seizure severity in the
treat-ment period did not show significant difference (CTRL: 4.4 ± 0.3; NPS: 2.5 ± 0.9; p = 0.1077) (Fig. 3C).
In the animals of the periodically stimulated (PS) group, there were no statistically significant differences between control and treatment periods regarding any of the analyzed parameters. Averages and their respective standard errors were the following: total seizure number (CRTL: 1.7 ± 1.7; PS: 5.4 ± 6.1; p = 0.1359) (Fig. 4A), seizure duration (CTRL: 20.2 ± 21.4; PS: 33.4 ± 27.1; p = 0.2675) (Fig. 4B), and seizure severity (CTRL: 3.1 ± 2.2; PS: 3.5 ± 1.7; p = 0.7984) (Fig. 4C).
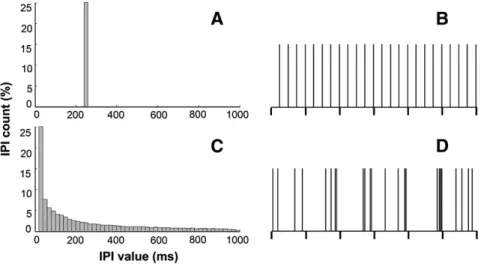
Fig. 1.Rats were stimulated with two different temporal patterns. Left column: interpulse interval (IPI) histograms for: (A) periodic (PS) and (C) nonperiodic (NPS) electrical stimulation. Right column: temporal distribution of pulses every second (vertical ticks) for PS (B) and NPS (D). Electrical stimulation for both patterns was bipolar pulses of 400 to 600μA amplitude,
100μs duration, and four-stimuli-per-second pulse count (see Cota et al.[18]for details).
4. Discussion
Our present results show that low pulse per second count, temporal-ly unstructured, electrical stimulation (nonperiodic or NPS) applied to the basolateral amygdala has a positive therapeutic effect on behavioral seizures of the pilocarpine animal model of temporal lobe epilepsy (TLE), being able to reduce their number and duration during the chron-ic phase. These results corroborate previousfindings from our group using the acute pentylenetetrazole (PTZ) animal model[18]and extend them to another important animal model used in epileptology (i.e., pilocarpine).
Previous studies reported the effect offixed frequency electrical stimulation on a pilocarpine or kainate animal model, but they were not accomplished in the chronic phase or did not show positive thera-peutic effects. Hamani et al. showed that bilateral stimulation (100 Hz) of the anterior thalamic nuclei was protective against SE in-duced by pilocarpine[42]. Jou et al. also found that anterior thalamic stimulation with high-frequency (200 Hz) and low-intensity currents may reduce the occurrence of seizure and SE[43]. Finally, Lado showed that high-frequency electrical stimulation (100 Hz) led to an increase in the frequency of seizures in animals with chronic epilepsy that received kainic acid[44]. To the best of our knowledge, there are no studies of electrical stimulation during the chronic phase of the pilocarpine animal
model targeting the amygdala or, most importantly, using unstructured temporal patterns with a low count of pulses per second.
The pathophysiology of the pilocarpine animal model is a complex, multivariate phenomenon, yet sharing many common mechanisms with human TLE[26]. Ultimately, molecular and cellular modifications lead to a state of highly excitable neural tissue, susceptible to developing spontaneous and recurrent seizures, that is similar to what is observed in humans affected with TLE[45]. Structures within the limbic system, prominently the hippocampus and the entorhinal cortex, are among the most affected neural substrates[23,46,47]. Aberrant epileptiform activity originating in a group of hyperexcitable neurons (epileptogenic focus) can propagate through aberrant pathological or, potentially, even apparently normal pathways in the central nervous system[48], cou-pling different brain areas for the full epileptic event by means of syn-chronization of activity[14,49,50]. In vitro studies in healthy neural tissue demonstrated that incoming activity from the entorhinal cortex enters the hippocampus through the perforant path passing the dentate gyrus to reach Ammon's horn via mossyfibers and Schaffer collaterals [51]. Suchflow of information seems to be powerfully modulated by
amygdala outputs to deep layers of the entorhinal cortex[26,27]. In pi-locarpine-treated animals, epileptiform activity may also propagate from the entorhinal cortex to the hippocampus laterally through the temporoammonic path to reach CA1[52,53]. Moreover, hippocampal outputs back to the entorhinal cortex form a reentrant circuit crucial for sustaining seizures in a reverberatory process[24,49,54–57]. Fig. 3.Number (A), duration (B), and severity (C) of seizures, according to the modified
Racine's scale, in the nonperiodically stimulated group during treatment period compared to its control period (CTRL). The number and duration of seizures was significantly decreased by NPS.
The present data do not allow elucidation of the mechanisms by which NPS attains its therapeutic effect. Yet, considering the importance of synchronization to ictogenesis and known correlations between be-havior and neural activation[58,59], we believe that NPS impairs the coupling of micro-oscillators within the entorhinal cortex to those in the hippocampus by imposing unstructured rhythms in the deep layers of the entorhinal cortex targeted by BLA outputs (connections in the limbic system of pilocarpine-treated animals are preserved[60]), and, thus, it suppresses seizures. Within this theoretical framework, the de-crease in the number and duration of seizures attained by NPS could be explained by a putative impairment of coupling and synchronization of micro-oscillators within the limbic system, crucial for limbic seizure initiation, propagation, and reverberation[61], by avoiding temporal co-incidence among activities of different substrates[14,49,62]. A real-time measurement of neural activity, such as recordings of localfield
poten-tial (or electroencephalogram), is necessary in order to clarify the role of each temporal pattern in the synchronization or desynchronization of these micro-oscillators and to more definitely state if such a mechanism is really underlying the observed effects.
Periodic stimulation (PS) did not show a clear proconvulsant effect, although there seemed to be an increasing trend in the number of sei-zures. It is plausible to believe that thefixed frequency used (4 Hz) is
not the proper stimulus design for precipitating seizures in the pilocar-pine animal model opposed to what has been demonstrated in PTZ-treated animals. On the other hand, the clear lack of an anticonvulsant effect of PS states that the therapeutic effect of NPS is not due to its low-frequency content.
NPS has both low frequency content and high frequency content as can be observed in its histogram inFig. 1. We ruled out the effect of high band in the anticonvulsant effect observed through experiments in a previous original work published in 2009[18]. There, we tested, in animals with PTZ-induced seizures, one-per-second 50-Hz bursts of electrical stimulation, with 4 pulses each, and they showed no anticon-vulsant effect. Finally, we also tested a variation (called LH) of the nonperiodic (temporally unstructured) pattern of electrical stimulation, obtained by a different computational algorithm, in addition to the one that is also used in the present work (called IH). We observed that LH variation results were no different from control, while IH had the anti-convulsant effect. It is important to stress that all these experiments used the very same pulse parameters (duration, amplitude, count per second, polarity, etc.) among groups and only the temporal coding was different. Particularly, this approach also rules out lesion-induced effects. If this were the case, all groups would display similar anticonvul-sant or even proconvulanticonvul-sant effects once the very same lesion-inducing charge (zero net charge in the present study once pulses are biphasic) was delivered in each case. It is also important to highlight that NPS had an anticonvulsant effect on dysfunctional neural tissue marked by aberrant exacerbated connectivity and excitability, including poor GABAergic inhibition[63]. This suggests that NPS does not act directly upon neurotransmitter systems; rather, it may have an effect on mech-anisms at the circuit level.
Taken together, these results led us to the conclusion that the temporal coding, rather than any other factor, is the responsible pa-rameter for the anticonvulsant effect. Moreover, these results and their plausible explanation are in agreement with previous results of our group, using noninvasive imaging, that synchronization and desynchronization of neural substrates may be determinant factors in ictogenesis and its suppression by NPS[37], respectively.
5. Conclusions
In this work, we showed that temporally unstructured (nonperiodic) electrical stimulation applied to the basolateral amygdala, even with a low count of pulses per second, is capable of decreasing the number and duration of spontaneous recurrent behavioral seizures of the rats during the chronic phase of the pilocarpine animal model, while periodic
stimulation has no clear effect. We believe these results to be of major importance because of three main reasons: 1) they corroborate previ-ousfindings of our group using the acute PTZ animal model[18], dem-onstrating that such temporal pattern has a robust effect in controlling seizures; 2) they add empirical evidence to the putative role of syn-chronization and desynsyn-chronization as underlying mechanisms of ictogenesis and its suppression, respectively; and 3) they clearly show that temporally unstructured electrical stimulation has a therapeutic effect even on dysfunctional hyperexcitable neural tissue susceptible to develop and sustain aberrant epileptiform activity.
Considering the common features of the pilocarpine animal model of epilepsy and human temporal lobe epilepsy (spontaneous recurrent sei-zures and limbic pathophysiology), these present results suggest that temporally unstructured electrical stimulation of the basolateral amyg-dala should be considered as a therapeutic approach in clinical trials.
Conflict of interest
The authors state that no other people or organization have inappro-priately influenced this work. Therefore, there is no pertinent claim of a conflict of interest.
Acknowledgments
We are grateful to Brazilian agencies CNPq (grants #484588/2011-7 and #484704/2012-5) and FAPEMIG (grant #APQ 01818-12) forfi nan-cial support. Márcio Flávio Dutra Moraes is a recipient of CNPq research fellowship.
References
[1]Fisher RS, van Emde Boas W, Blume W, Elger C, Genton P, Lee P, et al. Epileptic sei-zures and epilepsy: definitions proposed by the International League Against Epilep-sy (ILAE) and the International Bureau for EpilepEpilep-sy (IBE). Epilepsia 2005;46:470–2.
[2]World Health Organization. Epilepsy in the WHO Africa region, bridging the gap: the global campaign against epilepsy“out of the shadows”. Geneva: World health Organization; 2004.
[3]Téllez-Zenteno JF, Hernández-Ronquillo L. A review of the epidemiology of temporal lobe epilepsy. Epilepsy Res Treat 2012;2011:1–5.
[4]Wiebe S. Epidemiology of temporal lobe epilepsy. Can J Neurol Sci 2000;27:S20–1.
[5]Engel Jr J. Clinical neurophysiology, neuroimaging, and the surgical treatment of epilepsy. Curr Opin Neurol Neurosurg 1993;6:240–9.
[6]Theodore WH, Fisher RS. Brain stimulation for epilepsy. Lancet Neurol 2004;3: 111–8.
[7]E. Ben-Menachem. Vagus-nerve stimulation for the treatment of epilepsy. Lancet Neurol 2002;1:477–82.
[8]DeGiorgio CM, Shewmon DA, Whitehurst T. Trigeminal nerve stimulation for epilepsy. Neurology 2003;61:421–2.
[9]Mirski MA, Rossell LA, Terry JB, Fisher RS. Anticonvulsant effect of anterior thalamic high frequency electrical stimulation in the rat. Epilepsy Res 1997;28:89–100.
[10]Chabardès S, Kahane P, Minotti L, Koudsie A, Hirsch E, Benabid A. Deep brain stimu-lation in epilepsy with particular reference to the subthalamic nucleus. Epileptic Disord 2002;4:83–93.
[11]Vonck K, Boon P, Achten E, De Reuck J, Caemaert J. Long-term amygdalohippocampal stimulation for refractory temporal lobe epilepsy. Ann Neurol 2002;52:556–65.
[12]McIntyre CC, Savasta M, Kerkerian-Le Goff L, Vitek JL. Uncovering the mechanism(s) of action of deep brain stimulation: activation, inhibition, or both. Clin Neurophysiol 2004;115:1239–48.
[13]McIntyre CC, Grill WM, Sherman DL, Thakor NV. Cellular effects of deep brain stim-ulation: model-based analysis of activation and inhibition. J Neurophysiol 2004;91: 1457–69.
[14]Abeles M, Hayon G, Lehmann D. Modeling compositionality by dynamic binding of synfire chains. J Comput Neurosci 2004;17:179–201.
[15]Tass PA, Hauptmann C. Therapeutic modulation of synaptic connectivity with desynchronizing brain stimulation. Int J Psychophysiol 2007;64:53–61.
[16]Hebb DO. The organization of behavior. New York: Wiley; 1949.
[17]Medeiros DC, Oliveira LB, Mourão FAG, Bastos CP, Cairasco NG, Pereira GS, et al. Tem-poral rearrangement of pre-ictal PTZ induced spike discharges by low frequency electrical stimulation to the amygdaloid complex. Brain Stimul 2013;7:170–8.
[18]Cota VR, DdC Medeiros, MRSdP Vilela, Doretto MC, Moraes MFD. Distinct patterns of electrical stimulation of the basolateral amygdala influence pentylenetetrazole seizure outcome. Epilepsy Behav 2009;14:26–31.
[19]Clifford DB, Olney JW, Maniotis A, Collins RC, Zorumski CF. The functional anatomy and pathology of lithium–pilocarpine and high-dose pilocarpine seizures. Neurosci-ence 1987;23:953–68.
[21]Turski WA, Cavalheiro EA, Schwartz M, Czuczwar SJ, Kleinrok Z, Turski L. Limbic sei-zures produced by pilocarpine in rats: behavioural, electroencephalographic and neuropathological study. Behav Brain Res 1983:315–35.
[22]Turski WA, Cavalheiro EA, Bortolotto ZA, Mello LM, Schwarz M, Turski L. Seizures produced by pilocarpine in mice: a behavioral, electroencephalographic and mor-phological analysis. Brain Res 1984;321:237–53.
[23]Avoli M, Nagao T, Köhling R, Lücke A, Mattia D. Synchronization of rat hippocampal neurons in the absence of excitatory amino acid-mediated transmission. Brain Res 1996;735:188–96.
[24]Bertram EH, Zhang DX, Mangan P, Fountain N, Rempe D. Functional anatomy of limbic epilepsy: a proposal for central synchronization of a diffusely hyperexcitable network. Epilepsy Res 1998;32:194–205.
[25]Cavalheiro EA, Leite JP, Bortolotto ZA, Turski WA, Ikonomidou C, Turski L. Long term effects of pilocarpine in rats: structural damage of the brain triggers kindling and spontaneous recurrent seizures. Epilepsia 1991:778–82.
[26]Kajiwara R, Takashima I, Mimura Y, Witter MP, Iijima T. Amygdala input pro-motes spread of excitatory neural activity from perirhinal cortex to the entorhinal– hippocampal circuit. J Neurophysiol 2003;89:2176–84.
[27]Koganezawa N, Taguchi A, Tominaga T, Ohara S, Tsutsui KI, Witter MP, et al. Signif-icance of the deep layers of entorhinal cortex for transfer of both perirhinal and amygdala inputs to the hippocampus. Neurosci Res 2008;61:172–81.
[28]Lothman EW, Rempe DA, Mangan PS. Changes in excitatory neurotransmission in the CA1 region and dentate gyrus in a chronic model of temporal lobe epilepsy. J Neurophysiol 1995;74:841–8.
[29]Mangan PS, Rempe DA, Lothman EW. Changes in inhibitory neurotransmission in the CA1 region and dentate gyrus in a chronic model of temporal lobe epilepsy. J Neurophysiol 1995;74:829–40.
[30]Mello LE, Cavalheiro EA, Tan AM, Pretorius JK, Babb TL, Finch DM. Granule cell dis-persion in relation to mossyfiber sprouting, hippocampal cell loss, silent period and seizure frequency in the pilocarpine model of epilepsy. Epilepsy Res 1992:51–9.
[31]Mello LE, Cavalheiro EA, Tan AM. Circuit mechanisms of seizures in the pilocarpine model of chronic epilepsy: cell loss and mossyfiber sprouting. Epilepsia 1993:985–95.
[32]Nadler JV, Perry BW, Cotman CW. Selective reinnervation of hippocampal area CA1 and the fascia dentata after destruction of CA3–CA4 afferents with kainic acid. Brain Res 1980;182:1–9.
[33]Parent JM, Yu TW, Leibowitz RT, Geschwind DH, Sloviter RS, Lowenstein DH. Dentate granule cell neurogenesis is increased by seizures and contributes to aberrant network reorganization in the adult rat hippocampus. J Neurosci 1997:3727–38.
[34]Rempe DA, Mangan PS, Lothman EW. Regional heterogeneity of pathophysiological alterations in CA1 and dentate gyrus in a chronic model of temporal lobe epilepsy. J Neurophysiol 1995;74:816–28.
[35]Sloviter RS. Feedfoward and feedback inhibition of hippocampal principal cell activ-ity evoked by perforant path stimulation: GABA-mediated mechanisms that regulate excitabilityin vivo. Hippocampus 1991;1:31–40.
[36]McDonald AJ, Mascagni F, Guo L. Projections of the medial and lateral prefrontal cor-tices to the amygdala: aPhaseolus vulgarisleucoagglutinin study in the rat. Neurosci-ence 1996;71:55–75.
[37]Mesquita MBS, Castro Medeiros D, Cota VR, Richardson MP, Williams S, Moraes MFD. Distinct temporal patterns of electrical stimulation influence neural recruit-ment during PTZ infusion: an fMRI study. Prog Biophys Mol Biol 2011;105:109–18.
[38]Paxinos G, Watson C. The rat brain in stereotaxic coordinates. San Diego: Academic Press; 2007.
[39]Gerken GM, Judy MM. Electrode polarization and the detection of electrical stimula-tion of the brain. Physiol Behav 1977;18:825–32.
[40]Tehovnik EJ. Electrical stimulation of neural tissue to evoke behavioral responses. J Neurosci Methods 1996;65:1–17.
[41]Racine RJ. Modification of seizure activity by electrical stimulation: I. After-discharge threshold. Electroencephalogr Clin Neurophysiol 1972;32:269–79.
[42]Hamani C, Ewerton FIS, Bonilha SM, Ballester G, Mello LEAM, Lozano AM. Bilateral anterior thalamic nucleus lesions and high-frequency stimulation are protective against pilocarpine-induced seizures and status epilepticus. Neurosurgery 2004:54.
[43]Jou SB, Kao IF, Yi PL, Chang FC. Electrical stimulation of left anterior thalamic nucleus with high-frequency and low-intensity currents reduces the rate of pilocarpine-induced epilepsy in rats. Seizure 2013;22:221–9.
[44]Lado FA. Chronic bilateral stimulation of the anterior thalamus of kainate-treated rats increases seizure frequency. Epilepsia 2006;47:27–32.
[45]Curia G, Longo D, Biagini G, Jones RSG, Avoli M. The pilocarpine model of temporal lobe epilepsy. J Neurosci Methods 2008;172:143–57.
[46]Bernardo LS, Prince DA. Acetylcholine induced modulation of hippocampal pyrami-dal neurons. Brain Res 1981:227–34.
[47]Nagao T, Alonso A, Avoli M. Epileptiform activity induced by pilocarpine in the rat hippocampal–entorhinal slice preparation. Neuroscience 1996;72:399–408.
[48]Thom M, Bertram EH. Chapter 14—temporal lobe epilepsy. In: Stefan H, Theodore WH, editors. Handbook of clinical neurology epilepsy. Elsevier; 2012. p. 225–40.
[49]Avoli M, DAntuono M, Louvel J, Köhling R, Biagini G, Pumain R, et al. Network and pharmacological mechanisms leading to epileptiform synchronization in the limbic system in vitro. Prog Neurobiol 2002;68:167–207.
[50]Uhlhaas PJ, Singer W. Neural synchrony in brain disorders: relevance for cognitive dysfunctions and pathophysiology. Neuron 2006;52:155–68.
[51]Traub RD, Miles R. Neuronal networks of the hippocampus. Cambridge: Cambridge University Press; 1991.
[52]Barbarosie M, Louvel J, Kurcewicz I, Avoli M. CA3-released entorhinal seizures disclose dentate gyrus epileptogenicity and unmask a temporoammonic pathway. J Neurophysiol 2000;83:1115–24.
[53]Soltesz I, Jones RSG. Hippocampus forum: the direct perforant path input to CA1. Hippocampus 1995;5:101–46.
[54]Colder BW, Wilson CL, Frysinger RC, Chao LC, Harper RM, Engel J. Neuronal synchro-ny in relation to burst discharge in epileptic human temporal lobes. J Neurophysiol 1996;75:2496–508.
[55]de Guzman P, D'antuono M, Avoli M. Initiation of electrographic seizures by neuro-nal networks in entorhineuro-nal and perirhineuro-nal cortices in vitro. Neuroscience 2004;123: 875–86.
[56]Dutra Moraes MF, Galvis-Alonso OY, Garcia-Cairasco N. Audiogenic kindling in the Wistar rat: a potential model for recruitment of limbic structures. Epilepsy Res 2000;39:251–9.
[57]Si Imamura, Tanaka S, Akaike K, Tojo H, Takigawa M, Ji Kuratsu. Hippocampal tran-section attenuates kainic acid-induced amygdalar seizures in rats. Brain Res 2001;897:93–103.
[58]Eells JB, Clough RW, Browning RA, Jobe PC. Comparative fos immunoreactivity in the brain after forebrain, brainstem, or combined seizures induced by electroshock, pen-tylenetetrazol, focally induced and audiogenic seizures in rats. Neuroscience 2004;123:279–92.
[59]Garcia-Cairasco N, Wakamatsu H, Oliveira JAC, Gomes ELT, Del Bel EA, Mello LEAM. Neuroethological and morphological (Neo–Timm staining) correlates of limbic re-cruitment during the development of audiogenic kindling in seizure susceptible Wistar rats. Epilepsy Res 1996;26:177–92.
[60]Kemppainen S, Pitkänen A. Damage to the amygdalo-hippocampal projection in temporal lobe epilepsy: a tract-tracing study in chronic epileptic rats. Neuroscience 2004;126:485–501.
[61]Bertram EH. Neuronal circuits in epilepsy: do they matter? Exp Neurol 2013;244: 67–74.
[62]Womelsdorf T, Schoffelen JM, Oostenveld R, Singer W, Desimone R, Engel AK, et al. Modulation of neuronal interactions through neuronal synchronization. Science 2007;316:1609–12.