Universidade de Brasília Instituto de Biologia Departamento de Biologia Celular Pós-graduação em Biologia Microbiana
MICROBIOLOGIA DO CICLO DO NITROGÊNIO EM
SOLOS DO CERRADO
ELISA CATÃO CALDEIRA PIRES
ii
Universidade de Brasília Instituto de Biologia Departamento de Biologia Celular Programa de pós-graduação em Biologia Microbiana
Microbiologia do ciclo do nitrogênio em solos do Cerrado
ELISA CATÃO CALDEIRA PIRES
ORIENTADOR: RICARDO H. KRÜGER
Tese de doutorado apresentada ao Programa de Pós-Graduação de Biologia Microbiana do Departamento de Biologia Celular, Instituto de Biologia, Universidade de Brasília
iii
Dedico este trabalho àqueles que me
motivam todo dia a fazer o melhor de mim:
iv
Agradecimentos
Agradeço ao meu orientador, Ricardo Krüger, pelo apoio às nossas idéias de pesquisa, esdrúxulas ou não. Obrigada pela liberdade que você nos dá no laboratório. A meu ver, um local de trabalho com liberdade, cobranças medidas e bom humor ajuda muito na fluidez da pesquisa. Seu humor, que não é fácil de entender, sempre me fez sentir em casa, e algumas das suas ironias ficarão marcadas. Tal como os momentos de geekness, como a explicação do nome de 454 para a plataforma de pirosequenciamento.
Os trabalhos aqui realizados não existiriam sem o financiamento à pesquisa, especificamente pela FAP-DF, CNPq e CAPES. Agradeço especialmente à última, que financiou a minha bolsa de doutorado no país e no exterior.
Agradeço às bancas de qualificação (Professoras Cristine, Mercedes e Sérgio) e de defesa (Professores Cynthia, Mercedes, Alexandre e Helson) desta tese que auxiliaram na discussão e no refinamento deste documento.
Agradeço também aos professores colaboradores que fizeram parte mais diretamente das pesquisas apresentadas nesta tese: Gabriela Nardoto, Mercedes Bustamante e Jim Prosser. Entretanto, teria sido mais difícil de realizar esta tese se eu não tivesse ao meu lado parceiros de bancada e de computador: Fabyano, Ju, Re(nata) e Re(gina). E não tão diretamente relacionados ao meu dia-a-dia, mas também parte do grupo, agradeço à Débora, Paula e Samuel. E um especial obrigado à minha amiga que me chama de “orientadora -mirim”, Helena Magaldi, que me ajudou muito na leitura e correção dos meus textos.
Tenho um agradecimento especial a fazer ao Fabyano, parceiro científico, amigo, colega, nosso Thor do Cerrado. Aquele que me livrou de ataque de cupim e potencial ataque de suçuarana-pesquisador (?). Agradeço imensamente a oportunidade de termos trabalhado juntos. E espero que prospere o nosso plano de continuarmos colaborando em projetos futuros.
A pesquisa do sisbiota só foi possível porque conseguimos (Eu e Fabyano) reunir mão-de-obra amiga e voluntária para as várias coletas: Renata, Amanda, Lucas, Matias, Hugo e Huguinho.
De mesma forma, consegui carregar muita gente para campo para coletar solo na Fazenda Tabapuã dos Pirineus. Agradeço especialmente ao Marciano, que esteve em todas as coletas e me auxiliou com rigor nos meus primeiros experimentos de PCR em tempo real. Agradeço também à Cecília Kosmann, à Carol Benévolo, ao Lucas Pimenta, e novamente à Ju e ao Fabyano.
v
Quanto ao meu período de sanduíche na Escócia. Agradeço novamente ao Krüger, que me apoiou na minha escolha de ir lá bem para longe onde parcerias ainda não tinham sido formadas com o grupo.
Foi um sonho realizado trabalhar com o pesquisador que é Jim Prosser. Agradeço a ele pela oportunidade de trabalhar no seu laboratório, pelo acesso à infra-estrutura e às discussões científicas de alto nível. Agradeço ainda por me ensinar algumas palavras com o sotaque de Liverpool, e manter sempre um nível de descontração e ao mesmo tempo crítico nas reuniões.
Agradeço especialmente àquela que se tornou minha supervisora/colaboradora de pesquisa e amiga em todos os momentos, Cécile Thion e também à grande amiga Jessica Poirel. A bem dizer a experiência foi ótima com todos. O trabalho foi muito mais divertido por ser ao lado da lassie Heather Richmond, que falava comigo em bom escocês. As discussões geeks foram mais divertidas por causa da Eva Weber, e o cultivo de AOA e AOB só foi bem-sucedido por causa da ajuda da Jenna Ross, que me ajudou a entender várias palavras do sotaque escocês. Mas tenho que agradecer a todos do grupo: Graeme Nicol, Cécile GR, Laura Lehtorvita-Morley, Linda Hink, Heiko Nacke, Angus Mei e Marcus Bello. Agradeço também ao Michael e ao David pelas análises de FIA e à Annette e ao Professor Paul Hadley por me ensinarem e permitirem o uso da máquina de mensuração de potencial de água no solo.
Agradeço também àqueles que, fora do laboratório alegraram os meus dias, mesmo aqueles mais cinzas em “Aberdream”: Chiara, Lucas, Guilhem, Giulia, Ana, Babi e Mateus e Sarah. E Patricia Morcillo e Deboshree Gosh, melhores roommates ever. Também à Elitsa e à Gabi.
Por que uma tese é um trabalho constante, considerando que a nossa (a minha pelo menos) cabeça não pára um segundo de pensar ciência, agradeço ao suporte fora da Universidade. Mãe, pai, vocês me inspiram pelas carreiras que desenvolveram. Observo vocês para poder reproduzir na minha carreira um pouco dessa excelência que vocês têm. Obrigada ainda pelo apoio diferencial que vocês promovem na minha vida pessoal e profissional. Fran, você sabe que é a pessoa mais importante da minha vida e é aquele que sabe me alegrar e me escutar nas crises e nas alegrias.
Ainda fora do meio acadêmico, agradeço o suporte que recebo das minhas melhores amigas Renata, Rapha, Ju, Carol, Cecília e Sorriso.
vi
Resumo Geral
A interação entre as variáveis do solo e a microbiota influencia os processos que ocorrem no solo, tanto que, em ambientes terrestres o N é reciclado primariamente pela microbiota. No ciclo do N, a nitrificação é a etapa em que nitrato se torna disponível no solo para as plantas, mas também N é perdido por lixiviação de nitrato ou pela emissão de gases nitrogenados. Entretanto, as mudanças climáticas, a modificação do uso da terra e a aplicação de fertilizantes nitrogenados veem alterando a dinâmica de N. Um especial interesse é direcionado à maior savana na América do Sul, o bioma tropical sazonal seco que é o Cerrado, cuja paisagem vem sendo alterada pela agricultura. Fazendo uso da técnica de metagenômica, os atributos funcionais da microbiota do solo do Cerrado quanto ao ciclo do N foram comparados entre dois parques de conservação do bioma, distantes 500 km entre si, com variação na textura e no conteúdo de água do solo. Os tipos de vegetação amostradas dentro de cada parque mascararam os efeitos de altitude e distância entre os parques, e todas as amostras apresentaram uma maior abundância de genes para assimilação de amônia e amonificação. Isso corrobora a literatura encontrada sobre o metabolismo de amônia como forma principal de N no Cerrado. Em particular, o Campo limpo alagado, presente somente em um dos parques, apresentou a maior abundância de genes fixadores de nitrogênio. Ainda, foram detectados genes para denitrificação, mas somente dois hits foram observados para nitrificação. Sucessivamente, foi acessado o impacto do manejo do solo sobre a abundância de Archaea e Bacteria oxidantes de amônia por quantificação do gene marcador amoA ao longo do cultivo da soja no bioma Cerrado. A análise molecular, tal como as técnicas clássicas e de isótopos mostraram um maior conteúdo de C orgânico e de NH4+-N no pousio em comparação à área nativa de reserva
legal adjacente ao plantio da soja. De mesma forma, observou-se um aumento na abundância de oxidantes de amônia e da taxa de nitrificação no solo agrícola em comparação à área nativa, com a menor razão amônia/nitrato observada no solo após revolvimento. A abundância de AOB apresentou correlação com o aumento de pH ao longo do cultivo da soja. Experimentos seguintes testaram o efeito de água e de pH em microcosmos contendo solo do Cerrado, tal como a possível inibição de nitrificação em slurries contendo uma mistura de solo do Cerrado com um solo agrícola (Craibstone) com reconhecida atividade de oxidação de amônia. No entanto, o acúmulo de NO3- estava
vii
viii
General abstract
Interactions between soil characteristics and microbiota influence the processes in soil ecosystem, as the terrestrial N is primarily cycled by the microbiota. In the N cycle, nitrification enables plants’ access to nitrate, although N can be lost through nitrate leaching, or N trace gas emission. These N dynamics are being disturbed by climate change, land use modification and the employment of nitrogenous fertilizers. A special interest goes to the largest savanna in South America, the seasonally dry Cerrado biome, where agriculture is changing the biome landscape. Shotgun metagenomics was used to compare the functional attributes of N cycling from the soil microbiota present in two conservation parks of the Cerrado biome, 500 km distant from each other, with varying soil texture and water content. Types of vegetation sampled within each park masked the altitude and distance effects, but all samples showed higher abundance of genes for assimilation of ammonia and ammonification. This corroborates Cerrado literature of ammonia as the main soil N form. In addition, a flooded grassland presented the highest abundance of N fixation genes. Despite the detection of denitrification genes, only two hits for the nitrification process were described. Subsequently, we assessed the impact of soil management on the abundance of Archaea (AOA) and Bacteria (AOB) ammonia oxidizers by quantification of the marker gene (amoA) during different stages of soybean cultivation within the Cerrado. Molecular analysis and classic and isotope techniques exhibited higher content of organic C and NH4+-N during fallow than in the adjacent undisturbed field, and an increase in ammonia
oxidizers abundance and nitrification rates in the agricultural soil than in the undisturbed site, with the lowest ammonium/nitrate ratio in tilled soil. AOB abundance was correlated with the increase in pH during soybean cultivation. Further experiments tested the effect of moisture and pH in microcosms containing Cerrado soil, and the possible nitrification inhibition in slurries assembled with a mixture of Cerrado and agricultural soil known for actively oxidizing ammonia (Craibstone soil). Nevertheless, very little NO3- accumulation
was observed in Cerrado microcosms with either increasing moisture or pH, despite high ammonia concentration. Nitrification was not inhibited in the mixed soil slurries, and after 21 days it was possible to detect the activity of AOA with the quantification of amoA transcripts. Moreover, DGGE profiles showed a higher number of AOA amoA gene in the Craibstone-only slurries and similar to the mixed slurries, but lower in the Cerrado-only slurries. This was the first assessment of the N metabolism with metagenomic data and qPCR for ammonia oxidation in the Cerrado. However, the little accumulation of NO3- in the
ix
occurs in this ecosystem to preserve inorganic N preferentially in the NH3 form. Taken these
findings together, it is likely that not only the presence of ammonia oxidizers is fundamental for nitrification to occur, but that the microbial community composition and diversity affects the direction in which N process occur in soil. Most possibly there is a correlation between abiotic and biotic conditions that limits the abundance of autotrophic ammonia oxidizers, as for example the competition for NH4+ by plants or heterotrophic
x
General Index
LEGENDS OF FIGURES AND TABLES 9
MOTIVAÇÃO 11
OBJETIVOS E HIPÓTESES 13
ORGANIZAÇÃO DE CAPÍTULOS 15
CHAPTER 1 – N CYCLE, NITRIFICATION AND THE CERRADO BIOME: LITERATURE REVIEW 16
NITRIFICATION 17
HETEROTROPHIC NITRIFICATION 19
DENITRIFICATION 20
DENITRIFIERS GUILDS AND N TRACE GASES EMISSION 21
N FIXATION AND OTHER SOURCES OF N 22
LAND USE IMPACT ON MICROBIAL COMMUNITIES 23
CHAPTER 2 – DISTRIBUTION OF MICROBIAL COMMUNITIES IN TWO CERRADO CONSERVATION PARKS WITH A METAGENOMICS APPROACH, WITH SPECIAL FOCUS ON THE N METABOLISM 25
ABSTRACT 25
INTRODUCTION 26
MATERIAL AND METHODS 28
Soil sampling and physicochemical analyses 28
DNA extraction and sequencing 32
Statistical analysis 32
RESULTS 34
Study sites and soils characteristics 34
Phylogenetic and functional analyses 37
DISCUSSION 12
CHAPTER 3 - SHORT-TERM IMPACT OF SOYBEAN MANAGEMENT ON AMMONIA OXIDIZERS IN A BRAZILIAN SAVANNA UNDER RESTORATION AS REVEALED BY COUPLING DIFFERENT TECHNIQUES
11
ABSTRACT 11
INTRODUCTION 12
MATERIALS AND METHODS 14
Study sites and soil characteristics 14
Isotope analysis 18
DNA extraction 18
Real-time PCR 18
Statistical Analysis 19
RESULTS 20
Description of study sites and soil physicochemical characteristics 20
Ammonium and nitrate concentrations and soil δ15N 21
Abundance of archaeal and bacterial amoA genes 25
DISCUSSION 28
CHAPTER 4 – AMMONIA OXIDIZERS IN A NON-NITRIFYING BRAZILIAN SAVANNA SOIL 32
ABSTRACT 32
xi
MATERIALS AND METHODS 35
Soil sampling 35
Cultures with or w/o soil aqueous extracts 35
Soil incubation in slurries 36
Soil incubation in microcosms 36
Soil physicochemical analyses 37
Molecular analysis 37
Statistical analysis 38
RESULTS 40
Effects of soil extracts on ammonia oxidizer cultures 40 Effects of Campo sujo soil on nitrification in Craibstone soil 41
Effects of soil pH and moisture content 45
DISCUSSION 47
CHAPTER 5 – ABIOTIC AND BIOTIC FACTORS THAT AFFECT AMMONIA OXIDIZERS AND THEREFORE
NITRIFICATION: FINAL DISCUSSION 50
PH 51
NH4+ 53
HETEROTROPHIC NITRIFICATION 54
INHIBITION OF NITRIFICATION 55
FE 55
SOIL TEXTURE AND WATER CONTENTS 56
CERRADO VEGETATION COVER AND LAND USE CHANGE 60
FINAL CONSIDERATIONS AND NEW HYPOTHESES 61
CAPÍTULO 6 – CONCLUSÕES E PERSPECTIVAS 66
9
Legends of figures and tables
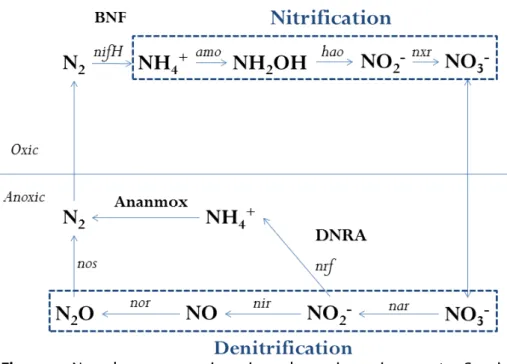
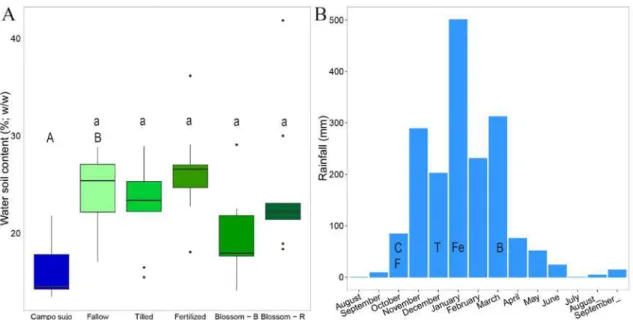
Figure 1. N cycle processes in oxic and anoxic environments. ... 18
Figure 2. Photographs of the sites sample in the two parks. ... 31
Figure 3. Boxplots on soils (A) NH4+-N and (B) NO3- -N concentration, (C) pH and (D) water
soil content. ... 34
Figure 4. Soil physicochemical variables in the two parks and their sites. ... 37
Figure 5. Principal component analysis (PCA) constructed with the relative abundance of annotated genes for (A) phylogenetic assignment of phyla and (B) subsystems functional 38
Figure 6. Bar plots for the relative abundance of SEED subsystems according to each site × park. (A) Most abundant SEED subsystems, (B) less abundant SEED subsystems. ... 9
Figure 7.Schematic representation of the N cycle according to the SEED subsystems
annotated genes performed with e!Sankey 2. ... 9
Figure 8. Boxplots of soils (A) ammonia assimilation, (B) nitrate and nitrite ammonification, (C) nitrogen fixation, (D) dissimilatory nitrite reductase, (E) denitrification, (F) nitrosative stress. ... 11
Figure 9. (A) Representation of the map (B) sampled parks PESA and PNCV marked in the Google Earth photography (C) Module experimental design scheme ... 9
Figure 10. Gravimetric soil water content. ... 14
Figure 11. Satellite view and photographs of the sample site on the Tabapuã dos Pireneus Farm. ... 16
Figure 12. Principal component analysis (PCA) of soil physicochemical properties ... 21
Figure 13. One-way ANOVA tests on soil N values, with Tukey–Kramer post hoc tests to compare group means (R with the ggplot2 package). ... 23
Figure 14. Relationship between soil δ13C and δ15N in ‰. Each point represents samples from
each soil condition, marked with different symbols. ... 24
Figure 15. Changes in (A) AOA amoA gene abundance, (B) AOB amoA gene abundance, (C) AOA:AOB amoA gene abundance ratio, and (D) archaeal 16S rRNA:amoA gene abundance ratio. ... 26
Figure 16. Graphical representation of the three experimental designs. ... 36
10
Figure 18. Changes in inorganic N concentration during incubation of slurries of Craibstone
and Campo sujo soils and mixtures of these soils. ... 43
Figure 19. Changes in (A) AOA amoA gene abundance, (B) AOB amoA gene abundance, (C) AOA:AOB amoA gene abundance ratio and (D) AOA amoA transcript abundance during incubation of slurries of Craibstone and Campo sujo soils and mixtures of these soils. ... 44
Figure 20. DGGE analysis of partial amoA gene products from triplicate soil slurries of (G) Campo sujo-only, (CG) 1:1 Campo sujo: Craibstone mixed and (C) Craibstone-only ... 45
Figure 21. Changes in (A) (NH4+-N + NO3--N) and (B) NO3--N during incubation of microcosms containing Campo sujo or Craibstone soil after manipulation of pH and moisture content. 46 Figure 22. Interaction between abiotic and biotic factors and their effect on the N cycle processes ... 63
Table 1. Physicochemical variables (mean ± SE) of the sampled sites in PNCV ... 29
Table 2. Physicochemical variables (mean ± SE) of the sampled sites in the PESA ... 30
Table 3. Coordinates and altitude of each sampled site ... 18
Table 4. Number of sequences for each metagenome, their identification and the number of reads that passed QC, that were annotated and to the N metabolism... 10
11
Motivação
Micro-organismos aprovisionam diversos serviços ecossistêmicos, tais como a reciclagem de nutrientes e a decomposição de matéria orgânica, a reciclagem de dejetos e o controle biológico de pestes. Na economia mundial atual, esses serviços representam um terço da contribuição anual dos serviços ecossistêmicos terrestres, significando uma média global estimada de 1,6 trilhões de dólares por ano. Além disso, a interação entre micro-organismos e plantas, especialmente na rizosfera, é responsável pela nutrição e saúde das plantas, que dependem de reações catalisadas pelos micro-organismos no solo. Consequentemente, o crescimento populacional mundial depende do fornecimento de comida pela agricultura e pecuária, por sua vez condicionado à reciclagem de nutrientes por micro-organismos no solo.
Todavia, o objetivo dos micro-organismos é o de obter energia para seu próprio metabolismo ou produção de biomassa. Por sua vez, se o substrato é provido em excesso no ambiente, uma maior concentração de produtos será liberada e não incorporada à biomassa microbiana (e de plantas). Por exemplo, o uso de fertilizantes nitrogenados em excesso na agricultura pode potencialmente levar ao aumento de emissão de gases nitrogenados causadores do efeito estufa (N2O), e também às perdas de nitrato que levam
à contaminação de cursos de água.
A aquisição de energia em solos não é tarefa simples: formas de vida diferentes competem para a viabilidade de substratos, ou também colaboram para a troca de substrato/produto. Essa competição acontece a todo momento, em micro hotspots do solo; uma batalha entre plantas e micro-organismos e entre diferentes micro-organismos.
12
Mudanças climáticas, pluviometria e o regime de fogo devem ser considerados nos estudos da savana tropical sazonalmente seca no Brasil Central. Esta, o Cerrado, é a savana de maior biodiversidade, e em grande parte endêmica. Ainda mais, sua área está em constante modificação devido à fronteira agrícola, envolvida na produção de commodities brasileiras.
13
Objetivos e hipóteses
Objetivos Hipóteses
Gerais Descrever a microbiota do solo
do Cerrado nativo e convertido à plantação da soja, fazendo uso de técnicas moleculares e com foco no metabolismo do N
Estudar as baixas taxas de nitrificação líquida observadas nesses solos ao analisar a comunidade microbiana
Analisar a relação entre biodiversidade microbiana e a provisão de serviços ecossistêmicos como a ciclagem de nutrientes
Determinar o impacto da agricultura na abundância de oxidantes de amônia e seu funcionamento
A comunidade de oxidantes de amônia será menos abundante nos solos do Cerrado, considerando as baixas taxas de nitrificação
A abundância relativa dos genes relativos ao ciclo do N irá variar conforme as qualidades físico-químicas dos solos amostrados
O solo agrícola apresentará uma estrutura diferente da comunidade de oxidantes de amônia em relação ao solo nativo
Específicos Capítulo 2 Analisar a diversidade taxonômica e funcional dos micro-organismos do solo do Cerrado, usando dados de metagenômica
Identificar genes dos grupos microbianos responsáveis pelo metabolismo do N
Estabelecer a correlação entre a abundância relativa dos genes do metabolismo do N e as características do solo e da vegetação entre e dentro dos parques de conservação
A comunidade microbiana irá diferir de acordo com a distância biogeográfica e as características físico-químicas dentro e entre os parques de conservação
A abundância dos genes anotados para o metabolismo do N irá refletir a razão C:N, o pH, o teor gravimétrico de água e os conteúdos de N e C dos solos amostrados dentro e entre os parques
Capítulo 3 Investigar a variação temporal e espacial da abundância de archaeas e bactérias oxidantes de amônia por PCR quantitativa ao longo do cultivo da soja no bioma Cerrado
Elucidar as variáveis físico-químicas que explicam a mudança na abundância dos oxidantes de amônia
A razão entre archaeas e bactérias oxidantes de amônia irá modificar ao longo do cultivo da soja devido ao aumento do pH e à adição de fertilizantes nitrogenados
A comunidade de archaeas oxidantes de amônia será maior em número que aquela de bactérias no solo nativo de Campo sujo e na área de manejo da soja durante o pousio devido ao pH mais ácido e à provisão de NH4+
14
Capítulo 4 Incubar solos em microcosmos para testar o efeito da água e do pH na habilidade do solo de acumular nitrato
Testar o potencial biológico de inibição para nitrificação em solos do Cerrado contra um solo exótico agrícola com alta capacidade de nitrificar
Exsudatos naturais de algumas plantas estarão relacionado à potencial inibição biológica e, portanto, à redução do crescimento e atividade de oxidantes de amônia
15
Organização de capítulos
Capítulo 1 – Introdução apresenta a revisão da literatura quanto ao conhecimento dos processos enzimáticos microbianos envolvidos na ciclagem de nitrogênio em solos, com uma perspectiva direcionada ao bioma Cerrado, que é o foco desta tese
Capítulo 2 – Análise metagenômica da microbiota do solo de Cerrado nativo, com especialmente interesse na abundância relativa de genes anotados para o metabolismo do nitrogênio
Capítulo 3 – Cultivo de soja na Fazenda Tabapuã dos Pirineus pela primeira vez foi escolhido para investigar o efeito a curto prazo do manejo agrícola sobre a abundância de archaeas e bactérias oxidantes de amônia
Capítulo 4 – Limitação da oxidação de amônia em solos do Cerrado foi avaliada em microcosmos e culturas puras para testar o efeito da água, pH e potenciais inibidores biológicos produzidos por plantas sobre a nitrificação
Capítulo 5 – Discussão com o objetivo de retornar aos principais pontos apresentados nos capítulos anteriores e também estabelecer novas considerações sobre regulações bióticas e abióticasdo ciclo do nitrogênio e, mais especificamente, da nitrificação, que ocorrem nos solos
16
Chapter 1 – N cycle, nitrification and the Cerrado biome: literature
review
1“We became scientists because we are curious – we are driven to solve the puzzles that nature presents.”
Joshua Schimel
itrogen cycling is mainly controlled by microorganisms in a multitude of processes and regulations. Advances in research presents novelties that were sometimes anticipated based in N thermodynamics. For example, just recently it has been discovered the “comammonas” process, which is the ability of ammonia oxidation to nitrite and subsequently to nitrate in a same organism; metabolism predicted by the higher gain of energy when are substrate- and spatial-limited (Daims et al., 2015; van Kessel et al., 2015).
Plants and microorganisms can assimilate N in the form of ammonia, nitrate, and sometimes organic N, or N2 for a few Bacteria and Archaea. N2 enters the lithosphere and is
biologically transformed to NH4+. In turn, NO3- is made available by the dissimilatory process
of ammonia oxidation by autotrophic Archaea, Bacteria or heterotrophic Bacteria and Fungi. Microorganisms compete within themselves and with plants to use the NO3- available in
soil, which can be either assimilated or used as electron donor. The balance of processes
1 A modified version of this thesis introduction and discussion will be submitted as a review on the N cycle of the Cerrado soils.
17
and the soil conditions determines the availability of N returning to NH4+ or being
completely reduced to N2.
N is essential to primary productivity and, in nature, is mainly dependent on biological nitrogen fixation, which produces reactive N. On the other hand, the non-natural chemical conversion of atmospheric N2 to NH3 in the Haber-Bosch process, increased the
reactive N concentration in the environment, presenting consequences due to N loss to the atmosphere as N trace gases, or to water courses as nitrate produced during nitrification (Galloway and Cowling, 2002).
Nitrification
Nitrification can be measured as gross or net rates; the first is quantified by the assimilatory or dissimilatory processes calculated for example by the 15N pool dilution
methods (Davidson et al., 1991). Net nitrification is obtained by the variation of NO3--N
concentration in incubated soil (either in laboratory or field conditions) during an established period of time. However, only the first method can assess if 15NO
3- pool is
diluted with 14NO
3- produced by autotrophic nitrifiers from 14NH4+ or by heterotrophic
organisms from organic 14N (Davidson et al., 1991). Net nitrification in native undisturbed
Cerrado soils is low and sometimes undetectable. These soils present high NH4+-N:NO3--N
ratio (Nardoto and Bustamante, 2003) and insignificant N2O emissions (Cruvinel et al., 2011;
Pinto et al., 2006; Pinto et al., 2002). Thus, the investigation of nitrification in the Cerrado biome is of particular interest for its N-limitation (Araujo et al., 2012), with higher rate of N immobilization than mineralization (Nardoto and Bustamante, 2003), which leads to a need of fertilizers and liming when land use is changed for agriculture.
Cerrado is the savanna of Central Brazil and, as such has a plant cover distribution dependent on the interaction between water and nutrient availability (Medina, 1987) in (Bustamante et al., 2006), with weathered soils with low nutrient availability (Reatto et al., 1998). The Cerrado presents a range of herbaceous and tree/shrub strata from grassland to savanna and forest formations, that are related to the type of soil (Reatto et al., 1998), which may present varying contents of nitrogen according to the tree-shrub layer density, the fire regime and the land use change (Bustamante et al., 2006).
18
associated with N metabolism. Microbial ecology has been used in the last couple of decades to improve knowledge on biogeochemical processes in the environment. The presence of genes can be directly measured by PCR quantification or the taxonomic categories can be assigned through sequencing (Figure 1). Metagenomics’ studies are primer-independent thus allowing a more holistic description of genes abundance in the ecosystem. Nevertheless, the current culture-independent methods depend on database search and a great number of genes is still unclassified. Therefore, classical microbiology approach with isolated microorganisms is complementary.
Nitrification involves two groups of specialized organisms phylogenetically unrelated: the ammonia-oxidizers and the nitrite-oxidizers. The oxidation of ammonia is often the focus of research, because it is the limiting-step for nitrification to occur. However, and as mentioned above, in the end of 2015 two groups were able to identify an organism, “Candidatus Nitrospira inopinata able to perform the complete oxidation of ammonia to nitrate, isolated from a biofilm in a pipe under hot water flow (Daims et al., 2015) and from an ammonium-oxidizing biofilm from an aquaculture system filter (van Kessel et al., 2015).
19
The presence of ammonia oxidizers is quantified by the amoA gene coding for the subunit A of the enzyme ammonia monooxygenase, and it is catalyzed by autotrophic Bacteria (AOB) – Nitrosomonas (β-proteobacteria), Nitrosospira (β-proteobacteria) (Kowalchuk and Stephen, 2001) and Nitrosococcus (γ-proteobacteria) – or autotrophic Archaea (AOA), phylum Thaumarchaeota. The ammonia monooxygenase is a protein of membrane that converts NH3 to NH2OH (hydroxylamine, HAO), then released into the
periplasm to be oxidized by HAO to NO2- in AOB (De Boer and Kowalchuk, 2001). No hao
gene has been detected in AOA genomes, but N. maritimus seems to produce NH2OH,
possibly through a different enzyme complex (Vajrala et al., 2013). AOA and AOB appear to be mechanistically similar even though differ, within other things, in the dependence of copper (AOB) rather than iron as the redox active for AOA (Stahl and de la Torre, 2012) and in the organization of the operon AMO. In AOB the AMO operon has a conserved organization as amoCAB (Bothe et al., 2000; Nicol G.W., 2006; Norton et al., 2002), while in Thaumarchaeota the organization as amoAxCB varies between lineages (Bartossek et al., 2012; Blainey et al., 2011).
The majority of studies with soils show AOA as more abundant than AOB and more frequently associated with nitrification rates (Leininger et al., 2006; Mao et al., 2011; Prosser and Nicol, 2012). In addition, AOA seem to prefer ammonia generated from the mineralization of organic N and are the predominant ammonia oxidizers in acidic soils (Levičnik-Höfferle et al., 2012; Prosser and Nicol, 2012; Zhang et al., 2012) or in environments with little availability of NH4+ (Gubry-Rangin et al., 2011; Gubry-Rangin et al., 2010; Nicol et al.,
2008). This apparent niche differentiation (Prosser and Nicol, 2012) might be important to consider in view of the economic and ecological costs of fertilization and nitrogen losses.
Heterotrophic nitrification
20
or inorganic N, but ammonia oxidation is not linked to cellular growth as in autotrophs. The presence of heterotrophic nitrifiers in soil can be presumed by the accumulation of nitrate in soils incubated with acetylene, a specific inhibitor of autotrophic nitrification, as suggested for Cerrado soils (Poth et al., 1995). On the other hand, nitrification in Fungi seems to involve the reaction of N compounds with hydroxyl radicals formed potentially during cell lysis or lignin degradation (De Boer and Kowalchuk, 2001).
Moreover, some of the bacteria able to perform nitrification heterotrophically can combine nitrification-denitrification processes; where denitrification is used by the organism to dissipate reducing equivalents (NADH) under low oxygen conditions, allowing a greater growth rate on an environment with substrate in excess (De Boer and Kowalchuk, 2001) , which is less likely in Cerrado soils.
Denitrification
Denitrification alone is represented as the reduction of NO3- to NO2-, and
subsequently to NO, N2O and N2 by the same organism or more commonly by different
organisms, thus considered a modular process (Graf et al., 2014). The reduction of NO2- to
NO is catalyzed either by a copper-containing enzyme, that can be identified by the measurement of the gene nirK abundance; or the nitrite reductase encoded by nirS which is a cytochrome cd1 (Mohan et al., 2004). These are dissimilatory enzymes associated with electron transport phosphorylation. However, nitrite reductases can also be assimilatory when the reduction of NO2- leads to NH4+. These use reduced pyrimidine nucleotides or
ferredoxin as electron donor: the cytoplasmatic NirB is more common in fermentative bacteria and the periplasmic nitrite reductase, deduced by the presence of the gene nrfA in the environment, is found in a wider range of bacteria than the above (Mohan et al., 2004).
Denitrification is dominant on nitrate-rich environment with low electron donors’ concentration; however, NO3- and NO2- reduction to NH4+ predominates on an electron-rich
environment where NO3- is in low concentration. Dissimilatory nitrite reduction to
ammonium (DNRA), known also as fermentative reduction of nitrate or ammonification, is the concurrent process to denitrification, representative in reduced and C-rich environments. Available soil literature is smaller for DNRA than denitrification, even though DNRA is also a process widespread among bacteria (Mohan et al., 2004). DNRA was suggested as a short-circuit of N cycle, returning NO3- to NH4+ (Cole and Brown, 1980), and
21
process, it can be relevant in soils (Rütting et al., 2011). On the other hand, anammox, the anaerobic ammonia oxidation to N2 seems to be strictly present in anoxic environments.
Denitrifiers guilds and N trace gases emission
A microbial guild is described as a group of organisms occurring in the same space and time, and that use same resources (Fauth et al., 1996). The relative abundance of different microbial guilds is dependent on soil characteristics. An increase in soil water content after the first rains that follow the dry season in the Cerrado promoted higher mineralization (Nardoto and Bustamante, 2003), reflecting a higher microbial activity and nitrification (da Silva, 2004). More specifically, AOA and AOB differ in their niches in soil according with different pH and ammonia availability. Similarly, denitrifier’s guilds, meaning organisms containing either nirK or nirS genes, also respond differently to the environment (Enwall et al., 2010; Jones and Hallin, 2010) as well as the nosZ organisms from clade I or II (Jones et al., 2013). The ratios of nirS/nirK type and nosZ clade I/clade II are related and have an effect on the soil N2O sink capacity, more significant in environments dominated by nosZ
clade II (Jones et al., 2014).
In turn, the balance between the processes described above controls N trace gases emissions (Conrad, 1996). Emission of NO and N2O can occur either during nitrification or
denitrification. A special attention is given to agricultural fields as fertilization increases the microbial transformation of reactive N (Galloway and Cowling, 2002). N2O is a significant
greenhouse gas after CO2 and CH4, and is also a relevant ozone depleting gas (Ravishankara
et al., 2009) when oxidized to NO, as reviewed recently (Kanter et al., 2013). In addition, N oxides (NO and NO2) are removed from the troposphere as nitric acid, contributing to
ecosystems acidification.
Emissions of nitric oxide (NO) represents 0.4 kg N ha-1 year-1 loss of N in the Cerrado
(Bustamante et al., 2006) and is emitted in higher concentration than N2O in those soils, as
expected by the dry and well-aerated characteristic of these soils (Pinto et al., 2002). The “hole-in-the-pipe” concept states that soil water content is the principal control on the balance of production, consumption and diffusive transport between NO, N2O and N2 in
soils (Davidson et al., 2000). The ratio of emission between N2O and NO should be 1 in soils
22
however, in a wet soil N2O is more prevalent and the analysis of only NO would lead to false
conclusions that nitrogen availability does not matter (Davidson et al., 2000).
Cerrado soils can experience short moments of flooding during the first rainfall after the dry season, but they are often described as well-drained, leached and oligotrophic soils (Ribeiro and Walter, 2008). The first rains after the dry season promote an increase of 100 fold on the emission of NO in the Cerrado, which does not continue during the wet season (Pinto et al., 2002). Although denitrification can occur in aerated soils (Braker et al., 2015), it is not expected in the Cerrado soils, especially because of the low accumulation of NO3- in these soils, and the dominance of N form as NH4+ is associated with low N trace
gases emission (Davidson et al., 2000).
N fixation and other sources of N
Reactive N enters the system through biological or chemical N fixation, which is the conversion of the inert gas N2 to NH4+. In the Cerrado soils, the biological nitrogen fixation
(16 a 44 kg N ha-1 year-1) exceeds the abiotic fixation through electrical discharges (4 kg N ha -1 year-1) (Bustamante et al., 2006; Cleveland et al., 1999). This important source of N is
possibly related with the high abundance of plant species from the Fabaceae family in the Cerrado (Filgueiras, 2002), even though very few studies have focused on the nodular activity of these plants (Bustamante et al., 2012c). It is recognized though that O2, P, Ca and
Al concentrations, soil moisture, bacterial density and plant needs of N determine the ability of nodulation by symbiotic dyazotrophs (Bustamante et al., 2006). As well as for denitrifiers, the genes encoding the enzyme nitrogenase (nifH being the gene used to quantify N fixation) are widespread in the Bacteria and Archaea domains. Although nitrogenase is an enzymatic complex sensible to oxygen, dyazotrophs are not necessarily anaerobic (Falkowski et al., 2008).
Organic matter mineralization recycles N in soils, which can then be assimilated by plants and microorganisms, or lost via NH3 volatilization, enzymatic denitrification and NO3
23
destruction, and nitrate causes eutrophication in water courses due to increased leaching. Furthermore, only half of N added in crops is used by the plants (Galloway and Cowling, 2002).
NO3- is considered the main form of nutrition used by plants in well aerated soils,
where nitrification is more prone to happen. However, in Cerrado soils, the greatest part of inorganic N is found in the form of ammonia (Nardoto and Bustamante, 2003), suggested to be related with the low pH found in these soils, or competition between plants and microorganisms. Furthermore, the availability of inorganic N in soil depends on organic matter mineralization, which is lower than N immobilization in Cerrado soils (Nardoto and Bustamante, 2003). For example, some forests in their climax are more efficient in N use, potentially by inhibiting nitrification, and so maintaining predominantly ammonia than nitrate in the soil solution, which leads to lower losses of N as reviewed recently (Subbarao et al., 2015). Therefore, the observation of dynamics between plants and microbial community in the belowground can help understand the balance in N transformations and N retention and therefore provide a model for a more sustainable crop productivity.
Land use impact on microbial communities
Brazil is the fourth worldwide country in agriculture production, which depends on inorganic fertilizers. The progressing frontier of agriculture and managed pasture for cattle breeding promoted the change of approximately 53% change of the Cerrado’s original area (Beuchle et al., 2015). Soybean, maize, cotton and sugarcane stand out as the major crops cultivated in the Cerrado region, in which only the first is partially independent on the addition of fertilizers (Mendes et al., 2003). A study published in 2010 showed that 81% of exported soybean was produced in Brazil, EUA and Argentina together. This reflects a global trade of biogeochemical N cycling represented in 25% by the soybean commodity (Lassaletta et al., 2014).
24
Then again, governmental initiatives are also concerned with preserving the Brazilian biomes biodiversity in conservation unities. However, only 2.2% of this biome is under unities of integral protection and other 1.9% in unities of sustainable use (Klink and Machado, 2005; Marris, 2005) ensuring its status of a hotspot for biodiversity conservation (Myers et al., 2000). In this context, it is important to have in mind that soil is the main actor in the ecosystem conservation, especially in seasonally dry environments as the Cerrado, where climatic change will probably modify rain distribution and regime, changing also the fire frequency and potentially nutrients and biomass loss (Bustamante et al., 2012c).
More specifically, soil microbial diversity contributes to the resistance/resilience of the system, which means that the lower diversity after land use change can alter the stability of the ecosystem. Mao et al. (2011) observed that N fertilization for bioenergy crops (Zea mays and Miscanthus giganteus) altered the microbial communities, and induced the modification on 15% to 30% of the relative abundance of nitrification and denitrification genes. This is an example of how agriculture impacts microbial potential ecological functions. Same results were observed in the Cerrado soils, where soybean cultivation reduced microbial N independent on the soil management or the plant growing stage in comparison to a soil under native Cerrado (Perez et al., 2005). Moreover, land use management in the Amazonian forest changed the composition and abundance of soil microbial communities, related with the modification in soil pH and OM (Paula et al., 2014).
25
Chapter 2 – Distribution of microbial communities in two Cerrado
conservation parks with a metagenomics approach, with special focus
on the N metabolism
2“everything is everywhere” and why do we care Baas Becking
Abstract
Nitrogen is the base for primary productivity, and primarily cycled by the soil microbiota. Climate change, land use side effects and nitrogenous fertilizers employment are changing the global N budget. The Cerrado is the largest savanna in South America, and as others savannas in the world, is suffering from the land use change to agriculture and pasture. Yet, little is known of how these changes affect soil microbial communities. Undisturbed areas are essential to understand the natural processes rates that occur in soil. We used shotgun metagenomics to compare the functional attributes of N cycling from the soil microbiota present in two parks for conservation of the Cerrado biome, 500-km distant from each other, with varying altitude, soil texture and water content. Types of vegetation sampled within each park masked the altitude and distance effects. The soils with greater and lower soil water content presented the highest levels of α-diversity, which may relate with greater evenness of species to overcome a less enabling environment. N fixation, nitrosative stress and ammonification from nitrate and nitrite differed significantly between the sites sampled. Across all soils, the assimilation of ammonia and ammonification were the most abundant subsystem of nitrogen cycle, corroborating the Cerrado literature that states ammonia as the main nitrogen form. We detected genes for denitrification enzymes, but only two hits for the nitrification process were described. This study suggests that the N cycle processes occurs differently between the sites. Furthermore, we suggest that each type of vegetation is relevant for N conservation in this biome.
Keywords: Brazilian savanna, Cerrado, N cycle, ammonia, nitrification, denitrification
26
Introduction
Savanna ecosystems hold almost one fifth of the world’s population. Cerrado is the main tropical savanna in the south hemisphere. It is a representative biome in central Brazil, the second largest in South America, and a wildlife corridor for species from the Amazon and Atlantic rainforests. As others savannas, Cerrado is controlled by the interaction between water and nutrient availability (Bustamante et al., 2006). Cerrado has an alternating wet and dry seasons and fire frequency that might change attributable to the global climatic changes, as higher temperatures, decreased rainfall and longer dry season may have an impact on net ecosystem exchanges and reduced nutrient stocks (Bustamante et al., 2012c).
The Cerrado is characterized by a continuous herbaceous layer over which stands a discontinuous tree/shrub stratum, resulting on a range of ecosystems from grassland to savanna and forest formations. This variation on the types of vegetation found in the Cerrado biome is related to the type of soil, mostly weathered with low nutrient availability (Reatto et al., 1998), which may present varying contents of nitrogen according to the tree-shrub layer density, the fire regime and the land use change (Bustamante et al., 2006). Plant type and soil texture influence microbial community structure in the rhizosphere soil (Tkacz et al., 2015).
Due to its progressively land use change - approximately 53% of the Cerrado landscape has been transformed (Beuchle et al., 2015) – the Cerrado is considered a hotspot for biodiversity conservation (Myers et al., 2000), and approximately 2% of this biome is under protection (Marris, 2005). However, conservation unities designated for environment protection are not necessarily continuous (Beuchle et al., 2015). In addition, a special attention for conservation is paid to forest formations bordering water courses in the Brazilian legislation. Nevertheless, ecological insurance theory assumes that a better occupation of space by higher diversity leads to a better system productivity (Yachi and Loreau, 1999), i.e. the distribution of Cerrado in different vegetation patches. Similarly, we suggest that microbial community performs differently in these patches, due to the different resources and soil characteristics.
27
linked to nutrient availability in soil and is often associated with the distribution of bacterial communities in soil (Bru et al., 2011; Griffiths et al., 2011; Kuramae et al., 2012; Rousk et al., 2010) (Lauber et al., 2009). However, in the Cerrado soil moisture is more strongly related with microbial community structure (Catão et al., 2014; Pereira de Castro et al., 2016; Viana et al., 2011), which can be associated with soil texture and its water retention capacity. Recently, Pereira de Castro et al. (2016) discussed the general metabolic potential distribution in the Cerrado biome besides the taxonomy approach. Nonetheless, until now no work has focused on the microbial genes associated with nitrogen cycling in the Cerrado, despite the need to understand microbial governed N pathways in undisturbed ecosystem and the use of high- throughput shotgun sequencing to characterize the N metabolism in other environmental samples (Andreote et al., 2012; Cobo-Díaz et al., 2015; Pfister et al., 2010).
Nitrogen is mainly recycled in soils through nitrogen fixation, SOM mineralization, ammonification, nitrification and denitrification. In undisturbed ecosystems, N leakage is minimized, and nitrification is restricted, but little is understood about this in the Cerrado biome. The ecology of N dynamics between compartments has been reviewed beforehand for this biome (Bustamante et al., 2006), which is characterized by a high NH4:NO3 ratio, low
nitrification and low N gas emission.
This work was conducted to investigate the variation of relative abundance of taxonomic and functional potential genes in the soil of Cerrado. It was considered the range of types of vegetation found in two 500-km distant parks of conservation with different altitudes, and pluviometry. The first hypothesis assumes that vegetation and edaphic characteristics, which vary within and between parks, will reflect on the microbial diversity, due to different resource use or environment constraints. Secondly, we hypothesized that genes related to N metabolism would vary with the soil characteristics specific to each vegetation type as carbon and NH4+ availability, pH and soil moisture. To
28
Material and methods
Soil sampling and physicochemical analyses
This study was performed in two sites: the National Park of the Chapada dos Veadeiros (PNCV) and State Park of Serra Azul (PESA) both located in Central Brazil (Figure 9), classified as the Cerrado biome and approximately 500 km distant (coordinates provided in Table S1). The two sites diverge in altitude (Table S1). The climate of these regions is classified as Koppen Aw and the annual mean rainfall is of 1500 mm mostly during the rainy season, which happens from October to May. Sampling was performed at the end of the rainy season: the accumulated rain and the mean temperature from the month of April (2013) until the sampling day in the PNCV was of 2.2 mm and 21 oC; for the PESA no rain was
measured on the month of May (2013) and an average 27 oC were measured. In total 8 areas
and 6 different vegetation types were sampled.
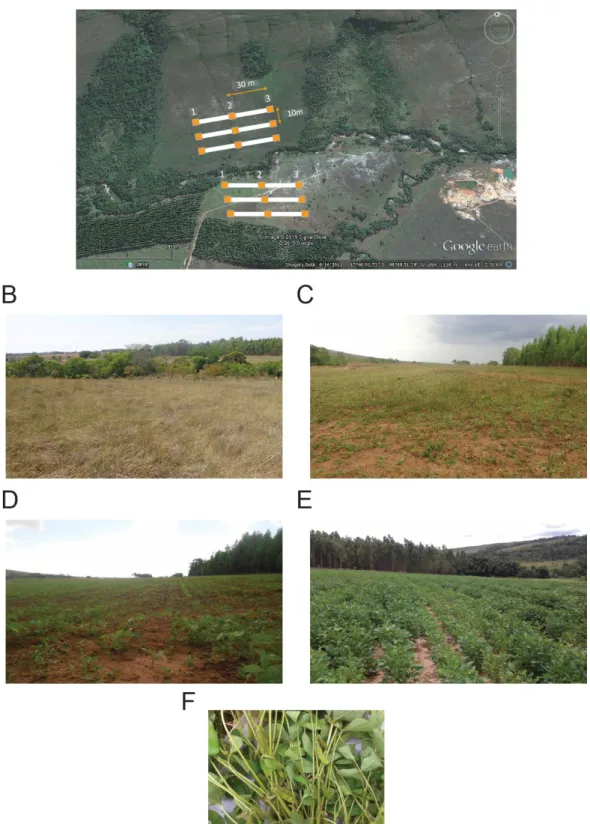
In the PNCV and the PESA, beside some other parks, it was installed modules of standardized sampling thanks to the project financed by CNPq “Diversidade biológica do Cerrado: estruturas e padrões”.. These modules were created within the “Rede ComCerrado” (Portaria MCT 319, 7 May 2009), which is a network founded by several research groups from public institutions in Brazil to monitor Cerrado’s biodiversity. These modules establish 5 km2 area bordered by 2 lines oriented east-west 1 km apart and 5 km
long as standardized in the literature to sample extensive biomes as the Amazon rainforest (Magnusson et al., 2005). Along these 5 km, 10 parcels (5 in each line) were established, one per km and a perpendicular line of 250 m was draw along the terrain level curve (Figure 9C).
Soil was sampled from a total of 24 points (8 sites in triplicates from the upper 10 cm. Replicates in each site were taken approximately 50 m apart (at 50, 100 and 150 m inside the parcel line (red line in Figure 9C), soil was sieved through a 2-mm mesh and stored on ice upon collection and on -20oC in the laboratory before physicochemical and
molecular analysis. Soil texture and content of macro and micronutrients were measured by using standard methods (Soils Embrapa–SNLCS) at SoloQuímica, Inc, Brasília, Brazil. Inorganic N was determined as described previously (Catão et al., 2016).
29
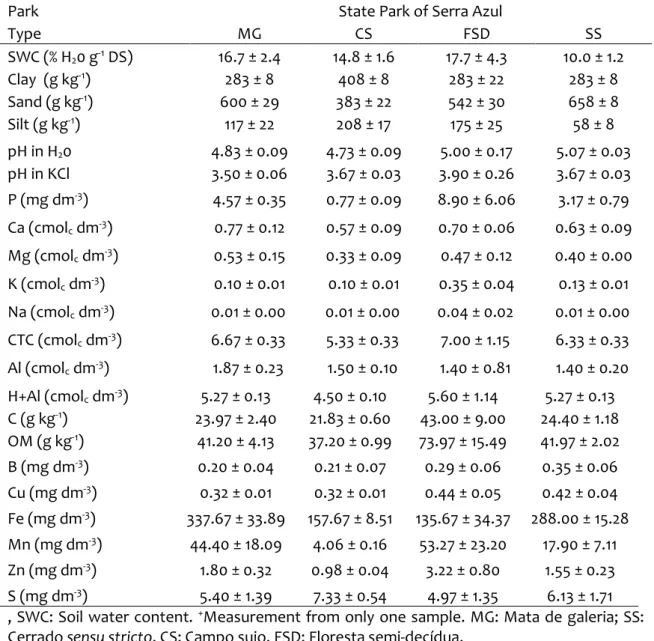
2007). In the PNCV, soil samples were obtained in a Cerrado sensu stricto (SS), in a riverbank gallery forest, hereafter called “Mata de galeria” (MG), a flooded grassland, hereafter named “Campo limpo” (CL) and a Cerrado “rupestre” (CR), (Figure 2). The physicochemical variables observed in the sampled soils are described in Table 1.
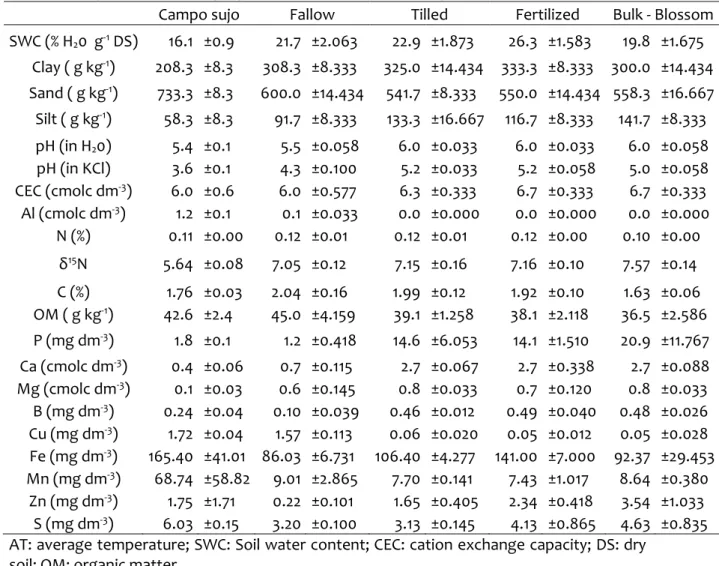
Table 1. Physicochemical variables (mean ± SE) of the sampled sites in PNCV
Park National Park of the Chapada dos Veadeiros
Type MG CL SS CR
SWC (% H20 g-1 DS) 45.8* 48.1 ± 8.8 17.8 ± 1.8 6.0 ± 0.6
Clay (g kg-1) 233 ± 8 167 ± 22 333 ± 22 133 ± 8
Sand (g kg-1) 617 ± 22 758 ± 22 608 ± 17 842 ± 8
Silt (g kg-1) 150 ± 29 75 ± 14 58 ± 8 25 ± 0
pH in H20 5.70 ± 0.06 5.27 ± 0.34 4.93 ± 0.09 5.00 ± 0.06
pH in KCl 3.97 ± 0.12 4.20 ± 0.06 3.73 ± 0.03 3.70 ± 0.06 P (mg dm-3) 10.83 ± 2.88 4.73 ± 0.66 1.17 ± 0.19 2.53 ± 0.20
Ca (cmolc dm-3) 1.10 ± 0.45 0.57 ± 0.12 0.50 ± 0.06 0.57 ± 0.15
Mg (cmolc dm-3) 0.53 ± 0.09 0.30 ± 0.00 0.33 ± 0.03 0.30 ± 0.00
K (cmolc dm-3) 0.17 ± 0.02 0.02 ± 0.01 0.09 ± 0.01 0.04 ± 0.00
Na (cmolc dm-3) 0.02 ± 0.01 0.02 ± 0.00 0.02 ± 0.00 0.01 ± 0.00
CTC (cmolc dm-3) 9.33 ± 0.33 6.00 ± 0.58 7.67 ± 0.33 5.67 ± 0.33
Al (cmolc dm-3) 2.33 ± 0.52 0.90 ± 0.15 1.80 ± 0.15 1.00 ± 0.10
H+Al (cmolc dm-3) 7.27 ± 0.64 5.13 ± 0.35 6.53 ± 0.17 4.43 ± 0.38
C (g kg-1) 182.07 ± 51.98 47.17 ± 13.20 29.33 ± 2.60 11.97 ± 1.24
OM (g kg-1) 313.17 ± 89.42 81.13 ± 22.68 50.43 ± 4.47 20.60 ± 2.15
B (mg dm-3) 0.63 ± 0.06 0.70 ± 0.01 0.69 ± 0.07 0.58 ± 0.10
Cu (mg dm-3) 0.13 ± 0.04 0.19 ± 0.04 0.17 ± 0.02 0.15 ± 0.03
Fe (mg dm-3) 72.47 ± 16.51 158.33 ± 9.02 246.00 ± 54.99 136.27 ± 27.79
Mn (mg dm-3) 5.68 ± 0.88 3.31 ± 0.10 3.41 ± 0.12 2.94 ± 0.03
Zn (mg dm-3) 0.85 ± 0.18 0.52 ± 0.17 0.40 ± 0.03 0.19 ± 0.02
S (mg dm-3) 25.03 ± 8.79 43.30 ± 7.40 12.70 ± 2.04 10.43 ± 1.21
SWC: Soil water content. *Measurement from only one sample. MG: Mata de galeria; CL:
Campo limpo; SS: Cerrado sensu stricto, CR: Cerrado rupestre
30
gallery forest, hereafter called “Mata de galeria” (MG), a semi-deciduous forest (FSD) and a shrubland (CD) (Figure 2).
Table 2. Physicochemical variables (mean ± SE) of the sampled sites in the PESA
Park State Park of Serra Azul
Type MG CS FSD SS
SWC (% H20 g-1 DS) 16.7 ± 2.4 14.8 ± 1.6 17.7 ± 4.3 10.0 ± 1.2
Clay (g kg-1) 283 ± 8 408 ± 8 283 ± 22 283 ± 8
Sand (g kg-1) 600 ± 29 383 ± 22 542 ± 30 658 ± 8
Silt (g kg-1) 117 ± 22 208 ± 17 175 ± 25 58 ± 8
pH in H20 4.83 ± 0.09 4.73 ± 0.09 5.00 ± 0.17 5.07 ± 0.03
pH in KCl 3.50 ± 0.06 3.67 ± 0.03 3.90 ± 0.26 3.67 ± 0.03 P (mg dm-3) 4.57 ± 0.35 0.77 ± 0.09 8.90 ± 6.06 3.17 ± 0.79
Ca (cmolc dm-3) 0.77 ± 0.12 0.57 ± 0.09 0.70 ± 0.06 0.63 ± 0.09
Mg (cmolc dm-3) 0.53 ± 0.15 0.33 ± 0.09 0.47 ± 0.12 0.40 ± 0.00
K (cmolc dm-3) 0.10 ± 0.01 0.10 ± 0.01 0.35 ± 0.04 0.13 ± 0.01
Na (cmolc dm-3) 0.01 ± 0.00 0.01 ± 0.00 0.04 ± 0.02 0.01 ± 0.00
CTC (cmolc dm-3) 6.67 ± 0.33 5.33 ± 0.33 7.00 ± 1.15 6.33 ± 0.33
Al (cmolc dm-3) 1.87 ± 0.23 1.50 ± 0.10 1.40 ± 0.81 1.40 ± 0.20
H+Al (cmolc dm-3) 5.27 ± 0.13 4.50 ± 0.10 5.60 ± 1.14 5.27 ± 0.13
C (g kg-1) 23.97 ± 2.40 21.83 ± 0.60 43.00 ± 9.00 24.40 ± 1.18
OM (g kg-1) 41.20 ± 4.13 37.20 ± 0.99 73.97 ± 15.49 41.97 ± 2.02
B (mg dm-3) 0.20 ± 0.04 0.21 ± 0.07 0.29 ± 0.06 0.35 ± 0.06
Cu (mg dm-3) 0.32 ± 0.01 0.32 ± 0.01 0.44 ± 0.05 0.42 ± 0.04
Fe (mg dm-3) 337.67 ± 33.89 157.67 ± 8.51 135.67 ± 34.37 288.00 ± 15.28
Mn (mg dm-3) 44.40 ± 18.09 4.06 ± 0.16 53.27 ± 23.20 17.90 ± 7.11
Zn (mg dm-3) 1.80 ± 0.32 0.98 ± 0.04 3.22 ± 0.80 1.55 ± 0.23
S (mg dm-3) 5.40 ± 1.39 7.33 ± 0.54 4.97 ± 1.35 6.13 ± 1.71
, SWC: Soil water content. +Measurement from only one sample. MG: Mata de galeria; SS:
31
32
DNA extraction and sequencing
DNA was extracted from 0.5 g of soil with the FastDNA Spin Kit (MP Biomedicals) with additional treatment using solutions steps 2 and 3 from the PowerSoil DNA Isolation Kit (MO Bio Laboratories Inc.) to achieve maximum DNA yields with least of organic contaminants. The extraction was evaluated in 1% agarose gel electrophoresis. The average concentration of each 24 DNA samples was of 100 ng/µL (Invitrogen Qubit fluorometer dsDNA BR Kit).
Approximately 2 µg of DNA was sent to sequence on 454 platform GS FLX + technology (Macrogen, Inc., South Korea) from each sample. Two 454 plates were used to sequence, one for each park; DNA from each site constituted one-quarter of the plate. Raw sequences were uploaded to the MG-RAST server, assigned to the projects SISBIOTA_PESA_2013 (ID 6701; accession numbers 4549601.3-4549612.3) and SISBIOTA_PNCV_april_13 (ID 5456; accession numbers 4530784.3-4530795.3), and processed with default quality control pipeline.
A total of 1,364,104 sequences (average size of 746bp and 515 bp, before and after quality control in MG-RAST) for PNCV and 992,685 sequences (average size of 659 bp and 382 bp, before and after quality control in MG-RAST) for PESA. After quality control, unassembled sequences were assigned to the taxonomic annotation with BLASTX against the M5NR non redundant databases, e-value of 10-5, 80% of identity cutoff and 50 bp
alignment. Functional annotation was performed against the metabolic subsystems SEED database with e-value of 10-5, 60% of identity cutoff and 15 bp alignment, as default. The
MG-RAST table format of sequences associated with total organism abundance (best hit classification), total bacteria assignment, total subsystems, and nitrogen metabolism were downloaded and transformed to wide format to R analysis.
In addition to the analyses of N metabolism annotated genes in PNCV and PESA soils, we compared our results with other metagenomes obtained in the Cerrado biome in a study of comparison between native and managed areas: MG-RAST ID’s 4577669.3 to 4577672.3, 4578924.3 to 4578927.3 and 4578714.3 (Souza et al., 2016).
Statistical analysis
33
34
Results
Study sites and soils characteristics
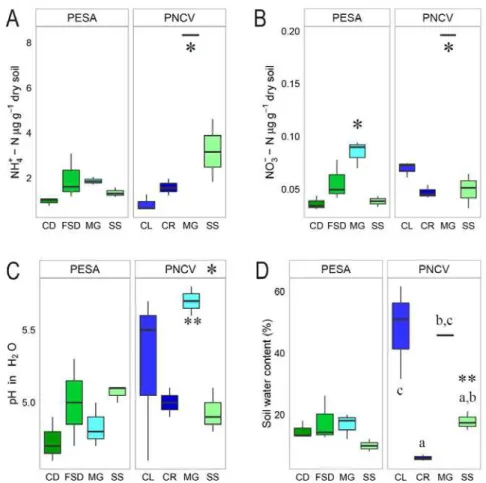
Soil NH4+-N and NO3- -N concentration, pH and water soil content were very similar in all 8
sites sampled in the two parks (Figure 3). The content of soil water, NO3- -N, NH4+-N, and
organic carbon, was measured in only one of the PNCV riverbank replicates due to the abundant presence of roots on the other replicates, which did not allow accurate measurements. The replicate of MG in PNCV had the highest NH4+-N and NO3- -N. NO3- -N
was higher in the PESA MG than CD or SS, but no difference compared to FSD. pH was higher in the sites sampled in PNCV than PESA, but were not different within each park. However, MG sites from the two parks differed in pH. Sites sampled in PESA did not differ in soil water content, but in PNCV, CL had higher soil water content than SS and CR. CR had the soil with the least water content in the two parks. Soil in the PNCV SS was slightly moister than the PESA SS.
Figure 3. Boxplots on soils (A) NH4+-N and (B) NO3- -N concentration, (C)
35
depict statistical difference between MG or between SS present in the two parks.
On the other hand, considering several physicochemical variables in a PCA, the two parks form segregated clusters (Figure 4). PNCV is a conservation unity representative of “Altitude Cerrado”, at 1200 m of altitude approximately, oppositely to PESA, that is at 650 m altitude. As altitude masked the effect of other variables, it was not considered in the PCA. Besides altitude, pH, clay (and sand), C content, Al+3, cation exchange capacity, S, Fe, K
and other micronutrients as B, Cu, Mn and Zn differ between the sampled vegetation, and consequently create two clusters according to the two parks.
The parks have different soil texture, PESA presenting a greater clay content then PNCV, except for the Cerrado sensu stricto, which had the highest clay content within the PNCV sites (Figure 4). Therefore, clay content in both SS from the two parks were not different. On the other hand, MG from PESA had higher clay content than MG in PNCV. In PESA, CD had the highest clay content.
Carbon content was similar along the sites sampled, except on the MG in PNCV. The soil in this same site had the highest cation exchange capacity (CEC), which was significantly different from the MG site in PESA (Figure 4). Similarly, SS sites differed in CEC between parks. Sulfur concentration was different between parks, especially due to S concentration in CL and MG in PNCV. Al+3 concentration was high in all sites sampled, but significantly
37
Figure 4. Soil physicochemical variables in the two parks and their sites. (A) Principal component analysis (PCA) of soil physicochemical properties based on a correlation matrix performed with R. Each vector points in the direction in which the respective value increases. Boxplots of soils (B) clay content, (C) C content, (D) cation exchange capacity, (E) S concentration, (F) Al+3 concentration, (G) Fe concentration. One-way ANOVA or T- tests
were performed in R. Tukey–Kramer post hoc tests to compare group means (R with the ggplot2 package) are represented with letters or with one asterisk (*) if only one site was significantly different from others. Two (or three) smaller asterisks depicted statistical difference between MG or between SS present in the two parks.
Phylogenetic and functional analyses
A total of 1,364,104 sequences were obtained from PNCV and 992,685 from PESA; an average of 7 and 11.7% did not pass on the quality control, respectively (Table S1), and 2.7 to 8.7%, respectively, were considered sequences’ replicates and were excluded from the analysis. The percentage of sequences annotated to known protein was 61.4 (± 3.5) % and 46.5 (± 1.4) % for the PNCV and the PESA, respectively, and only a small fraction (around 0.5%) of the reads was annotated as ribosomal, or to the N metabolism (around 1%).
The number of ribosomal sequences annotated varied from 88 to 775 and taxonomical assignment was against the non-redundant protein M5NR database. According to this database, most of the genes annotated were from Bacteria (around 97%), with the remaining being part either of the Domain Archaea (0,9%), the Domain Eukarya (1,6%) or unknown (0,18%). Archaea was mainly present in soil as Thaumarchaeota and Crenarchaeota; CR and MG from PNCV and FSM and SS from PESA – presented low values of Euryarchaeota. The most abundant phyla in the Bacteria domain were Actinobacteria, Proteobacteria and Firmicutes, especially the class Bacilli, Clostridia (both from Firmicutes), α-Proteobacteria, β-Proteobacteria and γ-Proteobacteria. Ascomycota, Basidiomycota, Streptophyta and Arthropodawere within the most Eukarya annotated sequences.
38
39
9
9
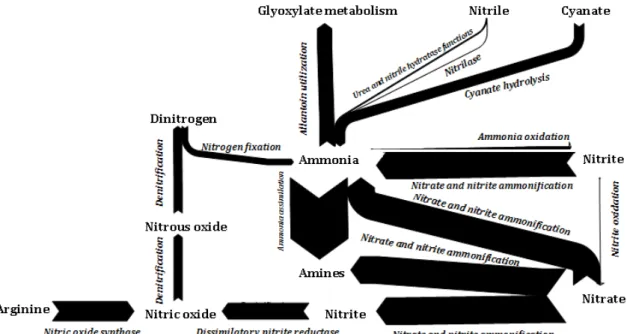
The greatest part of annotated genes to N metabolism were related to the ammonia assimilation (37%), followed by nitrate and nitrite ammonification (17%), nitric oxide synthase (12 %) and allantoin utilization (9%) as shown in Figure 7 that concatenates all genes annotated to N metabolism in the 24 metagenomes. The arrows are proportional to the number of genes annotated in our metagenomes. The least abundant were the cyanate hydrolysis (6%), the denitrification (5%), the dissimilatory nitrite reductase (5%), the nitrogen fixation (4%), the nitrosative stress (4%) and some genes related to the amidase clustered with urea and nitrile hydratase functions (1%) and nitrilase subsystems (<1%). Only two hits were found for ammonia monooxygenase, which is an enzyme part of nitrification process, but classified in the transport system according to SEED subsystems. No nitrite oxidoreductase was detected in the metagenomes, therefore both ammonia and nitrite oxidation were represented by slim arrows.
Figure 7.
Schematic representation of the N cycle according to the SEED subsystems annotated genes performed with e!Sankey 2. The total number of genes annotated from PESA and PNCV metagenomes. The arrows are proportional to the number of genes annotated for each process.10
11
12
Discussion
Here we present the first metagenomic description on the N cycling functional and phylogenetic genes from Cerrado soils microbiota in Central Brazil. This biome is composed by a gradient of trees/shrubs layer ranging from grasslands to forests and savannas. Analysis of phospholipid fatty acids and 16S rRNA genes have showed that vegetation cover influences the soil taxonomic microbial composition (Araujo et al., 2012; Mendes et al., 2012; Viana et al., 2011). Nevertheless, our first hypothesis was rejected, since we could not find patterns that explained distribution of functional guilds according to the macro distribution of Cerrado’s vegetation.
The types of vegetation sampled here differed in terms of soil physicochemical variables and were more similar within each park. The world literature shows pH as the factor that better explains soil microbial distribution (Lauber et al., 2009). Though, for the Cerrado it has been shown that the first rains on the beginning of the rainy season or experimentally the addition of water promote greater difference on the microbial community either with increase on microbial biomass (da Silva, 2004; Nardoto and Bustamante, 2003), microbial activity and nitrification rates (da Silva, 2004), or change on the bacterial composition with the transition between the dry and rain seasons (Bresolin et al., 2010; Nardoto and Bustamante, 2003; Pinto et al., 2006). Furthermore, water effect on the microbial community masks the fire effect (Viana et al., 2011). Truly, water promoted a change in the microbial community performing the N cycling, particularly in the Campo limpo. This confirms our second hypothesis that considers a variation in N metabolism according to soil characteristics between types of vegetation.
In fact, the soils in the PNCV had higher pH and S concentration than soils in PESA, which, in turn, presented higher clay content. However, no significant correlation was observed between pH and phylogenetic or functional genes relative abundance. Microbial community described with these metagenomes seem to differ more due to soil characteristics than the type of vegetation or the geographic distance. The similarity found between the Cerrado sensu stricto (SS) and the Mata de galeria (MG- riverbank) from both parks refutes the hypothesis of geographical differentiation between islands of Cerrado distant from each other in terms of soil microbial community. On the other hand, it reinforces the observed differences accordingly to type of soil and vegetation.
13
forest formations. However, they should be less similar to the savanna sites - SS and CR - or the sites with predominant herbaceous layer as the CS and the CD. On the other hand, geographic distance is informative, since microbial communities are similar in the parks, and are distant 500km, therefore, there is an indication that Cerrado biome has a particular soil microbial community. This will have to be confirmed in a biogeographical model, as for example the use of mantel test with other Cerrado areas and other biomes.
As microbes are confined to a thin layer of water in the soil particles, it is reasonable to think that water is the major limitation of prokaryotic life in soil (Fenchel, 2012). The soil texture influences the water retention according to the percentage of clay, sand and silt particles, which has an impact on the gravimetric soil water content and consequently on the microbial community. The Cerrado rupestre was the driest soil sampled with the greater composition of sand in comparison to clay. This type of vegetation is only present in some fragments of the biome Cerrado specially in higher altitudes between 800 and 2000 m, characterized by rocky outcrops with high vegetal endemism and usually found in Leptosols (neossolos litólicos, Brazilian soil classification). The metagenomes found in this vegetation had the greatest α-diversity of Shannon (data not shown), potentially a greater diversity in response to the nutrient and water stress.
The theory of pore connectivity favors the idea that low contact between organisms because of low water potential allows for greater microbial diversity (Carson et al., 2010). However, in the metagenomes, both the CR and the CL presented the highest Shannon diversities and they have also the two most distinct soil water content. Therefore, we considered that in this case, the higher diversity is due to disturbance (water lodging in the case of CL) promoting stochasticity for different groups to prevail instead of a higher abundance of one or another set of microorganisms.