REVISTA
PAULISTA
DE
PEDIATRIA
www.rpped.com.br
ORIGINAL
ARTICLE
Magistral
drugs
in
hospitalized
newborns
and
children
Agueda
Cabral
de
Souza
Pereira,
Elaine
Silva
Miranda,
Selma
Rodrigues
de
Castilho,
Débora
Omena
Futuro,
Lenise
Arneiro
Teixeira,
Geraldo
Renato
de
Paula
∗UniversidadeFederalFluminense(UFF),Niterói,RJ,Brazil
Received4November2015;accepted22February2016 Availableonline26July2016
KEYWORDS Medicationuse; Pediatric medications; Fractionateddrugs; Unlicenseddrugs
Abstract
Objective: Studytheuseofmagistraloralsolutionsandsuspensionsininfantsandchildrenat auniversityhospital.
Methods: Thisisadescriptivestudybasedontheanalysisoftheassessedhospital’smagistral drugrequestformsregardingthepatientsintheneonatalICU,Obstetrics,Pediatricsand Pedi-atricEmergencyfromJanuary 2012toDecember2013. Thefrequencyofdrugrequests and dispensationwasevaluatedandtheconsumptionofeachactiveingredientofthepreparations wasexpressedasnumberof‘‘infantdefineddailydose’’(iDDD)andofiDDD/100bed-days.
Results: Atotalof657formswereanalyzed---amonthlyaverageof27pediatricpreparations. TheneonatalICUaccountedfor69.6%oftheserequests.Twenty-onedrugitemswereused,of whichthemostcommonwerefolinicacid(88requests),sulfadiazine(85)andcaptopril(73). TheconsumptionoftheactiveprincipleinthesepreparationsvariedinnumberofiDDD,from 7.5(hydralazine)to16,520.0(folicacid),andinnumberofiDDD/100bed-daysintheneonatal ICU,from0.1(zincsulfate)to146.1(folicacid).
Conclusions: Theconstantconsumptionofmagistraloralsolutionsandsuspensionsbynewborns andchildrenoftheassessedhospitalindicatestheneedforsuchpreparationsasapediatric therapeuticalternativeinthishospital.
©2016SociedadedePediatriadeS˜aoPaulo.PublishedbyElsevierEditoraLtda.Thisisanopen accessarticleundertheCCBYlicense(http://creativecommons.org/licenses/by/4.0/).
∗Correspondingauthor.
E-mail:geraldopaula@vm.uff.br(G.R.Paula).
http://dx.doi.org/10.1016/j.rppede.2016.02.012
2359-3482/©2016SociedadedePediatriadeS˜aoPaulo.PublishedbyElsevierEditoraLtda.ThisisanopenaccessarticleundertheCCBY
PALAVRAS-CHAVE Usode
medicamentos; Medicamentos pediátricos; Medicamentos fracionados; Medicamentosnão licenciados
Medicamentosmagistraisemrecém-nascidosecrianc¸ashospitalizados
Resumo
Objetivo: Estudarousodesoluc¸õesesuspensõesoraismagistraisemrecém-nascidosecrianc¸as deumhospitaluniversitário.
Métodos: Foifeitoumestudodescritivoapartirdaanálisedosformuláriosdesolicitac¸ãode manipulac¸ãodohospitalestudadoreferentesaospacientesdaUTI-neonatal,obstetrícia, pedi-atria eemergência pediátricade janeirode 2012a dezembrode 2013. Asfrequências das solicitac¸õesedispensac¸õesdessesmedicamentosforamavaliadaseoconsumodecada princí-pioativodaspreparac¸õesforamexpressossobaformadenúmerodeinfantdefineddailydose
(iDDD)edeiDDD/100leitos-dia.
Resultados: Foramanalisados657formulários---médiamensalde27preparac¸õespediátricas.A UTI-neonatalfoiresponsávelpor69,6%dessassolicitac¸ões.Foramusados21itensde medica-mentos,destacou-seousodeácidofolínico(88solicitac¸ões),sulfadiazina(85)ecaptopril(73). Oconsumodeprincípio-ativonessaspreparac¸õesvariou,emnúmerodeiDDD,de7,5 (hidralaz-ina)a16.520(ácidofólico)eemnúmerodeiDDD/100leitos-diadaUTI-neonatal,de0,1(sulfato dezinco)a146,1(ácidofólico).
Conclusões: O consumo constante das soluc¸ões e suspensões orais magistrais pelos recém-nascidosecrianc¸asdohospitalestudadoindicaanecessidadedessaspreparac¸õescomoopc¸ão terapêuticapediátricanessehospital.
©2016SociedadedePediatriadeS˜aoPaulo.PublicadoporElsevierEditoraLtda.Este ´eum artigoOpenAccesssobumalicenc¸aCCBY(http://creativecommons.org/licenses/by/4.0/).
Introduction
Newborns and children go through physiological changes throughout their development, which interfere with the pharmacokineticsand,consequently,thesafetyand effec-tiveness of drug treatment in the pediatric age group. Therefore,studies areneeded in each pediatric subpopu-lationforwhichtheiruseisintended aimingatsafetyand efficacyassessmentofpediatricdrugs.1
However, thereis a shortageof pediatric drugs in the pharmaceuticalindustry, which can be explained by eco-nomic, ethical and technical issues. This fact makes the magistral preparations advantageous options for obtain-ingmedicationswithappropriatepharmaceuticalformfor pediatricuse, astheyallow dose flexibilityand easydrug administration.1,2
In Brazil, the preparation of compounded drugs must comply with the rules of the Collegiate Board Resolution (RDC)67/2007,whichprovidesforGoodCompounding Prac-ticesof Magistral and Compounded Drugs for Human Use inpharmacies.3 Toobtainmagistralsolutionsandoral
sus-pensions,thefirstchoiceisthecompoundingoftheactive principle;however,thesecanalsobeobtainedbydilutinga liquidformulation (e.g., an injectable dilution), provided it is compatible with oral administration, by pulverizing tabletsorremovingthepowderfromthecapsule.4
Thisstudyaimstoevaluatetheuseofmagistraloral solu-tionsandsuspensionsinhospitalizednewbornsandchildren.
Method
Adescriptive,retrospectivestudywascarriedoutbasedon theanalysisofrequestformsforcompoundingoforalliquid
preparationsfornewbornsandchildrenadmittedatHospital UniversitárioAntônioPedro(HUAP)ofUniversidadeFederal Fluminense(UFF),relatedtotheperiodofJanuary2012to December2013.This isatertiaryandquaternaryhospital, has287bedsandservesthepopulationoftheMetropolitan RegionIIoftheStateofRiodeJaneiro.
Thepediatricoralliquidpreparationsmentionedinthis studywerepreparedatthepharmacyoftheUFF,underthe responsibility and guidance of a pharmacist, with formu-lations beingbasedonscientific literature,withadequate validity and packaging, as well as correct storage rec-ommendations,qualitycontrolproceduresandmedication traceability.
The frequencies of requests for pediatric compounded oral liquid formulations in relation to: the month of request; their active principle; their Anatomical-Therapeutic-Chemical(ATC) classification,5 andrequesting
sectorswerecalculated.
Theadministrationfrequencyofmagistraloralsolutions andsuspensionswasanalyzedfortotaloraluseliquid phar-maceuticalformsdispensedbythehospitalpharmacytothe neonatalintensivecareunitandPediatricsin2013.
Theannualconsumptionofinfantdefinednumberofdaily dose(iDDD),whichcorrespondsto1/10ofthedefineddaily dose(DDD),wascalculatedforeachactiveprincipleofthese preparations, assuming the complete consumption of the compounded product. The number of iDDD/100 bed-days wasalsocalculatedforpatientsintheNICU.6Thetotal
occu-pationofbedsinthissectorwasconsideredtocalculatethe numberofbedsintheNICU.
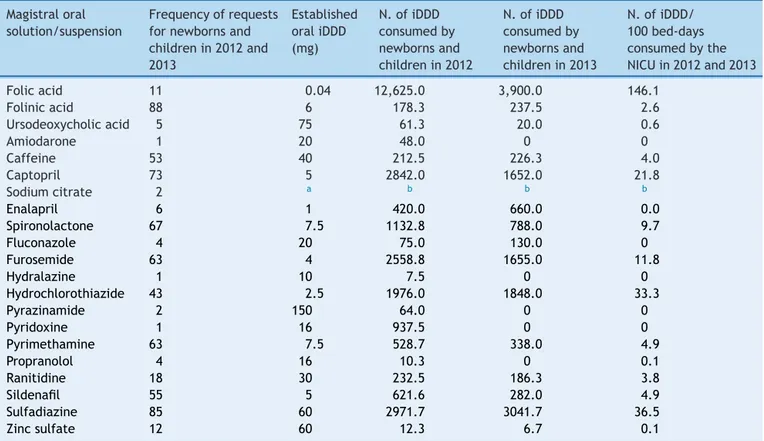
Table1 Useofnon-licensedmanipulatedoralliquidpreparationsfornewbornsandchildrenatahospitalin2012and2013 (n=657).
Magistraloral solution/suspension
Frequencyofrequests fornewbornsand childrenin2012and 2013
Established oraliDDD (mg)
N.ofiDDD consumedby newbornsand childrenin2012
N.ofiDDD consumedby newbornsand childrenin2013
N.ofiDDD/ 100bed-days consumedbythe NICUin2012and2013
Folicacid 11 0.04 12,625.0 3,900.0 146.1
Folinicacid 88 6 178.3 237.5 2.6
Ursodeoxycholicacid 5 75 61.3 20.0 0.6
Amiodarone 1 20 48.0 0 0
Caffeine 53 40 212.5 226.3 4.0
Captopril 73 5 2842.0 1652.0 21.8
Sodiumcitrate 2 a b b b
Enalapril 6 1 420.0 660.0 0.0
Spironolactone 67 7.5 1132.8 788.0 9.7
Fluconazole 4 20 75.0 130.0 0
Furosemide 63 4 2558.8 1655.0 11.8
Hydralazine 1 10 7.5 0 0
Hydrochlorothiazide 43 2.5 1976.0 1848.0 33.3
Pyrazinamide 2 150 64.0 0 0
Pyridoxine 1 16 937.5 0 0
Pyrimethamine 63 7.5 528.7 338.0 4.9
Propranolol 4 16 10.3 0 0.1
Ranitidine 18 30 232.5 186.3 3.8
Sildenafil 55 5 621.6 282.0 4.9
Sulfadiazine 85 60 2971.7 3041.7 36.5
Zincsulfate 12 60 12.3 6.7 0.1
a EitherDDDoriDDDwasnotidentifiedforsodiumcitrate.
b ItwasnotpossibletocalculatethenumberofiDDDconsumedbynewbornsandchildrenandthenumberofiDDD/100bed-days
consumedbytheNICUregardingtheoralsolutionsofsodiumcitrateduetolackofinformationonDDDoriDDDforsodiumcitrate.
Results
Thestudyanalyzed657compoundingrequestformsfororal liquidpreparationsintendedforinfantsandchildreninthe NICU,obstetrics(rooming-innewborns),pediatricsand pedi-atricemergencyservices.
Of theassessed newbornandinfant services,the NICU was the unit with the highest number of requests (457; 69.6%),followed bypediatrics(99; 15.1%),obstetrics (71, 10,8%);pediatricemergency(27;4.1%)andnon-identified sector(3,0.4%).
Permonth,anaverageof27magistraloralsolutionsand suspensions(standard deviation=9.8)wererequested. The months with the highest and lowest number of requests wereinMarch2013(45 requests)andDecember2012 and November2013(bothwith10requests),respectively.
The mostoftenrequesteddrugclassesduringthestudy period,accordingtotheATC, were:C---cardiovascular sys-tem(258requests---especiallycaptopril,spironolactoneand furosemide);J---anti-infectiveforsystemicuse(91 requests---mainly sulfadiazine) and V---varied (88 requests---corresponding to folinic acid). The least requested classwasB---bloodandblood-formingorgans(13 requests---correspondingtofolicacidandsodiumcitrate).
In 2013, 318 liquid pharmaceutical forms for oral use weredispensedtotheNICUand900tothePediatricsunit, ofwhich209(65.7%)and40(4.4%)werecompoundedoral solutionsandsuspensions,respectively.
The frequencies of requests for pediatric compounded oralsolutionsandsuspensionswerecalculatedinrelationto theactiveprinciplerequested forthesepreparations.The consumptionof active principlethrough theiDDDnumber ofthesemedicationsin infants andchildren wasalso cal-culated, as well asthe number of iDDD/100 bed-days of patientsintheNICU(Table1).
Discussion
Theneedfortheuseofmagistralpreparationstocopewith thelack oforalliquidformulationsfromthe pharmaceuti-calindustrywasdemonstratedinthisstudy,whichidentified 657magistraloral solutionsand suspensions,regarding21 different active principles, which have been used in the treatmentof newborns and childrenat HUAP in 2012and 2013. It is noteworthy that the main characteristics of theassessedhospitalare:being apublichospital; provid-ingmiddle-andhigh-complexitycare;providingspecialized outpatient care, urgency, emergency care and hospital admissionto patients from the Unified Health System; in additionto teaching (Graduation, Post-Graduation, medi-calandmultiprofessionalresidency)andresearchactivities, characteristicsthataresimilartootherBrazilianuniversity hospitals.
etal.andKhdouretal.,whoidentified,respectively,20.5% and 5.8% modifications in requests.7,8 Costa et al. found
119drugadaptationsfromsolidtoliquidformandGavrilov etal.identifiedtwodose modificationsin pharmaceutical forms.9,10DosSantosandHeineckfoundthat79prescription
itemsinvolvedextemporaneouspreparations.11
Extempora-neous compounded oral preparations are magistral drugs thatshouldbeusedwithin48hours.3
Important regulatory initiatives were developed in Europe,United States andAustralia to increase the num-ber of available pediatric drugs, but Brazil still lacks specific regulation for the registration and use of pedi-atric drugs and an incentive policy regarding pediatric research.9
Oralsolutionsandsuspensionscompoundedbythe hos-pital pharmacy or prepared through the modification of the pharmaceutical form performed by the nursing staff areoften studied in thecategory of unlicensed druguse. Thedefinitionofunlicenseddruguseinpediatricsincludes thoseoriginatedfromlicenseddrugsmodifications,suchas those that underwent pharmaceutical form modification, thosewithoutauthorizationtobesoldfromtheregulatory agency,importeddrugs,thecontraindicateddrugsandthose thathavenoinformationonuseinthispopulation---although therearedivergencesamongspecialists.12,13
Theuseofunlicensedandoff-labelmedications(ofwhich use is unauthorized by a regulatory agency) in pediatric patientshavebeenthesubjectofseveralstudies,astheyare oftenprescribedtothispopulation,particularlynewborns, despitethehighfrequencyofadversereactionsassociated withtheiruse.13
Magalhãesetal.foundthatunlicenseddrugusein hos-pitalizedpediatricpatientsoccursinmanycountries,such as Brazil, Sweden, Croatia, Turkey, Estonia, Netherlands, Palestine,France,Italy,Germany,Finland,Switzerland, Ser-bia, Spain, Israel and England.13 In Brazil, there are few
studies onthe use of magistral drugs in infants and chil-dren,whichareconcentratedin hospitalsfromonly three Brazilian cities: Fortaleza,9,14,15 Porto Alegre11,16 and Belo
Horizonte.17,18
Observing the profile of oral solutions and suspensions compounded for newborns and children of the stud-ied hospital, it was verified that solutions/suspensions of these active principles were identified in other studies: folic acid,9,19 folinic acid,9,20 ursodeoxycholic
acid,11,14 captopril,8,9,14,15,18,21 caffeine,12,16,19,22,23
spironolactone,9,24---28 fluconazole,17 furosemide,8,9,26
hydrochlorothiazide,8,9,18,26,27 propranolol,10 ranitidine,9
pyrimethamine17 and sulfadiazine.17 Therefore, it is
observed that the demand for oral solutions and suspen-sionsadministered tonewborns andchildren in thisstudy hasalsobeenobservedinother studiesandthatthe com-poundingofdrugsisanationalandinternationalnecessity duetothelackoforalliquiddrugsfromthepharmaceutical industry.
However,itisimportanttonotethatsomemedications that require compounding to be obtained in oral liquid formulationin a certaincountrymay be available in oth-ers.IntheUnitedStates,forinstance,thefollowingdrugs are approved by FDA and sold as oral solution: caffeine, enalapril,fluconazole,furosemide,propranolol,ranitidine, andsildenafil.29
Regardingtherecipientsof themagistraloral solutions and suspensions, it was observed in the studied hospital thatnewbornsarethemainrecipientsoftheseformulations andthattheNICUisresponsiblefor70%oftheserequests. This resultisin accordancewiththatidentifiedby Magal-hãesetal.,whichindicatethatmostunlicenseddrugsare prescribedfornewbornsandoftenforpreterminfants.13
Thecomparisonofresultsonmedicationuseinthisstudy withthoseof otherstudies washindered by methodologi-cal differences.Other authors chose toanalyze magistral drugsascasesofunlicensedmedicationuseandexpressed results as relative frequency (%) of unlicensed items in prescriptions,prescriptionsthatincluded unlicenseditems and/orpatientswhoreceivedunlicenseddrugs.Thepresent study concentrated onoral solutions and suspensions and expresseditsresults,mainlyregardingtheamountofactive principle consumedin milligrams pernumberof iDDDand numberof iDDD/100bed-days.The choice ofshowing the results ofthis study inrelation toiDDDwasbased onthe recommendation madeby the WHO on theuse of DDD in drugusestudies.
It should beobserved that the pharmaceuticalservice division,responsibleforadaptingactiveingredientsand/or drugsavailableonthemarketforadministrationin hospital-izedpatients,isscarceinBrazilianhospitals,30includingthe
studiedhospital.Thus,workingwiththecompounding phar-macyis veryimportanttoallowtheobtainingofmagistral drugsnecessaryforthetreatmentofnewbornsandchildren. Costa et al. reported that the lack of the drug in appropriatepresentationforpatientadministrationledthe nursing staff to make dosage form changes when treat-ing patients.9,17 Of the pharmaceuticalform adaptations,
75.63% had an inappropriate vehicle and therewere also casesofincorrectpackagingandconservation.9
Amongthestudylimitationsarethefactthatasingle hos-pitalwasstudied,assumingthateachrequestedpreparation wasconsumedinfullandthattheNICUoccupancyratewas 100%during thestudiedperiod.However,itis noteworthy thatthestudied hospitalprofileis similartothatofother universityhospitals.Additionally,thecompounded medica-tion was prepared for a specific patient and had a small volumeandnarrowexpirationdate,makingitreasonableto assumethatthecompoundedmedicationwasconsumedin full.Regardingoccupation,thestudiedhospitalisapublic referencehealth unit,which providescare tothe popula-tionofadenselypopulatedareaandtheoccupancyof100% ofbedsisjustifiable.Thus,thegeneralizationoftheresults musttaketheseaspectsintoaccount.
Itisnoteworthythattheuseofmagistraldrugsmaybea goodtherapeuticoptionforinfantsandchildren.However, regulatory incentives are needed for the pharmaceutical industrytoproducethesedrugsatadequatedosageforthis population.
Funding
Thisstudydidnotreceivefunding.
Conflicts
of
interest
References
1.MéndezEstebanME, Antequera Rodríguez-RabadánJ,Puebla García V, Pardo de Torres J, Gallego Lago V, Herreros de TejadaA. Formulacionesorales acuosas: uma administración másseguraparapediatría.RevOFIL.2006;16:15---28.
2.Pinto S, Barbosa MC. Medicamentos manipulados em pedi-atria: estado actual e perspectivas futuras. ARQUIMED. 2008;22:75---84.
3.Brazil---MinistériodaSaúde.AgênciaNacionaldeVigilância San-itária.RDC n◦. 67, de08 deoutubro de 2007. Dispõe sobre
BoasPráticasdeManipulac¸ãodePreparac¸õesMagistraise Ofic-inais paraUso Humano em farmácias. Brasília:Ministério da Saúde;2007.Availablefrom:http://189.28.128.100/dab/docs/ legislacao/resolucao67081007.pdf
4.RoqueMF.Desenvolvimentode formulac¸ões líquidasoraisde sildenafil paraadministrac¸ão em pediatria[master’s thesis]. Coimbra,PT:UC;2008.
5.WHOCollaboratingCentreforDrugStatisticsMethodology
Nor-wegian --- Institute of Public Health. ATC/DDD Index 2015.
Available from: http://www.whocc.no/atcdddindex/ [cited
06.04.15].
6.MeloDO,RibeiroE,StorpirtisS.Aimportânciaeahistóriados estudosdeutilizac¸ãodemedicamentos.RevBrasCienFarm. 2006;42:475---85.
7.OguzSS,KanmazHG,DilmenU.Off-labelandunlicenseddrug useinneonatalintensivecareunitsinTurkey:theold-innstudy. IntJClinPharm.2012;34:136---41.
8.KhdourM,HallakHO,AlayasaKS,AlShahedQ,HawwaAF, McEl-nayJC.Extentandnatureofunlicensedandoff-labelmedicine use in hospitalised children in Palestine. Int J Clin Pharm. 2011;33:650---5.
9.CostaPQ,LimaJE,CoelhoHL.Prescric¸ãoepreparode medica-mentossemformulac¸ãoadequadaparacrianc¸as:umestudode basehospitalar.BrazJPharmSci.2009;45:57---66.
10.GavrilovV,BerkovitchM,LingG,Brenner-ZaddaG,LifshitzM, GorodischerR.Unapprovedprescriptionsintwopediatric inten-sivecareunitsinIsrael.CurrTherResClinExp.2003;64:734---42.
11.DosSantos L, Heineck I. Drug utilization study in pediatric prescriptionsof a university hospital insouthern Brazil: off-label, unlicensed and high-alert medications. Farm Hosp. 2012;36:180---6.
12.NeubertA,WongIC,BonifaziA,CatapanoM,FelisiM,Baiardi P,et al. Defining off-label and unlicensed use of medicines for children: results of a Delphi survey. Pharmacol Res. 2008;58:316---22.
13.Magalhães J, Rodrigues AT, Roque F, Figueiras A, Falcão A, Herdeiro MT. Use of off-label and unlicensed drugs in hos-pitalisedpaediatricpatients:a systematicreview.EurJClin Pharmacol.2015;71:1---13.
14.SantosDB,ClavennaA,BonatiM,CoelhoHL.Off-labeland unli-censeddrugutilization inhospitalizedchildrenin Fortaleza, Brazil.EurJClinPharmacol.2008;64:1111---8.
15.LoureiroCV,NériED,DiasHI,MascarenhasMB,FontelesMM.Uso demedicamentosoff-label ounãolicenciados parapediatria emhospitalpúblicobrasileiro.RevBrasFarmHospServSaude. 2013;4:17---21.
16.CarvalhoCG,RibeiroMR,BonilhaMM,FernandesMJr,Procianoy RS,Silveira RC. Useof off-labeland unlicenseddrugsinthe neonatalintensivecare unitanditsassociationwithseverity scores.JPediatr(RioJ).2012;88:465---70.
17.Gonc¸alvesAC,Caixeta CM,ReisAM.Análise dautilizac¸ãode medicamentosantimicrobianossistêmicosem crianc¸ase ado-lescentesemdoishospitaisdeensino.RevCiencFarmBasica Apl.2009;30:177---82.
18.Ferreira LA, Ibiapina CC, Machado MG, Fagundes ED. A altaprevalência deprescric¸õesdemedicamentosoff-label e não licenciados em unidade de terapia intensiva pediátrica brasileira.RevAssocMedBras.2012;58:82---7.
19.KimlandE,NydertP,OdlindV,BöttigerY,LindemalmS. Paedi-atricdrugusewithfocusonoff-labelprescriptionatSwedish hospitals---anationwidestudy.ActaPaediatr.2012;101:772---8.
20.NguyenKA,ClarisO,KassaiB.Unlicensedandoff-labeldruguse inaneonatalunitinFrance.ActaPaediatr.2011;100:615---7.
21.PandolfiniC,ImpicciatoreP,ProvasiD,RocchiF,CampiR,Bonati M,etal.Off-labeluseofdrugsinItaly:aprospective, observa-tionalandmulticentrestudy.ActaPaediatr.2002;91:339---47.
22.JongGW,VultoAG,DeHoogM,SchimmelKJ,TibboelD,VanDen AnkerJN.Asurveyoftheuseofoff-labelandunlicenseddrugs inaDutchchildren‘shospital.Pediatrics.2001;108:1089---93.
23.LassJ,Käar R,JõgiK,Varendi H,MetsvahtT, LutsarI.Drug utilisationpatternandoff-label useofmedicinesinEstonian neonatalunits.EurJClinPharmacol.2011;67:1263---71.
24.O‘DonnellCP,StoneRJ,MorleyCJ.Unlicensedandoff-labeldrug useinanAustralianneonatalintensive careunit.Pediatrics. 2002;110:e52.
25.Bajcetic M,Jelisavcic M,Mitrovic J, DivacN, Simeunovic S, SamardzicR,etal.Offlabelandunlicenseddrugsusein paedi-atriccardiology.EurJClinPharmacol.2005;61:775---9.
26.LópezMartínezR,Caba˜nasPoyMJ,OliverasArenasM,Clemente BS.UtilizacióndemedicamentosenunaUCIneonatal:estudio prospectivo.FarmHosp.2005;29:26---9.
27.DiPaoloE,StoetterH,CottingJ,FreyP,GehriM,Beck-Popovic M,etal.Unlicensedandoff-labeldruguseinaSwisspaediatric universityhospital.SwissMedWkly.2006;136:218---22.
28.BellisJR,KikhamJJ,Nunn AJ,Pirmohamed M.Adversedrug reactionsandoff-labelandunlicensedmedicinesinchildren:a prospectivecohortstudyofunplannedadmissionstoa paedi-atrichospital.BrJClinPharmacol.2014;77:545---53.
29.USFoodandDrugAdministration.EstadosUnidosdaAmérica:
Drugs@FDA.Available from: http://www.accessdata.fda.gov/
scripts/cder/drugsatfda/[cited21.02.15].