ContentslistsavailableatScienceDirect
Neuroscience
Letters
j ou rn a l h om epa g e :w w w . e l s e v i e r . c o m / l o c a t e / n e u l e t
Research
paper
Gabapentin
attenuates
neuropathic
pain
and
improves
nerve
myelination
after
chronic
sciatic
constriction
in
rats
Carlos
C.
Câmara
a,b,
Celina
V.
Araújo
b,
Kalina
Kelma
Oliveira
de
Sousa
b,
Gerly
A.C.
Brito
c,
Mariana
L.
Vale
c,
Ramon
da
Silva
Raposo
e,
Fabiana
Evaristo
Mendonc¸
a
d,
Bruno
S.
Mietto
d,
Ana
Maria
B.
Martinez
d,
Reinaldo
B.
Oriá
b,∗ aLaboratoryofNeurophysiology,FederalandRuralUniversityoftheSemiArid—UFERSA,Mossoro,RN,BrazilbLaboratoryofTissuehealing,OntogenyandNutrition,DepartmentofMorphology,FacultyofMedicine,FederalUniversityofCeara,Fortaleza,CE,Brazil cLaboratoryofInflammationandCancer,DepartmentofPhysiologyandPharmacology,FacultyofMedicine,FederalUniversityofCeara,Fortaleza,CE, Brazil
dDepartmentofPathology,FacultyofMedicine,HUCFF,HealthScienceCenter,FederalUniversityofRiodeJaneiro,RiodeJaneiro,RJ,Brazil eExperimentalBiologyCore,UniversityofFortaleza,Fortaleza,CE,Brazil
h
i
g
h
l
i
g
h
t
s
•Gabapentinimprovesmyelinbasicproteinexpressionintheinjuredsciaticnerve.
•Gabapentinamelioratesneuropathicpainbehaviors.
•Gabapentinhasadualroleinimprovingneuropathicpainandnervemyelinationfollowingsciaticnerveinjury.
a
r
t
i
c
l
e
i
n
f
o
Articlehistory:
Received23February2015
Receivedinrevisedform11July2015
Accepted16September2015
Availableonline25September2015
Keywords:
Gabapentin
Sciaticnerve
Neuropathicpain
Nervemyelination
a
b
s
t
r
a
c
t
Gabapentin(GBP)isananti-convulsivedrugoftenusedasanalgesictocontrolneuropathicpain.This
studyaimedatevaluatingoralGBPtreatment(30,60,120mg/kg,60minpriortochronicconstriction
ofthesciaticnerve(CCSN)along15-daytreatmentpost-injury,12h/12h)bymonitoringspontaneous
andinduced-painbehaviorsinWistarratson5thand15thdayspost-injuryduringearlyneuropathic
events.CCSNanimalsreceivingsalinewereusedascontrols.Anotheraimofthisstudywastoevaluate
GBPeffectsonmyelinbasicprotein(MBP)onthe5thand15thdayspost-injuryandnerve
morphol-ogybytransmissionelectronmicroscopytoaddressnerveregeneration.Onthe5thand15thdays,GBP
(60mg/kg)reducedneuropathicpainbehaviors(scratchingandbiting)intheipsilateralpawand
allevi-atedmechanicalallodyniaincomparisonwiththeneuropathicsalinegroup.GBPsignificantlyincreased
climbingandrearingbehaviorsinCCSNandCCSN-freeanimalssuggestingincreasedmotoractivityrather
thansedation.Wefoundthree-foldsignificantincreaseinMBPexpressionbywesternblotsonthe15th
daywhencomparedtocontrols.Inaddition,GPB(60mg/kg)improvednerveaxonal,fiberandmyelinarea
15dayspost-surgery.Inconclusion,GBPalleviatedmechanicalandthermalallodyniaandspontaneous
pain-relatedbehaviorsandimprovedlaternervemorphology.OurfindingssuggestthatGBPimprove
nerveremyelinationafterchronicconstrictionofthesciaticnerve.
©2015ElsevierIrelandLtd.Allrightsreserved.
1. Introduction
Peripheralnerveinjury(PNI)isanescalatingproblem
world-wide mostly due to a growing prevalenceof societalviolence,
∗Correspondingauthorat:InstituteofBiomedicineandDepartmentof
Morphol-ogy,SchoolofMedicine,FederalUniversityofCeara.RuaCoronelNunesdeMelo,
1315,CEP:60430-270Fortaleza,CE,Brazil.
E-mailaddress:rbo5u@virginia.edu(R.B.Oriá).
occupational and traffic accidents, especially in more
densely-populated urban areas. PNI may lead to a chronic condition
associatedwithneuropathicpainandnervefunctionloss,witha
tremendousimpactinhospitalcostsandincapacity[1].
Although peripheral nerve regeneration is possible, current
treatmentisstillunsatisfactory,especiallyfortheelderly.Novel
therapies to accelerate nerve regeneration are needed in
asso-ciationwithbenefitsinreducing neuropathicpain.Mechanisms
underlyingpainreliefwiththeuseofanticonvulsantsarea
promis-http://dx.doi.org/10.1016/j.neulet.2015.09.021
ingtargetfortherapyimprovements[2],howevertodatenostudies
haveaddressedtheirpotentialdualbenefitinimprovingpainand
nerveregeneration.
The anticonvulsivant gabapentin (GBP) (1-(aminomethyl)
cyclohexaneaceticacid),onestructuralderivativeofthe
gamma-aminobutyricacid(GABA),isnowfurtherconsideredasaneffective
therapyforsomeformsofneuropathicandpost-surgicalpain[3].
GBP-specificinhibitionofcalcium–voltagedependent␣2␦subunits
[4]oractivationofproteinkinaseG-K+channels[5]hasbeen
impli-catedinreducingallodyniabyalteringneurotransmitterrelease.
Ourpreviousstudyhasdocumentedthebenefiteffectoforal
GBP (60 and 120mg/kg) treatment in improving heat-induced
hyperalgesiawithnervepro-inflammatory effectsina modelof
sciaticnerveconstrictioninWistarrats[6].However,thepotential
nerveregenerativeeffectofGBPhasbeenpoorlyexplored.
InthisstudyweexploredwhetherprolongedGBP treatment
improvessciaticnervemyelinationbyassessingnervemyelinbasic
protein (MBP) (a constitutive myelin protein)[7], spontaneous
motor-relatedactivity,andfinenervemyelinmorphology,
follow-ingsciaticnerveconstrictioninWistarrats.Inaddition,wediscuss
whetherbetternerveremyelinationandreducedmyelinprotein
debriscouldimproveneuropathicpainfollowingGBPtreatment.
2. Materialsandmethods
ProtocolsfromthisstudywereinaccordancewiththeBrazilian
CollegeforAnimalExperimentation(COBEA)andtheInternational
AssociationfortheStudyofPain(IASP)andwereapprovedbythe
AnimalCareandUseCommitteeofDepartmentofPhysiologyand
Pharmacology,FederalUniversityofCeara.
2.1. Animalstudies
160maleWistarratsweighingbetween250and300gfromthe
DepartmentofPhysiologyandPharmacologyvivariumatthe
Fed-eralUniversityofCearawereusedinthisstudy.Ratswerehoused
inatemperature-controlledroom(26±2◦C)withfreeaccessto
waterandchowdietina12h/12hlight/darkcycles.Allsurgical
procedureswereperformedinthelaboratoryofExperimental
Neu-rologyintheDepartmentofPhysiologyandPharmacologyatthe
FederalUniversityofCeara.
2.2. Sciaticnervechronicconstriction
Inordertoinducetheexperimentalneuropathy,weusedthe
chronicconstrictionofthesciaticnerve(CCSN)model,describedby
BennettandXie[8]andmodifiedbySommeretal.[9].Animalswere
anesthetized with intraperitoneal injection of tribromoethilene
(25mg/kg),following trichotomyand anti-sepsisof thesurgery
field.A15-mmlongitudinalincisionoftherightthigh,atthelevel
ofthefemoraltrocateroftheposteriorlimb,wasusedtoaccessand
exposethesciaticnerveaftergluteusandfemoralbicepsdissection.
Weusedthree4–0cat-gutlooseligaturesontherightpawsciatic
nerve,1-mmawayfromthesciatictrifurcationinducingaslight
nerveischemia.Intheleftthigh,thesciaticnervewasexposed,
butremaineduntouchedandsurgeryclosedafterwards.Skinand
muscularlayersweresuturedwitha5–0mononylonthread.
2.3. Drugsandtreatmentregimens
Gabapentin (GBP) (1-(aminomethyl) cyclohexaneacetic acid,
C9H17NO2)(Neurontin®
,Pfizer)capsulesweredissolvedin0.9%
salinesolutionandthengivenorallybygavageevery12hduring
eithera5or 15-daytreatmentcourse.GBPdosesof30,60 and
120mg/kgwereusedbasedonpreviousstudieswithgoodclinical
response[10].AsthemaximumGBPeffectoccurs60minafteroral
administration[11],thefirstdosewasgiven60minpriortothe
nervesurgeryandthelastdosewasgiven60minbeforebehavior
tests.
2.4. Neuropathicpainassessment
InordertoassesswhetherGBPtreatmentcouldameliorate
neu-ropathicpainfollowingsciaticnerveinjury,cohortanimalswere
evaluatedinspontaneousandinducedbehaviors.
2.4.1. Spontaneouspainbehaviors
Ratswerekeptinawoodencage(100×50×50cm)fora5-min
acclimationtimeandtesting.Theobservationswereconductedas
describedelsewhere[6].Eachexperimentaland controlratwas
observedduringatimeof30min.Positionedinfrontofthecage,
theobservercouldidentifyeachbehavioralcomponentandrecord
itusingacomputersoftware(Comporta®
)designedbyProf.
Mar-cusVale(FederalUniversityofCeara,Brazil).Experimenterswere
unawareoftheidentityoftheexperimentalgroups.
Thefirstobservationwasperformedbeforethesurgery
(base-line)and then onthe5th and 15thpost-surgery.Adeltamean
behaviorvaluewasderivedfrombaseline.Formeasurements(in
seconds),weconsideredthefollowingpain-relatedspontaneous
behaviors:(1)scratching:timespentraisingthehindleftorright
pawtoscratchpartsofthebodywithrapidmovementsofthepaw
andclaws;(2)biting:timespentpiercingtheskinwiththemouth
andteethontheleftorrightsideofthebody.
2.4.2. Inducedpainbehaviors
2.4.2.1. Mechanicalallodynia. InordertoassesstheGBP chronic
effectonCCSNmechanicalallodynia,thevonFreyassessmentwas
usedaccordingtoAzevedoandcolleagues[12].Briefly,the
test-ingconsistedofpokingthehindpawtoprovokeaflexionreflex
followed by a clearflinch response afterpawwithdrawal. Paw
stimulationwasrepeateduntiltheanimalpresentedthreesimilar
measurements(thedifferencebetweenthehighestandthe
low-estmeasurementshouldbelessthan10g).Animalsweretested
onthe5thand15thdaypost-CCSN.Theresultsarereportedasthe
withdrawalthreshold(g).
2.4.2.2. Coldacetoneallodynia.InordertoassesstheGBPchronic
treatment effect on CCSN-induced cold allodynia, the method
describedbyBennettandFlatters[13]wasused.Briefly,ratswere
placedinacryliccageswithawiregridfloor15–30minbeforethe
beginningofthetestsinaquietroom.Adrop(0.05mL)of
ace-tonewasplacedagainstthecenterofhindpawventralsideanda
stopwatchwasstarted.Responsestoacetoneweregradedtothe
following4-pointscale:0,noresponse;1,quickwithdrawal,flick
orstampofthepaw;2,prolongedwithdrawalorrepeatedflicking
ofthepaw;3,repeatedflickingofthepawwithlickingdirectedat
theventralsideofthepaw.Acetonewasappliedalternatelythree
timestoeachpawandtheresponsesscoredcategorically.Animals
weretestedonthe5thand15thdaypost-CCSN.
2.5. Spontaneousmotor-relatedbehaviors
InordertodifferentiateGBPtreatmentanalgesiceffectsfrom
sedation, we observed the control and experimental rats, as
describedabove(Section2.4.1),measuringtherearing(timespent
suspendingtheforefeetandsupportingthebodyonthehindpaws
andclimbingbehaviors(timespentclimbinga2-cmhighthreestep
2.6. MyelinnervemorphologyandMBPexpression
In order to investigate nerve remyelination following CCSN
injury,weassessultra-structuralmyelinfeaturesandnervefine
histologyandMBPexpressionbywesternblottingand
immuno-histochemistryonday15thpost-challenge.
2.6.1. Finenervehistologyandmorphometry
Anesthetizedratswereperfusedintracardiallywithasolution
containing4%paraformaldehydeand2%glutaraldehydein0.1M
phosphatebuffer(pH 7.4).After,a 5mm segment(5mmdistal
fromtheinjurysite)ofthe15thday-post-injurysciaticnervewas
removedandfixedwith2.5%glutaraldehydein0.1Mcacodilate
buffer(pH 7.4)for 2h and processedfor transmission electron
microscopy.Afterthat,segmentswerepost-fixedin1%osmium
tetroxideplus 0.8% potassium ferrocyanide and 5mM CaCl2 in
0.1Mcacodilatebuffer(pH7.4)during1h.Next,segmentswere
dehydrated in increasing concentrations of acetoneand finally
embeddedin Embed-812 resin (Electron Microscopy Sciences).
Semithin(1m)andultrathin(60m)sectionswereobtainedina
RMCMT-6000ultramicrotome.Thesemithinsectionswerestained
with1%toluidinebluesolutionandobservedandphotographed
underaZeissAxioskop2Pluslightmicroscopewith20×and40×
objectives.Theultrathinsectionswerecollectedoncoopergrids
andstained30mininuranylacetatefollowedby10mininlead
citrate.ElectronmicrographswereacquiredusingZeiss900
trans-missionelectronmicroscopeoperatedat120kV.
2.6.2. Nervemorphometry
Inordertoidentifymyelinatedfibers,fiveareaswere
systemati-callyselectedfromthesemi-thincrosssectionsofthenerveofeach
animalandwereimage-capturedinhighmagnificationby
Axiovi-sionRel.4.5(CarlZeissMicroimaging,Thornwood,NY,USA).The
totalof30areasfromeachgroupwasanalyzed.Thisquantification
wasperformedusingImageJavasoftware(Bethesda,MD,USA).
2.6.3. MBPimmunohistochemistry
Onthe15thdaypost-CCSN and underdeepanesthesia, rats
weretranscardiallyperfusedwithsalineandthenthedistalsciatic
nervesegmentwasharvested5mm-distaltotheligatures,
imme-diatelyimmersedinbufferedformaline(pH7.4)for24handsentto
thehistologycoreforimmunohistochemistryprocessing.
Immuno-histochemistryforMBP(anti-MBPdiluted1:200,SantaCruz,CA)
wasperformedusingthestreptavidin-biotin-peroxidasemethod
informalin-fixed,paraffin-embeddedtissuesections(4mthick)
aftercitratebufferantigenretrieval,accordingtomanufacturer’s
instructions.Immunolabelingwasvisualizedwiththechromogen
3,3-diaminobenzidine(DAB).Negativecontrolsectionswere
pro-cessedsimultaneously,asdescribedabovebutwiththeprimary
antibodybeingreplacedby5%PBS-BSA.Noneofthenegative
con-trolsshowedimmunoreactivity.Slideswerecounterstainedwith
Harry’shaematoxylin.
2.6.4. MBPimmunoblotting
A2.5cm-longnervetissueoftheproximalsciaticnervestump
washarvestedtoassesstheMPBonthe15thdayspost-surgery
andimmediatelysnap-frozeninliquidnitrogenandprocessedfor
westernblotting,asdescribedelsewhere[14].MBPhasbeenshown
tobehighlyexpressedintheproximalstumpthanthedistalone
14days-post-CCSN[15].
2.7. Statisticalanalyses
NormaldistributionwasassessedusingKolmogorov–Smirnov’s
test. Effects amongst groups were assembled using
one-way ANOVA with post-hoc Tukey for parametric data and
Kruskal–Walliswithpost-hocDunn’sfornon-parametricdata.In
nerve morphometry, we used Mann–Whitney test. Values are
shownasmean±SEM.
3. Results
3.1. GBPeffectonneuropathicpainbehaviors
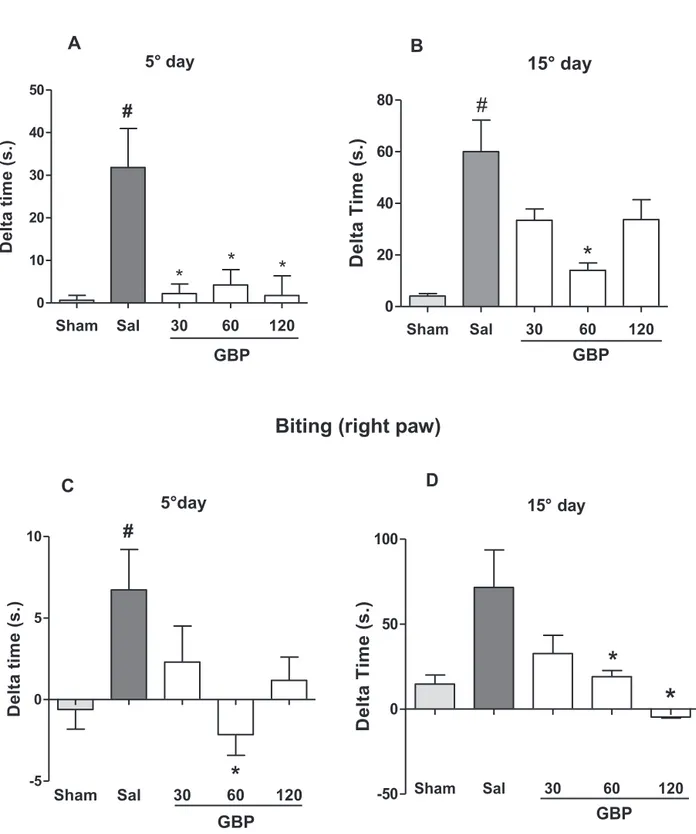
After5-days of GBP treatment, all tested doses (30, 60 and
120mg/kg)causedsignificantreductionsinthescratching
behav-ioroftherightpaw(93,86.7,94.4,and83.6%,respectively)when
comparedtoneuropathicsalinegroup(p<0.01).GBP(60mg)lowed
thebitingbehavior(131.8%),asopposedtotheneuropathicsaline
group(p<0.01).InCCSN-freerats,thosebehaviorswerebarelyseen
(N=12).Onthe15thdaypost-surgery,allneuropathicpain-related
behaviors(scratchingandbiting)wereworse(∼2and10-foldmore,
respectively).GBP(60mg)markedlyimprovedthescratchingand
bitingbehaviors (76 and 73%,respectively) compared withthe
untreatedchallenged-group(Fig.1A–D).
3.1.1. GBPeffectonvonFrey’sandcoldacetoneallodynia
Onthe5thdaypost-constriction,allGBPdosescaused
signifi-cantincreaseintherightpawwithdrawalthresholdinrelationto
theneuropathicsalinegroup.GBPatdosesof60and120mgwere
responsibleforthemaximum effect,providingthreshold
catch-upof45.9%and57.7%,respectively,inrelationtotheneuropathic
salinegroup,Fig.1E(p<0.00001).Onthe5thdaypost-constriction,
thescoreforcoldallodyniawasreduced(42.2%)by120mgofGBP
incomparisonwiththeneuropathicsalinegroup.The30and60mg
dosesleadtoreductiontrendsbutneitherreachedastatistical
dif-ference(Fig.1G).Onthe15thday,GBPtreatment(60mg)improved
mechanicalandthermalallodyniacomparedwiththeuntreated
CCSN-group(p<0.01)(Fig.1FandH).GBPtreatmentdidnotinduce
significantdifferencesinthecontra-lateralCCSN-freepaw(datanot
shown).
3.2. GBPeffectonspontaneousmotor-relatedbehaviors
GBP(60mg)raisedthedeltascoresofrearingcomparedwith
the untreated CCSN-rats on day 5 post-nerve constriction. No
differences were found for the rearing behavior on the 15th
post-challenge.60and120mgofGBP-treatedCCSNratsshowed
significantincreaseinclimbingwhencomparedwiththe
neuro-pathicsalinegroup,p<0.05 ontheday5 post-surgery.AllGBP
testeddosesimprovedclimbing15dayspost-surgerycompared
withtheuntreatedchallenged-group(SupplementaryFig.1).
Supplementarymaterial related tothis article found, in the
onlineversion,athttp://dx.doi.org/10.1016/j.neulet.2015.09.021.
3.3. Nervemorphology
Onthe15thday,qualitativeobservationofsemi-thincross
sec-tionsofthesciaticnerve showedhighernumber ofmyelinated
fiberswithinthechallengedandGBP-treatedgroupasopposedto
thenervesoftheneuropathicsalinegroup.Ultra-thinnerve
cross-sectionsrevealedthicker and higherdensity ofmyelinsheaths.
Inaddition, thenerves oftheneuropathic salinegroupshowed
nervefiberswithsignsofdegeneration,includingseveralmyelin
ovoids,amongpreservedand/orregeneratedfibersandsomefibers
withthinmyelinlamellae.InjuredsciaticnervetreatedwithGBP
showed many preserved and/or regenerated fibers among few
myelinovoids(Supplementary Figs.2 and3).Furthermore,oral
GBP(60mg/kg)improvedby63.3,32.8and28.2%insciaticnerve
axonal and fiber areas and myelin area, respectively, in
CCSN-challenged rats when compared with untreated nerve-injured
Scratching (right paw)
Sham
Sal
30
60
12
0
0
10
20
30
40
50
GBP
*
*
*
#
A
5° d
ay
D
e
lta
tim
e
(s
.)
Biting
(righ
t paw)
Sham
Sal
30
60
12
0
-5
0
5
10
C
#
*
GBP
5°day
De
lt
a t
im
e
(
s.
)
Sham
Sa
l
30
60
12
0
-50
0
50
100
*
GBP
15° day
D
*
De
lt
a T
ime
(s
.)
Sham
Sal
30
60
120
0
20
40
60
80
#
*
B
GBP
15° day
De
lt
a T
ime
(s
.)
Fig.1.Effectofchronicgabapentin(GBP)treatmentonscratching(A,B),biting(C,D)behaviorsandonvonFrey’spressurestimulus(E,F),andcoldacetone(G,H)testing
oftheipsilateralhindpawinCCSN-ratsoneitherthe5◦or15thday.Atleastn=8animalspergroupwereused.GBP30,60,120mg/gwasgivenbydailygavage(12/12h).
Behavioraldata(mean±SEM)areexpressedindeltavalues.Inpressurestimulus,dataareexpressedinthreshold(g).Incoldstimulustest,dataareexpressedinscores.
Pressur
e stimulus
Sham
Sa
l
30
60
12
0
0
20
40
60
80
#
*
*
*
GBP
E
5°day
Th
re
sh
ol
d
(g
.)
15° day
Sham
Sa
l
30
60
12
0
0
20
40
60
80
GBP
*
*
#
F
Th
re
sh
ol
d (
g
.)
Cold stimulus (10°C)
Sham
Sal
30
60
120
0
2
4
6
8
10
GBP
*
#
15° day
H
Sc
or
e
Sham Sa
l
30
60
12
0
0
2
4
6
8
#
*
GBP
G
5° day
Sc
o
re
CCSN-challengedratswereworsecomparedwiththeshamgroup
(SupplementaryFig.4).TheGratiowasnotfounddifferentbetween
theGBP-treatedinjurednervesasopposed tothecontrols,
sug-gestingboththemyelinandtheaxonproportionallyimprovedin
diameter.
Supplementarymaterial related tothis article found, in the
onlineversion,athttp://dx.doi.org/10.1016/j.neulet.2015.09.021.
3.4. GBPeffectonthesciaticnerveMBPexpression
Atthetimepointofthe15thdaypost-injury,GBP(60mg/kg)
significantlyincreased(overthreefold,p<0.05)MBPnerve
expres-sion,ascomparedwiththeneuropathicsalinegroup.Inaddition,
theMBPimmunostainingwasmarkedlyseenintheGBP(60mg/kg)
treatedratsafter15daysoftreatmentfollowingsciaticnerve
con-striction(SupplementaryFig.5).Atthetimepointofthe5thday
post-injuryanincreasedinMBPnerveexpressionwasseeninthe
GBP(60mg)treatedgroupalbeitnotreachingstatistically
signifi-cance(datanotshown).
Supplementarymaterial related tothis article found, in the
onlineversion,athttp://dx.doi.org/10.1016/j.neulet.2015.09.021.
4. Discussion
Inthis study,weaddressedGBP effects onneuropathic pain
behavior (5th and 15thdaypost-sciatic ligature)and on nerve
myelinationparametersfollowingchronicsciaticnerve
constric-tioninrats.
Ourdatahaveshownreducedbitingandscratchingbehaviors
inchallengedrats,followingGBPtreatmentonthe5thand15th
daypost-nerveconstriction.Earlystudieshavedemonstratedthat
ipsilateralscratchingtotheinjuredhindlimbisarecognized
hall-markfeatureofneuropathicpain[16]withapeakonthe14thday
post-nerveconstriction[17].Bitinglikewisehasbeenimplicated
inneuropathicdistressbutwithadistinctevolutioninmodelsof
chronicarthritis-associatedneuropathy[18].
OralGBPtreatment(12/12h)atdosesof30, 60,and120mg
inducedsignificantreductionsinthescratchingtime(intheright
ipsilateralpaw)spentbytheneuropathicratsincomparisonwith
theuntreatedgroup.However,thisfindingwasnotseenina
dose-dependentmanner,in factthebesteffectwasseen witha GBP
doseof 30mg/kg.Interestingly,Kayser andChristensenfounda
benefitwiththesameGBPdose(30mg/kgi.p.)onthevocalization
thresholds(asupraspinal-derivedbehavior)followingsciaticnerve
constrictioninrats[11].Ontheotherhand,ourdatashowthatGBP
(60mg/kg)wasmoreeffectiveinreducingthebitingbehavior.
Bagriyaniketal.havefounddeficitsinmotor-relatedbehaviors
(openfieldandrotarodtest)14dayspost-nerveconstrictioninrats
[19].DivergingfromGustafssonetal.findingsthatshowdecreased
locomotionandrearingbehaviorsafteracuteandcumulativedoses
ofGBP(200mol/kg,∼40mg/kg)[20],wefoundthatprolonged
GBPtreatmentincreasedmotor-relatedbehaviordeltascores
(rear-ingandclimbing),ascomparedtocontrolsthereforesuggesting
thatoralGBPdidnotinducesedativeeffectsintheseanimals.
How-ever,wecannotruleoutanexcitatoryeffectofGBPenhancingthese
behaviors.Morestudiesareneededtodissectbetterthesefindings.
In accordance withour data, LaBuda and Little [10] used a
spinalnerve(L5)ligaturemodelandfoundincreasedpaw
with-drawn threshold(tactile von Frey) withGBP treatment(30, 60
and120mg/kgi.p.)ascomparedwiththesalinecontrolafter1h
post-surgery.Theanti-allodynicGBPeffectcouldbeexplainedby
blockageofthe␣2␦intheCav2.1calciumchannelsubunits[21].In
addition,GBPcansuppressinvivoandinvitroectopicdischargesin
thesciaticnerveafferentfibersfromneuropathicanimalsbutnot
fromnormalanimals[22].
MBPandPLPconstitute85%oftheproteinfoundinthemyelin
sheathandhelptostabilizethemyelinstructurescaffoldingthe
lipid component [23]. Setton-Avruj et al. studied the temporal
course of MBP nerve expression (3, 7 and 14 days), after
sci-aticnerveconstrictionin adultrats,distallytotheligature,and
foundthat therewas amaxiumpeak of MBPexpression seven
dayspost-constriction,reducingthereafter.Intheproximalnerve
stump, a maximum peak of MBP was found on the 14th day
post-constriction[24].SimilarlytoSetton-Avruj findings,aMBP
redistribution was seen in the distal stump of injured nerves,
suggesting an increased cellularphagocytosis and scatteringof
MBP debris. MBP redistribution maybe ongoing during
simul-taneous processes of distal Wallerian degeneration and nerve
regeneration.Noteworthy,axon-releasedMBPmaybedigestedby
metaloproteinase-9togenerateMBP84-104andMBP68-86
frag-mentpeptidesthatarestronglyimmunogenic,activatingT-cells
andcausingT-cell-mediatedmechanicalallodynia[25].Our
pre-vious[6]andcurrentfindings suggestthatGBP-induced myelin
debrisremovalfromtheinjurednervesiteandbetterregenerating
axonscouldbebeneficialtoimproveneuropathicpainbyreducing
MBPdigestedfragmentsofthenervemillieu.
MacrophagesandSchwanncellsbothcooperateduringmyelin
degeneration,removingmyelinandaxondebristhatmayfacilitate
nerveregeneration[26].Inaddition,accordingtoHall(1978),rapid
Schwanncellproliferation(3–4dayspost-nerveinjury)iskey
fac-tortopromoteaxonalregeneration.Removalofmyelindebrisby
macrophage-likeactivatedcellsisanimportantfactor
beneficiat-ingnerverecoveryaftersciaticnerveconstriction,asmyelindebris
couldbeinhibitorytonerveregeneration, sinceseveral
myelin-associatedinhibitorsofaxonregenerationhavebeenfoundinthe
peripheralmyelin[27,28].
Onestudyhasfoundnonerveregenerativeeffectwithchronic
injectedpregabalin,aGBPanalogous,(10mg/kgbydaily
subcuta-neousinjection)atthe21thdayfollowingsciaticnervecrush[29],
thismaybeduetoadifferentdoseused.Inlightofourfindings,
Machadoetal.havedocumentedbenefitsofa highdoseoforal
GBP(300mg/kg)inimprovingtheareaanddensityofmyelinated
fibers30daysfollowingstretch-inducednerveinjuryinWistarrats
[30].
Morestudiesarewarrantedtoappreciate inmoredetail the
mechanisms,time-courseandfinehistologyofmyelinremovaland
remodelingduringGBPpro-myelinationeffectsintheirassociation
withtheameliorationofneuropathicpain.
5. Conclusion
In summary, altogether our findings reinforce the analgesic
effectsofGBPandsuggestabeneficialroleofGBPonnerve
mor-phologyfollowingsciaticnerveinjury,throughmodulationofMBP
expressionandmyelinremodeling.
Funding
ThisstudywassupportedbyFUNCAPandCNPq.
References
[1]C.Perez,A.Navarro,M.T.Saldana,M.Figueras-Balsells,M.Munoz-Tuduri,J. Rejas,Costsavingsassociatedwithearlyinitiationofpregabalininthe managementofperipheralneuropathicpain,Clin.J.Pain29(2013)471–477.
[2]Y.B.Martin,G.Herradon,L.Ezquerra,Uncoveringnewpharmacological targetstotreatneuropathicpainbyunderstandinghowtheorganismreacts tonerveinjury,Curr.Pharm.Des.17(2011)434–448.
[3]J.M.Zakrzewska,Medicalmanagementoftrigeminalneuropathicpains, ExpertOpin.Pharmacother.11(2010)1239–1254.
[5]T.Mixcoatl-Zecuatl,F.J.Flores-Murrieta,V.Granados-Soto,Thenitric oxide-cyclicGMP-proteinkinaseG-K+channelpathwayparticipatesinthe antiallodyniceffectofspinalgabapentin,Eur.J.Pharmacol.531(2006)87–95.
[6]C.C.Camara,H.F.Ramos,A.P.daSilva,C.V.Araujo,A.S.Gomes,M.L.Vale,A.L. Barbosa,R.A.Ribeiro,G.A.Brito,C.M.Costa,R.B.Oria,Oralgabapentin treatmentaccentuatesnerveandperipheralinflammatoryresponses followingexperimentalnerveconstrictioninWistarrats,Neurosci.Lett.556 (2013)93–98.
[7]M.Simons,J.Trotter,Wrappingitup:thecellbiologyofmyelination,Curr. Opin.Neurobiol.17(2007)533–540.
[8]G.J.Bennett,Y.K.Xie,Aperipheralmononeuropathyinratthatproduces disordersofpainsensationlikethoseseeninman,Pain33(1988)87–107.
[9]C.Sommer,A.Lalonde,H.M.Heckman,M.Rodriguez,R.R.Myers,Quantitative neuropathologyofafocalnerveinjurycausinghyperalgesia,J.Neuropathol. Exp.Neurol.54(1995)635–643.
[10]C.J.LaBuda,P.J.Little,Pharmacologicalevaluationoftheselectivespinalnerve ligationmodelofneuropathicpainintherat,J.Neurosci.Methods144(2005) 175–181.
[11]V.Kayser,D.Christensen,Antinociceptiveeffectofsystemicgabapentinin mononeuropathicrats,dependsonstimuluscharacteristicsandleveloftest integration,Pain88(2000)53–60.
[12]M.I.Azevedo,A.F.Pereira,R.B.Nogueira,F.E.Rolim,G.A.Brito,D.V.Wong,R.C. Lima-Junior,R.R.deAlbuquerque,M.L.Vale,Theantioxidanteffectsofthe flavonoidsrutinandquercetininhibitoxaliplatin-inducedchronicpainful peripheralneuropathy,Mol.Pain9(2013)53.
[13]S.J.Flatters,G.J.Bennett,Ethosuximidereversespaclitaxel-and
vincristine-inducedpainfulperipheralneuropathy,Pain109(2004)150–161.
[14]H.C.Pan,D.Y.Yang,Y.C.Ou,S.P.Ho,F.C.Cheng,C.J.Chen,Neuroprotective effectofatorvastatininanexperimentalmodelofnervecrushinjury, Neurosurgery67(2010)376–388.
[15]C.P.Setton-Avruj,J.B.Aquino,C.J.Goedelman,E.F.Soto,M.J.Villar,P0and myelinbasicprotein-likeimmunoreactivitiesfollowingligationofthesciatic nerveintherat,Neurochem.Res.27(2002)1293–1303.
[16]R.C.Kupers,D.Nuytten,M.DeCastro-Costa,J.M.Gybels,Atimecourse analysisofthechangesinspontaneousandevokedbehaviourinaratmodel ofneuropathicpain,Pain50(1992)101–111.
[17]L.M.Batista,I.M.Batista,J.P.Almeida,C.H.Carvalho,S.B.Castro-Costa,C.M. Castro-Costa,Preemptiveanalgesiceffectoflidocaineinachronic neuropathicpainmodel,Arq.Neuropsiquiatr.67(2009)1088–1092.
[18]C.M.DeCastro,S.P.De,J.Gybels,H.J.Van,Adjuvant-inducedarthritisinrats:a possibleanimalmodelofchronicpain,Pain10(1981)173–185.
[19]H.A.Bagriyanik,N.Ersoy,C.Cetinkaya,E.Ikizoglu,D.Kutri,T.Ozcana,L.G. Kamanga,M.Kiray,Theeffectsofresveratrolonchronicconstrictioninjuryof sciaticnerveinrats,Neurosci.Lett.561(2014)123–127.
[20]H.Gustafsson,K.Flood,O.G.Berge,E.Brodin,L.Olgart,C.O.Stiller,Gabapentin reversesmechanicalallodyniainducedbysciaticnerveischemiaand formalin-inducednociceptioninmice,Exp.Neurol.182(2003)427–434.
[21]J.Hendrich,A.T.VanMinh,F.Heblich,M.Nieto-Rostro,K.Watschinger,J. Striessnig,J.Wratten,A.Davies,A.C.Dolphin,Pharmacologicaldisruptionof calciumchanneltraffickingbythealpha2deltaligandgabapentin,Proc.Natl. Acad.Sci.U.S.A105(2008)3628–3633.
[22]H.L.Pan,J.C.Eisenach,S.R.Chen,Gabapentinsuppressesectopicnerve dischargesandreversesallodyniainneuropathicrats,J.Pharmacol.Exp.Ther. 288(1999)1026–1030.
[23]A.Asipu,G.E.Blair,Regulationofmyelinbasicprotein-encodinggene transcriptioninratoligodendrocytes,Gene150(1994)227–234.
[24]C.P.Setton-Avruj,J.B.Aquino,C.J.Goedelman,E.F.Soto,M.J.Villar,P0and myelinbasicprotein-likeimmunoreactivitiesfollowingligationofthesciatic nerveintherat,Neurochem.Res.27(2002)1293–1303.
[25]H.Liu,S.A.Shiryaev,A.V.Chernov,Y.Kim,I.Shubayev,A.G.Remacle,S. Baranovskaya,V.S.Golubkov,A.Y.Strongin,V.I.Shubayev,Immunodominant fragmentsofmyelinbasicproteininitiateTcell-dependentpain,J. Neuroinflammation.9(2012)119.
[26]K.Hirata,H.Mitoma,N.Ueno,J.W.He,M.Kawabuchi,Differentialresponseof macrophagesubpopulationstomyelindegradationintheinjuredratsciatic nerve,J.Neurocytol.28(1999)685–695.
[27]S.Shim,G.L.Ming,Rolesofchannelsandreceptorsinthegrowthconeduring PNSaxonalregeneration,Exp.Neurol.223(2010)38–44.
[28]M.E.Vargas,B.A.Barres,WhyisWalleriandegenerationintheCNSsoslow? Annu.Rev.Neurosci.30(2007)153–179.
[29]E.L.Whitlock,A.Moradzadeh,D.A.Hunter,S.E.Mackinnon,Pregabalindoes notimpactperipheralnerveregenerationaftercrushinjury,J.Reconstr. Microsurg.23(2007)263–268.