w w w . s b f g n o s i a . o r g . b r / r e v i s t a
Original
Article
Intraspecific
variation
of
meroditerpenoids
in
the
brown
alga
Stypopodium
zonale
guiding
the
isolation
of
new
compounds
Angelica
R.
Soares
a,b,∗,
Heitor
M.
Duarte
b,
Luzineide
W.
Tinnoco
c,
Renato
C.
Pereira
a,d,
Valéria
L.
Teixeira
a,daProgramadePós-graduac¸ãoemQuímicaOrgânica,InstitutodeQuímica,UniversidadeFederalFluminense,Niterói,RJ,Brazil
bGrupodeProdutosNaturaisdeOrganismosAquáticos,NúcleoemEcologiaeDesenvolvimentoSócioambientaldeMacaé,UniversidadeFederaldoRiodeJaneiro,Macaé,RJ,Brazil cInstitutodePesquisasdeProdutosNaturais,UniversidadeFederaldoRiodeJaneiro,RiodeJaneiro,RJ,Brazil
dDepartamentodeBiologiaMarinha.InstitutodeBiologia,UniversidadeFederalFluminense,Niterói,RJ,Brazil
a
r
t
i
c
l
e
i
n
f
o
Articlehistory:
Received1May2015 Accepted7September2015 Availableonline23October2015
Keywords:
Phaeophyceae Dictyotales Meroterpenoids PCA
Biodiversity Seaweed
a
b
s
t
r
a
c
t
IntraspecificvariationonmeroditerpenoidsproductionbythebrownmarinealgaStypopodiumzonale atfourdifferentpopulationsalongtheBraziliancoastwasanalyzedusingPrincipalComponent Anal-ysisoverhigh-performanceliquidchromatographyprofilesfromalgaeextracts.Theordinationofthe samplesbythe similaritiesof theirchromatographic traitsshowed theexistenceofthree chemo-types:(i)thepopulationsBúziosandAbrolhoswhichwerecharacterizedbythepresenceofatomaric acid(1),(ii)thepopulationAtoldasRocaswhichcontainedthecompoundstypoldione(2),and(iii) thepopulationMarataízeswhichwascharacterizedbyotherpeaksthatguidedtheisolationofthree newmeroditerpenoidsstypofuranlactone(3),10,18-dihydroxy-5′a-desmethyl-5′-acetylatomaricacid
(4),andthe10-keto-10-deisopropyliden-5′a-desmethyl-5′-acetylatomaricacid(5)togetherwiththe
knowncompoundthe10-keto-10-deisopropyliden-atomaricacid(6).Thestructuresandrelative stere-ochemistryof3,4and5wereelucidatedbyNMRandMStechniques.Theobservedchemicaldifferences amongpopulationsofS.zonalecanberelatedtoitsgeographicdistributionandcanopenanavenueto thediscoveryofnewcompoundsinalgae.
©2015SociedadeBrasileiradeFarmacognosia.PublishedbyElsevierEditoraLtda.Allrightsreserved.
Introduction
Thetermmeroterpenoidsisappliedtodescribenatural
prod-uctsofmixedbiosyntheticorigin,whicharepartiallyderivedfrom
terpenoids.Theprefixmero(fromGreekmerus)means“part,
par-tialorfragment”(GerisandSimpson,2009).Therefore,anumberof
secondarymetabolitescanbedescribedasmeroterpenoids,which
assemblesahugerangeofstructuraldiversity,oftenlinkedwith
variedfunctionalities,arisingfromintra-andintermolecularring
closuresand/orrearrangementsoftheterpenechains(Gerisand
Simpson,2009;Macíasetal.,2014).Thisclassofnaturalproductsis
widelydistributedamongmarineorganisms,buttheyare
particu-larlyabundantinmarinebrownalgae,microorganismsandsponge
(Vallimetal.,2005;Mennaetal.,2013).
Within the marine brown algae, order Fucales and the
genusStypopodium(Dictyotales)havebeenrecognizedasarich
sourceofstructurallyunique,biologicallyandecologicallyactive
∗ Correspondingauthor.
E-mail:angelica@iq.ufrj.br(A.R.Soares).
meroditerpenoids(Vallsetal.,1993;Mesguicheetal.,1997;Dorta
et al.,2003;Soares etal.,2007; Mendeset al.,2011)thathave
beennamedbysomeauthorsasstypols(Macíasetal.,2014).Some
stypols,suchasstypoldiol,stypoldioneandepitaondiol,havebeen
tested for theircell proliferation inhibitoryactivity in different
celllines(WhiteandJacobs,1983;Sabryetal.,2005;Pereiraetal.,
2011),thusmakingthempromisingcandidatesasanticancerdrugs.
Stypoldiolandstypoldionehaveremarkableantioxidantproperties
throughradical-scavengingtogetherwithstructurallyrelated
com-pounds,taondiolandisoepitaondiol(Nahasetal.,2007).Inaddition
tothevarietyofbiologicalactivity,theecologicalfunctionas
defen-sivechemicalsagainstherbivoryof thesemeroditerpenoidshas
beendocumented(GerwickandFenical,1981;Pereiraetal.,2004).
Interestingly,theanalysesofmeroditerpenoidscompositionand
concentrationinStypopodiumrevealedanintra-specificvariation
amonggeographicallocations(GerwickandFenical,1981;Gerwick
etal.,1985;Soaresetal.,2003;Pereiraetal.,2004).
Inter- and intrapopulational chemical differences in marine
organisms(orchemotypes)havebeendescribedinseveralstudies
(e.g.Machadoetal.,2014),andthesevariationsareexplainedby
combinationofgenetic(Wrightetal.,2004),developmental(Paul
http://dx.doi.org/10.1016/j.bjp.2015.09.006
andVanAlstyne,1988),andenvironmental(Sudattietal.,2011) factors.
Hereweinvestigatedthespatialvariabilityofthe
meroditer-penoids in Stypopodium zonale (Lamouroux) Papenfuss
(Dictyotales),theuniquespecieofStypopodiumgenusdescribed
toBrazil(GuiryandGuiry,2015).PrincipalComponentAnalysis
(PCA) of the chemical profiles was employed in this study to
accessthechemicalvariabilityamongthepopulationsandguided
theisolationofthreenewcompounds(3–5),inadditiontoother
threeknownmeroditerpenoids(1,2and6).Thestructureswere
elucidated based on spectroscopic and spectrometric analysis
including1Hand13CNMR,1H–1HCOSY,HSQC,HMBC,ROESYand
HREIMS.
Materialsandmethods
Generalexperimentalprocedures
OpticalrotationsweremeasuredonaPerkin-Elmermodel241
polarimeter(Perkin-Elmer,USA)usingaNalampat25◦C.IR
spec-tra wereobtainedwitha Perkin-Elmer1650/FTIR spectrometer
in CHCl3 solutions. HREIMS spectrum was taken on a Waters
MicromassAutospecmassspectrometer(Waters,EUA).1HNMR,
13CNMR,HSQC,HMBCand1H–1HCOSYspectraweremeasured
employingaVarianUnity300instrumentoperatingat300MHzfor
wereacquiredwiththestandardBrukerDRXpulsesequence
oper-ating at 400MHz for 1H NMR. The 2D ROESY experiment was
performedwithmixingtimeof300ms,with16scansat25◦C.
Sil-icagel(Merck,70–230and230–400mesh)wasusedincolumn
chromatography.Thinlayerchromatographywascarriedoutwith
silicagel60GF254(Merck).Oncedeveloped,spotsontheplates
werevisualizedbysprayingwith2%cericsulphateinsulfuricacid,
followedbygentleheating.
Algalmaterial
SpecimensofStypopodiumzonale(Lamouroux)Papenfuss
(Dic-tyotales)werecollectedbysnorkelingandSCUBAdivingatfour
distinctareas of theBrazilian coast:Forno inlet,Búzios,Rio de
JaneiroState(22◦ 45′S,41◦ 52′W),atthedepthsofthe3–6min
April,2002;Marataízes,EspíritoSantoState(21◦02′S,40◦49′W),
atthedepthsofthe13–15minApril,2002;AtoldasRocas,Rio
GrandedoNortestate(03◦51′S,33◦40′W),at6–10minJune2002
andAbrolhos,Bahiastate(14◦46′S,39◦01′W),at10–12minApril
1995.Thealgaewerewashedinseawatertoeliminateassociated
organismsandair-dried. Voucherspecimens (Búzios:HRJ8643;
Marataízes:HRJ5543;Abrolhos:HRJ5593andAtoldasRocas:HRJ
8678)weredepositedattheHerbariumoftheRiodeJaneiroState
University,Brazil(HRJ).
Extractspreparation
Adultandwell-developedspecimensofS.zonalefromBúzios
(115galgadryweight,dw),Marataízes(12.8gdw),Abrolhos(173g
dw)and Atoldas Rocas(106gdw) were air-dried, milled and
extractedthreetimeswithCH2Cl2(using100mlofsolventtoeachg
ofdw)atroomtemperature.Thesolventwasremovedinvacuum
toyieldadarkgreentarinallcases(19g,16.5%dw;1.8g,14.2%
dw;31g,17.9%dw;12g,12.9%dw,respectively).Individual
spec-imens(n=3)fromeachpopulationwereindependentlyextracted
inanidenticalfashionandhadtheirchemicalprofilesanalyzedby
HPLC-DAD.
HPLC-DADchemicalprofiles
Thecrudeextractsandpurecompounds(1)and(2)were
ana-lyzedbyhigh-performanceliquidchromatography(HPLC)and
car-riedoutonaVP-ODSShim-packcolumn(5M,250mm×4.6mm)
usinga Shimadzu HPLCchromatographequipped witha Diode
Array Detector (DAD, Shimadzu, Japan) and a coupled system
consistingof a vacuumdegasser,pumpLC-20AT, and detectors
SPD-M20Aand CBM-20A.Abinarygradientelutionatflowrate
1.0ml/min wasemployedusingwater (pH3.0 withphosphoric
acid)assolventAandacetonitrileassolventB,asfollows:60–100%
Bat 0–25min.Theeluent remainedat100%acetonitrileduring
5min,thenchangeddirectlytotheinitialgradientconditions
dur-ing10minforthecolumnre-conditioningbetweentwosequential
injections.TheUV–Visabsorbancespectrawereregistered.Crude
extractswere dilutedin acetonitrileto a final concentrationof
10mg/mlandfilteredina0.45MPTFEsyringefilter(Millipore,
USA)prior injection.Analiquotof 20lof filtratesolutionwas
injectedforHPLCanalysis.
PrincipalComponentAnalysis(PCA)
ThefirststepofthedatapreparationforthePCAanalysiswas
theremovalofthefirst5minoftheretentiontime(tR)fromthe
two-dimensionalchromatogrammatricesfromeachextract
sam-plefromspecimensofS.zonaleobtainedbytheHPLC-DADsystem,
sinceitcontainsaverylowpeakresolutioncausedbythestrong
overlapping signal from polar compounds. The chromatograms
werethenbaselinealignedandcorrectedforthetimeshiftofpeaks
among them using the Correlation Warping Algorithm (COW),
asdescribed by Nielsenetal. (1998),usingthesoftware “COW
tool” (http://www2.biocentrum.dtu.dk/mycology/analysis/cow/).
Data fromwavelengthof 280nm andtR between5 and27min
wereextractedfromeachofthetwo-dimensionalchromatograms
andplacedinamatrixcontainingallsamples.Eachchromatogram
wasnormalizedbythehighestpeakfoundineachsampleinother
toenhancetheproportionalinformationamonghighpeaks.The
matrixwithallchromatogramswasthen meancenteredbefore
runningthePCAroutine,whichwasconductedusingtheRlanguage
and environment (http://www.R-project.org) withthe installed
package“ChemometricsWithR”(Wehrens,2011).
Isolationandidentificationofthemeroditerpenes
Toobtainthepurestandardsoftheknowncompounds1and
2previouslydescribedbySoaresetal.(2003),theextractsfrom
specimenscollectedatBúziosandAtoldasRocaswere
fraction-ated.FollowingthemethodusedbySoaresetal.(2003),partofthe
extractofthespecimenscollectedatBúzios(2.1g)wassubmittedto
anacidic–basicextraction.Theacidfraction(198mg)was
fraction-atedonsilicagelvacuumliquidchromatographytoyield8mgof1.
TheextractobtainedfromalgaecollectedatAtoldasRocas(2.5g)
waselutedina columnchromatographyover silicagelwithan
increasinggradientofhexane,dichloromethane(CH2Cl2)andethyl
acetate(EtOAc)toprovideaslightlyimpurefractionofcompound
2.Thisfractionwaspurifiedbyvacuumliquidchromatographyover
silicagelandelutedwithhexane:EtOAc(1:1,v/v)toyield2(6mg).
Theextract(1.8g)fromMarataízeswasfractionatedbysilicagel
vacuumliquidchromatographyusingastepwisegradientsolvent
systemofincreasingpolaritystartingfrom100%hexaneto100%
methanol(twelve fractions,A–M).The fractioneluted with15%
EtOAcinCH2Cl2(fractionG,247mg)wasfurtherpurifiedwithsilica
gelcolumnchromatographyusingastepwisegradientofmethanol
(MeOH)inCH2Cl2 toafford3(12.0mg).Thefractionelutedwith
50%EtOAcinCH2Cl2(fractionI,126mg)wasre-chromatographed
usingsilicagelcolumnchromatographywithEtOAc20%inCH2Cl2
toyield4mgofthecompound4.Finally,thefractionelutedwith
5%MeOHinEtOAc(91mg,fractionL)waspurifiedwithsilicagel
columnchromatographyelutedwithincreasingamountofEtOAc
inCH2Cl2togive38mgofmixtureof5and6.
Thestructureof allthecompoundswaselucidatedbasedon
thespectral data (opticalrotation,IR, EIMS and/orHREIMS,1D
and2DNMR).Theknowncompoundswereidentifiedcomparing
thesespectroscopicdatawiththeliterature(MoriandKoga,1992;
Wesselsetal.,1999;Dortaetal.,2003)and, whenavailable,by
directcomparisonwiththeoriginalpurifiedstandards.
Resultsanddiscussion
Marine algae of the Stypopodium genus are a rich source
of meroditerpenoids, which very often display potent
biologi-cal activities in addition to their ecological roles (Valls et al.,
1993; Mesguiche et al., 1997; Dorta et al., 2003; Soares et al.,
2007;Mendesetal.,2011).Thesecompoundshavebeenusedas
chemotaxonomicmarkerstodistinguishtherepresentativesofthe
genuswithintheDictyotales(Vallimetal.,2005).Thepresenceof
thesecompoundsinStypopodiumcanbeusefulfordereplication
purposeshelping onthedetectionof newcompounds amonga
plethoraofknowncompounds.
Here we compared four populations of S. zonale collected
alongofBraziliancoastlineaccordingtotheirHPLC-DADdata.The
ordinationofthepopulationsscoresbythefirsttwocomponents
PCA scores
CP2 (35.0%)
CP1 (62.6%)
Atol das Rocas Abrolhos
Búzios
Marataízes
5
4
3
2
1
0
–1
–2
–3
–4 –2 0 2 4 6
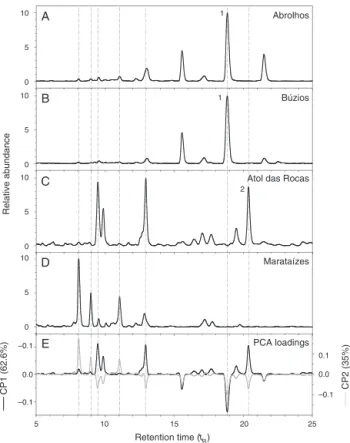
Fig.1.OrdinationofthefirsttwoPCAscores(CP1andCP2)obtainedfromthe analysisofchemicalprofilesfromfourdifferentpopulationsofStypopodiumzonale.
Atol das Rocas Abrolhos
A
B
C
D
E
Búzios
Marataízes
PCA loadings
CP2 (35%)
CP1 (62.6%)
Relative abundance
Retention time (tR)
5 10 15 20
2 1
1
25 –0.1 0.0 0.1 –0.1
0 5 10 0 5 10 0 5 10 0 5 10
–0.1 0.0
Fig.2. Meanchemicalprofiles(n=3)offourdifferentpopulationsofStypopodium zonaleandthePCAloadingsplottedagainsttheretentiontime.Eachchemical pro-filewasnormalizedbyitsmaximalpeakintensityinothertoenhancetherelative differenceamongpeaks.(1)and(2)markthepeaksofatomaricacidandstypoldione respectively.
variation, revealed that thesamples from Abrolhos and Búzios
sitesweregroupedcloselytogetherat thenegativeside ofCP1
(Fig.1).Thisisaresultoftheirsimilarchromatograms(Fig.2Aand
B),differentonlybypresenceofonepeak(retentiontime,tR=22.5)
intheextractsfromAbrolhos specimensofS.zonale.Thesetwo
populationsdifferfromtheotherpopulationsmostlybythe
pres-enceofapeakattR=18.9min,whichhadthestrongestloadingon
samplevariation(CP1,Fig.2E).Incontrast,thesamplesfromAtol
dasRocas,placedatthepositivesideofCP1(Fig.1),donothave
thispeakandweremostlycharacterizedbythreepeaks(tR’s=9.5,
12.9and20.4min)accordingtothePCAloading(Fig.2CandE).
Theextractof samplesfromMarataízeshad a weakcorrelation
withtheotherthreeanditwascompletelyseparatedfromthem
atCP2(Fig.1),withthestrongestpeakloadingsattR’s=8,9and
11min(Fig.2DandE),notobservedinotherpopulations.
Standardsofthemeroditerpenesatomaricacid(Soaresetal.,
2003)(1)andstypoldione(Soaresetal.,2003)(2)wereanalyzed
byHPLCinthesamewayastheextracts.Themajor
chromato-graphicpeakinFig.2AandB(tR=18.9min)wasidentifiedasthe
atomaricacid(1)in Búziosand Abrolhos extracts.In the
chro-matogramofAtoldasRocas(Fig.2C),thepeakattR=20.4minwas
identifiedasstypoldione(2).Tounambiguouslyassignthesepeaks
totheatomaric acid(1)and stypoldione (2), theextractsfrom
BúziosandAtoldasRocaswerefractionatedandthecompounds
wereidentifiedbythecomparisonoftheirspectroscopicdatawith
theliterature(Moriand Koga,1992; Wesselset al.,1999).
Nei-therofthesecompoundswasidentifiedinthechromatogramof
theMarataízessamples,whichwerequitedifferentfromtheother
populationsasshownbyPCAanalysis.1HNMRdataofthisextract
indicatedthepresenceofdifferentmeroditerpenes.Then,inorder
toevaluatetheextractofMarataízesforthepresenceofnew
sec-ondarymetabolites,itwasfractionatedandthreenewcompounds
(3–5)in additionof theknowncompound 6wereisolated and
identified.
Compound 3 wasobtained as an opticallyactive yellow oil
([␣]D26+6.36◦,c0.04,CH2Cl2).The1Hand13CNMRdata(Table1)
combinedwiththe[M]+peakatm/z484.2713(calc.forC
29H40O6,
484.2814) in the HREIMS suggested a molecular formula of
C29H40O6,indicatingthatthiscompoundpossesses10degreesof
unsaturation.TheIRspectrumdisplayedstrongabsorptionbands
forahydroxylgroupat3435cm−1andtwocarbonylgroupat1749
and1740cm−1.The13CNMRspectrumshowedsignalsforall29
carbons:sevenmethylgroups,sixmethylenegroups(allsp3),six
methinegroups(4sp3and2sp2)andtenquaternarycarbons(4
sp3and6sp2).Amongthequaternarycarbons,twoofthemwere
resonatingatıCcarbonylgroupsofesterorlactonefunctionalities
atıC172.3andıC169.9.The1HNMRspectrumshowedsignalsfor
twometa-coupledaromaticprotonsatıH6.75(d,2.7)andıH6.72
(d,2.7)thattogetherwiththecharacteristicaromaticcarbon
sig-nalsindicatedthepresenceofatetrasubstitutedphenylring.Inthe
up-fieldregionsignalsappearforfivemethylgroupsatıH1.46(s),
ıH1.43(s),ıH1.18(d,6.9),ıH0.98(s)andıH0.80(s).Thesesignals,
inadditiontothebenzylicmethylenegroupatıH3.18(d,13.5)and
ıH2.12(d,13.5),suggestedaditerpenemoietyofthecompound3.
Additionally,theanalysisofthe1HNMRspectrumalsoindicated
anaromaticmethylgroupatıH2.19(s),anacetylgroupatıH2.26
(s)andadeshieldedmethineresonanceatıH4.80(dd,1.2and4.2)
that,byHSQC,correspondedtoanoxygen-bondedmethine.
Theinspectionofthe1H–1HCOSYspectrumallowedusto
con-necttheprotonatıH4.80(H-13)tothemethylenegroupatıH1.48
(H-12)andthistothemethinegroupatıH1.93(H-11).HMBC
cor-relationoftheprotonatıH4.80(H-13)withthecarbonatıC81.3
(C-10)establishedanoxygenbridgebetweenthesecarbons(atıC
81.3and79.9).TheprotonatıH 4.80(H-13)alsoshowedHMBC
crosscorrelationtocarbonatıH41.4(C-11),whichcombinedwith
further1H–1HCOSYcorrelationsestablishedthefive-membered
ringfragmentcontainingaheteroatom.Finally,theHMBC
correla-tionsofıH1.43(H-19)tothequaternarycarbonsatıC81.3(C-10),
89.1(C-15),andthemethylgroupatıC26.4(C-20)inadditionto
thesecondcarbonylgroupatıC172.3(C-14)allowedtoestablish
theunusualstructureof3.Interpretationof1H–1HCOSY,HSQCand
HMBCexperimentsassignedallof1Hand13CNMRsignals(Table1)
Table1
NMR spectroscopic data for stypofuranlactone (3), 10,18-dihydroxy-5′a-desmethyl-5′-acetylatomaric acid (4) and 10-keto-10-deisopropyliden-5′a-desmethyl-5′ -acetylatomaricacid(5)inCDCl3(300.0MHz).
Position 3 4 5
ıC.m ıH(JinHz) HMBCa ıC,m ıH(JinHz) HMBCa ıC,m ıH(JinHz) HMBCa
1 38.4,CH2 2.12,d(13.5)
3.18,d(13.5)
17,1′,2′,6′ 35.7,CH2 2.31,d(13.8) 2.86,d(13.8)
2,3,17,1′,2′,3′ 35.8,CH2 2.31,d(12.9) 3.03,d(12.9)
6′
2 39.8,C 40.0,C 40.6,C
3 37.1,CH 1.75,m 35.5,CH 1.73,m 35.5,CH 1.75,m
4 24.8,CH2 1.31,m
1.82,m
16 25.3,CH2 1.75,m 15 25.4,CH2 1.42,m
1.82,m
5 31.2,CH2 1.37,d(6.6) 35.2,CH2 1.34,m
1.65,m
31.8,CH2 1.44,m 1.64,m
6 35.1,C 38.9,C 42.8,C
7 45.9,CH 2.46,m 46.8,CH 1.31,m 47.4,CH 1.99,d(10.2) 16,17
8 18.0,CH2 1.54,d(4.8)
2.03,m
19.1,CH2 1.38,m 24.1,CH2 1.62,m
2.03,m
9 29.8,CH2 1.85,d(9.3)
2.50,d(9.3)
28.7,CH2 2.47,m 2.54,m
42.3,CH2 2.36,m 2.40,m
10 81.3,C 87.5,C 212.5,C
11 41.4,C 1.93,ddd(1.2,
4.2,13.2)
45.0,C 1.60,m 63.4,CH 2.24,m
12 24.9,CH2 1.48,d(4.2) 17.4,CH2 1.89,d(12.3)
2.25,m
17.2,CH2 1.62,m 1.93,m
13 79.9,CH 4.80,dd(1.2,
4.2)
10,11 35.1,CH2 1.34,m 32.5,CH2 2.44,m
2.49,m
14 172.3,C 175.0,C 179.2,C
15 89.1,C 20.2,CH3 0.92,s 2,3,7 20.1,CH3 0.91,s 2,3
16 20.8,CH3 0.98,s 16.3,CH3 1.08,d(6.9) 16.4,CH3 1.15,d(6.9)
17 15.9,CH3 1.18,d(6.9) 19.5,CH3 1.07,s 6,11 16.9,CH3 0.78,s 5,11
18 16.2,CH3 0.80,s 5,6,7,11 76.9,C
19 22.7,CH3 1.43,s 10,15,20 25.5,CH3 1.29,s 10,18
20 26.4,CH3 1.46,s 27.2,CH3 1.27,s 10,18,19
1′ 130.0,C 126.8,C 126.7,C
2′ 150.2,C 150.3,C 150.5,C
3′ 126.7,C 123.7,C 123.7,C
4′ 120.9,CH 6.72,d(2.7) 3′,6′,7′ 121.0,CH 6.73,d(2.7) 2′,5′,7′ 121.0,CH 6.71,d(2.4) 2′,5′,7′
5′ 143.6,C 143.1,C 143.0(C)
6′ 121.3,CH 6.75,d(2.7) 3′,4′,6′ 121.4,CH 6.81,d(2.7) 2′,5′ 121.4,CH 6.82,d(2.4) 2′,4′,5′
7′ 16.6,CH3 2.19,s 2′,3′ 16.3,CH3 2.23,s 2′ 15.9,CH3 2.21,s 2′,3′
8′ 169.9,C 169.8,C 170.0,C
9′ 21.0,CH3 2.26,s 8′ 20.9,CH3 2.26,s 8′ 21.0,CH3 2.26,s 8′
OH 4.58,s 4.56,s
aHMBCcorrelationsarefromproton(s)statedtotheindicatedcarbon.
Therelativestereochemistryof3wasachievedbyROESY
exper-iment(Fig.3)alongwithbiogeneticconsiderations(Gonzálezetal.,
1982).IntheROESYspectrum,correlationsof17-Me(ıH1.18)with
H-7(ıH2.46),H-11(ıH1.93)andH-13(ıH 4.80),inadditionto
thecorrelationofthehydrogenH-13(ıH4.80)and20-Me(ıH1.46)
indicatedthatthesehydrogenatomsarespatiallyclose,and
accord-ingtoDortaetal.(2003),thesewereproposedina␣-configuration.
Ontheotherhand,ROESYcorrelationsofH-3(ıH1.75)and16-Me
(ıH0.98)and18-Me(ıH0.80),andalsothecorrelationof18-Me
(ıH0.80)and16-Me(ıH0.98)and19-Me(ıH1.43)suggestedthat
allofthemwere-oriented.
Thus, the structure of 3 was determined and named as
stypofuranlactone,aderivativecompoundofthe5′
a-desmethyl-5′-acetylatomaricacid,isolatedoftheS.zonalefromPacific,north
Atlanticand Caribbean area(Gerwicket al., 1985;Dorta et al.,
2003).Although moleculeswiththisfuran-lactonemoiety have
beenobtainedfromplantsandfungi(Macíasetal.,2014),thisis
thefirsttimethatcompoundswiththismoietyhavebeenobtained
frommarinebrownalgae(MarinLit,2015).
Compound4wasisolatedasapaleyellowoil([␣]D26+4.55◦,
c0.02,CH2Cl2)withthemolecularformula C29H44O7,as
deter-minedbyitsHREIMSatm/z504.3087 [M]+ (calc.forC
29H44O7,
504.3081),20massunitsmorethancompound3.TheIRshowed
theabsorptionat3434cm−1forhydroxylgroupandat1698cm−1
forcarbonylgroup.The1Hand13CNMRdatafor4(Table1)were
quitesimilartothoseof3,exceptbyabsenceoftheoxymethine
group,thedifferenceobservedinthechemicalshiftstothetwo
oxygenatedquaternarycarbonsatıC87.5(C-10)andıC76.9
(C-18),and tothegeminaldimethylgroups atıH 1.27(H-20)and
ıH1.29(H-19).IntheHMBCspectrum,thesetwomethylgroups
showedcross-peakswithbothoxygenatedquaternarycarbonsat
ıC87.5(C-10)andıC76.9(C-18).Thesedataindicateda
hydrox-yisopropylmoietyadjacenttothesecondhydroxygroupbonded
tothemainbackboneof4.Thecompleteanalysisofthe2DNMR
experimentsincluding1H–1HCOSY,HSQCandHMBCallowedthe
determinationoftheplanarstructureofthecompound4,which
wasnamedas10,18-dihydroxy-5′a-desmethyl-5′-acetylatomaric
acid.Therelativestereochemistryofallchiralcentersofthe
com-pound4,exceptforC-10(ıC87.5),wasdeducedbycomparisonof
theROESYcorrelations(Fig.3)withthoseofthecompound3.
The 10-keto-10-deisopropyliden-5′a-desmethyl-5′
-acetyl-atomaric acid(5)and the10-keto-10-deisopropyliden-atomaric
acid (6) were isolated as a homogeneous mixture by TLC.The
compound5wasobtainedasthemajorcomponentin the
mix-turewith6.Themolecularformulaofthemajorcompoundwas
determinedtobeC26H36O6 byHREIMS(m/z[M]+444.2511)and
NMRdata.Comparisonofspectroscopicdataof5(Table1)with
the literature (Gerwick et al., 1985) suggested that compound
5 was closely related to 5′a-desmethyl-5′-acetylatomaric acid,
exceptbyreplacementofisopropylene groupof5′
a-desmethyl-5′-acetylatomaric acid by a ketone group at ıC 212.5 (C-10).
Table2
IsolatedandidentifiedmeroditerpenoidsfoundinfourpopulationsofStypopodiumzonalealongtheBraziliancoast.
Meroditerpenoids Populations
Abrolhos Búzios AtoldasRocas Marataízes
Atomaricacid(1) X X
Stypoldione(2) X
Stypofuranlactone(3) Xa
10,18-Dihydroxy-5′a-desmethyl-5′-acetylatomaricacid(4) Xa
10-Keto-10-deisopropyliden-5′a-desmethyl-5′-acetylatomaricacid(5) Xa
10-Keto-10-deisopropyliden-atomaricacid(6) X
aNewcompounds.
Fig.3.KeyROESYcorrelationsofthemetabolites3–5.
assigningallof1Hand13CNMRsignals(Table1)tothecompound
5.Theminorcompoundinthemixturewasidentifiedasthe
10-keto-10-deisopropyliden-atomaricacid(6)byanalysesofthe1H
NMRand13CNMRdataandcomparisonthemwiththeliterature
(Dorta et al., 2003). By comparison of the ROESY correlations
(Fig.3)ofthe5and6compoundswiththoseofthecompound3,
wecoulddeducethattheyhavethesamerelativestereochemistry
of3.
Table2showsthemeroditerpenoidsidentifiedinthefour
stud-iedpopulations,inwhichwecoulddistinguishthreechemotypes
constitutedby algaefrom(i) Abrolhos andBúzios,(ii)Atol das
Rocasand(iii) Marataízes.Inthelast one,thenewcompounds
(3),(4)and(5)werefound.Chemicaldiversityamongpopulations
ofS.zonalewasreportedinsmallandlargespatialscale.Gerwick
etal.(1985)isolateddifferentcompoundsofthisspecieoccurring
atSouthAtlantic(Palao)andattheCaribbeanSea(Belize).Within
thecollectionsiteofBelize,theseauthorshavealsofoundchemical
diversitydifferencesbetweenpopulationsfromdeepandshallow
waters.DifferentandnovelcompoundsofS.zonalewerealsofound
inotherstudiescomparingpopulationsfromdistantgeographic
locations(Dortaetal.,2002,2003).Additionally,previousstudies
fromourresearchgrouphave demonstratedintraspecific
varia-tioninS.zonalefromBrazilianpopulations.Collectioncampaigns
in1998/1999showedthepresenceofatomaricacid(1)relatedto
otherpopulationsfromArchipelagoofAbrolhosandBúzios,Riode
JaneiroState,aswellasthepresenceofstypodione(2)in
popula-tionsfromAtoldasRocasandArchipelagoofFernandodeNoronha
(Soaresetal.,2003;Pereiraetal.,2004).Thesefindingsare
congru-entwiththechemotypesfoundinthiswork,wherethepopulations
fromAbrolhosandBúziosstillshowedthepresenceofatomaric
acidandAtoldasRocas,thestypodione.Thispatternwas
indepen-dentofthecollectiontime,indicatingatemporalconservationof
thechemotypesfound.
ThedifferencesobservedusingPCAonHPLCprofilesamongthe
samplesclearlyshowedthatthepopulationsofS.zonalealongthe
Braziliancoastcouldbeuniqueinrelationtosecondary
metabo-litecomposition.Thechemicaldiversityinalgaecouldarrivefrom
bioticandabioticfactors,andalsobytheinteractionbetweenthem.
CrossingexperimentswithspeciesofLaurenciagenusrevealedthat
geneticvariationwasresponsibleforthediversityofhalogenated
metabolitesinnaturalpopulationsofL.nipponica(Masudaetal.,
1997).Nevertheless, the intraspecific variations of halogenated
compoundsarerelatedtoalgalsexandlifehistoryphasesinthe
redalgaAsparagopsisarmata,whichresultsinaselectiveherbivory
byseahare(Vergésetal.,2008),andstresstheimportance
intraspe-cific variation on ecological and evolutionary consequences of
plant–herbivoreinteractions.Furthermore,abioticfactorssuchas
temperature(Sudattietal.,2011),salinity(Kuwanoetal.,1998),
lightexposure(Yuanetal.,2009),inductionofchemicaldefenses
againstherbivoresand spacecompetitionpressure (Amade and
Lemée,1998)arealsorelatedtointraspecificdifferencesin
chemi-calcompositionofmarineorganisms.Althoughthespecificsource
ofintraspecificvariationwasnotconsideredhere,thehigh
chem-icaldiversityofStypopodiumzonaleisoncemoresuggestedtobe
relatedtogeographicdistribution.
Moreover,theapplicationofanalyticalmethodstogetherwith
multifactorialstatisticshasproventobeausefultolltoordinate
samplesbythesimilaritiesoftheircomplexchromatographictraits,
andguidetheprioritization,whichhelpsonisolationand
identifi-cationofnewmeroditerpenesinS.zonale.
Author’scontribution
ARS collectedpart of thebiological material, performedthe
experiments,analyzedthedataandwrotethepaper.HMDwas
responsibleforthePCAandwrotethepaper.LTandVLTcontributed
workanddidcriticalreadingofthemanuscript.Alltheauthorshave
readthefinalmanuscriptandapprovedthesubmission.
Conflictsofinterest
Theauthorsdeclarenoconflictsofinterest.
Acknowledgements
ThisresearchwasfinanciallysupportedbyCNPqandFAPERJ.
ARS,RCPandVLTthankCNPqforprovidingResearchGrants.We
arealsogratefultoFAPERJforthe“Nota10”doctoralfellowship
toARS.Dr.RobertoC.Villac¸a(UFF)identifiedthespecimensfrom
allpopulationsandalsokindlycollectedthealgaeatMarataízes
site.The authorsthankLorenaSigilianofor technicalassistance
withtheHPLCanalysis.WealsothanktheNMRLaboratory
(Insti-tutode Química.UFF), Thomson MassSpectroscopy Laboratory
(InstitutodeQuímica,UNICAMP)and CNRMN(Institutode
Bio-química Médica, UFRJ) for analyses.We greatly appreciate the
helpfulcommentsofHosanaDebonsi(FCFRP-USP)thatimproved
themanuscript.
References
Amade,P.,Lemée,R.,1998.ChemicaldefenceoftheMediterraneanalgaCaulerpa taxifolia:variationsincaulerpenyneproduction.Aquat.Toxicol.43,287–300. Dorta,E.,Cueto,M.,Brito,I.,Darias,J.,2002.Newterpenoidsfromthebrownalga
Stypopodiumzonale.J.Nat.Prod.65,1727–1730.
Dorta,E.,Diaz-Marrero,A.R.,Cueto,M.,Darias,J.,2003.Ontherelative stereochem-istryofatomaricacidandrelatedcompounds.Tetrahedron59,2059–2062. Geris,R.,Simpson,T.J.,2009.Meroterpenoidsproducedbyfungi.Nat.Prod.Rep.26,
1063–1094.
Gerwick,W.H.,Fenical,W.,1981.Ichthyotoxicandcytotoxicmetabolitesofthe trop-icalbrownalgaStypopodiumzonale(Lamouroux)Papenfuss.J.Org.Chem.46, 22–27.
Gerwick,W.H.,Fenical,W.J.,Norris,J.N.,1985.Chemicalvariationinthetropical seaweedStypopodiumzonale(Dictyotaceae).Phytochemistry24,1279–1283. González,A.G.,Alvarez,M.A.,Martin,J.D.,Norte,M.,Pérez,C.,Rovirosa,J.,1982.
Diter-penoidsofmixedbiogenesisinPhaeophytabiogenetic-typeinterconversions. Tetrahedron38,719–728.
Guiry,M.D., Guiry,G.M., 2015. AlgaeBase. World-wide ElectronicPublication. NationalUniversity ofIreland,Galwayhttp://www.algaebase.org (accessed 10.03.15).
Kuwano,K.,Matsuka,S.,Kono,S.,Ninomiya,M.,Onishi,J.,Saga,N.,1998.Growth andthecontentoflaurinterolanddebromolaurinterolinLaurenciaokamurae (Ceramiales,Rhodophyta).J.Appl.Phycol.10,9–14.
Machado,F.L.S.M.,Lima,W.P.,Duarte,H.M.,Rossi-Bergmann,B.,Gestinari,L.M., Fujii,M.T.,Kaiser,C.R.,Soares,A.R.,2014.Chemicaldiversityandantileishmanial activityofcrudeextractsofLaurenciacomplex(Ceramiales,Rhodophyta)from Brazil.Rev.Bras.Farmacogn.24,635–643.
Macías,F.A.,Carrera,C.,Galindo,J.C.G.,2014.Brevianesrevisited.Chem.Rev.114, 2717–2732.
MarinLit, 2015. MarinLit, a database of marine natural products literature. http://www.pubs.rsc.org/marinlit/.
Masuda,M.,Abe,T.,Sato,S.,Suzuki,T.,Suzuki,M.,1997.Diversityofhalogenated secondarymetabolitesintheredalgaLaurencianipponica(Rhodomelaceae, Ceramiales).J.Phycol.33,196–208.
Mendes,G.,Soares,A.R.,Sigiliano,L.,Machado,F.,Kaiser,C.,Romeiro,N.,Gestinari, L.,Santos,N.,Romanos,M.T.V.,2011.Invitroanti-HMPVactivityof meroditer-penoidsfrom marinealgaStypopodiumzonale (Dictyotales).Molecules16, 8437–8450.
Menna,M.,Imperatore,C.,D’Aniello,F.,Aiello,A.,2013.Meroterpenesfrommarine invertebrates:structures,occurrence,andecologicalimplications.Mar.Drugs 11,1602–1643.
Mesguiche,V.,Valls,R.,Piovetti,L.,Banaigs,B.,1997.MeroditerpenesfromCystoseira
amentaceavar.StrictacollectedofftheMediterraneancoasts.Phytochemistry
45,1489–1494.
Mori,K.,Koga,Y.,1992.Synthesisandabsoluteconfigurationof(−)-stypoldione. Bioinorg.Med.Chem.Lett.2,391–394.
Nahas,R.,Abatis,D.,Anagnostopoulou,M.A.,Kefalas,P.,Vagias,C.,Roussis,V., 2007.Radical-scavengingactivityofAegeanseamarinealgae.FoodChem.102, 577–581.
Nielsen,N.P.V.,Carstensen,J.M.,Smedsgaard,J.,1998.Aligningofsingleand multi-plewavelengthchromatographicprofilesforchemometricdataanalysisusing correlationoptimizedwarping.J.Chromatogr.A805,17–35.
Paul,V.J.,VanAlstyne,K.L.,1988.Chemicaldefenseandchemicalvariationinsome tropicalPacificspeciesofHalimeda(Chlorophyta,Halimedaceae).CoralReefs6, 263–270.
Pereira,D.M.,Cheel,J.,Areche,C.,San-Martin,A.,Rovirosa,J.,Silva,L.R.,Valentao, P.,Andrade,P.B.,2011.Anti-proliferativeactivityofmeroditerpenoidsisolated fromthebrownalgaStypopodiumflabelliformeagainstseveralcancercelllines. Mar.Drugs9,852–862.
Pereira,R.C.,Soares,A.R.,Teixeira,V.L.,Villac¸a,R.,daGama,B.A.P.,2004.Bot.Mar. 47,202–208.
Sabry, O.M.M., Andrews, S., McPhail, K.L., Goeger, D.E., Yokochi, A., LePage, K.T.,Murray,T.F.,Gerwick, W.H.,2005. Neurotoxicmeroditerpenoidsfrom thetropicalmarinebrownalgaStypopodiumflabelliforme. J.Nat.Prod.68, 1022–1030.
Soares,A.R.,Abrantes,J.L.,Souza,T.M.L.,Fontes,C.F.L.,Pereira,R.C.,Frugulhetti, I.C.D.P.,Teixeira,V.L.,2007.Invitroantiviraleffectofmeroditerpenesisolated fromtheBrazilianseaweedStypopodiumzonale(Dictyotales).PlantaMed.73, 1221–1224.
Soares,A.R.,Teixeira,V.L.,Pereira,R.C.,Villac¸a,R.C.,2003.Variationonditerpene productionbytheBrazilianalgaStypopodiumzonale(Dictyotales,Phaeophyta). Biochem.Syst.Ecol.31,1347–1350.
Sudatti, D.B., Fujii, M.T.,Rodrigues, S.V., Turra, A., Pereira,R.C., 2011. Effects of abiotic factors on growth and chemical defenses in cultivated clones
ofLaurenciadendroideaJ.Agardh(Ceramiales,Rhodophyta).Mar.Biol.158,
1439–1446.
Vallim,A.,DePaula,J.C.,Pereira,R.C.,Teixeira,V.L.,2005.Thediterpenesfrom Dicty-otaceaenmarinebrownalgaeinthetropicalAtlanticAmericanregion.Biochem. Syst.Ecol.33,1–16.
Valls,R.,Piovetti,L.,Praud,A.,1993.Theuseofditerpenoidsaschemotaxonomic markersinthegenusCystoseira.Hydrobiologia260–261,549–556.
Vergés,A.,Paul,N.A.,Steinberg,P.D.,2008.Sexandlife-historystagealterherbivore responsestoachemicallydefendedredalga.Ecology89(5),1334–1343. Wehrens,R.,2011.ChemometricswithR–MultivariateDataAnalysisintheNatural
SciencesandLifeSciences.Spring-Verlag,Berlin,Heidelberg.
Wessels,M.,König,G.M.,Wright,A.D.,1999.Anewtyrosinekinaseinhibitorfrom themarinebrownalgaStypopodiumzonale.J.Nat.Prod.62,927–930. White,S.J.,Jacobs,R.S.,1983.Effectsofstypoldioneoncellcycleprogression,DNA
andproteinsynthesis,andcelldivisioninculturedseaurchinembryos.Mol. Pharmacol.24,500–508.
Wright, J.T., De Nys, R., Poore, A.G.B., 2004. Chemical defense in a marine alga:heritabilityandthepotentialforselectionbyherbivores.Ecology 58, 2946–2959.