No mullet, no gain: cooperation between dolphins and cast net fishermen
in southern Brazil
Mauricio L. Santos
1, Valéria M. Lemos
1, João P. Vieira
11Laboratório de Ictiologia, Instituto de Oceanografia, Universidade Federal do Rio Grande. Avenida Itália km 8,
96203-900 Rio Grande, RS, Brazil.
Corresponding author: Mauricio L. Santos (mlang.oceano@gmail.com)
http://zoobank.org/80CE3581-5D36-4575-A911-513924AD987B
ABSTRACT. We report on the interaction between common bottlenose dolphins, Tursiops truncatus (Montagu, 1821) and cast net fishermen in southern coast of Brazil. The fishery was monitored in the mouth of the Tramandaí River Estuary to investigate the seasonality of catches and their relationships with a set of variables: presence/absence and number of bottlenose dolphins, fishing area, temperature, salinity, wind and water flow direction in the channel. The mullet, Mugil liza Valenciennes, 1836 is the target species and was the dominant fish in the catches (77% of total catch; 50% in frequency; 0.2 ind. x f-1). The use of GLM models helped to reveal that the number of the bottlenose dolphins, time of year (months) and spatial variation of fishing activity were the main factors explaining the presence and abundance of mullet in the fishermen’s catches. The presences of bottlenose dolphins in the fishing area raise the probability of fishermen catch larger number of mullets with smaller fishing effort. However, the size of the mullet is influenced basically by seasonality. The mullets are the “currency” of bottlenose dolphins and fishermen interaction. There are reasons for concern about the sustainability of the southern Brazilian M. liza stock, once the decrease of this fishing resource can lead this rare and traditional fishery to the extinction. KEY WORDS. Fishing activity, generalized linear models, Mugil liza, seasonality, Tramandaí River Estuary, Tursiops truncatus.
INTRODUCTION
In many fisheries, fishers look for signs of animals, such as birds or mammals, in order to find fish schools (Chilvers and Corkeron 2001, Clua and Grosvalet 2001). However, the interaction between cetaceans and artisanal fishermen is a rare phenomenon described in few places, including parts of Australia, Mauritania, Myanmar, India and Brazil (Fairholme 1856, Busnel 1973, Pryor et al. 1990, Smith et al. 2009, Kumar et al. 2012). Certain species of Delphinidae, such as Orcaella brevirostris (Owen in Gray, 1866), Tursiops truncatus (Montagu, 1821) and Sousa
chi-mensis (Osbeck, 1765) are known to aid fishermen in their catches
(Simões-Lopes 1991, Kumar et al. 2012, D’Lima et al. 2014). The interactive fisheries with dolphins can be performed from land or by boat, using passive fishing gear (gill nets and traps) or active fishing gear (cast nets, hand nets and spears) (Fairholme 1856, Busnel 1973, Peterson et al. 2008, D’Lima et al. 2014). Generally, the interaction occurs in two different ways: initiated by the fishermen (Busnel 1973, Fairholme 1856) or initiated by the cetaceans (Simões-Lopes et al. 1998). In the land-based fishery the interaction typically takes the form when
cetaceans driving shoals of fish towards shallower waters as a food capture strategy. Usually occurs near shorelines adjacent to the mouth of estuaries, where fishermen are waiting for the arrival of the shoals to catch fish. Fish of the family Mugilidae, which generally use estuaries as nursery and feeding grounds be-fore eventually migrating to marine environments for spawning (Vieira and Scalabrin 1991, Whitfield et al. 2012, Lemos et al. 2014), are the most common target species of these interactive fisheries (Fairholme 1856, Busnel 1973, Pryor et al. 1990, Kumar et al. 2012, D’Lima et al. 2014).
In Brazil, the interaction between the common bottlenose dolphins, T. truncatus, and cast net fishermen, called ‘tarrafeiros’, is restricted to the southern coast (Simões-Lopes 1991) and has been observed and described in the estuarine systems of Laguna (28°S; 48°W) and Tramandaí River Estuary (TRE) (29°S; 50°W) (Pryor et al. 1990, Simões-Lopes 1991, Simões-Lopes et al. 1998, Zappes et al. 2011). In these regions the T. truncatus interact with tarrafeiros to catch the mullet Mugil liza Valenciennes, 1836. This interaction begins when the bottlenose dolphins herd shoals of mullet to shallower areas of the estuarine banks (Pryor et al. 1990, Simões-Lopes 1991, Simões-Lopes et al. 1998).
RESEARCH ARTICLE
Fishermen stand side-by-side along the banks, waiting for the dolphin signals to cast their nets toward the school. The theory of interaction is that the tarrafeiros increase their catch and cap-ture larger individuals by casting their nets over mullet schools herded by bottlenose dolphins, whereas the bottlenose dolphins are able to catch mullets more easily because the throwing nets disrupts the schools (Simões-Lopes et al. 1998). This interspecific relationship can be considered cooperation (Pryor et al. 1990, Simões-lopes 1991, Simões-Lopes et al. 1998, Simões-Lopes et al. 2016), being called “cooperative fishery”.
Acquiring fishery data (e.g., fishing effort, catch rates, species size distribution) during an extensive period, especially in inaccessible fishing grounds, with unpredictable spatial and temporal dynamics is not an easy task (Berkes et al. 2001, Cetra and Petrere 2014, Oviedo and Bursztyn 2017). It is expected that the assessment of artisanal cooperative fishery between the common bottlenose dolphins and tarrafeiros at TRE to be even more complex. There can be, at least four groups of variables that interact with each other and oscillate in time and space: fisher-men, bottlenose dolphins, mullet and environmental conditions. The few fishery studies available at TRE did not cover a one-year period and have focused on the interaction between bottlenose dolphins and fishermen per se, particularly the behav-ior of the bottlenose dolphins and their relationship with the fisherman (Simões-Lopes 1991, Simões-Lopes et al. 1998, Zappes et al. 2011, Simões-Lopes et al. 2016). The temporal and quan-titative assessment of catches and interspecific relationships of cooperative fishery with environmental factors are fundamental to support sustainable management proposals that ensure the maintenance of this rare artisanal fishery. In this context, our goal therefore was to study the cooperative fishery at TRE over a one-year period to assess the relative importance of M. liza in
the cast net fishery and to understand the degree of dependence between tarrafeiros and bottlenose dolphins under the influence of environmental conditions.
MATERIAL AND METHODS
The Tramandaí River Estuary (TRE) (30-km2) is located in southern Brazil (29°58’33.93”S; 50°7’16.78”W) and is con-nected to the Atlantic Ocean by a permanent channel (1.5 km long and 100 m wide) (Würdig 1988). The main tributary is the Tramandaí River, which has a drainage basin of approximately 2500 km2. The average salinity in the estuary varies from 0 to 11 and exhibits daily variation (Chomenko and Schäfer 1984, Kapusta et al. 2006). Water temperature varies seasonally, with highest and lowest average values occurring in austral summer (29 °C) and winter (16 °C), respectively (Kapusta et al. 2006). The semi-diurnal astronomical tide (mean amplitude of 0.25 m) plays a secondary role to meteorological tides (Villwock and Tomazelli 1995, Tabajara and Dillenburg 1997), which may reach as high as 2 m but usually average 1.20 m (Toldo et al. 2006, Guimarães et al. 2015). The estuarine system is shallow (1.0–1.4 m depth), except the main channel (2.5–5 m depth) (Tabajara and Dillenburg 1997).
Sampling was conducted in an area of approximately 700 m in length located on the south bank of the mouth of the TRE, which was sectioned into five adjacent fishing sectors (i.e., sam-pling sectors) of similar sizes: S1 (29°58’38.22”S, 50°7’10.27”W), S2 (29°58’36.30”S; 50°7’15.97”W), S3 (29°58’39.86”S; 50°7’20.67”W), S4 (29°58’42.84”S; 50°7’24.21”W) and S5 (29°58’46.30”S; 50°7’29.04”W) (Fig. 1).
Fishermen activities were observed weekly between June 2014 and May 2015. Each observation period had an average
duration of three hours and was always conducted during day-light from 8:00 to 11:00 a.m. Each sample comprised 15 minutes of observation without interruption, for a total of 12 samples per day. The following variables were recorded during each sampling period: the fishing sector (S1, S2, S3, S4 or S5) where the fishermen were operating (Fig. 1); the number of active fishermen; the total number of cast nets thrown by the group of active fishermen; the total number of fish caught; the number of bottlenose dolphins present in the fishing sector sampled; and the wind and water flow directions (ebb, flood, slack water) in the entrance channel. Water temperature and salinity were mea-sured at the end of each sampling period using a thermometer and an optical refractometer, respectively.
The fish caught by the fishermen in each sample were counted and identified at the minor taxonomic rank possible
in situ or in laboratory according with Figueiredo and Menezes
(1978, 1980, 2000), Menezes and Figueiredo (1980, 1985), Fischer et al. (2004). The total length (LT) of individual fish caught was measured in millimeters (mm).
Catch per unit effort (CPUE) is defined as the ratio between the total number of captured individuals (ind.) of a species and the fishing effort (f): CPUE = ind. × f-1. The CPUE was used as a relative abundance index. Fishing effort (f) was defined as the to-tal number of cast nets thrown by the toto-tal number of fishermen per hour: f = [(number of cast nets thrown × number of fishermen in the sample-1) × 1 h-1]. Species frequency of occurrence (%FO) was expressed as the ratio between the occurrence of each fish species and the total number of samples. Both CPUE and %FO were calculated for each austral season (summer = January to March; autumn = April to June; winter = July to September; spring = October to December).
Species were classified as abundant and frequent (CPUE ≥ µ CPUE, %FO ≥ µ %FO), abundant and infrequent (CPUE ≥ µ CPUE, %FO <µ %FO), frequent and not abundant (CPUE <µ CPUE, %FO ≥ µ%FO), or not abundant and infrequent (CPUE <µ CPUE, %FO <µ %FO), where µ denotes the average CPUE or FO for each species during each season. Species classified as abundant and frequent were considered dominant in each sea-son (Artioli et al. 2009, Rodrigues et al. 2014, Ceni et al. 2016).
Generalized linear models (GLM) were used to investigate the relationship between mullet catch and a set of predictor variables, including month, season, fishing sector, number of bottlenose dolphins in the sampled sector, temperature, salinity, wind and water flow direction in the channel. Predictive vari-ables were tested for collinearity using the Spearman coefficient prior to model formulation (Beger and Possingham 2008). Thus, when collinearity was detected between two variables, the variable interpreted to have greater ecological importance was maintained in the analysis. Only season and month were highly correlated, and month was selected to increase the accuracy of the models (Table 1). In addition, the interactions between predictive variables were tested. The seasonal variation of the predictive variables is available in Figs 2–7.
The high frequency of zeros in the data matrix (> 40%) required the construction of two models (McCullagh and Nelder 1989, Courrat et al. 2009). The first model was a coupled sub-model testing for the presence or absence of mullet in the samples and used the binomial family with the “logit” link function (McCullagh and Nelder 1989). The second sub-model was used to test the positive abundance of mullet catches, for which a gamma distribution model with the “log” link function was employed (Myers and Pepin 1990).
To choose the best model, we followed the “backward stepwise” procedure by selecting the template that had the lowest Akaike Information Criterion (AIC) value (Burnham and Anderson 2002). In addition, a drop1 function in software “R” was used to compare the full model with a model in which the interaction was dropped using a chi-square test (Zuur et al. 2007). All models were tested for overdispersion, and the final model was fitted only with significant predictor (p < 0.05) (Rodrigues et al. 2014). The percentage of the total deviance explained, and the relative contribution of each predictor were independently verified for each model (binomial and gamma) (França et al. 2011, Rodrigues et al. 2014).
CPUE of each season, for 10-mm length class (CPUE-LC%) of mullet individuals was calculated following the formula: y = ni
×Ni −1×f i
−1, where (y) is the CPUE-LC%, (n) is the total number
of individuals caught on each sample (i), (N) is the total number
Table 1. Spearman (roh) correlations among variables.
Month Fishing sector Season Temperature Salinity Wind Regime channel Number of
dolphins
Month 1
Fishing sector -0.02 1
Season 0.94* -0.12* 1
Temperature -0.49* -0.17* -0.39* 1
Salinity 0.29* -0.08 0.40* -0.25* 1
Wind 0.03 -0.06 0.09 -0.04 0.03 1
Regime channel -0.23* -0.01 -0.22* -0.01 -0.30* -0.19* 1
Number of dolphins -0.09 0.41* -0.08 -0.02 0.06 -0.01 0.01 1
Figures 2–7. Seasonal variation of the predictive variables: (2) Mean and standard deviation of water temperature, min. 15 °C and max. 27 °C; (3) Mean and standard deviation of salinity, min. 1 PSU and max. 36 PSU; (4) Mean and standard deviation of number of dolphins, min. 0 and max. 5; (5) Frequency of occurrence% of water flow direction (ebb, flood, slack water) in the entrance channel; (6) Frequency of occurrence% of wind direction (Northerly, Southerly, Easterly); (7) Frequency of occurrence% of the fishing sector (S1, S2, S3, S4 or S5).
of measured individuals and (f) is the fishing effort (Garcia et al. 2001, Vieira 2006, Ceni et al. 2016).
The factorial ANOVA was used to test the influence of season and the presence and absence of bottlenose dolphins on the dependent variables of fishing effort and total length of
M. liza. Post-hoc significant differences were evaluated using
Tukey’s test (Zar 1984).
Statistical analyzes were performed with R software (R De-velopment Core Team 2015). We used 95% confidence intervals and a significance level of p < 0.05 in all analyses.
RESULTS
From June 2014 to May 2015, based on a total of 443 samples, 815 fish of 16 species were identified. The number of species varied seasonally. The species richness (S) was highest in autumn (S = 12) and lowest in winter (S = 3). The seasonal vari-ations of Total CPUE (spring = 0.40 ind × f-1; summer = 0.11 ind
× f-1) were not significant (ANOVA, F = 0.59; P > 0.05) (Table 2).
Mugilliza composed 77% of the total fish caught and was
the only species classified as dominant. Mullet abundance was highest in autumn (0.25 ind × f-1) and lowest in summer (0.09 ind
× f-1), but no statistically significant differences among seasons were detected (Table 2; ANOVA, F = 0.83; P > 0.05). The remaining species were occasional (not abundant and infrequent), except for Argentine menhaden Brevoortia pectinata (Jenyns, 1842), which was abundant but not frequent in autumn and spring (Table 2).
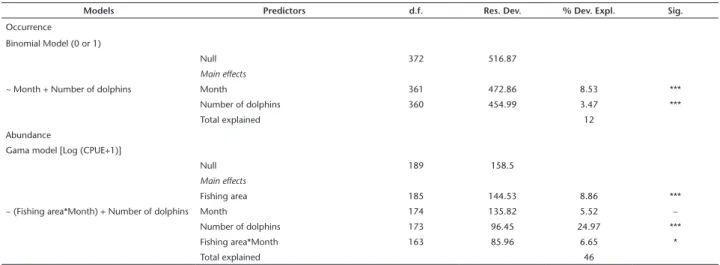
The best GLM model accounting for the presence or absence of mullet in the catches explained 12% of the total de-viance (Table 3). The model consisted of two predictors: month, which contributed 8.5% of the deviance, and the number of bottlenose dolphins, which contributed 3.5%. The probability of catching mullet was highest in April, May and Jun (autumn) and October, November and December (spring) (Fig. 9) and con-sistent with the increase in the numbers of bottlenose dolphins in the fishing sectors (Fig. 8).
The best model for explaining M. liza abundance (CPUE) consisted of three predictors that were responsible for 46% of the total explained deviance: fishing sector, month and number of bottlenose dolphins (Table 3). The single predictor with the highest level of explicability was number of bottlenose dolphins (24.9%). The interaction between fishing sector and month was significant, with the two variables accounting for 8.8% and 5.5% of the total explained deviance, respectively (Table 3). The expect-ed abundance of mullet increasexpect-ed with the number of bottlenose dolphins (Fig. 10) and can be associated with the displacement of fishermen toward the inner portion of the sampling area (Fig. 12). The GLM modeling did not reveal monthly significant variations in the expected abundances of the mullet (Fig. 11).
Table 2. List of species caught by cast net fishermen in cooperation with dolphins at the entrance channel of the ESTR, along with fre-quency of occurrence (%FO) and number of individuals per unit of effort (CPUE). Species were classified as: abundant and frequent (++), abundant and infrequent (+–), frequent and not abundant (–+), infrequent and not abundant (absence of the signals) and absent (–).
Species Summer Autumn Winter Spring
Frequency of Occurrence
CPUE Frequency of Occurrence
CPUE Frequency of Occurrence
CPUE Frequency of Occurrence
CPUE
FO% (Ind × f-1) FO% (Ind × f-1) FO% (Ind × f-1) FO% (Ind × f-1)
Mugil liza 38.33++ 0.093++ 52.81++ 0.25++ 48.00++ 0.24++ 62.71++ 0.19++
Brevoortia pectinata 3.33 0.002 1.12 0.05+– – – 6.78 0.1 8+–
Genidens sp. – – 1.12 0.001 1.33 0.0002 5.08 0.01
Eucinostomus melanopterus 1.67 0.005 1.12 0.002 – – 3.39 0.003
Centropomus sp. 1.67 0.004 0.56 0.002 – – 3.39 0.002
Mugil curema 3.33 0.002 1.12 0.003 – – 3.39 0.003
Paralichthys orbignyanus 3.33 0.001 0.56 0.001 – – 3.39 0.004
Caranx latus 1.67 0.002 – – – – – –
Selene vomer 1.67 0.001 – – – – – –
Acestrorhynchus pantaneiro – – – – 1.33 0.001 – –
Menticirrhus americanus – – – – – – 1.69 0.001
Elops saurus – – 0.56 0.001 – – – –
Trachinotus marginatus – – 0.56 0.001 – – – –
Menticirrhus littoralis – – 0.56 0.001 – – – –
Trachinotus carolinus – – 0.56 0.001 – – – –
Pomatomus saltatrix – – 0.56 0.0004 – – – –
Total 0.11 0.31 0.24 0.40
Number of species 8 12 3 8
Table 3. Analyses of deviances for the generalized linear model that best explains the occurrence (binomial model) and abundance (gamma model) of mullets. Degrees of freedom (d.f.); residual deviance (Res. Dev); deviance explained in percentage (%Dev. Expl); significance (Sig.).
Models Predictors d.f. Res. Dev. % Dev. Expl. Sig.
Occurrence Binomial Model (0 or 1)
~ Month + Number of dolphins
Null 372 516.87
Main effects
Month 361 472.86 8.53 ***
Number of dolphins 360 454.99 3.47 ***
Total explained 12
Abundance
Gama model [Log (CPUE+1)]
~ (Fishing area*Month) + Number of dolphins
Null 189 158.5
Main effects
Fishing area 185 144.53 8.86 ***
Month 174 135.82 5.52 –
Number of dolphins 173 96.45 24.97 ***
Fishing area*Month 163 85.96 6.65 *
Total explained 46
(*) p < 0.05; (**) p < 0.01; (***) p < 0.001; (–) p > 0.05.
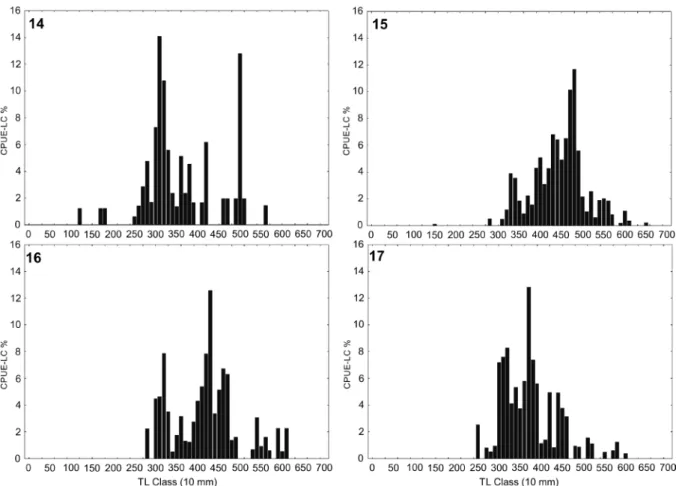
Mullets ranged in size from 121 to 655 mm (TL) (Figs 14–17) and exhibited statistically significant seasonal differ-ences in size distribution (Table 5). Larger individuals were captured in autumn (mean = 444 ± 72 mm TL) and winter (mean = 423 ± 73 mm TL) and smaller in summer (mean = 348 ± 94 mm TL) and spring (mean = 381 ± 67 mm TL) (Figs 14–17).
Figures 8–9. Expected occurrence of Mugil liza for predictors of the binomial model. Number of dolphins (8) and month (9); the black
lines indicate the moving averages; dark gray areas represent the 95% confidence intervals of the predictions.
Figures 10–12. Expected abundance of Mugil liza for predictors of the gamma model. Number of dolphins (10), month (11) and fishing area (12); the black line indicates the moving average; dark gray areas represent the 95% confidence intervals of the predictions.
Figure 13. Seasonal variation in fishing effort. Means and standard deviations of the effort in the presence or absence of dolphins.
Table 4. Seasonal comparisons of fishing effort and relationships with the presence or absence of dolphins. Factorial ANOVA results indicate sum of squares (SSQ), degrees of freedom (d.f.), average square (MS), F value and Tukey’s test.
Factorial Anova (Fishing Effort) Tukey Test Effect SSQ d.f. MS F p Comparisons p
Dolphins 188.5 1 188.5 177.552 *** Summer (Presence) x Summer (Absence) ***
Season 5.4 3 1.8 1.698 – Autumn (Presence) x Autumn (Absence) ***
Season *
Dolphins 7.6 3 2.54 2.389 – Winter (Presence) x Winter (Absence) ***
Residuals 386.5 364 1.06 Spring (Presence) x Spring (Absence) –
Figures 14–17. Catch per unit effort (CPUE-LC%) by body size classes (10 mm) of the mullet. (14) summer, (15) autumn, (16) winter and (17) spring.
Figure 18. Seasonal variation in total length (mean and standard de-viation) of Mugil liza caught in the presence or absence of dolphins.
Table 5. Seasonal comparison of body size (total length, mm) of mullets with the presence or absence of dolphins. Factorial ANOVA results indicate sum of squares (SSQ), degrees of freedom (d.f.), average square (MS), F value and Tukey’s test.
Factorial Anova (Total Lenght) Tukey Test
Effect SSQ d.f. MS F p Comparisons p
Season 541669 3 180556 35.642 *** Summer (Presence) x Summer (Absence) ***
Dolphins 14041 1 14041 2.772 – Autumn (Presence) x Autumn (Absence) – Season*
Dolphins 141467 3 47156 9.309 *** Winter (Presence) x Winter (Absence) –
Residuals 2502548 494 5066 Spring (Presence) x Spring (Absence) –
DISCUSSION
Cast net is a traditional fishing method that has been used since the Neolithic Age (Edo 2007). Although Taylor and Gerking (1978) suggested that cast net have low efficiency, artisanal fish-ermen adapted the technique to improve capture effectiveness (Berkes et al. 2001). In southern Brazil, the primary target species of cast net fishermen are mullet, silversides (Atherinidae) and shrimp (Harayashiki et al. 2011). More than 70% of the fish caught by cast net fishermen (tarrafeiros) in the mouth of the Tramandaí River Estuary (TRE) are M. liza, suggesting that this fishing method is highly selective for this species in this region.
Mugil liza is an important traditional fishery resource for artisanal
fishermen in the southern states of Brazil and is considered of high economic, cultural and social relevance (Diegues 2004, Klippel et al. 2005, Peres et al. 2007, Kalikoski and Vasconcellos 2013). Approximately 40 tarrafeiros are officially licensed to fish in the TRE (Zappes et al. 2011). Mullet is the main target species, and source of income and food security for this group of fishermen, locally.
According to Zappes et al. (2011), tarrafeiros in Tramandaí city believe that the presence of bottlenose dolphins increases mullet capture efficiency by reducing fishing effort and increas-ing total catch. Simões-Lopes et al. (1998) reported a positive relationship between fishing efficiency and the presence of bottlenose dolphins, although their study was based on a limited three-month sampling period. Our results carried out over a one-year period and considering a wide set of variables (wind, water temperature, salinity, channel regime and spatial distribution of tarrafeiros) are consistent with these preliminary observations. Moreover, our extended and standardized obser-vations allowed us to statistically demonstrate that the presence of bottlenose dolphins reduces fishing effort and increases the capture probabilities of mullet.
According to Simões-Lopes et al. (1998) the tarrafeiros wait for signals from the bottlenose dolphins, which usually consist of “head slaps” (when a dolphin raises its head out of the water and slaps the surface with its throat), indicating the appropriate time to throw the cast nets and the location of the mullet shoals, an interaction that seems to increase fish capture efficiency. We also observed that in the absence of bottlenose dolphins fishermen tend to randomly throw their nets, targeting no specific location in the water, thereby significantly reducing fish capture efficiency and, consequently, increasing fishing effort.
Bottlenose dolphins use the estuaries for feeding, shelter-ing and restshelter-ing area (Irvine et al. 1981, Hanson and Defran 1993, Simões-Lopes and Fabian 1999). The presence of bottlenose dolphins at TRE does not necessarily indicate that they are in-teracting with the fishermen. However, our results demonstrated that the presence of bottlenose dolphins change the fishing strategy of the tarrafeiros. We can assume that the presence and the number of bottlenose dolphins in a given sector may be related to the availability of mullets. The fishermen look at the
bottlenose dolphins and run to the sector where they are and, consequently, increase mullet catch probabilities. Therefore, we cannot consider that only the interactions between fishermen and bottlenose dolphins generate effects on fishing. The simple fact of the presence of the bottlenose dolphins in the TRE posi-tively influences the fishing behavior of the tarrafeiros.
Only in spring the fishing effort was not influenced by the presence/absence of bottlenose dolphins, which may be due to the reduction in the occurrence of bottlenose dolphins in the sampling area (Fig. 4). The highest number of samples in the absence of bottlenose dolphins was recorded in spring, although there was a high frequency of occurrence of mullet in the catches (%FO > 62). Tursiops truncatus is an opportunistic predator, and their occurrence in coastal areas and estuaries varies seasonally, typically associated with the availability of food resources (Defran et al. 1999, Hanson and Defran 1993, Irvine et al. 1981, Shane et al. 1986). Simões-Lopes and Fabian (1999) demonstrated that the residence pattern of T. truncatus
in the Laguna estuary (about 250 km north of the TRE) varies seasonally, with fewer bottlenose dolphins observed in spring/ summer than in autumn/winter. The mullet M. liza inhabits the estuaries of southern Brazil throughout the year (Lemos et al. 2014) and cast net fishermen of the TRE catch mullet all year round regardless of the presence of bottlenose dolphins. Therefore, although the presence of bottlenose dolphins in-creases mullet capture efficiency by tarrafeiros, these fishermen are also able to catch mullet when bottlenose dolphins are less frequent or absent.
Our results reveal that the body size distribution of mul-lets caught varies significantly with season in the study area. In the coastal regions of southern Brazil, the adult mullets move in large schools from the estuaries to the marine environment during the reproductive migration in austral autumn and winter (Vieira and Scalabrin 1991, Vieira et al. 2008, Lemos et al. 2014). Most M. liza individuals caught in autumn and winter in the TRE are adults, and larger than the average total length of first maturity (Lm = 408-mm; Lemos et al. 2014). Also, many of the females caught showed well-developed ovaries. The average size of mullets caught in autumn/winter was larger than in spring and summer.
are present. This can be explained by the fact that after the reproductive period (autumn/winter) adult individuals of M. liza move from the marine environment to the estuaries and coastal region of southern Brazil in small and sporadic schools, mixing with juvenile individuals (Herbst and Hanazaki 2014, Lemos et al. 2014). In this work, the size analysis of individuals caught in summer showed a predominance of juveniles and the occurrence of few adults, only. We believe that this mixture of cohorts associated with the opportunistic behavior of T. truncatus
and the tarrafeiros is the explanation for this result. Therefore, fishermen and bottlenose dolphins capture mullets that are available in the environment, where their sizes vary seasonally according to the life cycle of M. liza.
Several authors demonstrate the importance of water temperature variation and salinity in the reproductive/migratory cycle of M. liza (Vieira and Scalabrin 1991, Vieira 1991, Vieira et al. 2008, Lemos et al. 2014, 2016). According to them, the decrease in water temperature and the increase in salinity in the estuaries, associated with the southern wind that promote the intrusion of marine water in estuaries during the austral autumn act as “triggers” for sexually mature individuals of M. liza runs to the ocean. In this reproductive migration period, called the ‘corrida da tainha’, artisanal fishermen intensify fishing efforts throughout the southern Brazilian coast, raising the quantities of mullets caught within the estuaries (Vieira et al. 2008, Herbst and Hanazaki 2014, Lemos et al. 2016, Sant’Ana et al. 2017). Nevertheless, water temperature and salinity did not have repre-sentative effects on the explicability of the models of occurrence and abundance of mullet in the cast net fishery at TRE.
The interactions between mullets, bottlenose dolphins, fishermen and environmental variables are complex, and diffi-cult to distinguish the possible cause-and-effect relationships. The use of GLM models helped to reveal that the time of year (months) followed by the number of bottlenose dolphins were the main factors explaining the presence of mullet in the fish-ermen’s catches. The number of bottlenose dolphins and the spatial distribution of fishermen along the banks of the entrance channel of TRE together explained more than 30% of mullet CPUE. Fishermen follow the movement of bottlenose dolphins over the channel, and thus the fishing sector used appears to vary seasonally: during autumn and winter the fishing occurs in the interior of the channel in the estuary, whereas during spring and summer the fishing occurs primarily near the adjacent coastal zone. Therefore, the higher the number of bottlenose dolphins in a specific fishing sector, the higher the probability that tarrafeiros will catch mullet in higher quantities.
No significant seasonal variations of mullet abundance were observed. This is unusual because it is known that in periods of reproductive aggregation of M. liza the catches of artisanal fisheries increased notably (Vieira et al. 2008, Herbst and Hanazaki 2014, Lemos et al. 2014). This may be associated with the way cast nets operate and the fishing dynamics. Unlike gillnet fishing, for example, where large sets of nets remain
an-chored for 24 hours away from the coast in estuarine channels (Kalikoski and Vasconcellos 2013), the tarrafeiros remain in the sand with limited throwing distance and often performed blindly. Thus, the mullets available for the tarrafeiros are in the shallow areas, while the dense shoals move in deeper and inaccessible areas of the channel. The importance of bottlenose dolphins (demonstrated in the models) is precisely to make mullets accessible for fishermen, bringing the fish from deeper regions to shallow areas, increasing the chances of catching them (Simões-Lopes et al.1998). This behavior is pronounced during the autumn, when the concentration of mullets in the estuary increases. On the other hand, when the abundance of mullet is reduced in the estuary (spring and summer) tarrafeiros direct their efforts to the estuarine mouth near the ocean (sector S1 and S2; Figs 1–7), usually without the presence of the bottlenose dolphins. In this situation mullet schools are a mixture of large adults, returning from marine reproductive areas, and mid-size juveniles that use the surf zone as transient nursery area (Vieira and Scalabrin 1991, Lemos et al. 2014). Therefore, catches and fishing effort tend to remain constant throughout the year.
Historically, estuaries around the world are being degraded by anthropic activities, resulting in the depletion of aquatic life (Lotze et al. 2006). The TRE is located between two vocational towns, influenced by tourism, real estate business and oil in-dustry, challenging the resilience of the system. Local artisanal fishermen report that environment protection of the estuary is neglected and should be one of the main causes of mullet abun-dance reduction and low frequency of bottlenose dolphins in the region. Zappes et al. (2011) reported on the apprehensiveness of tarrafeiros and suggested that the main factors that hamper the cooperative fishing in TRE are: lack of vessel traffic control, the presence of illegal (unregulated) fishermen and the growing of industrial fishing activity in southern Brazil.
According to Daura-Jorge et al. (2012) social connections between T. truncatus individuals facilitate the maintenance of cooperative behavior with fishermen through social learning and may be interpreted as a “cultural process”. In southern Brazil, fishing for mullet is a cultural tradition, also. Both bottlenose dolphins and fishermen visit coastal and estuarine environments because those are the preferential habitats of their common prey. The mutual benefit of this cooperation is catching mullet, but the implications of mullet population dynamics and fishery exploitation for this complex human-dolphin relationship have been neglected in prior studies.
Mugil liza migration period overlaps with the industrial
the species (MPA/MMA 2014) is still not effective. According to Lemos et al. 2016, it is imperative to manage the mullet resource at adequate levels to prevent the stock collapse. There is strong scientific evidence that the southern population of M. liza (Mai et al. 2014) is overfished, particularly due to fishing pressure during the reproductive migration period (Gonzáles-Castro et al. 2015, Lemos et al. 2014, Sant’Ana et al. 2017). Increasing concern about the viability of the southern population of M. liza has led Brazilian authorities to enact stricter regulations on fishing, but these measures have been largely ineffective, systematically transgressed and, apparently, inadequate for ensuring the maintenance of mullet population abundance, as showed by the results of the latest published stock assessment of this species (Sant’Ana et al. 2017). There is, therefore, a clear need for better management to safeguard the sustainability of the southern population of M. liza.
Although the coastal population of T. truncatus is not endangered (Hammond et al. 2012) their conservation is rele-vant to tarrafeiros at TRE. The cast net fishermen in this estuary should also be protected. The artisanal fishermen suffer from economic exclusion, social marginalization, class exploitation, political disempowerment, environmental change, ecological marginalization, loss of identity, and disconnection from resources and from other fishermen (Nayak et al. 2014). There-fore, the management measures for the population of M. liza
in southern Brazil is fundamental to ensure the maintenance of the basis of the rare and beneficial interaction between humans and bottlenose dolphins.
ACKNOWLEDGMENTS
We thank the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) and Conselho Nacional de Desenvolvi-mento Científico e Tecnológico (CNPq) for financial support for the development work. This work is a contribution of Fundação de Amparo a Pesquisa do estado do Rio Grande do Sul (FAPERGS, process 2327-2551/14-6) and Brazilian Long Term Ecological Research Program (PELD; CNPq, process 403805/2012-0). Thanks to all of the cast net fishermen of the Tramandaí.
LITERATURE CITED
Artioli LGS, Vieira JP, Garcia AM, Bemvenuti MD (2009) Dis-tribuição, dominância e estrutura de tamanhos da assem-bléia de peixes da lagoa Mangueira, sul do Brasil. Iheringia, Série Zoologia 99: 409–418. https://doi.org/10.1590/S0073-47212009000400011
Beger M, Possingham HP (2008) Environmental factors that influ-ence the distribution of coral reef fishes: modeling occurrinflu-ence data for broad-scale conservation and management. Marine Ecology Progress Series 361: 1–13. https://doi.org/10.3354/ meps07481
Berkes F, Mahon R, McConney P, Pollnac R, Pomeroy R (2001) Managing small-scale fisheries alternative directions and methods. IDRC, Ottawa, 308 pp.
Burnham PK, Anderson DR (2002) Models Selection and Multi-Model Inference: practical information – theoretic ap-proach. Springer-Verlag, New York, 2nd ed., 488 pp.
Busnel RG (1973) Symbiotic relationship between man and dolphins. Transactions of the New York Academy of Scienc-es 35: 112–31. https://doi.org/10.1111/j.2164-0947.1973. tb01511.x
Ceni G, Fontoura NF, Cabral HN (2016) The freshwater artisanal fishery of Patos Lagoon. Journal of Fish Biology 89: 337–354. https://doi.org/10.1111/jfb.13004
Cetra M, Petrere M (2014) Seasonal and annual cycles in ma-rine small-scale fisheries (Ilhéus – Brazil). Fisheries Manage-ment and Ecology 21: 244–249. https://doi.org/10.1111/ fme.12070
Chilvers BL, Corkeron PJ (2001) Trawling and bottlenose dol-phins’ social structure. Proceedings of the Royal Society of London, Series B, Biological Sciences 268: 1901–905. https:// doi.org/10.1098/rspb.2001.1732
Chomenko L, Schäfer A (1984) Interpretação da distribuição do gênero Littoridina (Hydrobiidae) nas lagoas costeiras do Rio Grande do Sul, Brasil. Amazoniana 9: 127–146.
Clua É, Grosvalet F (2001) Mixed-species feeding aggregation of dolphins, large tunas and seabirds in the Azores. Aquatic Living Resources 14: 11–18. https://doi.org/10.1016/S0990-7440(00)01097-4
Courrat A, Lobry J, Nicolas D, Laffargue P, Amara R, Le Pape LM (2009) Anthropogenic disturbance on nursery func-tion of estuarine areas for marine species. Estuarine, Coastal and Shelf Science 81: 179–190. https://doi.org/10.1016/j. ecss.2008.10.017
D’Lima C, Marsh H, Hamann M, Sinha A, Arthur R (2014) Pos-itive interactions between Irrawaddy dolphins and artisanal fishers in the Chilika Lagoon of eastern India are driven by ecology, socioeconomics, and culture. Journal of the Human Environment 43: 614–624. https://doi.org/10.1007/s13280-013-0440-4
Daura-Jorge FG, Cantor M, Ingram SN, Lusseau D, Simões-Lopes PC (2012) The structure of a bottlenose dolphin society is coupled to a unique foraging cooperation with artisanal fish-ermen. Biology Letters 8: 702–705. https://doi.org/10.1098/ rsbl.2012.0174
Defran RH, Weller DW, Kelly DL, Espinosa MA (1999) Range char-acteristics of Pacific coast bottlenose dolphins (Tursiops trunca-tus) in the Southern California Bight. Marine Mammal Science 15: 381–393. https://doi.org/10.1111/j.1748-7692.1999. tb00808.x
Edo H (2007) Cast Nets. In: Johnson DH, Shrier BM, O’Neil JS, Knutzen JA, Augerot X, O’Neil TA, Pearsons TN (Eds) Salmo-nid field protocols handbook: techniques for assessing status and trends in salmon and trout populations. American Fisher-ies Society, Bethesda, 87–94.
Fairholme JKE (1856) The blacks of Morenton Bay and the por-poises. Proceedings of the Zoological Society 24: 353–354. Figueiredo JL, Menezes NA (1978) Manual de peixes marinhos
do sudeste do Brasil, II Teleostei (1). São Paulo, Universidade de São Paulo, Museu de Zoologia, 110 pp.
Figueiredo JL, Menezes NA (1980) Manual de peixes marinhos do sudeste do Brasil. II Teleostei (3). São Paulo, Universidade de São Paulo, Museu de Zoologia, 110 pp.
Figueiredo JL, Menezes NA (2000) Manual de peixes marinhos do sudeste do Brasil, VI Teleostei (5). São Paulo, Universidade de São Paulo, Museu de Zoologia, 110 pp.
Fischer LG, Pereira LED, Vieira JP (2004) Peixes estuarinos e costeiros. Rio Grande, Editora Ecoscientia, 127 pp.
França S, Costa MJ, Cabral HN (2011) Inter- and intra-estuarine fish assemblage variability patterns along the Portuguese coast. Estuarine, Coastal and Shelf Science 91: 262–271. https://doi.org/10.1016/j.ecss.2010.10.035
Garcia AM, Vieira JP, Winemiller KO (2001) Dynamics of the shal-low-water fish assemblage of the Patos Lagoon estuary (Brazil) during cold and warm ENSO episodes. Journal of Fish Biology 59: 1218–1238. https://doi.org/10.1111/j.1095-8649.2001. tb00187.x
Gonzáles-Castro M, Vieira JP, Peres MB, Albieri RJ, Mendonça JT, Miranda LV, Fadre NN, Padovani BF, Da Silva FMS, Rodrigues AMT, Chao L (2015) Mugil liza. The IUCN Red List of
Threat-ened Species 2015: e.T190409A1951047.
Guimarães PV, Farina L, Toldo EJ, Diaz-Hernandez G, Akhmatskaya E (2015) Numerical simulation of extreme wave run up during storm events in Tramandaí Beach, Rio Grande do Sul, Brazil. Coastal Engineering 95: 171–180. https://doi.org/10.1016/j. coastaleng.2014.10.008
Hammond PS, Bearzi G, Bjørge A, Forney KA, Karkzmarski L, Kasuya T, Perrin WF, Scott MD, Wang JY, Wells RS, Wilson B (2012) Tursiops truncatus. The IUCN Red List of Threatened Species 2012: e.T22563A17347397.
Hanson MT, Defran RH (1993) The behavior and feeding ecolo-gy of the Pacific coast bottlenose dolphin, Tursiops truncatus. Aquatic Mammals 19: 127–142.
Harayashiki C, Furlan FM, Vieira JP (2011) Perfil sócio-econômico dos pescadores da ponte dos franceses, Rio Grande, RS, Brasil. Boletim do Instituto de Pesca 37: 93–101.
Herbst DF, Hanazaki N (2014) Local ecological knowledge of fishers about the life cycle and temporal patterns in the mi-gration of mullet (Mugil liza) in Southern Brazil. Neotropical Ichthyology 12: 879–890. https://doi.org/10.1590/1982-0224-20130156
Irvine AB, Scott MD, Wells RS, Kaufman JH (1981) Movements and activities of the Atlantic Bottlenose dolphin, Tursiops trun-catus, near Sarasota, Florida. Fishery Bulletin 79: 671–688. Kalikoski DC, Vasconcellos M (2013) Case study of the technical,
socio-economic and environmental conditions of small-scale fish-eries in the estuary of Patos Lagoon, Brazil: a methodology for as-sessment. FAO, Rome, Fisheries and Aquaculture Circular, #1075. Kapusta SC, Wurdig NL, Bemvenuti CE, Pinto TK (2006) Spatial
and temporal distribution of Nematoda in a subtropical estu-ary. Acta Limnologica Brasiliensia 18: 133–144.
Klippel S, Peres MB, Vooren CM, Lamónaca AF (2005) A pesca artesanal na costa da Plataforma Sul. In: Vooren CM, Klippel S (Eds) Ações para a conservação de tubarões e raias no sul do Brasil. Igaré, Brasil, 179–197.
Kumar AB, Smrithy R, Sathasivam K (2012) Dolphin-assisted cast net fishery in the Ashtamudi Estuary, south-west coast of In-dia. Indian Journal of Fisheries 59: 143–148.
Lemos VM, Troca DFA, Castello JP, Vieira JP (2016) Tracking the southern Brazilian schools of Mugil liza during reproductive migration using VMS of purse seiners. Latin American Journal of Aquatic Research 44: 238–246. https://doi.org/10.3856/ vol44-issue2-fulltext-5
Lemos VM, Varela Jr AS, Schwingel PR, Muelbert JH, Vieira JP (2014) Migration and reproductive biology of Mugil liza (Te-leostei: Mugilidae) in south Brazil. Journal of Fish Biology 85: 671–687. https://doi.org/10.1111/jfb.12452
Lotze HK, Lenihan HS, Bourque BJ, Bradbury RH, Cooke RG, Kay MC, Kidwell SM, Kirby MX, Peterson CH, Jackson JB (2006) Depletion, degradation, and recovery potential of estuar-ies and coastal seas. Science 312: 1806–1809. https://doi. org/10.1126/science.1128035
Mai ACG, Miño CI, Marins LFF, Monteiro-Neto C, Miranda LV, Schwingel PR, Lemos VM, Gonzalez-Castro M, Castello JP, Vie-ira JP (2014) Microsatellite variation and genetic structuring in
Mugil liza (Teleostei: Mugilidae) populations from Argentina
and Brazil. Estuarine, Coastal and Shelf Science 149: 80–86. https://doi.org/10.1016/j.ecss.2014.07.013
McCullagh P, Nelder JA (1989) Generalized Linear Models. Chapman and Hall, London, 2nd ed., 506 pp. https://doi. org/10.1007/978-1-4899-3242-6
Menezes NA, Figueiredo JL (1980) Manual de peixes marinhos do sudeste do Brasil, VI Teleostei (3). São Paulo, Universidade de São Paulo, Museu de Zoologia, 96 pp.
Menezes NA, Figueiredo JL (1985) Manual de peixes marinhos do sudeste do Brasil, V Teleostei (4). São Paulo, Universidade de São Paulo, Museu de Zoologia, 105 pp.
MPA/MMA (2014) Plano de gestão para o uso sustentável da tainha, Mugil liza Valenciennes, 1836, no Sudeste e Sul do Bra-sil. Proposta elaborada pelo grupo técnico de trabalho-GTT tainha, instituído pela Portaria Interministerial N°01, de 28 de junho de 2012. Ministério da Pesca e Aquicultura, Ministério do Meio Ambiente, Brasília, 137 pp.
Myers RA, Pepin P (1990) The robustness of lognormal based estimators of abundance. Biometrics 46: 1185–1192. https:// doi.org/10.2307/2532460
Nayak PK, Oliveira LE, Berkes F (2014) Resource degradation, marginalization, and poverty in small-scale fisheries: threats to social-ecological resilience in India and Brazil. Ecology and So-ciety 19: 73–82. https://doi.org/10.5751/ES-06656-190273 Oviedo AFP, Bursztyn M (2017) Community-based monitoring of
small-scale fisheries with digital devices in Brazilian Amazon. Fisheries Management and Ecology 24: 320–329. https://doi. org/10.1111/fme.12231
Peres MB, Klippel S, Vianna MAC (2007) Áreas de exclusão de pesca propostas no processo de gestão participativa da pes-ca artesanal no litoral norte do Rio Grande do Sul: um rela-to experiência. In: Ministério do Meio Ambiente (Ed.) Áreas aquáticas protegidas como instrumento de gestão pesqueira. Brasília, Ministério do Meio Ambiente, 131–147.
Peterson D, Hanazaki N, Simões-Lopes PC (2008) Natural re-source appropriation in cooperative artisanal fishing between fishermen and dolphins (Tursiops truncatus) in Laguna, Bra-zil. Ocean & Coastal Management 51: 469–475. https://doi. org/10.1016/j.ocecoaman.2008.04.003
Pryor K, Lindbergh J, Lindbergh S, Milano R (1990) A dolphin-hu-man fishing cooperative in Brazil. Marine Mammal Science 6: 77–82. https://doi.org/10.1111/j.1748-7692.1990.tb00228.x R Development Core Team (2015) ‘R: a Language and Environ-ment for Statistical Computing.’ R Foundation for Statistical Computing, Vienna. http://www.R-project.org
Rodrigues FL, Cabral HN, Vieira JP (2014) Assessing surf-zone fish assemblage variability in southern Brazil. Marine Ecolo-gy Progress Series 66: 106–119. https://doi.org/10.1071/ MF13210
Sant’Ana R, Kinas PG, Miranda LV, Schwingel PR, Castello JP, Vie-ira JP (2017) Bayesian state-space models with multiple CPUE data: the case of a mullet fishery. Scientia Marina 81: 361– 370. https://doi.org/10.3989/scimar.04461.11A
Shane SH, Wells RS, Würsig B (1986) Ecology, behavior and social organization of the bottlenose dolphin: A re-view. Marine Mammal Science 2: 34–63. https://doi. org/10.1111/j.1748-7692.1986.tb00026.x
Simões-Lopes PC (1991) Interaction of coastal populations of
Tursiops truncatus (Cetacea, Delphinidae) with the mullet arti-sanal fisheries in southern Brazil. Biotemas 4: 83–94.
Simões-Lopes PC, Fabian ME (1999) Residence patterns and site fidelity in bottlenose dolphins, Tursiops truncatus (Montagu) (Cetacea, Delphinidae) off Southern Brazil. Revista Brasileira
de Zoologia 16: 1017–1024. https://doi.org/10.1590/S0101-81751999000400012
Simões-Lopes PC, Fabian ME, Menegheti JO (1998) Dolphin in-teractions with the mullet artisanal fishing on southern Bra-zil: a qualitative and quantitative approach. Revista Brasileira de Zoologia 15: 709–726. https://doi.org/10.1590/S0101-81751998000300016
Simões-Lopes PC, Daura-Jorge FG, Cantor M (2016) Clues of cultural transmission in cooperative foraging between arti-sanal fishermen and bottlenose dolphins, Tursiops truncatus
(Cetacea: Delphinidae). Zoologia (Curitiba) 33: e20160107. https://doi.org/10.1590/s1984-4689zool-20160107
Smith BD, Tun TM, Chit AM, Winb H, Moeb T (2009) Catch com-position and conservation management of a human-dolphin cooperative cast-net fishery in the Ayeyarwady River, Myan-mar. Biological Conservation 142: 1042–1049. https://doi. org/10.1016/j.biocon.2009.01.015
Tabajara LLCA, Dillenburg S (1997) Batimetria e sedimentos de fundo da laguna de Tramandaí – RS. CECO/IG/UFRGS, Porto Alegre, 21–33.
Taylor W, Gerking W (1978) Potential of the Ohrid rifle minnow, Alburnoides Bipunctatus ohridanus, as an indicator of pollu-tion. Verhandlungen des Internationalen Verein Limnologie 20: 2178–2181.
Toldo E, Nicolodi J, Almeida L, Corrêa I, Esteves L (2006) Coastal dunes and shore face width as a function of longshore trans-port. Journal of Coastal Research 39: 390–394.
Vieira JP (1991) Juvenile mullets (Pisces: Mugilidae) in the estuary of Lagoa dos Patos, RS, Brazil. Copeia 2: 409–418. https://doi. org/10.2307/1446590
Vieira JP (2006) Ecological analogies between estuarine bot-tom trawl fish assemblages from Patos Lagoon, Rio Grande do Sul, Brazil and York River, Virginia, USA. Revista Brasileira de Zoologia 23: 234–247. https://doi.org/10.1590/S0101-81752006000100017
Vieira JP, Scalabrin C (1991) Migração reprodutiva da Tainha (Mugil platanus, Günther, 1880) no sul do Brasil. Atlântica 13: 131–141. Vieira JP, Garcia AM, Grimm AM (2008) Evidences of El Niño ef-fects on the mullet fishery of the Patos Lagoon Estuary. Brazil-ian Archives of Biology and Technology 51: 433–440. https:// doi.org/10.1590/S1516-89132008000200025
Villwock JA, Tomazelli LJ (1995) Geologia Costeira do Rio Grande do Sul. Centro de Estudos de Geologia Costeira e Oceânica, UFRGS, Porto Alegre, 45 pp.
Whitfield AK, Panfili J, Durand J (2012) A global review of the cosmopolitan flathead mullet Mugil cephalus Linnaeus 1758 (Teleostei: Mugilidae), with emphasis on the biology, ge-netics, ecology and fisheries aspects of this apparent species complex. Journal of Fish Biology 22: 641–681. https://doi. org/10.1007/s11160-012-9263-9
Rio Grande do Sul, Brasil. Acta Limnologica Brasiliensia 11: 701–721.
Zappes CA, Andriolo A, Simões-Lopes PC, Di Beneditto APM (2011) ‘Human-dolphin (Tursiops truncatus Montagu, 1821) cooperative fishery’ and its influence on cast net fishing activities in Barra de Imbé/Tramandaí, Southern Brazil. Ocean & Coastal Management 54: 427–432. https://doi. org/10.1016/j.ocecoaman.2011.02.003
Zar JH (1984) Biostatistical Analysis. Prentice Hall, Englewood Cliffs, 2nd ed., 718 pp.
Zuur AF, Ieno EN, Smith GN (2007) Analysing Ecological Data. Springer, Dordrecht, 672 pp. https://doi.org/10.1007/978-0-387-45972-1
Submitted: February 13, 2018 Accepted: June 14, 2018 Available online: October 19, 2018
Editorial responsibility: Cassiano Monteiro Neto
Author Contributions: MLS VML and JPV designed the experiment, analyzed the data, wrote the paper; MLS conducted the experiment.
Competing Interests: The authors have declared that no competing
interests exist.