ww w . r e u m a t o l o g i a . c o m . b r
REVISTA
BRASILEIRA
DE
REUMATOLOGIA
Review
article
Treatment
of
postmenopausal
osteoporosis:
a
literature-based
algorithm
for
use
in
the
public
health
care
system
Ellen
Luz
Pereira
Caires
a,
Mailze
Campos
Bezerra
b,c,
Ana
Flávia
Torquato
de
Araújo
Junqueira
a,c,
Sheila
Márcia
de
Araújo
Fontenele
b,c,
Silvana
Cristina
de
Albuquerque
Andrade
c,d,
Catarina
Brasil
d’Alva
a,c,∗aUniversidadeFederaldoCeará(UFC),FaculdadedeMedicina,Servic¸odeEndocrinologiaeDiabetes,Fortaleza,CE,Brazil bUniversidadeFederaldoCeará(UFC),FaculdadedeMedicina,Servic¸odeReumatologia,Fortaleza,CE,Brazil
cUniversidadeFederaldoCeará(UFC),FaculdadedeMedicina,NúcleodeAtendimentoMultidisciplinaràsDoenc¸asOsteometabólicas,
Fortaleza,CE,Brazil
dUniversidadeFederaldoCeará(UFC),FaculdadedeMedicina,Servic¸odeNefrologiaeTransplanteRenal,Fortaleza,CE,Brazil
a
r
t
i
c
l
e
i
n
f
o
Articlehistory:
Received3July2016 Accepted5December2016 Availableonline15February2017
Keywords:
Osteoporosistreatment Bisphosphonates Publichealthcare
a
b
s
t
r
a
c
t
Bisphosphonatesareconsideredfirst-lineagentsinthetreatmentofpostmenopausal osteo-porosisbased on extensive experience ofuse, safety, and proven efficacy in reducing vertebral,non-vertebralandfemurfractures.However,post-marketingreportsbasedon thetreatmentofmillionsofpatients/yearoverlengthyperiodsoftimehaverevealedthe occurrenceofinitiallyunexpectedadverseeffects,suchasosteonecrosisofthejawand atypicalfemoralfracture,leadingtotherestrictionoftreatmentdurationwith bisphospho-natesbyglobalregulatoryagencies.However,despitetheassociationbetweentheseeffects andbisphosphonates,thisriskshouldbeanalyzedinthecontextofosteoporosistreatment, alongsidethebenefitofpreventingosteoporoticfracturesandtheirclinicalconsequences. Therefore,weconsideritplausibletodiscusstherestrictiontotheuseofbisphosphonates, possibleindicationsforprolongedtreatmentandalternativetherapiesfollowingthe suspen-sionofthisdrugclassforpatientswithpersistenthighriskoffractureafterinitialtreatment, especiallyconsideringtheproblemsofpublichealthfundinginBrazilandtheshortageof drugsprovidedbythegovernment.Thus,tostandardizethetreatmentofosteoporosisinthe publichealthcaresystem,weaimtodevelopaproposalforascientifically-based pharmaco-logicaltreatmentforpostmenopausalosteoporosis,establishingcriteriaforindicationand allowingtherationaluseofeachpharmacologicalagent.Wediscussthedurationofthe ini-tialbisphosphonatetreatment,thetherapeuticoptionsforrefractorypatientsandpotential indicationsofotherclassesofdrugsasfirst-choicetreatmentinthesphereofpublichealth, inwhichassessingriskandcosteffectivenessisapriority.
©2017ElsevierEditoraLtda.ThisisanopenaccessarticleundertheCCBY-NC-ND license(http://creativecommons.org/licenses/by-nc-nd/4.0/).
∗ Correspondingauthor.
E-mails:cbdalva@terra.com.br,majaco@terra.com.br(C.B.d’Alva). http://dx.doi.org/10.1016/j.rbre.2017.01.001
Tratamento
da
osteoporose
pós-menopáusica:
um
algoritmo
baseado
na
literatura
para
uso
no
sistema
público
de
saúde
Palavras-chave:
Tratamentodaosteoporose Bisfosfonatos
Saúdepública
r
e
s
u
m
o
Combasenavastaexperiênciadeuso,seguranc¸aeeficáciacomprovadanareduc¸ãode fraturasvertebrais,nãovertebraisefemorais,osbisfosfonatossãoconsideradosagentes deprimeiralinhanotratamentodaosteoporosepós-menopáusica.Noentanto,osrelatos pós-vendabaseadosnotratamentodemilhõesdepacientes/anoduranteperíodos prolon-gadosdetemporevelaramaocorrênciadeefeitosadversosinicialmenteinesperados,como osteonecrosedamandíbulaefraturaatípicadofêmur.Issolevouasagênciasreguladoras globaisarestringiremadurac¸ãodotratamentocombisfosfonatos.Noentanto,apesarda associac¸ãoentreessesefeitoseosbisfosfonatos,esseriscodeveseranalisadonocontexto dotratamentodaosteoporose,paralelamenteaobenefícionaprevenc¸ãodefraturas osteo-poróticasesuasconsequênciasclínicas.Portanto,considera-seplausíveldiscutirarestric¸ão aousodosbisfosfonatos,possíveisindicac¸õesparaotratamentoprolongado eterapias opcionaisapósasuspensãodessaclassedefármacoparapacientescomaltorisco persis-tentedefraturaapósotratamentoinicial,especialmenteseconsiderarmososproblemas financeirosdesaúdepúblicanoBrasileaescassezdefármacosfornecidospelogoverno. Assim,parapadronizarotratamentodaosteoporosenosistemapúblicodesaúde pretende--sedesenvolverumapropostadetratamentofarmacológicocientificamentefundamentada paraaosteoporosepós-menopáusica,estabelecercritériosdeindicac¸ãoepermitirouso racionaldecadaagentefarmacológico.Discutem-seadurac¸ãodotratamentoinicialcom bisfosfonatos,asopc¸õesterapêuticasparapacientesrefratáriosepotenciaisindicac¸õesde outrasclassesdemedicamentoscomotratamentodeprimeiralinhanaesferadasaúde pública,emqueaavaliac¸ãodoriscoecusto-efetividadeéumaprioridade.
©2017ElsevierEditoraLtda.Este ´eumartigoOpenAccesssobumalicenc¸aCC BY-NC-ND(http://creativecommons.org/licenses/by-nc-nd/4.0/).
Introduction
Osteoporosisischaracterizedbylossofbonemassand dete-riorationoftissuemicroarchitecture,leadingtobonefragility andincreasedriskoffractures,theclinicalconsequencesof whicharedeformities,chronicpain,disabilityanddeath.1It isacommondiseasewithincreasingprevalenceamongmen andwomen duetoincreasedlifeexpectancy andanaging population.
Bisphosphonatesrepresentthe first-line therapy forthe preventionofosteoporoticfractures.2 These drugsare syn-thetic analogs of inorganic pyrophosphate obtained by replacingtheoxygenatomwithacarbon(P-C-P),makingthem resistant tobiologicaldegradation,and byadding twoside chains(R1and R2),responsible forskeletalbindingaffinity andpower,respectively.Thischemicalstructurehasthe prop-ertyofforming compoundswithdivalent cations,showing greataviditywithhydroxyapatitecrystals ofbonesurfaces, particularlyofactiveremodelingsites.In theacid environ-mentofresorption,bisphosphonates arereleasedfrom the boneandabsorbedbytheosteoclast,causingtheinhibition ofthe enzyme farnesyl pyrophosphate synthase, which is importantfortheintegrityofitscytoskeletonandcell func-tion.Thisleadstoalossinresorptivefunctionandpotential osteoclastapoptosis. Considering that boneformation and resorptionare coupledprocesses, reducedresorptionis fol-lowed by a decrease in bone formation, thus achieving a
new stateof decreasedbone remodeling afterstarting the treatment.3
Thefirst drug ofthisclass wassynthesized inthe 19th century,butitsclinicalrelevancewasonlyrecognizedinthe late1960s,whenbisphosphonatesstartedbeingusedinthe treatmentofvariousbonemetabolicdiseases.4However,the widespreaduseofbisphosphonatesinosteoporosistherapy occurredafter1993,whenWorldHealthOrganization(WHO) established the diagnosis ofosteoporosis bythe technique ofbone densitometryby dual-energy X-rayabsorptiometry (DEXA).5
Effectiveness
of
bisphosphonates
in
the
prevention
of
osteoporotic
fractures
55–81years,neckT-score≤−2.1,2.9-year follow-up),which showedareductionof47%,55%and51%intheriskof radio-graphic vertebral fractures, clinical vertebral fractures and femurfractures,respectively,inthisgroupofpostmenopausal womenwithpreviousvertebralfractures.7
Thesecond bisphosphonateapproved forthetreatment of osteoporosis in the US was risedronate, in 2000. The use of risedronate (VERT study, n=2458 women, age <85 years, 2 prior vertebral fractures or 1 prior vertebral frac-tureplusspineT-score≤−2.0, 3-yearfollow-up) resultedin 41%and39%reductionsinvertebralandnon-vertebral frac-tures,respectively.9Then,a40%reductionofhipfracturerisk wasshownbyMcLunget al.inalargestudy thatincluded 5445womenaged70–79,withneckT-score≤−4.0orneckT -score≤−3.0plus1riskfactorforhipfracture,inatwo-year follow-up.10
Ibandronate,approvedin2005forthetreatmentof osteo-porosis,reducedtheriskofvertebralfractureby62%(BONE study,n=2946women,age55–80years,T-score≤−2.0inat leastonevertebrapluspriorvertebralfracture,3-year follow-up). In this study, there was no reduction in the risk of non-vertebralfractures,exceptforaposthocanalysisofthe subgroupofwomen withneck T-score<−3.0.11 In order to estimatethe effectonnon-vertebralfractures,afew meta-analyzes of randomized studies that evaluated individual patientdatawerepublishedsuggestingabeneficialeffectof higherdosesofibandronate(correspondingto150mg/month orallyor 12mg/yeari.v.).12,13 However,thereisno evidence from placebo-controlled studies showinga reduced risk of non-vertebralfracturewiththeuseofibandronate.
Zoledronicacid,abisphosphonatewithgreater antiresorp-tivepotency,wasapprovedforthetreatmentofosteoporosis in2007.Anannualinfusionofzoledronicacidfor3 consec-utiveyears(HORIZONPFT,n=7765women,age65–89years, neck T-score≤−2.5 or neck T-score≤−1.5 plus 1 vertebral fracture,3-yearfollow-up)waseffectiveinreducingby70%, 41%and25%theriskofvertebral,femurandnon-vertebral fractures,respectively.14
Safety
of
bisphosphonates
in
osteoporosis
treatment
Given that osteoporosis is a chronic disease, along with thegoodsafetyprofileofbisphosphonatesdemonstratedby placebo-controlled studies, osteoporosis treatment should, conceptually,beextendedthroughoutthepatient’swholelife. However,post-marketingreportsbasedonthetreatmentof millionsofpatients/yearoveralengthyperiodoftimerevealed theoccurrenceofinitiallyunexpectedadverseeffectsof bis-phosphonatetreatmentsuchasosteonecrosisofthejaw(ONJ) andatypicalfemoralfracture.15–17
ONJ is characterized by exposure ofbone tissue in the maxillofacialregionwithouthealingafter8weeks.18Evidence suggeststheexistenceofacausalrelationshipbetweenthe useofbisphosphonatesandONJ,withadose-responseeffect duetothegreater incidenceofthis complicationincancer patientsreceivinghighcumulativedosesofbisphosphonates. Theprevalencewasestimatedat0.4%amongcancerpatients and0.001%inpatientswithosteoporosisinasurveyofcases
observed byCanadianmaxillofacial surgeons.19 A random-ized, double-blind, placebo-controlled study involving 2046 patientswithbreastcancerobservedtheoccurrenceofONJ in2.0%and 1.4%ofthose treatedwithhighdosesof deno-sumaborzoledronicacid,respectively(p=0.39),revealingthat thiseffectisnotspecifictobisphosphonates,buttotreatment withpotentantiresorptives.20Therefore,ONJisveryrareinthe treatmentofosteoporosisanddiscontinuationof antiresorp-tivetherapyinosteoporoticpatientspriortodentalprocedures possiblydoesnothaveanyimpactinreducingthisrisk.
However,recently,the TaskForce onONJ recommended tostopantiresorptivetherapy,ifitispossibletodoso with-out adverseconsequencesforbonehealth,inpatientswho requireextensiveinvasiveoralsurgeryaswellasthosewith multipleriskfactorsforONJ,althoughthereislittleevidence tosupportthisrecommendationasbisphosphonatesremain in bone foryears.21 Therefore, clinical judgmentis always essential.
Afurthercomplicationobservedfollowingthemarketing ofbisphosphonateswasatypicalfemoralfracturedefinedas noncomminutedtransverseorshortobliquefractures,which occurinthesubtrochantericregionafterminimaltrauma.22 Despitetheinconclusivestudiesonbonephysicalproperties inbisphosphonatesusers,itisbelievedthatsuchfracturesare theresultofexcessiveandprolongedsuppressionof remod-eling,causinglossofbonequalityandmechanicalfunction, whichleadstotheaccumulationofmicrofracturesand skele-tal fragility.The resultis the development ofinsufficiency fractures atthe maximum mechanical overloadpoint rep-resentedbythesubtrochantericordiaphyseal regionofthe femur.Hencethetermatypicalfracture,sinceitinvolvesthe strongest regionofthe femur, unlikeosteoporotic fracture, whichcommonlyoccursinthefemoralneck.23
Afterreviewing12,777femurfracturescasesthatoccurred in Sweden in 2008,Schilcher et al.24 identified 59 atypical fractures,78%ofwhichoccurred inbisphosphonatesusers. Despitethisassociation,theabsoluteriskofatypicalfracture relatedtotheuseofbisphosphonatesislow(50cases/100,000 patients-year). Dell et al.25 analyzed approximately 15,000 subtrochantericfracturesinCaliforniabetween2007and2009, identifying 102 atypical fractures, 97 of which in patients usingbisphosphonatesforanaverageof5.5years.However, when analyzingthe useofbisphosphonates overtime, the absoluteriskwas2cases/100,000patients-yearin2yearsof treatment and 78 cases/100,000 patients-year in8 yearsof treatment.
Warnings
from
drug
regulatory
agencies
worldwide
Impact
of
the
regulatory
restriction
on
duration
of
bisphosphonates
use
in
the
Brazilian
public
health
system:
a
critical
analysis
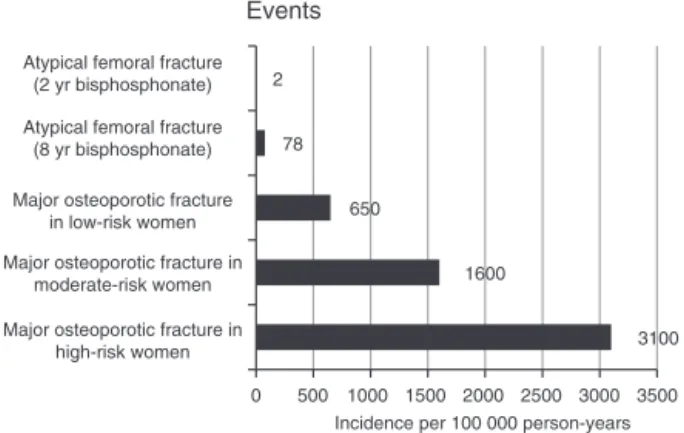
Despite the association demonstrated between atypical femoralfracturesand bisphosphonates, thisriskshould be analyzedinthecontextofosteoporosistreatment,alongside thebenefitofpreventingosteoporoticfractures.Itisestimated thatforeach100typicalfemoralfracturespreventedby bis-phosphonates,1atypicalfractureoccurs.29Forpatientswith severeosteoporosisandhighriskoffracture,3300osteoporotic fractures are prevented per 100,000 patients-year treated, whileforpatientswithmoderaterisk,1700osteoporotic frac-tures are prevented per 100,000 patients-year treated with bisphosphonates.22Therefore,theeffectivenessofthisclass ofdrugsinthepreventionofosteoporoticfracturesinpatients withmoderatetohighriskosteoporosisoutweighstheriskof atypicalfractures(Fig.1).30
In Brazil, where the constitution guarantees universal accesstohealthcare,onlyraloxifeneandalendronateare reg-ularlyavailableinourpublichealthsystem.Inordertoobtain drugssuchaszoledronicacidandteriparatide,patientsturn tothecourts,obligingthegovernmenttoprovidethe medica-tions,whichdisruptsitsbudgetandimpairshealthpolicies. Thus,itisreasonabletoquestiontherestrictionontheuseof bisphosphonates,especiallywhenweconsiderpublichealth fundingproblemsinBrazilandthescarcityofdrugssupplied bythegovernment.
Therefore, given the potential severity of osteoporosis, theabsolutelowriskofatypicalfracture andtherestricted drug supply by our public health system, it is reason-able to propose long-term bisphosphonates treatment for women with osteoporosis and moderate to high risk of fracture.
Withtheobjectiveofstandardizingthetreatmentof osteo-porosis in Brazilian public health care system, as well as reducing the phenomenon of judicialization of health, we proposetodevelopascientifically-basedprotocolforthe treat-ment ofpostmenopausalosteoporosis, establishingcriteria forindicationandallowingtherationaluseofeach pharma-cologicalagent.
3100 1600
650 78 2
3500 3000 2500 2000 1500 1000 500 0 Major osteoporotic fracture in
high-risk women Major osteoporotic fracture
in low-risk women
Major osteoporotic fracture in moderate-risk women Atypical femoral fracture
(8 yr bisphosphonate) Atypical femoral fracture
(2 yr bisphosphonate)
Incidence per 100 000 person-years Events
Fig.1–Riskofosteoporoticfracturesandatypicalfemoral fractures.
AdaptedfromBrownetal.30
Efficacy
of
prolonged
treatment
with
bisphosphonates
Itisworthexaminingwhetherprolongeduseof bisphospho-nates offers benefits, sincethese drugs accumulate in the skeleton and continue to bereleased for months to years afterthetreatmentissuspended,resultinginaresidual anti-fractureeffect.31Belowaredescribedsomeextensionstudies oftreatmentwithbisphosphonates.
TheextensionoftheFITstudy(FractureInterventionalTrial Long-term Extension,FLEX), inwhich, followingthe first 5 yearswithalendronate,patientsinthetreatedgroupwere ran-domizedinto5moreyearsofalendronateorplacebo,showed nodifferenceintheriskofnon-vertebralfractureand mor-phometricvertebralfracturesbetweenthegroups.However, it demonstratedareducedriskofclinicallyapparent verte-bralfractures(RR:0.45;95%CI0.24–0.85)inpatientscontinuing treatmentfor10 years.32 Itisnoteworthythat, intheFLEX study, many women had osteopenia only, and those with a femurneck T-score<−3.5 were excluded, indicating that partofthemalreadypresentedlowriskandhadnoneedto prolongtreatment.Moreover,asubsequentanalysisofFLEX datashowedthatmaintenanceofalendronatefor10yearsin the subgroupofwomenwithafemoralneckT-score≤−2.5 decreasedby50%theriskofnon-vertebralfractures(RR:0.50, CI95%0.26–0.96).33Theseresultsindicatethatsomewomen, especiallythosewithhighriskoffracture,canbenefitfrom themaintenanceoftreatmentwithalendronatefor10years. Inotherwords,theeffectivenessoftheprolongedtreatment dependsonthefracturerisk,whichcanbeevaluated,among otheraspects,byBoneMineralDensity(BMD).
To investigate the long-term effects of zoledronic acid, patientswho hadbeen treatedfor3yearsintheHORIZON studywererandomizedinto3moreyearsofzoledronicacid or placebo. This study showed a reduction in the risk of morphometricvertebralfractures(OR:0.51;p=0.035)with con-tinuedtreatment.Thisfindingledtotheconclusionthatmany patientscansafelydiscontinuethemedicationaftertheinitial 3yearsoftreatment,whilesomeofthemmaybenefitfromthe maintenanceofzoledronicacidforanother3years.34
Wecanconcludethatsuspensionofthebisphosphonate after3(zoledronicacid)to5years(alendronate) isjustified for patients who, at the end of this period, present low risk of fracture. However, those who persist with femoral
T-score≤−2.5 after the initial course of treatment should havethis treatmentcontinued forup to6(zoledronic acid) to10 years(alendronate).Moreover,despitethe absenceof evidence-based recommendations, it is likely that women who persistwithmoderate tohighrisk offractures dueto factorsindependentoffemoralT-scoremayalsobenefitfrom treatmentmaintenance.
Transition
studies:
what
to
do
in
the
case
of
women
who
persist
with
high
risk
of
fracture
following
10
years
of
treatment
with
bisphosphonates?
bisphosphonates, becoming candidates for treatment with otheranti-osteoporoticdrugs.However,bisphosphonatesare potentsuppressorsofboneremodelingandhavehighaffinity andretentiontimeinthebone,inhibitingturnoverforyears aftertheirsuspension.Therefore,theimpactoftheswitchto otherdrugsmustbecarefullyanalyzed.35,36
Giventhegreaterpotencyofthezoledronicacid,McLung et al.37 studied the effect ofa single dose of this drug in menopausalwomenpreviouslytreatedwithalendronatefor anaveragetimeof4years(n=225,age46–79years,spineor neckT-score≤−2.0).BMDremainedessentiallystableafter12 monthsofalendronateorzoledronicacid,withnodifference betweenthegroups.
Denosumab,thefirstbiologicaldrugapprovedforthe treat-mentofosteoporosis,isamonoclonalantibodythatbindsto receptoractivatorofnuclearfactorkappaligand(RANKL), a cytokine secreted bythe osteoblast considered essential forthe differentiation, activity and survival ofosteoclasts, potentlyreducing boneresorptionand therisk ofvertebral (68%),non-vertebral(20%)andfemur(40%)fracture.38,39
Two studies investigated the effects of denosumab in patients previously treated with bisphosphonates.40,41 Kendleretal.,40analyzingpatientstreatedwithalendronate foranaverageof36months(n=504,age≥55years,−4.0≤T -score≥−2.0), observed that the transition to denosumab resulted in a significantly greater increase of BMD in the totalfemurandlumbarspineafter12monthscomparedto themaintenanceofalendronate (1.9%vs.1.0%totalfemur,
p<0.0001;3.0%vs.1.8%lumbarspine,p<0.0001).
In patients using alendronate irregularly for a median of 20 months (n=870, age≥55 years), there was a signifi-cantlygreaterincreaseinBMDatallbonesitesfollowingthe transitiontodenosumabcomparedwiththetransitionto rise-dronate(totalfemur2.0%vs.0.5%,neck1.4%vs.0%;spine3.4% vs.1.1%,p<0.0001atallsites).41
Denosumabandbisphosphonatesarebothantiresorptive drugs,buthavedifferentmechanismsofaction.Theydecrease osteoclast activity and survival, but RANKL inhibition by denosumabalsopreventsthedifferentiationofthesecells.42 Furthermore,itispossiblethatboneformationina resorption-independentprocess,known as bone-modeling, persistsat a lesser extentin adultskeletons and is preserved during denosumabtreatment, as it was recentlydemonstrated in cynomolgusmonkeys treatedwithdenosumab.43 Detection ofendocorticalandperiostealsurfacefluorochromelabeling reflectedcontinuedboneformationatspecificsites,outside thetrabecularcompartment, providingpreclinical evidence forapotentialmechanismthatcouldcontributetotheeffects ofdenosumabinBMDandfracturerisk.43Thedifferent mech-anismofactionmayberesponsibleforthefurtherincreasein bonemasscausedbydenosumabinpreviousbisphosphonate users.40
However, when BMD was analyzed according to the lengthofprioralendronate use, agreater increase inBMD withdenosumabwasobservedingroupswithshorter alen-dronatetreatmentduration,whichcanbeexplainedbythe filling of the bone remodeling units during the previous antiresorptivetreatment.40Thishypothesiscanalsoexplain the greater BMD gain with teriparatide in treatment-naïve patients.40
Inthiscontext,itisworthnotingthattheseauthorsstudied theeffectofdenosumabinwomenreceivingbisphosphonates foramaximumperiodof4years,while,inourproposal,we intendtojustifytheuseofotherdrugsafter10yearsof bis-phosphonates,whichwesuggestasatherapeuticstrategyfor ourpublichealthreality.Giventhelackoftransitionalstudies aftersuchalongtherapeuticcoursewithbisphosphonates,it isourassumptionthattheswitchtoanotherantiresorptive drugmightnotofferanyadditionalbenefitinthisparticular circumstance.
Unlike the antiresorptive drugs discussed above, teri-paratide, the 1-34 N-terminal fragment of parathyroid hormone (PTH),isananabolic agent(inducerofosteoblast boneformation)whoseintermittentadministrationresultsin theincreaseinthenumberandactivityofosteoblasts, caus-ing rapidbonemassincreaseand improvedtrabecularand corticalarchitecture.44 Itistheonlyclassofanabolicdrugs currentlyusedinthetreatmentofosteoporosis.Itcausesa significantreductionintheriskofvertebral(RR:0.35;CI95% 0.22–0.55)andnon-vertebral(RR:0.47;CI95%0.25–0.88) frac-tures in menopausal women withprior vertebral fractures (n=1637),althoughreductionoffemurfracturehasnotbeen demonstratedsofar.45,46
An importantquestion is whether the prior antiresorp-tivetreatmentmodifiestheanabolicresponsetoteriparatide. Thebenefitsofteriparatideinpatientspreviouslyexposedto antiresorptivedrugsoverlongperiodsoftimeweretestedby someauthors.47–52
Ettinger et al.47 studied theeffect of18 monthsof teri-paratide in women previously treated with raloxifene or alendronateforaperiodof18–36months(EUROFORSstudy,
n=59,age60–87years,T-score≤−2.0).Bothgroupsshowed astatisticallysignificant increaseinboneturnovermarkers (BTM)(P1NP,boneFAandosteocalcin)asearlyastheendof the firstmonthofteriparatide,withatendency forfurther increaseinthegrouppreviouslytreatedwithraloxifene.BMD increaseoccurredearlierinraloxifeneusers,butbytheend oftreatmentBMDincreaseinthelumbarspinewasobserved inbothgroups,again of10.2% forprevioususers of ralox-ifeneandof4.1%forprevioususersofalendronate(p<0.001). However, BMDincreaseintotal femurwas significantonly in previoususers ofraloxifene(0.5% inprevious raloxifene usersand–1.8%inpreviousalendronateusers,p=0.002).The authorsconcludedthatteriparatidestimulatesboneturnover inwomenpreviouslytreatedwithraloxifeneoralendronate for18–36months,althoughpreviousexposuretoalendronate slowsskeletalresponsetoteriparatide.Thisdelayedeffecton BMDandmorelimitedresponseafterprioruseof bisphos-phonatesisprobablyduetotheabsenceoftargetcellsforthe anabolic effectofteriparatide.Afterfewyearsoftreatment withapotentantiresorptive,theextremelylowboneturnover reducestheavailabilityofpre-osteoblasts,osteoblastsand lin-ingcellstobeconvertedintoosteoblasts.35,48
followingitssuspension,comparedtotheinitial6monthsof thistreatment).
In addition, clinical studies examining bone mass by histomorphometry, high-resolution peripheral quantitative computed tomography (HR-pQCT) and finiteelement anal-ysis in HR-pQCT showed that the suppressive effect of bisphosphonatesmaybeoffsetbycontinuedtreatmentwith teriparatide.50–52
Inshort,thesestudiesshoweffectiveanabolicresponseto teriparatideafterprevioustreatmentwithbisphosphonates foranaverageperiodof36months.Again,thereisnoevidence oftheuseofteriparatidefollowingmoreprolongedtreatment withbisphosphonates,asweproposeinourtherapeutic strat-egy.Theremaybegreaterdelayandmorelimitedresponseof BTMandBMD,andclinicaltrialsareneededtoevaluatethis therapeuticscenario.However,giventheavailableevidence, weconsideritacceptabletousedrugswithanaboliceffectin previoususersofantiresorptivesoveraprolongedtimespan that,aftersuchtreatment,persistwithsevereosteoporosis andhighriskoffracture.
Protocol
for
pharmacological
treatment
of
postmenopausal
osteoporosis
in
the
public
health
care
system
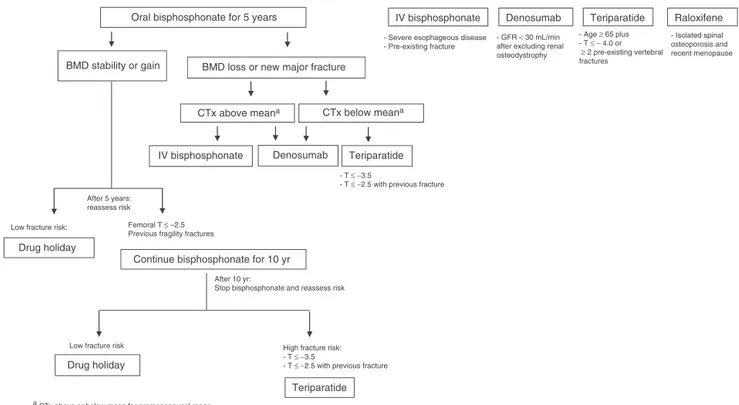
Alendronateasfirst-linetreatment
Due to the extensive experience of use, safety and anti-fracture efficacy, bisphosphonates are the mainstay of osteoporosistreatmentandshouldgenerallybeusedasdrugs offirst choice (Fig. 2). Considering the availability of alen-dronateintheBrazilianpublichealthservice,mostpatients with an indication of pharmacological treatment should
receivealendronate,aprioriforaperiodoffiveyears, accord-ingtothepresentedevidence.
Duringtreatment,norandomizedstudyassessedthevalue ofserialbonedensitometryontheriskoffracture,butthistest canbeusefulifusedcorrectly.53Therefore,treatment moni-toringbysequentialBMDassessmentisindicated,withbone massstabilityandabsenceofnewfracturesinmajorbonesites asindicatorsoftherapeuticsuccess.54Inaddition, demonstra-tionofacross-linktelopeptideoftype1collagen(CTx)decline of25%frombaselinelevelsafter3–6monthsoftreatmentcan beusedasearlyevidenceofinhibitionofboneresorptionand goodtherapeuticresponse.53,54
Following the first 5 years oftreatment, stopping alen-dronate therapy is suitable for most patients and a drug holidayshouldbeconsideredwithreassessmentoffracture risk after 2 or 3 years. However, women with a femoral
T-score≤−2.5shouldhavethistreatmentcontinued, consid-eringtheevidenceofbenefitinthissubgroupofwomenby FLEX study.33 Another important consideration is whether thepatienthadexperiencedpreviousosteoporoticfractures, especiallyinmajorbonesites.Sincesuchfracturesincrease substantiallyfuturefracturerisk,thesepatientsshouldalso havebisphosphonatetreatmentcontinued.Itisworth consid-eringthatthebenefitoutweighstheriskofatypicalfemoral fracturesinthesegroupsofindividuals,aswellasthegreat impactofanosteoporoticfractureonmortality,qualityoflife andcoststothehealthsystem.55Duringthisprolonged treat-mentperiod,drugsuspensionshouldbedeterminedbythe periodicassessmentoftheindividualriskoffragilityfractures. After10yearsusingalendronate,theriskofosteoporotic fracture should be reassessed. In view of the absence of evidence for fracture reduction and safety beyond such a long-termtherapy,maintenanceofbisphosphonatemaynot beappropriate.Then,inlower-riskpatients,adrugholiday
CTx above meana
Oral bisphosphonate for 5 years
CTx below meana
IV bisphosphonate Denosumab Teriparatide
Drug holiday
Continue bisphosphonate for 10 yr
After 10 yr:
Stop bisphosphonate and reassess risk
Drug holiday
Teriparatide
After 5 years: reassess risk
BMD stability or gain BMD loss or new major fracture
Teriparatide Denosumab
- T ≤ −3.5
- T ≤ −2.5 with previous fracture
IV bisphosphonate
Femoral T ≤ –2.5 Previous fragility fractures Low fracture risk:
Low fracture risk
- Age ≥ 65 plus - T ≤ − 4.0 or ≥ 2 pre-existing vertebral fractures
- GFR < 30 mL/min after excluding renal osteodystrophy - Severe esophageous disease
- Pre-existing fracture
Raloxifene
- Isolated spinal osteoporosis and recent menopause
High fracture risk: - T ≤ −3.5
- T ≤ −2.5 with previous fracture
a CTx above or below mean for premenopausal range.
shouldbeconsideredandalendronatediscontinued.However, for the non-negligible percentage of women with persis-tent high risk of fragility fractures, stopping osteoporosis treatmentisnotadvisable.Theoptionsaretocontinue treat-ment with alendronate or switch to another anti-fracture medication.Atthispoint, weproposeadrugwithanabolic mechanismofactionforthesehigh-riskpatientswith persist-ingsevereosteoporosis,althoughtherehavenotbeenstudies evaluatingefficacy ofsuchan approach.Theonlyanabolic drugavailableintheBrazilianmarketisteriparatide,approved forthetreatmentlengthof24months.Becauseofitshighcost and limitedevidence aftersuchaprolonged antiresorptive treatment,wesuggestselectingforitsuseonlythesubgroup ofwomenwithsevereosteoporosisandhigherrisk.
ThedefinitionofsevereosteoporosisbyWHOincludes pre-existingfragilityfractures inthepresenceofT-score≤−2.5. Theriskofanewvertebral fracture isfivetimeshigher in patientswithpriorvertebralfractures.56Moreover, consider-ingBMDasanimportantdeterminantoffracturerisk,high riskcanalsobearbitrarilydefinedasT≤−3.5score,evenin theabsenceoffractures.46
Patientsrefractorytotreatmentwithalendronate
Somepatientsareconsideredrefractorytothetreatmentwith alendronate,whichmustbeverifiedbyadeclineinBMDin atleasttwoserialBMDmeasurementsoroccurrenceofnew fragilityfracturesinmainbonesites.54Insuchcases,reviewof complianceandasearchforoccultsecondarycausesof osteo-porosisaremandatory.Ifadherencecannotbeimprovedand secondaryosteoporosisisexcluded,switchingtoan alterna-tivetherapyisindicated.54
Mostclinicalpracticeguidelines havenotrecommended themeasurementofBTMinthemanagementofosteoporosis largely because they demonstrate high degrees of pre-analyticalandanalyticalvariability.57However,theyprovide asurrogatemeasureoftherateofboneturnoverand there isgrowingevidencethattheyarepotentiallyusefulin deter-miningfractureriskandresponsetotherapy.57 Thereisno reference tosupport their measurementto assess fracture riskafterlong-termbisphosphonate,butthisisnotthecase. Consideringthewiderangeofqualityofavailable bisphospho-nateformulationsaswellasthepooradherencetotreatment, serum CTx levels may help to identify patients with high boneturnover,inwhom bisphosphonateisnotexertingits effects.Therefore, duringbisphosphonatetreatment,aCTx drop lower than 25% from baseline or, in the absence of pre-treatment values, a CTx above the mean of the pre-menopausalreferenceinterval areindicativeofactivebone reabsorption.54,58Therefore,inthiscaseoftreatmentfailure alongwithCTxexceedingthelower halfofpremenopausal range,thetransitiontootheranti-resorptivedrugs,zoledronic acidordenosumab,wouldbeindicatedduetofullabsorption andgreateranti-resorptiveeffect.
For patients who failto alendronatedespite adequately suppressedCTx,webelievethat zoledronic acidmight not offerbenefitbasedonitsabsenceofsuperiorityonBMDin womenwithpostmenopausalosteoporosispreviouslytreated with alendronate, although this effect was demonstrated regardless of CTx.37 Therefore, in patients with treatment
failure along with adequately suppressed CTx, we infer that denosumab and teriparatide may represent the most appropriateagents.Onceagain,becauseofthehighcostof teriparatide, the most severe subgroup of women with T -score≤−3.5orT-score≤−2.5withprevious fractureswould alsohaveanindicationforteriparatide.46
AlthoughitisimpracticaltoobtainCTxlevelsroutinelyin thepublichealthsystem,weconsideritsmeasurement specif-icallyincasesoftreatmentfailuremayhelpclinicianinthe decisionofalternativetherapy.
Useofotherdrugsasfirstchoice
Finally,indicationofotherdrugsasfirst-linetreatmentshould alsobeconsideredinsomeclinicalsettings.
Zoledronicacid,owingtoitsgreatefficacyinreducing ver-tebral,non-vertebralandfemoralfractures,aswellasforits ease ofadministration and guaranteed absorption, can be the drug ofchoicein allscenarios wherebisphosphonates areindicated.However,duetoitsunavailabilityinthe pub-lic healthsystem, wecan arbitrarilyreserveit forpatients withcontraindicationfororalbisphosphonates,patientswith moresevereesophagealdiseaseorthosewithpriorfractures forhavingahigherriskofnewfractures.Itisestimatedthat adherence totreatmentwithoralbisphosphonatesislower than40%in1year.59
Denosumab is the only drug indicated for the treat-ment of osteoporosisin patientswith creatinine clearance <30mL/min.However,thecharacterizationofosteoporosisin patients with chronickidney disease is complex,requiring theexclusionofrenalosteodystrophythroughlaboratorytests andoftenbonehistomorphometry.
Raloxifene,aselectiveestrogenreceptormodulatorwith antiresorptivemechanismofaction,discretelyincreasesBMD andreducesby30%theriskofvertebralfractures,notacting onnon-vertebralandfemurfractures.60Duetoitslower effi-cacyinreducingfractures,wesuggestitsuseinwomenwith isolatedspinalosteoporosis,inperimenopausalage.
Finally,giventhefastimprovementinbonemassand archi-tecture seeninresponsetoteriparatide,this drugcouldbe indicated asfirst-line therapy inindividuals atparticularly high-riskforfractures,whichincludesthesamemoresevere subgroupofwomenwithT-score≤−3.5orT-score≤−2.5with pre-existingfractures.
highcost,wearbitrarilyrelocateitsuseasinitialtherapyinthe groupofwomenwithverylowBMDwithoutpreviousfractures tothosewithT-score≤−4.0.
Conclusion
Inconclusion, alendronateisan appropriatefirst-linedrug to be used for a period of five years, with recommended extensionforpatientswithpersistentfemoralT-score≤−2.5 andforthosewithpreviousfragilityfractures.After10years of bisphosphonate treatment, there have not been clini-calstudies evaluatingdifferent approaches.Atthis time, a drugwithanabolicmechanismofactionmaybeappropriate forthe high-riskpatientswithpersistentsevere osteoporo-sis.Furthermore,drugsasdenosumab,zoledronicacid and teriparatideareoptionsincasesofrefractorinesstooral bis-phosphonatesaswellasfirst-linetherapyinspecificclinical settings.
Itisimportanttomentionthatcurrently,inviewofthe limitedevidence,wedonothaveanswerstomanyofour clin-icalquestions,butwetake advantageofthe best scientific knowledgeavailabletoproposecriteriafortherationaluseof pharmacologicaltreatmentofpostmenopausalosteoporosis inthesphereofpublichealth.
Conflicts
of
interest
Theauthorsdeclarenoconflictofinterest.
r
e
f
e
r
e
n
c
e
s
1. NIHConsensusDevelopmentPanelonOsteoporosis
Prevention,Diagnosis,andTherapy.Osteoporosisprevention, diagnosis,andtherapy.JAMA.2001;285:785–95.
2. PapaioannouA,MorinS,CheungAM,AtkinsonS,BrownJP, FeldmanS,etal.2010clinicalpracticeguidelinesforthe diagnosisandmanagementofosteoporosisinCanada: summary.CMAJ.2010;182:1864–73.
3. DominguezLJ,DiBellaG,BelvedereM,BarbagalloM. Physiologyoftheagingboneandmechanismsofactionof bisphosphonates.Biogerontology.2011;12:397–408. 4. WattsNB,DiabDL.Long-termuseofbisphosphonatesin
osteoporosis.JClinEndocrinolMetab.2010;95:1555–65. 5. OrganizationWH.WorldHealthOrganizationAssessmentof
fractureriskandapplicationtoscreeningforpostmenopausal osteoporosis.Geneva,Switzerland;1994.
6. LibermanUA,WeissSR,BröllJ,MinneHW,QuanH,BellNH, etal.Effectoforalalendronateonbonemineraldensityand theincidenceoffracturesinpostmenopausalosteoporosis.N EnglJMed.1995;333:1437–43.
7. BlackDM,CummingsSR,KarpfDB,CauleyJA,ThompsonDE, NevittMC,etal.Randomisedtrialofeffectofalendronateon riskoffractureinwomenwithexistingvertebralfractures. Lancet.1996;348:1535–41.
8. CummingsSR,BlackDM,ThompsonDE,ApplegateWB, Barrett-ConnorE,MuslinerTA,etal.Effectofalendronateon riskoffractureinwomenwithlowbonedensitybutwithout vertebralfractures:resultsfromtheFractureIntervention Trial.JAMA.1998;280:2077–82.
9. HarrisST,WattsNB,GenantHK,McKeeverCD,HangartnerT, KellerM,etal.Effectsofrisedronatetreatmentonvertebral
andnonvertebralfracturesinwomenwithpostmenopausal osteoporosis:arandomizedcontrolledtrial.JAMA.
1999;282:1344–52.
10.McClungMR,GeusensP,MillerPD,ZippelH,BensenWG, RouxC,etal.Effectofrisedronateontheriskofhipfracture inelderlywomen.NEnglJMed.2001;344:333–40.
11.ChesnutCH,SkagA,ChristiansenC,ReckerR,StakkestadJA, HoisethA,etal.Effectsoforalibandronateadministered dailyorintermittentlyonfractureriskinpostmenopausal osteoporosis.JBoneMinerRes.2004;19:1241–9.
12.HarrisST,BlumentalsWA,MillerPD.Ibandronateandtherisk ofnon-vertebralandclinicalfracturesinwomenwith postmenopausalosteoporosis:resultsofameta-analysisof phaseIIIstudies.CurrMedResOpin.2008;24:237–45. 13.CranneyA,WellsG,YetisirE,AdamiS,CooperC,DelmasP,
etal.Ibandronateforthepreventionofnonvertebral fractures:apooledanalysisofindividualpatientdata. OsteoporosInt.2009;20:291–7.
14.BlackDM,DelmasPD,EastellR,ReidIR,BoonenS,CauleyJA, etal.Once-yearlyzoledronicacidfortreatmentof
postmenopausalosteoporosis.NEnglJMed. 2007;356:1809–22.
15.SirisES,PasqualeMK,WangY,WattsNB.Estimating bisphosphonateuseandfracturereductionamongUS womenaged45yearsandolder,2001–2008.JBoneMinerRes. 2011;26:3–11.
16.GohS-K,YangK,KohJ,WongM,ChuaS,ChuaD,etal. Subtrochantericinsufficiencyfracturesinpatientson alendronatetherapy:acaution.JBoneJointSurgBr. 2007;89:349–53.
17.AliT,JayRH.Spontaneousfemoralshaftfractureafter long-termalendronate.AgeAgeing.2009;38:625–6. 18.KhoslaS,BurrD,CauleyJ,DempsterDW,EbelingPR,
FelsenbergD,etal.Bisphosphonate-associatedosteonecrosis ofthejaw:reportofataskforceoftheAmericanSocietyfor BoneandMineralResearch.JBoneMinerRes.
2007;22:1479–91.
19.KhanAA,RiosLP,SándorGK,KhanN,PetersE,RahmanMO, etal.Bisphosphonate-associatedosteonecrosisofthejawin Ontario:asurveyoforalandmaxillofacialsurgeons.J Rheumatol.2011;38:1396–402.
20.StopeckAT,LiptonA,BodyJ-J,StegerGG,TonkinK,deBoer RH,etal.Denosumabcomparedwithzoledronicacidforthe treatmentofbonemetastasesinpatientswithadvanced breastcancer:arandomized,double-blindstudy.JClinOncol. 2010;28:5132–9.
21.KhanAA,MorrisonA,HanleyDA,FelsenbergD,McCauleyLK, O’RyanF,etal.Diagnosisandmanagementofosteonecrosis ofthejaw:asystematicreviewandinternationalconsensus.J BoneMinerRes.2015;30:3–23.
22.ShaneE,BurrD,AbrahamsenB,AdlerRA,BrownTD,Cheung AM,etal.Atypicalsubtrochantericanddiaphysealfemoral fractures:secondreportofataskforceoftheAmerican SocietyforBoneandMineralResearch.JBoneMinerRes. 2014;29:1–23.
23.LenartBA,LorichDG,LaneJM.Atypicalfracturesofthe femoraldiaphysisinpostmenopausalwomentaking alendronate.NEnglJMed.2008;358:1304–6.
24.SchilcherJ,MichaëlssonK,AspenbergP.Bisphosphonateuse andatypicalfracturesofthefemoralshaft.NEnglJMed. 2011;364:1728–37.
25.DellR,GreeneD,OttS,SilvermanS,EisemonE,FunahashiT, etal.Aretrospectiveanalysisofallatypicalfemurfractures seeninalargeCaliforniaHMOfromtheyears2007to2009.J BoneMinerRes.2010;25:61.
http://www.cvs.saude.sp.gov.br/up/ALERTA%20TERAP%C3 %8AUTICO%2011%20Bisfosfonatos.pdf[accessed16.01.16]. 27.USFoodandDrugAdministration.FDADrugSafety
Communication:safetyupdateforosteoporosisdrugs, bisphosphonates,andatypicalfractures.SilverSpring,MD: USFoodandDrugAdministration;2010.Availablein: www.fda.gov/Drugs/DrugSafety/ucm229009.htm[accessed 16.01.16].
28.EuropeanMedicinesAgency.EuropeanMedicinesAgency concludesclassreviewofbisphosphonatesandatypical fractures.Rareatypicalfracturesofthefemur:aclasseffect ofbisphosphonates.London;2011.Availablein:
http://www.ema.europa.eu/ema/index.jsp?curl=pages/news andevents/news/2011/04/newsdetail001245.jsp&mid= WC0b01ac058004d5c1[accessed16.01.16].
29.WangZ,BhattacharyyaT.Trendsinincidenceof
subtrochantericfragilityfracturesandbisphosphonateuse amongtheUSelderly,1996–2007.JBoneMinerRes. 2011;26:553–60.
30.BrownJP,MorinS,LeslieW,PapaioannouA,CheungAM, DavisonKS,etal.Bisphosphonatesfortreatmentof osteoporosisexpectedbenefits,potentialharms,anddrug holidays.CanFamPhysician.2014;60:324–33.
31.PapapoulosSE,CremersSC.Prolongedbisphosphonate releaseaftertreatmentinchildren.NEnglJMed. 2007;356:1075–6.
32.BlackDM,SchwartzAV,EnsrudKE,CauleyJA,LevisS,Quandt SA,etal.Effectsofcontinuingorstoppingalendronateafter5 yearsoftreatment:theFractureInterventionTrialLong-term Extension(FLEX):arandomizedtrial.JAMA.2006;296:2927–38. 33.SchwartzAV,BauerDC,CummingsSR,CauleyJA,EnsrudKE,
PalermoL,etal.Efficacyofcontinuedalendronatefor fracturesinwomenwithandwithoutprevalentvertebral fracture:theFLEXtrial.JBoneMinerRes.2010;25:976–82. 34.BlackDM,ReidIR,BoonenS,Bucci-RechtwegC,CauleyJA,
CosmanF,etal.Theeffectof3versus6yearsofZoledronic acidtreatmentofosteoporosis:arandomizedextensionto theHORIZON-PivotalFractureTrial(PFT).JBoneMinerRes. 2012;27:243–54.
35.ChavassieuxPM,ArlotME,RedaC,WeiL,YatesAJ,Meunier PJ.Histomorphometricassessmentofthelong-termeffectsof alendronateonbonequalityandremodelinginpatientswith osteoporosis.JClinInvest.1997;100:1475.
36.ToninoRP,MeunierPJ,EmkeyR,Rodriguez-PortalesJA, MenkesC-J,WasnichRD,etal.Skeletalbenefitsof alendronate:7-yeartreatmentofpostmenopausal osteoporoticwomen1.JClinEndocrinolMetab. 2000;85:3109–15.
37.McClungM,ReckerR,MillerP,FiskeD,MinkoffJ,KriegmanA, etal.Intravenouszoledronicacid5mginthetreatmentof postmenopausalwomenwithlowbonedensitypreviously treatedwithalendronate.Bone.2007;41:122–8.
38.CummingsSR,MartinJS,McClungMR,SirisES,EastellR,Reid IR,etal.Denosumabforpreventionoffracturesin
postmenopausalwomenwithosteoporosis.NEnglJMed. 2009;361:756–65.
39.PapapoulosS,RouxC,BoneH,DakinP,CzerwinskiE,FreyD, etal.Denosumabtreatmentinpostmenopausalwomenwith osteoporosisforupto9years:resultsthroughyear6ofthe freedomextension.OsteoporosInt.2015;26:S37–9. 40.KendlerDL,RouxC,BenhamouCL,BrownJP,LillestolM,
SiddhantiS,etal.Effectsofdenosumabonbonemineral densityandboneturnoverinpostmenopausalwomen transitioningfromalendronatetherapy.JBoneMinerRes. 2010;25:72–81.
41.RouxC,HofbauerL,HoP,WarkJ,ZillikensM,
Fahrleitner-PammerA,etal.Denosumabcomparedwith risedronateinpostmenopausalwomensuboptimally
adherenttoalendronatetherapy:efficacyandsafetyresults fromarandomizedopen-labelstudy.Bone.2014;58:48–54. 42.HofbauerLC,SchoppetM.Clinicalimplicationsofthe
osteoprotegerin/RANKL/RANKsystemforboneandvascular diseases.JAMA.2004;292:490–5.
43.OminskyMS,LibanatiC,NiuQT,BoyceRW,KostenuikPJ, WagmanRB,etal.Sustainedmodeling-basedboneformation duringadulthoodinCynomolgusmonkeysmaycontributeto continuousBMDgainswithdenosumab.JBoneMinerRes. 2015;30:1280–9.
44.ReginsterJ-Y,TaquetA,FraikinG,GossetC,ZegelsB. Parathyroidhormoneinthetreatmentofinvolutional osteoporosis:backtothefuture.OsteoporosInt.1997;7:163–8. 45.NeerRM,ArnaudCD,ZanchettaJR,PrinceR,GaichGA,
ReginsterJ-Y,etal.Effectofparathyroidhormone(1-34)on fracturesandbonemineraldensityinpostmenopausal womenwithosteoporosis.NEnglJMed.2001;344:1434–41. 46.HodsmanAB,BauerDC,DempsterDW,DianL,HanleyDA,
HarrisST,etal.Parathyroidhormoneandteriparatideforthe treatmentofosteoporosis:areviewoftheevidenceand suggestedguidelinesforitsuse.EndocrRev.2005;26:688–703. 47.EttingerB,MartinSJ,CransG,PavoI.Differentialeffectsof
teriparatideonBMDaftertreatmentwithraloxifeneor alendronate.JBoneMinerRes.2004;19:745–51. 48.JilkaRL,WeinsteinRS,BellidoT,RobersonP,ParfittAM,
ManolagasSC.Increasedboneformationbypreventionof osteoblastapoptosiswithparathyroidhormone.JClinInvest. 1999;104:439–46.
49.JakobF,OertelH,LangdahlB,LjunggrenO,BarrettA,KarrasD, etal.Effectsofteriparatideinpostmenopausalwomenwith osteoporosispre-treatedwithbisphosphonates:36-month resultsfromtheEuropeanForsteoObservationalStudy.EurJ Endocrinol.2012;166:87–97.
50.StepanJ,BurrD,LiJ,MaY,PettoH,SiposA,etal. Histomorphometricchangesbyteriparatidein alendronate-pretreatedwomenwithosteoporosis. OsteoporosInt.2010;21:2027–36.
51.GraeffC,TimmW,NickelsenTN,FarreronsJ,MarínF,Barker C,etal.Monitoringteriparatide-associatedchangesin vertebralmicrostructurebyhigh-resolutionCTinvivo:results fromtheEUROFORSstudy.JBoneMinerRes.2007;22:1426–33. 52.GraeffC,ChevalierY,CharleboisM,VargaP,PahrD,Nickelsen
TN,etal.Improvementsinvertebralbodystrengthunder teriparatidetreatmentassessedinvivobyfiniteelement analysis:resultsfromtheEUROFORSstudy.JBoneMinerRes. 2009;24:1672–80.
53.LewieckiE,WattsN.Assessingresponsetoosteoporosis therapy.OsteoporosInt.2008;19:1363–8.
54.Diez-PerezA,AdachiJ,AgnusdeiD,BilezikianJ,CompstonJ, CummingsS,etal.Treatmentfailureinosteoporosis. OsteoporosInt.2012;23:2769–74.
55.McClungM,HarrisST,MillerPD,BauerDC,DavisonKS,Dian L,etal.Bisphosphonatetherapyforosteoporosis:benefits, risks,anddrugholiday.AmJMed.2013;126:13–20.
56.LindsayR,SilvermanSL,CooperC,HanleyDA,BartonI,Broy SB,etal.Riskofnewvertebralfractureintheyearfollowinga fracture.JAMA.2001;285:320–3.
57.AdlerRA,FuleihanGEH,BauerDC,CamachoPM,ClarkeBL, ClinesGA,etal.Managingosteoporosisinpatientson long-termbisphosphonatetreatment:reportofataskforceof theAmericanSocietyforBoneandMineralResearch.JBone MinerRes.2016;31:16–35.
58.VasikaranSD,ChubbSP.Theuseofbiochemicalmarkersof boneturnoverintheclinicalmanagementofprimaryand secondaryosteoporosis.Endocrine.2016;52:222–5. 59.ModiA,SirisES,TangJ,SenS.Costandconsequencesof
60.EttingerB,BlackDM,MitlakBH,KnickerbockerRK,Nickelsen T,GenantHK,etal.Reductionofvertebralfractureriskin postmenopausalwomenwithosteoporosistreatedwith raloxifene:resultsfroma3-yearrandomizedclinicaltrial. JAMA.1999;282:637–45.