Anibais a genus that comprises 41 species of shrubs and trees and is primarily a lowland group with its centre of di-versity in Central Amazonia and Guiana. However, this genus extends into the Andes, the mountains of northern Venezuela, the lesses Antilles and eastern and southern Brazil. One of its species, Aniba riparia (NEES) MEZ, from the Lauraceae family, is popularly known as “louro” in Brazil and occurs in the Humaitá region of the Amazonas state of Brazil.1)
From the unripe fruit of Aniba riparia, collected from the Amazonas state of Brazil, were isolated three substances with broad spectrum antimicrobial activity: methyl ethers of
N-(benzoyl) tyramine (riparin I), N-(2-hydroxybenzoyl) tyra-mine (riparin II) and N-(2,6-dihydroxybenzoyl) tyramine (ri-parin III)2)(Fig. 1) which were later synthesized.3,4)
It was reported that one of the above compounds, (O -methyl)-N-(2,6-dihydroxybenzoyl) tyramine (riparin III), has potent smooth muscle relaxant activity.5,6) Thus, in concen-trations from 8 to 30mM, riparin III antagonized acetyl-choline- and histamine-induced contractions of guinea-pig ileum, and oxytocin- and bradykinin-induced contractions of the rat uterus. Further, in the guinea-pig trachea, riparin III inhibited the spontaneous tone (IC507.7mM) and carbachol-induced contractions (IC5010mM). The spasmolytic effect of
riparin III, was investigated7)concerning the involvement of the compound in relation to Ca2⫹metabolism. It was demon-strated that riparin III produces an inhibition of Ca2⫹ influx and release of intracellular Ca2⫹. These results lead to the re-duction of intracellular Ca2⫹concentration and possibly con-tribute to the drug spasmolytic effect.
Recently, our group has made a central nervous system pharmacological screening of riparin III, which presented anxiolytic and antidepressant effects in mice, when adminis-tered intraperitonally.8)Thus, the general purpose of the pres-ent study was to analyze whether the orally administration of riparin III promotes anxiolytic effects in male mice.
MATERIALS AND METHODS
Animals Male Swiss mice (25—30 g) provided by the Animal House of the Federal University of Ceará (Brazil) were used in each experiment. The animals were housed, groups of 30, in plastic cages with sawdust as beddings and kept in a room with controlled temperature (23⫾1 °C) and a 12 h light/dark cycle, with food and water ad libitum, except during the experiments. Animals were treated in accordance to the current law and the NIH Guide for Care and Use of Laboratory Animals.
Drugs Riparin III was emulsified with 2% Tween 80 (Sigma-U.S.A.). Animals were treated with the substance at doses of 25 and 50 mg/kg, orally, one hour before the experi-ments. Controls received 2% Tween 80 (Sigma-U.S.A.) dis-solved in distilled water at the same volume as the treated groups (10 ml/kg). Diazepam (DZP) 1 and 2 mg/kg, in-traperitoneally, (União Química Brazil) was used as standard. Experimental Protocol The animals were tested during the light period and were observed in a closed room at con-stant temperature (23⫾1 °C) and poorly illuminated with a
March 2006 Biol. Pharm. Bull.29(3) 451—454 (2006) 451
Anxiolytic-Like Effects of (
O
-Methyl)-
N
-2,6-dihydroxybenzoyl-tyramine
(Riparin III) from
Aniba riparia
(N
EES) M
EZ(Lauraceae) in Mice
Carla Thiciane Vasconcelos de MELO,aAndreisa Paiva MONTEIRO,aCaroline Porto LEITE,a Fernando Luiz Oliveira de ARAÚJO,aVera Targino Moreira LIMA,aJosé Maria BARBOSA-FILHO,b Marta Maria de FRANÇAFONTELES,aSilvânia Maria Mendes de VASCONCELOS,a
Glauce Socorro de BARROSVIANA,aand Francisca Cléa Florenço de SOUSA*,a
aDepartment of Physiology and Pharmacology, Faculty of Medicine, Federal University of Ceará; Rua Cel. Nunes de Melo
1127, 60430–270, Fortaleza, Brazil: and bLaboratory of Pharmaceutics Technology, Federal University of Paraíba; João Pessoa, Brazil. Received July 4, 2005; accepted December 1, 2005
This work presents behavioral effects of (O-methyl)-N-2,6-dihydroxybenzoyl-tyramine (riparin III) isolated from the unripe fruit of Aniba riparia(NEES) MEZ(Lauraceae) in animal models of open field, rota rod, elevated
plus maze and hole board tests in mice. Riparin III (ripIII) was administered orally, in male mice, at single doses of 25 and 50 mg/kg. The results showed that ripIII, at both doses, had no effects on the spontaneous motor activ-ity in the rota rod test nor in the number of squares crossed in the open field test. However, riparin III decreased the number of grooming and rearing. In the plus maze test, ripIII, at both doses increased the following parame-ters: percentage of entries in the open arms (PEOA), time of permanence in the open arms (TPOA) and percent-age of time of permanence in the open arms (PTOA) and at the dose of 50 mg/kg, increased the number of entries in the open arms (NEOA). Similarly, ripIII, at both doses, showed an increase in the number of head dips into the holes of the hole board test. These results show that riparin III presents anxiolytic effects in the plus maze and hole board tests which are not influenced by the locomotor activity in the open field test.
Key words Aniba riparia; riparin III; anxiolytic effect
© 2006 Pharmaceutical Society of Japan ∗To whom correspondence should be addressed. e-mail: clea@ufc.br.
15-V red light. One hour after the treatment, the open field and rota rod tests were performed with the same animals in the manner described below: Firstly, the animal was placed in the open field area for 5 min. Immediately after the open field test, the animal was removed to the rota rod where it was evaluated for 1 min. All the other tests were performed in dif-ferent days with other groups of animals.
Open Field Test The open field area was made of acrylic (transparent walls and black floor, 30⫻30⫻15 cm) di-vided into nine squares of equal area. The open field was used to evaluate the exploratory activity of the animal.9)The observed parameters were: number of squares crossed (with the four paws) and numbers of grooming which consists of licking the paws, washing movements over the head, fur lick-ing and tail/genitals cleanlick-ing10—12) and rearing which is the number of times an animal stood erect on its hind legs with forelegs in the air of against the wall. The animals were di-vided into four groups with 7—20 animals per group.
Rota Rod Test In this test13) the animals divided into four groups with 7—20 mice per group were placed with the four paws on a 2.5 cm diameter bar, 25 cm above the floor, which was turning at 12 rpm. For each animal, the number of falls (up to three falls) and time of permanence on the bar for 1 min were registered.
Elevated Plus Maze Test (EPM) The elevated plus maze for mice14) consisted of two opposed open arms (30⫻5 cm) and two closed arms (30⫻5⫻25 cm) also in op-posed position. The open and closed arms were connected by a central platform (5⫻5 cm). The lateral walls of the closed arms were made of transparent acrylic and the floor was made of black acrylic. The maze was 45 cm above the floor. One hour after the treatment, the animal was placed at the center of the plus maze facing one of the enclosed arms, and observed for 5 min, according to the following parameters: number of entries into the open and closed arms, and time of permanence in each of them. The ratios “number of entries into open arms/number of entries into all (i.e., open and closed) arms” and “time spent in the open arms/time spent in all arms” were calculated and multiplied by 100 to yield the percentages of entries into open arms (PEOA) and the per-centage of time of permanence in the open arms (PTOA). Anxiolytic compounds reduce the animal’s aversion to the open arms and promotes the exploration thereof. On the other hand, the forced or voluntary passages of the animal into the open arms of the elevated plus maze are associated with hormonal and behavioral changes indicative of in-creased anxiety. The animals were divided into four groups with 8—17 per group.
Hole Board Test The hole-board test for exploratory be-havior in mice was used as described previously by Clark et al. (1971).15) The apparatus used was an Ugo Basile of 60 cm⫻30 cm with 16 evenly spaced holes with in built infra red sensors. In brief, adult male mice were randomly divided into four groups with 8—20 mice per group. Two groups re-ceived graded doses of riparin III (25, 50 mg/kg, p.o.). One group received diazepam (1 mg/kg, i.p.) and the remaining group received vehicle orally to serve as control. One hour later, the number of head dips into the holes was counted for each animal for 5 min. An increase in the head dipping re-sponse reveals a positive anxiolytic-like effect.16)
Statistical Analysis Data were analyzed by Graphpad
Prism version 4.0 software and presented as mean⫾S.E.M. values. The statistical tests used were one way analysis of variance (ANOVA) followed by Tukey as post hoc test, ex-cept in the parameter of number of falls of rota rod test, in which was used a nonparametric test (Kruskal–Wallis) fol-lowed by Dunns as post hoc. Results were considered signifi-cant at p⬍0.05.
RESULTS
Open Field Test RipIII, at both doses, p.o., decreased the number of rearing [control: 10.1⫾0.46 (15); ripIII-25: 6.1⫾1.35 (15); ripIII-50: 6.7⫾0.73 (15)] and the number of grooming [control: 3.5⫾0.32 (16); ripIII-25: 2.1⫾0.34 (16); ripIII-50: 1.7⫾0.21 (16)] comparing to respective controls. The substance did not alter the number of squares crossed. Diazepam 2 mg/kg (DZP-2), i.p., decreased all the parame-ters compared to controls (Table 1).
Rota Rod Test The absolute values of number of falls and time of permanence are presented in Table 2. RipIII, p.o., showed no alteration in both parameters unlikely DZP-2, i.p., which decreased both parameters compared to respective controls.
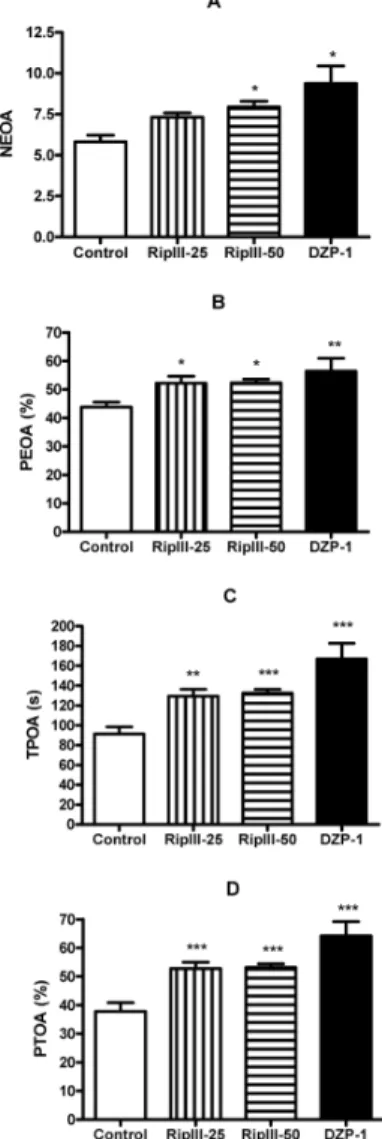
Elevated Plus Maze Test In this test (Fig. 2), riparin III 50 mg/kg, p.o., and diazepam 1 mg/kg, i.p., significantly increased NEOA [control: 5.81⫾0.41 (16); ripIII-50: 7.94⫾0.35 (18); DZP-1: 9.37⫾1.06 (8)]. Both doses of ri-parin III and diazepam increased all other parameters ana-lyzed, PEOA, TPOA and PTOA compared to respective controls: PEOA [control: 43.83⫾1.70 (16); ripIII-25: 52.23⫾2.37 (16); ripIII-50: 52.28⫾1.35 (16); DZP-1: 56.43⫾4.49 (8)]; TPOA [control: 91.35⫾7.12 (17); ripIII-25: 129.4⫾7.01 (17); ripIII-50: 132.6⫾3.84 (17); DZP-1: 167.0⫾15.70 (8)] and PTOA [control: 37.81⫾3.07 (16); ripIII-25: 52.80⫾2.18 (16); ripIII-50: 53.15⫾1.30 (17); DZP-1: 64.17⫾5.04 (8)].
Hole Board Test Similarly to diazepam (1 mg/kg; i.p.), riparin III at both doses, p.o., (Fig. 3) increased the number
452 Vol. 29, No. 3
Table 1. Effects of Riparin III and Diazepam in the Open Field Test
Groups Number of squares Rearingb) Groomingc) crosseda)
Control 50.6⫾4.66 (19) 10.1⫾0.46 (15) 3.5⫾0.32 (16) RipIII-25 50.6⫾2.43 (19) 6.1⫾1.35 (15)** 2.1⫾0.34 (16)** RipIII-50 57.4⫾2.83 (19) 6.7⫾0.73 (15)* 1.7⫾0.21 (16)*** DZP-2 24.3⫾7.6 (7)** 0*** (9) 1.1⫾0.29 (8)*
Each value represents the mean⫾S.E.M. of the number of animals in parenthesis.
∗p⬍0.05, ∗∗p⬍0.01, ∗∗∗p⬍0.001 as compared with the respective control group. a—c) One way ANOVA followed by Tukey as the post hoc.
Table 2. Effects of Riparin III and Diazepam in the Rota Rod Test
Groups Number of fallsa) Time of permanenceb)(s)
Control 1.45⫾0.21 (20) 51.75⫾2.43 (20) RipIII-25 1.90⫾0.22 (20) 46.05⫾3.18 (20) RipIII-50 1.70⫾0.26 (20) 49.05⫾3.18 (20) DZP-2 2.71⫾0.18 (7)* 35.25⫾6.68 (8)*
Each value represents the mean⫾S.E.M. of the number of animals in parenthesis.
of head dips [control: 30.60⫾1.25 (20); ripIII-25: 40.87⫾2.44 (15); ripIII-50: 40.00⫾2.83 (15); DZP-1: 44.88⫾1.83 (8)] as compared to control.
DISCUSSION
In this work, the effects of riparin III from A. ripariawere
studied in animal models of anxiety, such as open field, rota rod, EPM, and hole board tests. These tests are classical models for screening central nervous system actions provid-ing information about psychomotor performance, myorelax-ant activity and anxiety.
The assessment of anxiety-related behavior in animal models is based on the assurance that anxiety in animals is comparable to anxiety in humans. Although, as a matter of fact, it cannot be proven that an animal experiences anxiety in the same way as a human being, it is undisputed that dis-tinct behavior patterns in rodents indicate anxiety, i.e. behav-ioral and peripheral changes presumed to accompany high sympathetic nervous activity.17)Thus, an analogy, if not a ho-mology, between anxiety in humans and rodents may be as-sumed.
Taking into consideration the various ways to keep the ani-mal under stress, it is well known from both field studies and laboratory observations that rodents tend to avoid the unpro-tected area of a novel environment when first entering it.18,19) In an experimental setup, usually represented by a defined area, as the open field, rodents will typically start to explore the environment along the walls while avoiding the open, i.e.
unprotected area. In order to guarantee that the animal would explore the unprotected area, we placed the animal in the centre of the open field instead of the lateral. Another point of stressful event is that the aversive character of an area can be modulated by illumination levels, with a brightly lit area being more aversive for rodents, thus producing a more pro-nounced avoidance behavior than a dark area. Likewise, we placed the animals in a closed room poorly illuminated with a 15-V red light. Another possibility to increase a rodent’s aversion against an unprotected area is by elevating it and by enabling the animal to see the edge, as we can see in the EPM and hole board tests respectively.
The EPM is based on the observation that rodents tend to avoid elevated areas.20) Following the concept of avoidance behavior in rodents, avoidance of the open arms is inter-preted as anxiety.21—23) Moreover, the EPM test is the most popular test to search for new benzodiazepine-like anxiolytic agents.21,22)Our results showed that riparin III decreased the avoidance to open arms, thereby increasing the number of entries into open arms (NEOA) with the higher dose and also increased, at both doses, the time of permanence in the open arms (TPOA), the percentage of entries into open arms (PEOA) and the percentage of time of permanence in the open arms (PTOA), similar to the effects observed after ad-ministration of the reference anxiolytic drug diazepam (1 mg/kg).
The hole board test is a measure of exploratory behav-ior.24) Exploration is gradually inhibited by anxiety, thereby representing an indirect measurement of anxiety.22,25)The in-hibition of exploration behavior can be reversed by anxiolytic compounds26—28)indicated, in the case of hole board test, by the increase of number of head dips.29) The animals treated with riparin III at both doses showed an increase in the num-ber of head dips as the same as diazepam (1 mg/kg), indicat-ing an anxiolytic activity.
Data in literature demonstrated that drugs that alter gen-eral motor activity may give false-positive/negative results in the plus maze. In this way, we decided to study the effects of riparin III in the open field test, a classical animal model that
March 2006 453
Fig. 2. Plus Maze Test of Groups of Mice Which Received Vehicle, Ri-parin III (25, 50 mg/kg) and DZP (1 mg/kg)
(A) NEOA: number of entries in the open arms; (B) PEOA (%): percentage of entries in the open arms; (C) TPOA (s): time of permanence in the open arms. The results are presented as mean⫾S.E.M. Significant difference compared with control (∗p⬍0.05; ∗∗p⬍0.01; ∗∗∗p⬍0.001). ANOVA and Tukey as the post hoctest.
Fig. 3. Hole Board Test of Groups of Mice Which Received, Riparin III (25, 50 mg/kg), and DZP (1 mg/kg)
allows measurements through which is possible to evaluate autonomic effects of drugs and general activity of animals.30) Our findings show that riparin III (25, 50 mg/kg) did not alter the locomotor activity different from diazepam 2 mg/kg which decreased this parameter showing a sedative effect. Likewise, it is unlikely that these effects of riparin III ob-served in the plus maze and hole board tests are based on the stimulation of general motor activity. Further, in the rota rod test, riparin III, different from diazepam (2 mg/kg), had no effect on the motor co-ordination suggesting that the anxi-olytic-like effect of the substance might not be exerted through peripheral neuromuscular blockade, but rather, elicited centrally.31,32)
It is well known that benzodiazepines act as anxiolytics (at low doses), anticonvulsants, and also produce sedation and a myorelaxant effect at higher doses,33—36) thereby our group has used diazepam at 1 mg/kg in EPM and hole board tests and 2 mg/kg in open field and rota rod tests.
In this work, we could observe that riparin III seems to be more active after oral administration as compared to the in-traperitoneal administration as detected in previous work8)in the plus maze test. However, the apparent discrepancy could be due to the different number of animals used in each condi-tion. For instance, in the first condition (after oral administra-tion) number of animals used were higher. It is largely ac-cepted that behavioral tests require a great number of ani-mals due to tests variability. In this way, in the second condi-tion (intraperitoneal administracondi-tion), although not detected statistically, a clear tendency was observed. Furthermore, re-cent data from our laboratory using riparin I (methyl ethers of N-benzoyl tyramine) also isolated from unripe fruit of
Aniba ripariademonstrated anxiolytic activity in both routes of administration.37)
The anxiolytic effect of riparin III detected in the plus maze test was also observed in the hole board test with both routes of administration. Similarly, in the open field test, it could be seen that riparin III had the same effects comparing i.p.8)and p.o.(present study) administrations.
In conclusion, we showed that acute oral administration of riparin III presented anxiolytic-like effects in the elevated plus maze and hole board tests and was devoid of sedative ef-fect as assessed by the open field test.
REFERENCES
1) Castelo-Branco U. V., Castelo-Branco U. J. V., Thomas G., Araújo C. C., Barbosa-Filho J. M., Acta Farm. Bonaerense, 19, 197—202 (2000). 2) Barbosa-Filho J. M., Yoshida M., Gottlieb O. R., Barbosa R. C. S. B. C., Giesbrecht A. M., Young C. M., Phytochemistry, 26, 2615—2617 (1987).
3) Barbosa-Filho J. M., Yoshida M., Gottlieb O. R. Rev., Latinoamer. Quim., 21, 5—7 (1990).
4) Barbosa-Filho J. M., Silva E. C., Bhattacharyya J., Química Nova, 13, 332—334 (1990).
5) Castelo Branco U. J. V., Thomas G., Araújo C. C., Barbosa-Filho J. M., Resume VI Reunião Anual de Federação de Sociedades de Biologia Experimental. Abstr., 302, 6—69 (1991).
6) Castelo Branco U. J. V., “Estudos Farmacológicos do Éter Metílico de N-(2,6-Dihydroxibenzoyl)-tyramine,” MSc Thesis, ed. by Federal Uni-versity of Paraíba, Brazil, 1992.
7) Thomas G., Castelo-Branco U. J. V., Barbosa-Filho J. M., Bachelet M., Vargaftig B. B., J. Pharm. Pharmacol., 46, 103—107 (1994). 8) Sousa F. C. F., Melo C. T. V., Monteiro A. P., Lima V. T. M., Gutierrez
S. J. C., Pereira B. A., Barbosa-Filho J. M., Vasconcelos S. M. M., Fonteles M. F., Viana G. S. B., Pharmacol. Biochem. Behav., 78, 27— 33 (2004).
9) Archer J., Anim. Behav., 21, 205—235 (1973).
10) Kalueff A. V., “Grooming and Stress,” ed. by Kiv, KSF Publishers, 2002.
11) Kruk M. R., Westphal K. G. C., VanErp A. M. M., VanAsperen J., Cave B. J., Slater E., Neurosci. Biobehav. Rev., 23, 163—177 (1998). 12) VanErp A. M. M., Kruk M. R., Meelis W., Willekens-Bramer D. C.,
Behav. Brain Res., 65, 7—55 (1994).
13) Dunham N. W., Miya T. S., J. Am. Pharm. Assoc., 46, 208 (1957). 14) Lister R. G., Psycopharmacol., 92, 180—185 (1987).
15) Clark G., Koster A. G., Person D. W., Psychopharmacology, 20, 169— 171 (1971).
16) File S., Pellow S., Br. J. Pharmacol., 86, 729—735 (1985). 17) Hall C. S., J. Comp. Physiol. Psychol., 22, 345—352 (1936). 18) Barnett S. A., “The Rat: a Study in Behavior,” ed. by University of
Chicago Press, Chicago, 1975.
19) Treit D., Fundytus M., Pharmacol. Biochem. Behav., 31, 959—962 (1989).
20) Montgomery K. C., J. Comp. Physiol. Psychol., 48, 254—260 (1958). 21) Rodgers R. J., Cao B. J., Dalvi A., Holmes A., Braz. J. Med. Biol. Res.,
30, 289—304 (1997).
22) Pellow S., Chopin P., File S. E., Briley M., J. Neurosci. Methods, 14, 149—167 (1985).
23) Lister R. G., Pharmacol. Ther., 46, 321—340 (1990). 24) Crawley J. N., Neurosci. Behav. Rev., 9, 37—44 (1985).
25) Crawley L. N., Goodwin F. K., Pharmacol. Biochem. Behav., 13, 167—170 (1980).
26) Belzung C., Berton F., Behav. Pharmacol., 8, 541—548 (1997). 27) Griebel G., Moreau J. L., Jenck F., Martin J. R., Misslin R., Behav.
Proc., 29, 37—48 (1993).
28) Rodgers R. J., Cole J. C., Cobain M. R., Behav. Pharmacol., 3, 621— 624 (1992).
29) Takeda H., Tsuji M., Matsumiya T., Eur. J. Pharmacol., 350, 21—29 (1998).
30) Novas M. L., Wolfman C., Medina J. H., De Robertis E., Pharmacol. Biochem. Behav., 30, 331—336 (1988).
31) Perez G. R. M., Perez L. J. A., Garcia D. L. M., Sossa M. H., J. Ethnopharmacol., 62, 43—48 (1998).
32) Amos S., Kolawole E., Akah P., Wambebe C., Gamaniel K., Phy-tomedicine, 8, 356—361 (2001b).
33) Onaivi E. S., Maguiri P. A., Tsai N. F., Davies M. F., Locu G. H., Pharmacol. Biochem. Behav., 43, 825—831 (1992).
34) Tang A. H., Code R. A., Himes C. S., Eur. J. Pharmacol., 236, 1—5 (1993).
35) Davies M. F., Onaivi E. S., Chen S. W., Maguiri P. A., Tsai N. F., Loew G. H., Pharmacol. Biochem. Behav., 49, 47—56 (1994).
36) Wolffgramm J., Mikolaicz K. C., Coper H. Naunyn-Schmiedebergs, Arch. Pharmacology, 349, 279—286 (1994).
37) Sousa F. C. F., Monteiro A. P., Melo C. T. V., Oliveira G. R., Vasconce-los S. M. M., Fonteles M. M. F., Gutierrez S. J. C., Barbosa-Filho J. M., Viana G. S. B., Phytother. Res., 19, 1005—1008 (2005).