w ww . e l s e v i e r . c o m / l o c a t e / b j p
Original
Article
Antiviral
activity
of
Myracrodruon
urundeuva
against
rotavirus
Alzira
B.
Cecílio
a,∗,
Pollyana
de
C.
Oliveira
a,
Sérgio
Caldas
a,
Priscilla
R.V.
Campana
b,
Fernanda
L.
Francisco
b,
Maria
Gorette
R.
Duarte
c,
Lorena
de
A.M.
Mendonc¸
a
b,
Vera
L.
de
Almeida
baDivisãodePlataformasTecnológicas,DiretoriadePesquisaeDesenvolvimento,Fundac¸ãoEzequielDias,BeloHorizonte,MG,Brazil
bDivisãodeDesenvolvimentoTecnológicoFarmacêutico,DiretoriadePesquisaeDesenvolvimento,Fundac¸ãoEzequielDias,BeloHorizonte,MG,Brazil cDivisãodeEpidemiologiaeControledeDoenc¸as,DiretoriadoInstitutoOctávioMagalhães,Fundac¸ãoEzequielDias,BeloHorizonte,MG,Brazil
a
r
t
i
c
l
e
i
n
f
o
Articlehistory:
Received26December2014 Accepted7October2015 Availableonline2December2015
Keywords: Medicinalplant Myracrodruonurundeuva Crudeextract
Leaves
Antiviralactivityrotavirus
a
b
s
t
r
a
c
t
MyracrodruonurundeuvaAllemão,Anacardiaceae,isamedicinalplantwidelyfoundinBrazil,especially inthenorthernregion.Inourpreviousstudy,theethanolicextractfromleavesofM.urundeuvashowed antiviralactivityagainstsimianrotavirusSA-11.Here,thecrudeextractwassubjectedtofractionationsin ordertosubsequentlyworkwithmoreconcentratedandpurebioactivecompounds,whichwereanalyzed byTLCandHPLCmethodstosupportabetterunderstandingoftheirvirucidaleffect.Theantiviralactivity wasevaluatedusingarotavirusinfectionmodelinMA-104cellstreatedwiththemaximumnon-cytotoxic concentrationofthecrudeextractanditsfractions.Datawereexpressedasthepercentageinhibitionof viralreplicationcalculatedbytheinhibitionofcytopathiceffectinthetreatedcellscomparedtountreated controlsafter48hofincubation.First,weconductedafractionation,generatingfivefractions(F1–F5) whichweresubmittedtoantiviralassay.Then,thefractionthatshowedthehighestvirucidaleffect(F3, PI=75%)wassubjectedtoalargerpartition,yieldingeighteensubfractions,whichweresubmittedto newantiviralassays.Terpenes,flavonoidsandtanninswerethemajorsecondarymetabolitesdetected byTLCanalysisinF3.SF1,aflavonoid-enrichedfraction,showedthestrongestinvitroactivityagainst rotavirus(PI=92%),preventingcytopathiceffect.ChromatographicprofileswereobtainedbyHPLCforthe crudeextractandSF1,themostpotentsubfraction.Overall,ourdatapointtothepotentialanti-rotavirus activityofflavonoid-enrichedfraction(SF1)ofM.urundeuvaleaves,corroboratingthetraditionaluseof thisspeciestotreatdiarrheaandbroadeningourperspectivesoninvivoassaysinmicewithSF1isolated orassociatedwithotherfractions.
©2015SociedadeBrasileiradeFarmacognosia.PublishedbyElsevierEditoraLtda.Allrightsreserved.
Introduction
Rotavirusesarerecognizedasamajorcauseofnon-bacterial
gastroenteritisespeciallyininfantsandyoungchildrenworldwide
(Parasharetal.,2006;Junaidetal.,2011).Itisanunpredictable
diseasehighlycontagiousandmayleadtoseveredehydrationand evendeath(Offit andClark,2000).Itstransmissionispersonto personthroughthefecal-oralroute.Controlandpreventionofthis infectionaredifficultduetothelackofanyeffectivetreatment
otherthanpalliativemeasuresandthepresenceofasymptomatic
children shedding virus(Dennehy, 2000). Despite two liveoral rotavirusvaccinesthathavealreadybeenlicensed,amonovalent
human rotavirus vaccine (Rotarix, GlaxoSmithKline Biologicals)
and a pentavalent bovine-human reassortant rotavirus vaccine
(RotaTeq,Merck),theireffectivenessindevelopingcountrieshas
∗ Correspondingauthor.
E-mail:alzira.cecilio@funed.mg.gov.br(A.B.Cecílio).
shownapooledefficacyofabout51%requiringeffortstooptimize
(Jiangetal.,2010).
Nevertheless,inseveralcases,wherethehostissufferingfrom prolongeddiarrheaandfever,virus-specifictreatmentwillbe nec-essaryifpossible(Takahashietal.,2001).Naturalproductshave
beenthe sourceofmostof theactive ingredientsof medicines
andmorethan80%ofdrugsubstanceswerenaturalproductsor
inspiredbyanaturalcompound(Harvey,2008).Inthiscontext, invitroassays have beenestablishedand usedby ourresearch grouptoscreenantiviralactivitiesofextracts,fractionsandnatural substanceswithpotentialtherapeuticalaction.
Inpreviouswork,usingsimilarmethodologyemployedhere,
weevaluatedethanoliccrudeextractsofdifferentBrazilian medic-inalplantsandweobservedaninvitroanti-rotavirusactivityof Myracrodruon urundeuva Allemão, Anacardiaceae (Cecílio et al., 2012),amedicinalplantwidelyusedinBrazil,mainlyduetoits
anti-inflammatory, antimicrobialand wound healing properties
(Monteiroetal.,2006;Souzaetal.,2007;Sáetal.,2009a).Thisplant
isarepresentativespeciesoftheAnacardiaceaefamilyoccurringin
http://dx.doi.org/10.1016/j.bjp.2015.10.005
BrazilandotherSouthAmericancountries,notablyintheCerrado region(Leite,2002).
Analgesic, anti-inflammatory, antioxidant, antifungal, and
antibacterial, among other activities, have been reported from
preparationsmadewithM.urundeuvaextracts.Vianaetal.(1997,
2003)haveobserved analgesicandanti-inflammatoryeffects of
thetannin and chalcones fractions isolated from M.urundeuva
barksinstudiesconductedinmice.Souzaetal.(2007)havealso shownthattannin-enrichedfractionfromstembarkofthisplant presentsanti-inflammatoryandantiulcer effectsin mice,partly duetoitsantioxidantaction,knowntobepresentinpolyphenols, includingtannins.Interestingly,DeMendonc¸aAlbuquerqueetal.
(2011)demonstrated inhibitionofmyeloperoxidaseactivityand
antioxidanteffectsofchalconesfromM.urundeuvastembarkson anallergicconjunctivitismodelinguineapig,indicatingthemas candidatesforthetreatmentofallergicconjunctivitisandother
inflammatoryconditions.
AccordingtoSáetal.(2009a),lectinsisolatedfromM. urun-deuvaheartwoodshowedantimicrobialactivityagainstbacteria andfungithatattackplants,includingwoods.Inaddition,stembark hydroethanolicextractwasactiveagainstStaphylococcusaureus, Klebsiellapneumonia,EnterococcusfaecalisandCandidaspp.(Alves
etal.,2009;Gomesetal.,2013),andtheseactivitieswereattributed
tothepresenceofbioactivecompoundssuchastannins,flavonoids andalkaloids.
Furthermore,experimentsinratsthatpointtoanti-diarrheal
(Chavesetal.,1998)andneuroprotectiveactivities(Nobre-Júnior
etal.,2009),colonicanastomotichealing(Goesetal.,2005),and evenlarvicidaleffectagainstAedesaegypti(Napoleãoetal.,2012) andtermiterepellentaction(Sáetal.,2009b)showthe biologi-calpotentialofthisplant,whichhidesarichsourceofcompounds thatcouldbeemployedtotherapeuticandbiotechnological appli-cations,amongotherpurposes.
ConsideringthepharmacologicalpotentialofM.urundeuvaand ourpreviousdataofanti-rotavirus activity(Cecílioetal.,2012), thecrudeextractfromtheleavesofthisplantwasfractionatedand subjectedtoantiviralassay,beingtheactivefractionscharacterized
byTLCmethodinanattempttoidentifythebioactivecompounds
involvedinthevirucidaleffect.
Materialandmethods
Plantmaterial
MyracrodruonurundeuvaAllemão,Anacardiaceae,leaveswere collectedfromadultplantsinthe“cerrado”areaofSantanado Pira-pama,intheStateofMinasGerais,Brazil,betweenSeptember2006 andFebruary2007.Theplantwasidentifiedasavoucherspecimen
andwasdepositedattheHerbárioPAMGdaEmpresadePesquisa
AgropecuáriadeMinasGeraisunderthenumberPAMG53312.
Preparationofextractandfractionation
Thecrudeextract (MUL)waspreparedby percolationofthe
driedandpowderedmaterialwithethanol95GL(VetecQuímica
Fina)untilexhaustionatroomtemperatureandevaporatedunder
reducedpressureat40◦C.Theethanolicextract(250g)was
frac-tioned by filtrationchromatography in silica gel (silica gel 60, 0.040–0.063mm,Merck),givingfivefractionsafterelutionwith
hexane(F1,30g),dichloromethane (F2,21g),
dichloromethane-ethyl acetate (1:1) (F3, 14.7g), ethyl acetate (F4, 45.5g) and
methanol(F5, 120g). A portionof themostpotentfraction, F3
(10g), was further fractioned in a silica gel column (silica gel
60,0.040–0.063mm,Merck),withsolventsofincreasing
polari-ties(dichloromethane,ethylacetate,ethylacetate,methanol,water
andformicacid1%andmixturesofthese),yieldingeighteen sub-fractions.SF1(47mg),obtainedwithDCM:EtOAc(1:1),showedthe bestantiviralactivityanditschemicalcompositionwasevaluated byTLCandHPLCprofiles.Thelowamountobtainedprevented fur-therfractionation.
Phytochemicalscreening
MUL,fractions(F1–F5)andthesubfractionSF1weresubjected tophytochemicalscreeningtodeterminethepresenceofdifferent classesofnaturalproductsusingmethodsdescribedbyWagnerand
Bladt(2001).Theanalysiswasperformedbythin-layer
chromatog-raphy(TLC)onMercksilicagel60F254aluminumplates,which
weredevelopedaccordingtoTable1.Thepresenceoftanninswas determinedusingaproteinprecipitationtest(Matos,1997).
HPLCanalysis
MULand SF1were preparedat10mg/mland at5mg/mlin
MeOHHPLCgrade,respectively(VetecQuímicaFina).After
cen-trifugation at 9300×g (Eppendorf, model, 5415D) thesolution
wasinjected in an Agilent Technologies 1200 seriesHPLC
sys-temequippedwithDADdetector.Chromatographicanalysiswas
obtainedinaZorbaxXDBC18column(50×4.6mm,1.8m),at
40◦C,flowrateof0.3ml/minanddetectionat210nm.Theprofiles
wereobtainedinlineargradientelutionofwater(A)and acetoni-trile(B),from15to90%ofBfrom0to50min,andfrom90to95%
ofBbetween50and60min.TheUVspectrawererecordedonline
inthe190–400nmrangeforallretentiontimes.
Biologicalassays
Samplepreparation
For thebioassays, each samplewas solubilizedin
dimethyl-sulfoxide(DMSO,Sigma–Aldrich)at50mg/mlandcentrifugedat
9300×g(Eppendorf,model,5415D).Thesamplesweredilutedto
workconcentrationusingculturemedia.
Cellsandviruses
MA-104cells(arhesusmonkeykidneycellline)werecultivated
inDulbecco’smodifiedEaglemedia(DMEM)supplementedwith
10%fetalbovineserum(FBS),100g/mlofstreptomycin(Gibco)
and100U/mlpenicillinG(Invitrogen).Thecellcultureswere main-tainedat37◦Cinahumidified5%CO2atmosphere.Simianrotavirus
SA11wereactivatedwith10g/mltrypsinfor60minat37◦Cand
propagatedinMA-104cellsmonolayersinthepresenceof10g/ml
trypsin.Thevirustiterswere estimatedfromcytopathogenicity
bythelimit-dilutionmethodandexpressedas50%tissueculture infectiousdoseperml(TCID50/ml)(ReedandMuench,1938).
Cytotoxicity
Thecytotoxicityofthesamples(MUL,fractions and
subfrac-tions)wasdeterminedusingthemethoddescribedbyMirandaetal.
(1999)basedoncellularmorphologicalterations.Several
concen-trations(5000,500,50,5and0.5g/ml)ofsampleswereplacedin
contactwithconfluentMA-104cellsmonolayerspreparedin
12-wellmicroplatesandwereincubatedat37◦Cinahumidified5%
CO2atmospherefor48h.Aftertheincubationperiod,thecellswere examinedusinganinvertedopticalmicroscope(Nikon)andtreated
anduntreatedcultures(control)werecompared.Thehigher
con-centrationofeachextractshowingnocellularmorphologicchanges
Table1
TLCconditionstotestthepresenceofdifferentclassesofnaturalproductsintheethanolicextract.
Class Eluent Spray
Tannins Toluene:aceticacid:formicacid(70:167:14) K3Fe(CN)61%:FeCl32%(1:1)
Flavonoids Ethylacetate:methanol:water(176:22:22) AlCl3reagent(2%)
Anthraquinones Toluene:acetone:chloroform(40:25:35) KOHreagent(5%)
Terpenes Hexane:ethylacetate(1:1) Anisaldehyde-sulfuricacidreagent
Cardiotonicglycosides Ethylacetate:methanol:water(20:3:2) Keddereagent Alkaloids Ethylacetate:formicacid:aceticacid:water:ethylmethylcetone(86:16:23:47:78) Dragendorffreagent
andwasconfirmedusingPromega’sCytoTox96®Non-Radioactive CytotoxicityAssay,accordingtothemanufacturer’sinstructions.
Antiviralassay
Antiviralactivitywasevaluatedbasedontheabilityofsamples todiminishthemultiplication of thevirus, includinginhibiting thecytopathiceffect(CPE)of rotavirusontreatedMA-104 cells monolayers(4×104cells/well)cultivatedin96-wellmicroplates.
Thus,MULandfractions(atMNTC)wereaddedtothecells, fol-lowedbytheadditionof10TCID50ofactivatedrotaviruses,and theplateswereincubatedina5%CO2atmosphereat37◦Cfor48h. Theantiviralactivitywasexpressedasapercentageofinhibition
(PI)(Mirandaet al.,1999)usingantilogarithmvalues ofTCID50
for rotavirus,as follows:PI=[1−(antilogarithm T/antilogarithm
C)]×100being“T”viraltiteroftreatedcellsand“C”viraltiterof
untreatedcells(positivecontrol).Experimentswerecarriedoutin octoplicate.ThepresenceofrotaviruswasalsoverifiedbyRT-PCR.
RNAextraction
SupernatantsfromtheantiviralassaysweresubmittedtoRNA
extractionbyamodifiedsilicamethod(Boometal.,1990).Briefly,
60lofeach sample wastreated with200llysis buffer(60g
guanidineisothiocyanate,Invitrogen,50mlof0.1MTris–HClpH 6.4,Invitrogen, 11mlof 0.2MEDTApH8.0, Invitrogen, 1.3gof TritonX-100,PackardInstrumentCo.)and50lofsterilized sil-icasolution(preparedaccordingtoBoometal.,1990).Afterbeing
centrifuged,thesilica waswashed witha washing buffer(60g
guanidine isothiocyanate) (Invitrogen), 50ml of 0.1M Tris–HCl
pH 6.4 (Promega), followed by two washes with 70% ethanol
(Merck)and acetone(Merck).Thematerial wasresuspendedin
watertreatedwithdiethylpyrocarbonate0.1%,andafterbeing cen-trifuged,theRNAwascollectedintheupperphaseandmaintained at−80◦Cuntilrequired.
cDNAsynthesis
ThecDNAsynthesiswasconductedina20lreaction contain-ing7%dimethylsulfoxide,7lofviralRNAand1Moftheprimers
RotaA–Fwd1:5′GGATGTCCTGTACTCCTTGTCAAAA3′andRotaA
–Rev1:5′TCCAGTTTGGAACTCATTTCCA3′,whichamplifya144-bp
product(Loganetal.,2006)fromrotavirusVP6region.The reac-tionwasincubatedat95◦Cfor5minandthenchilledonicefor
5min.Next,1×reactionbufferwasadded,3mMMgCl2,0.5mMof eachdNTPand1lImpromIITMReverseTranscriptase(Promega). Finally,thereactionwasincubatedfor5minat25◦C,60minat
42◦C, and theenzyme wasinactivatedfor 15minat 70◦C. The
cDNAsweremaintainedat−80◦Cuntilrequired.
PCRassay
ThePCRassaywasconductedinareactionof50lcontaining 5lofcDNA,22mMTris–HClpH8.4,55mMKCl,1.65mMMgCl2,
220Meachdeoxynucleosidetriphosphate(dNTP),800nMofthe
sameprimersusedinthecDNAsynthesisand0.5Urecombinant
TaqDNAPolymerase(Invitrogen).Thecyclingprogramconsistedof aninitialdenaturationat95◦Cfor10min,followedby30cyclesof
95◦Cfor15s,60◦Cfor1minand70◦Cfor1min.Eachreactionset
containedthereferencesampleSA11asapositivecontrol. Nega-tivecontrolconsistedofareactionwithallreagentsincludedand waterinsteadofcDNA.Then,10lofthePCRamplifiedproductwas analyzedonasilver-stained8%polyacrylamidegelelectrophoresis (PAGE).
Results
Determiningthemaximumnon-cytotoxicconcentration
Tocarryouttheinvitroantiviralassays,wefirstdeterminedthe concentrationofMULandfractionatedsamplesthatcouldbeused withoutcausingdamagetothecell,basedoncellularmorphologic
alterationsandPromega’sCytoToxAssaydata.Themaximum
non-toxicconcentrations(MNTC)observedwere500g/mlforMUL,
and50g/mlformostofthegeneratedfractions,exceptforF2and SF3,4,6and15,whichshowedMNTCat5g/ml(Table2).
Fig.1Ashowsacytotoxiceffectcharacterizedbytypical
mor-phological alterations including detachmentof cells from their
substratum and disruption of cell monolayer, compared with
healthycontrolcells(Fig.1C).
Table2
Maximumnon-toxicconcentration(MNTC)andpercentinhibitionofrotavirus(PI) inMA-104cellstreatedwithcrudeextract(MUL)fromMyracrodruonurundeuvaleaf anditsfractions(F)andsubfractions(SF).
Selectedmaterials MNTC(g/ml) PI(%)
Crudeextract
MUL 500 100
Fractions
F1 50 0
F2 5 21
F3 50 75
F4 50 21
F5 50 68
Subfractions
SF1 50 92.06
SF2 50 0
SF3 5 80.05
SF4 5 0
SF5 50 68.38
SF6 5 36.90
SF7 50 20.57
SF8 50 20.57
SF9 50 0
SF10 50 0
SF11 50 0
SF12 50 20.57
SF13 50 0
SF14 50 0
SF15 5 0
SF16 50 0
SF17 50 60.19
Fig.1.TherhesusmonkeykidneycelllineMA-104.(A)Typicalcytotoxiceffectinducedbytoxicconcentrationofextract.(B)Typicalcytopathiceffectobservedincells infectedwiththesimianrotavirusSA11.(C)Uninfectedanduntreatedcontrolcells(magnification,20×).
Antiviralassays
TheMNTCofthesampleswereusedtoconducttheantiviral assaystoassesstheirpotentialactivityagainstrotavirus.Basedon cytopathiceffect,wedeterminedthepercentinhibition(PI)ofthe samplesonthereplicationofrotavirus.Therewerenocytopathic effects(CPE)incellstreatedwiththeMUL.However,weobserved CPE(datanotshown)anddifferentPI(rangingfrom0to75%)inthe cellstreatedwiththefractions(Table2).Thus,thefractionwiththe highestPI(F3,PI=75%)wasselectedforfurtherfractionation.The testswiththesubfractionsrevealedthatnoneofthemwereable toinhibit100%ofviralreplication,andmostofthemshowedno antiviraleffect.Interestingly,SF1(asubfractionfromF3)showed thehighestPI(92%),evenhigherthanF3(Table2).Fig.1Bshowsthe typicalCPEinducedbythevirusinMA-104cells.The morpholog-icaldataobservedinopticalmicroscopywereconsistentwiththe moleculardataassayedbyRT-PCR.Theresultsoftheamplifications werevisualizedinasilver-stained8%polyacrylamidegelshowing thebandcorrespondingtothe144-bpfromrotavirusVP6region.
PhytochemicalandHPLCanalysis
MUL,F1–F5andSF1weresubjectedtophytochemical
analy-sistodeterminethemainclassesofnaturalproductswhichcould beinvolvedintheantiviralactivity.Tannins,flavonoidsand
ter-penes were detected in MUL. F1 and F2 showed terpenes, F4
andF5showedflavonoidsandtannins,andF3showedterpenes,
flavonoidsandtannins(Table3).BecauseF3showedthehighest PI,it wasselectedforanadditionalfractionation,yielding eigh-teensubfractions.Then,SF1,thesubfractionwiththehighestPI, wasalsoanalyzed,revealingflavonoidsandterpenes.Finally,
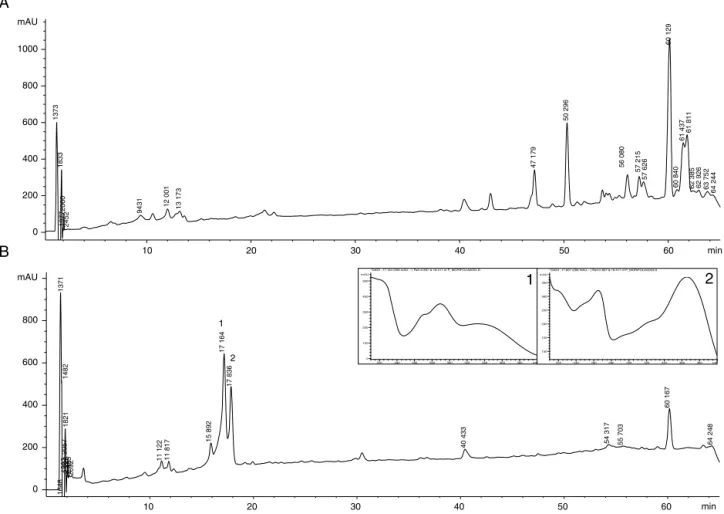
chro-matographicprofilesofMULandSF1wereobtainedbyHPLC.MUL
showedapredominanceofcompoundsoflowpolarityasshownin
Fig.2A.SF1,whichhadthehighestantiviralactivity,showedpeaks distributedthroughoutthechromatogram,indicatingthepresence
ofcompoundswithdifferentpolarities.Mostofthecompounds
wereelutedwithretentiontimelowerthan20min.Inthistime
range,weobservedtwomajorpeaks(r.t.of17.16and18.84min) withUVprofilecharacteristicofflavonoids(Fig.2B).Thisfraction
alsoshowedpeakswithretentiontimehigherthan40min,which
hadlowUVabsorption,withmaximumabsorptionatca.210nm.
Discussion
Our tests were conductedusing a simple but very effective
modelfordetectionofbioactivesamples.Ourstrategywasto deter-minetheworkingconcentrationofcrudeandfractionatedextract, andthendeterminethepercentageinhibitionofrotavirus replica-tioninMA104cells.TheSimianrotavirusstrainSA11waschosen becausethis strainis wellcharacterizedasa groupArotavirus andisone ofthemostwidelyusedreferencestrains(Gutiérrez etal.,2010).Rotavirusesaredifferentiatedintosevengroups(A–G).
Groups A,B, and C areassociated withacute gastroenteritisin
humansandanimalswhilegroupsD,E,F,andGonlyinfectanimals
(SaifandJiang,1994;Westermanetal.,2006;EstesandGreenberg,
2013).Besides,groupA rotavirusesare themost commonviral
agentscausingdiarrhealinfectionsinchildrenyoungerthanthree years(Parasharetal.,2006;Junaidetal.,2011).Thesevirusesare internalizedintoMA104 cellsshowingtypicalcytopathiceffects
(MalherbeandStrickland-Cholmley,1967;LópezandArias,1992;
Gonc¸alvesetal.,2005;Loganetal.,2006;Gutiérrezetal.,2010;
Wolfetal.,2011)whichareessentialfeaturesforthesuccessofour test.
Rotaviruses are nonenvelopedviruses composedof a
triple-layeredproteincapsidthatsurroundstheviralgenome. Nonstruc-turalglycoprotein4(NSP4)encodedbyrotavirusistheonlyviral proteincurrentlybelievedtofunctionasanenterotoxin. Identifi-cationofNSP4asthefirstviralenterotoxinisofinterestbecause itshowsthisproteinhaspleiotropicpropertiesbesidesits intra-cellularroleinviralreplication.Theoutermostlayeriscomposed oftheproteinVP7 andprotrudingspikesoftrimericVP4(López
andArias,2006;EstesandGreenberg,2013).TheVP4cleavageby
trypsinpromotesrearrangementsintheviralparticlesthatrigidify thespikesandisrequiredforreceptorbindingandcellpenetration
(Lópezetal.,1985;Ariasetal.,1996).
In ourwork,thechemical fractionationof thecrude extract
resultedinfivefractions,andoneofthem(F3)showedpotential antiviralactivitywithCPEinhibitionat50g/ml(datanotshown) andasatisfactoryPI(75%),althoughlowerthantheactivityfound tothecrudeextract(PI=100%).Asexpected,thefractionation
pro-ducedsampleswithhighercytotoxicityduetotheconcentration
ofsomecompoundclasses,forcingtheevaluationoftheantiviral activityatlowerconcentrationsthanthatusedforthecrudeextract.
Table3
Phytochemicalscreeningandyieldofcrudeethanolicextract(MUL),fractions(F)andsubfractions(SF)fromMyracrodruonurundeuvaleaf.
Selectedmaterials Extractyield(%,w/w) Tan Fla Ant Ter CarGly Alk
MUL 19.4 + + − + − −
F1 12.0 − − ND + ND ND
F2 8.4 − − ND + ND ND
F3 5.9 + + ND + ND ND
F4 18.2 + + ND − ND ND
F5 48.0 + + ND − ND ND
SF1 0.5 − + ND + ND ND
min
10 20 30 40 50 60
mAU
0 200 400 600 800 1000
1373
1833
1977
2060
2452
9431
12 001 13 173
47 179
50 296
56 080
57 215
57 626
60 129
60 840
61 437
61 811
62 385 62 926 63 752 64 244
min
10 20 30 40 50 60
mAU
0 200 400 600 800
1371
1482
1646
1821
1983
2057
2.225 2.455 2692
11
122
11
817
15
892
17
164
17
836
40
433
54
317
55
703
60
167
64
248
A
B
1
2
Fig.2.ChromatographicprofileofcrudeextractMUL(A)andfractionSF1(B)withUVspectraobtainedonlineforpeaks1and2.
Interestingly,afurtherF3fractionationyieldedtwoactive subfrac-tions(SF1andSF3)withPIvaluesgreaterthanF3(92%and80%, respectively).InSF1,whichshowedthehighestactivity,flavonoids werethemajor class ofcompounds foundin additiontosmall amountsofterpenes,asevidencedbyTLC(datanotshown)and HPLCprofiles.TheTLCanalysisofthisfractionshowed predomi-nanceofspotswithyellowfluorescenceunderUV365nmlight,which wasintensifiedwiththeuseofNP/PEGsprayreagent.Thisfinding wascorroboratedbytheHPLCprofile,whichshowedpeaks asso-ciatedwithUVspectracharacteristicofflavonoidsandmaximum absorptionatca.275and350nm(Fig.2).Terpeneswereevidenced
bythepresenceofspotsofpurplecolorwhentheTLCplatewas
sprayed with anysaldehyde/sulfuric acid and these compounds
maybetheresponsibleforthepeaksobservedinthechromatogram ofSF1withretentiontimesinthe40–65minrange.Thefinal purifi-cationofthechemicalconstituentsofSF1wasnotperformeddue tothelowamountobtainedforthissample(47mg).
The antiviral activities of flavonoids have been extensively
reported.Massspectrometricdatahavedemonstrated the
pres-ence of gallotannins in M. urundeuva (Da Silva et al., 2011).
Interestingly,gallotanninsarepotentcalcium-activatedCl−
chan-nel inhibitors whose biological activity provides antisecretory
benefits(Namkunget al., 2010).On the otherhands, there are
evidencesthatNSP4,anonstructuralglycoproteinreleasedfrom
rotavirus-infected cells, induces the release of Ca2+ from the
endoplasmicreticulum,resultinginincreasedparacellular perme-abilityinenterocytes,aswellasincreasedsecretioninthecrypt cells mediated by activation of Cl− transporter (Ramig, 2004).
Thus,thesefindingspoint toapossiblecourseofactionforthe anti-rotavirusactivityofM.urundeuva.
Anotherinteresting findingin literature, which corroborates withthepotentantiviraleffectofflavonoid-enrichedfractionSF1, istheinhibitoryactivityrelatedtoseveralflavonoidsonreverse transcriptasesandproteasesevenatlowconcentrations(Koetal., 2009).Trypsininhibitoryactivitieshavealsorecentlybeen
iden-tified in flavonoids extracted from orange peel and green tea
leaves(Shahwaretal.,2013).Sincetheinfectivityofrotavirusesis increasedbytrypsinandthevirusesreplicateprimarilyin intesti-nalenterocytesduringanaturalinfection,itispossiblethatthe
flavonoids present in SF1, may be disrupting viral entry by a
proteaseinhibitormechanism.Therefore,ourdataprovideinsights forfurtherstudieswiththisflavonoid-enrichedsubfraction, focus-ingonthesuppressiveeffectofviralamplificationintheearlyphase ofinfection,andonaninhibitoryactivityonapossiblemechanism ofactioninvolvingtheNSP4enterotoxin.
Finally,itisimportanttoconsiderthatasinglecompoundcould notberesponsibleforthefullantiviralactivityandthecombination ofcompoundswithbalancedproportionsincrudeextractcouldbe actinginanadditiveorsynergisticmoderesultinginmoreeffective virucidalactivity.Theseconsiderationsencourageustoperform
additionalstudieswithSF1andMULusingothercomplementary
testsinvitroand invivoassaywithmurinerotavirusstrainsin mice.
Ethicaldisclosures
Protectionofhumanandanimalsubjects. Theauthorsdeclare
thatnoexperimentswereperformedonhumansoranimalsfor
Confidentialityofdata. Theauthorsdeclarethatnopatientdata appearinthisarticle.
Righttoprivacyandinformedconsent. Theauthorsdeclarethat nopatientdataappearinthisarticle.
Authorscontributions
ABC,coordinationoftheresearchandwritingofthemanuscript; PCO,biologicalassays;SC,PCRassayandanalysis;PRVC, phyto-chemicalscreeningandfractionation;FLF,preparationofextract;
MGRDandVLA,HPLCanalysis;LAMM,preparationofextract.
Conflictsofinterest
Theauthorsdeclarenoconflictsofinterest.
Acknowledgements
TheauthorsthanktheFundac¸ãodeAmparoàPesquisadoEstado deMinasGerais(FAPEMIG)forfinancialsupport(N◦EDT-3253/06)
ofthisresearchandtheFundac¸ãoEzequielDias(Funed).
References
Alves,P.M.,Queiroz,L.M.,Pereira,J.V.,Pereira,M.S.V.,2009.Invitroantimicrobial, antiadherentandantifungalactivityofBrazilianmedicinalplantsonoralbiofilm microorganismsandstrainsofthegenusCandida.Rev.Soc.Bras.Med.Trop.42, 222–224.
Arias,C.F.,Romero,P.,Alvarez,V.,López,S.,1996.Trypsinactivationpathwayof rotavirusinfectivity.J.Virol.70,5832–5839.
Boom,R.,Sol,C.J.,Salimans,M.M.,Jansen,C.L.,Wertheim-vanDillen,P.M.,VanDer Noorda,J.,1990.Rapidandsimplemethodforpurificationofnucleicacids.J. Clin.Microbiol.28,495–503.
Cecílio,A.B.,deFaria,D.B.,deC.Oliveira,P.,Caldas,S.,deOliveira,D.A.,Sobral,M.E., Duarte,M.G.,Moreira,C.P.,Silva,C.G.,deAlmeida,V.L.,2012.Screeningof Brazil-ianmedicinalplantsforantiviralactivityagainstrotavirus.J.Ethnopharmacol. 141,975–981.
Chaves,M.C.,Santos,F.A.,Menezes,A.M.S.,Rao,V.S.N.,1998.Experimental eval-uationof Myracrodruonurundeuvabarkextract forantidiarrhoealactivity. Phytother.Res.12,549–552.
DaSilva,V.C.,Napolitano,A.,Eletto,D.,Rodrigues,C.M.,Pizza,C.,Vilegas,W., 2011.CharacterizationofgallotanninsfromAstroniumspeciesbyflow injec-tionanalysis-electrosprayionization-iontrap-tandemmassspectrometryand matrix-assistedlaserdesorption/ionizationtime-of-flightmassspectrometry. Eur.J.MassSpectrom.(Chichester,Engl.)17,365–375.
De Mendonc¸a Albuquerque, R.J., Leal, L.K.A.M., Bandeira, M.A., Viana, G.S.B., Rodrigues,L.V.,2011.ChalconesfromMyracrodruonurundeuvaareefficacious inguineapigovalbumin-inducedallergicconjunctivitis.Rev.Bras.Farmacogn. 21,953–962.
Dennehy,P.H.,2000.Transmissionofrotavirusandotherentericpathogensinthe home.Pediatr.Infect.Dis.J.19,S103–S105.
Estes,M.K.,Greenberg,H.B.,2013.Rotaviruses.In:Knipe,D.M.,Howley,P.M.(Eds.), FieldsVirology,vol.2.WoltersKluwer,LippincottWilliams&Wilkins, Philadel-phia,PA,pp.1347–1401.
Goes,A.C.,Rodrigues,L.V.,deMenezes,D.B.,Grangeiro,M.doP.,Cavalcante,A.R., 2005.Histologicanalysisofcolonicanastomotichealing,inrats,undertheaction of10%Aroeira-do-sertao(Myracrodruonurundeuvafr.all.)enema.ActaCir.Bras. 20,144–151.
Gomes,V.T.L.,Chaves,T.P.,Alencar,L.C.B.,Dantas,I.C.,DeMedeiros,A.C.D., Felis-mino,D.C.,2013.AntimicrobialactivityofnaturalproductsfromMyracrodruon urundeuvaAllemão(Aroeira-do-sertão).Rev.Cub.Plant.Med.18,529–533. Gonc¸alves,J.L.,Lopes,R.C.,Oliveira,D.B.,Costa,S.S.,Miranda,M.M.,Romanos,M.T.,
Santos,N.S.,Wigg,M.D.,2005.Invitroanti-rotavirusactivityofsomemedicinal plantsusedinBrazilagainstdiarrhea.J.Ethnopharmacol.99,403–407. Gutiérrez,M.,Isa,P.,Sánchez-SanMartin,C.,Pérez-Vargas,J.,Espinosa,R.,Arias,
C.F.,López,S.,2010.DifferentrotavirusstrainsenterMA104cellsthrough dif-ferentendocyticpathways:theroleofclathrin-mediatedendocytosis.J.Virol. 84,9161–9169.
Harvey,A.L.,2008.Naturalproductsindrugdiscovery.DrugDiscov.Today.13, 894–901.
Jiang,V.,Jiang,B.,Tate,J.,Parashar,U.D.,Patel,M.M.,2010.Performanceofrotavirus vaccinesindevelopedanddevelopingcountries.Hum.Vaccin.6,532–542.
Junaid,S.A.,Umeh,C.,Olabode,A.O.,Banda,J.M.,2011.Incidenceofrotavirus infec-tioninchildrenwithgastroenteritisattendingJosuniversityteachinghospital, Nigeria.Virol.J.8,233.
Ko,Y.-J.,Oh,H.-J.,Ahn,H.-M.,Kang,H.-J.,Kim,J.-H.,Ko,Y.H.,2009.Flavonoidsas potentialinhibitorsofretroviralenzymes.J.KoreanSoc.Appl.Biol.Chem.52, 321–326.
Leite,E.J.,2002.State-of-knowledgeonMyracrodruonurundeuvaFr.Allemão (Anac-ardiaceae)forgeneticconservationinBrazil.Perspect.PlantEcol.5,193–206. Logan,C.,O’Leary,J.J.,O’Sullivan,N.,2006.Real-timereversetranscription-PCRfor
detectionofrotavirusandadenovirusascausativeagentsofacuteviral gastroen-teritisinchildren.J.Clin.Microbiol.44,3189–3195.
López,S.,Arias,C.F.,1992.SimianrotavirusSA11strains.J.Virol.66,1832. López,S.,Arias,C.F.,2006.Earlystepsinrotaviruscellentry.Curr.Top.Microbiol.
Immunol.309,39–66.
López,S.,Arias,C.F.,Bell,J.R.,Strauss,J.H.,Espejo,R.T.,1985.Primarystructureofthe cleavagesiteassociatedwithtrypsinenhancementofrotavirusSA11infectivity. Virology144,11–19.
Malherbe,H.H.,Strickland-Cholmley,M.,1967.SimianvirusSA11andtherelatedO agent.Arch.Ges.Virusforsch.22,235–245.
Matos,F.J.A.,1997.Introduc¸ãoafitoquímicaexperimental.Edic¸õesUFC,Fortaleza. Miranda,M.M.,Almeida,A.P.,Costa,S.S.,Santos,M.G.,Lagrota,M.H.,Wigg,M.D.,
1999.InvitroactivityofextractsofPersea americanaleaveson acyclovir-resistantandphosphonoaceticresistantHerpessimplexvirus.Phytomedicine 4,347–352.
Monteiro,J.M.,Albuquerque,U.P.,LinsNeto,E.M.F.,Araújo,E.L.,Amorim,E.L.C.,2006. Usepatternsandknowledgeofmedicinalspeciesamongtworuralcommunities inBrazil’ssemi-aridnortheasternregion.J.Ethnopharmacol.105,173–186. Namkung,W.,Thiagarajah,J.R.,Phuan,P-W.,Verkman,A.S.,2010.InhibitionofCa2+
-activatedCl−channelsbygallotanninsasapossiblemolecularbasisforhealth
benefitsofredwineandgreentea.FASEBJ.24,4178–4186.
Napoleão,T.H.,Pontual,E.V.,deAlbuquerqueLima,T.,deLimaSantos,N.D.,Sá,R.A., Coelho,L.C.,doAmaralFerrazNavarro,D.M.,Paiva,P.M.,2012.Effectof Myracro-druonurundeuvaleaflectinonsurvivalanddigestiveenzymesofAedesaegypti
larvae.Parasitol.Res.110,609–616.
Nobre-Júnior,H.V.,Oliveira,R.A.,Maia,F.D.,Nogueira,M.A.S.,Moraes,M.O.,Bandeira, M.A.M.,Andrade,G.M.,Viana,G.S.B.,2009.Neuroprotectiveeffectsofchalcones fromMyracrodruonurundeuvaon6-hydroxydopamine-inducedcytotoxicityin ratmesencephaliccells.Neurochem.Res.34,1066–1075.
Offit,P.A.,Clark,M.F.,2000.Reoviruses.In:Mandell,G.L.,Bennett,J.E.,Dolin,R.(Eds.), PrincipleandPracticeofInfectiousDiseases.ChurchillLivingstone,Philadelphia, PA,pp.1696–1703.
Parashar,U.D.,Gibson,C.J.,Bresee,J.S.,Glass,R.I.,2006.Rotavirusandsevere child-hooddiarrhea.Emerg.Infect.Dis.12,304–306.
Ramig,R.F.,2004.Pathogenesisofintestinalandsystemicrotavirusinfection.J.Virol. 78,10213–10220.
Reed,L.J.,Muench,H.,1938.Asimplemethodofestimatingfiftypercentendpoints. Am.J.Epidemiol.27,493–497.
Sá,R.A.,Gomes,F.S.,Napoleão,T.H.,Santos,N.D.L.,Melo,C.M.L.,Gusmão,N.B.,Coelho, L.C.B.B.,Paiva,P.M.G.,Bieber,L.W.,2009a.Antibacterialandantifungalactivities ofMyracrodruonurundeuvaheartwood.WoodSci.Technol.43,85–95. Sá,R.A.,Argolo,A.C.C.,Napoleão,T.H.,Gomes,F.S.,Santos,N.D.L.,Melo,C.M.L.,
Albu-querque,A.C.,Xavier,H.S.,Coelho,L.C.B.B.,Bieber,L.W.,Paiva,P.M.G.,2009b. Antioxidant,FusariumgrowthinhibitionandNasutitermescornigerrepellent activitiesofsecondarymetabolitesfromMyracrodruonurundeuvaheartwood. Int.Biodeter.Biodegr.63,470–477.
Saif,L.J.,Jiang,B.,1994.Non-groupArotavirusesofhumansandanimals.Curr.Top. Microbiol.Immunol.185,339–371.
Shahwar,Raza,D.,Atta-Ur-Rahman.,M.A.,2013.Identificationofflavonoidswith trypsininhibitoryactivityextractedfromorangepeelandgreentealeaves.J. Sci.FoodAgric.93,1420–1426.
Souza,S.M.,Aquino,L.C.,MilachJr.,A.C.,Bandeira,M.A.,Nobre,M.E.,Viana,G.S., 2007.AntiinflammatoryandantiulcerpropertiesoftanninsfromMyracrodruon urundeuvaAllemão(Anacardiaceae)inrodents.Phytother.Res.21,220–225. Takahashi,K.,Matsuda,M.,Ohashi,K.,Taniguchi,K.,Nakagomi,O.,Abe,Y.,Mori,
S.,Sato,N.,Okutani,K.,Shigeta,S.,2001.Analysisofanti-rotavirusactivityof extractfromSteviarebaudiana.Antivir.Res.49,15–24.
Viana,G.S.,Bandeira,M.A.,Matos,F.J.,2003.Analgesicandantiinflammatoryeffects ofchalconesisolatedfromMyracrodruonurundeuvaAllemão.Phytomedicine10, 189–195.
Viana,G.S.B.,Bandeira,M.A.M.,Moura,L.C.,Souza-Filho,M.V.P.,Matos,F.J.A.,Ribeiro, R.A.,1997.Analgesicandantiinflammatoryeffectsofthetanninfractionfrom
MyracrodruonurundeuvaFr.All.Phytother.Res.11,118–122.
Wagner,H.,Bladt,S.,2001.PlantDrugAnalysis:AThinLayerChromatographyAtlas, 2nded.Springer,Berlin/Heidelberg/NewYork.
Westerman,L.E.,Jiang,B.,Mcclure,H.M.,Snipes-Magaldi,L.J.,Griffin,D.D.,Shin,G., Gentsch,J.R.,Glass,R.I.,2006.Isolationandcharacterizationofanewsimian rotavirus,YK-1.Virol.J.3,40–48.