w w w . r b h h . o r g
Revista
Brasileira
de
Hematologia
e
Hemoterapia
Brazilian
Journal
of
Hematology
and
Hemotherapy
Review
article
Splenic
marginal
zone
lymphoma:
a
literature
review
of
diagnostic
and
therapeutic
challenges
Tayse
Silva
dos
Santos,
Renato
Sampaio
Tavares,
Danielle
Leão
Cordeiro
de
Farias
∗HospitaldasClínicas,UniversidadeFederaldeGoiás(UFG),Goiânia,GO,Brazil
a
r
t
i
c
l
e
i
n
f
o
Articlehistory: Received25May2016 Receivedinrevisedform 26July2016
Accepted9September2016 Availableonline22December2016
Keywords:
Lymphoma,non-Hodgkin Splenicmarginalzonelymphoma Spleniclymphoma
Indolentlymphoma Rituximab
a
b
s
t
r
a
c
t
Splenicmarginalzonelymphoma(SMZL)isalow-gradeB-cellnon-Hodgkin’slymphoma characterizedbymassivesplenomegaly,moderatelymphocytosiswithorwithoutvillous lymphocytes,rareinvolvementofperipherallymphnodesandindolentclinicalcourse. Asararedisease,withnorandomizedprospectivetrials,thereisnostandardofcarefor SMZLsofar.Splenectomyhasbeendoneformanyyearsasanattempttocontroldisease, butnowadaysithasnotbeenencouragedasfirstlinebecauseofnewadvancesin ther-apyasrituximab,thatareaseffectivewithminimaltoxicity.Facingthesecontroversies, thisreviewhighlightsadvancesintheliteratureregardingdiagnosis,prognosticfactors, treatmentindicationsandtherapeuticoptions.
©2016Associac¸ ˜aoBrasileiradeHematologia,HemoterapiaeTerapiaCelular.Published byElsevierEditoraLtda.ThisisanopenaccessarticleundertheCCBY-NC-NDlicense (http://creativecommons.org/licenses/by-nc-nd/4.0/).
Introduction
and
clinical
features
Splenicmarginalzonelymphoma(SMZL) isa rareindolent non-Hodgkinlymphoma(NHL)subtypethatoriginatesfrom Bmemorylymphocytespresentinthemarginalzoneof sec-ondarylymphoidfollicles.1–3
Patientsusuallypresentmassivesplenomegalyandbone marrowinvolvementwithminimal orabsent lymphadeno-pathyexceptfor the spleenhilum.There isno extranodal involvement,exceptforthebonemarrowandliver.3,4About
25%ofthepatientsareasymptomaticandthepresenceofB symptomsorhighlactatedehydrogenaselevels(LDH)at diag-nosisisnotusual.5,6
∗ Correspondingauthorat:AlamedadasEspatodias,qd44,lt9,AldeiadoVale,74680-160Goiânia,GO,Brazil. E-mails:danileao10@gmail.com,danielleleao@uol.com.br(D.L.Farias).
Lymphocytosisiscommonlypresent.Cytopeniasarefound in25%ofthecasesmostlyrelatedtohypersplenism,andless frequentlytoauto-antibodiesorbonemarrowinfiltration.3,4
Small amounts(less than2g/dL)ofmonoclonalprotein, usuallyimmunoglobulin(Ig)Mkappa,aredetectedin approx-imately onethird of patients.5,7 Hyperviscosity syndromes
are not usual,3 but 20% of patients present autoimmune
hemolytic anemia and other autoimmune disorders, such as thrombocytopenia, cold agglutinin disease, circulating anticoagulantsandevenangioedemabecauseofacquired C1-esteraseinhibitordeficiency.5,7,8
The rarity ofthis disease and its indolent course are a challenge todeterminestandardcareinthe treatmentand managementofpatients.Therearenorandomizedtrials,most
http://dx.doi.org/10.1016/j.bjhh.2016.09.014
oftheliteratureareretrospectiveseriesofcasesfromsingle centersandfewprospectivestudieshavebeencompletedor areongoing.8
Epidemiology
SMZListhesecondmostcommonsubtypeofmarginalzone lymphoma,comprisingabout20%ofthecases.Itrepresents about0.9%ofallNHLandwasconsideredaspecific patholog-icalentityonlyin1991.1,5,9
Medianage atdiagnosis ofSMZLis 69 years.The over-allage-adjustedincidenceis0.13/100,000habitantsperyear. Thepercentagechange inage-adjustedincidence is4.81%, withmostofthepatientsbeingWhite.8 Genderprevalence
iscontroversial,6,7 but thereisanincreasingtrendtomale
predominance.8,10,11
TheassociationofSMZLwithhepatitisC(HCV)iscommon inthesouthofEurope,3,12,13andlymphomadevelopmentis
usuallytriggeredbytheglycoproteinE2ofthevirusthat sti-mulatesCD81inBcells.5,6,13Althoughtherearecontroversial
datainBrazilregardingtheassociationofHCVandlymphoma, nostudieshaveevaluatedthisassociation.14,15
The International Lymphoma Epidemiology Consortium Non-HodgkinLymphomaSubtypesProject,withadatabaseof 17,471NHLcasesand23,096controls,identifiedanassociation betweenSMZLandBcellactivatingautoimmuneconditions, asthmaanduseofhairdye.16
Diagnosis
ThediagnosisofSMZLcanbebytheanalysisofpathological cellspresentinbonemarrowwithbloodandspleenanalysis notbeingessential.
Bone marrow infiltration is a very common finding (83–100%),althoughcirculatingcellsaredetectedmuchless frequently(29–75%).7Duringthecourseofthedisease,75%of
thepatientswillpresentlymphocytosis,withcharacteristic, butnotpathognomonic,villouscells.4,17 Bonemarrow
aspi-rateisnotsufficientfordiagnosis;atrephinehistologywith immunohistochemicalanalysisisrequired.5
Pathological cells of SMZL are small- to medium-sized matureBcellswithroundorovalnucleiandcondensed chro-matin,basophiliccytoplasm,andmostofthecasespresent with typical unequal membrane projections (villi), the so-calledvillous cells (Figure 1).4–7 Marrow infiltration can be
nodular,interstitialorintrasinusoidal.5
ThereisnospecificimmunophenotypicpatternforSMZL. PathologicalcellsareusuallypositiveforCD19,CD20,CD22, CD79a,CD79b,FMC7 and IgMand negativefor CD5,CD10, CD43,BCL6, cyclin D1or CD103.The expressionsofCD23, IgDandcytoplasmaticIgarevariable,6,18 usuallyscoring0–2
points in the Modified Matutes scoring system.19 CD5 are
weakly positive in 10–25%of the cases, evenwith the co-expressionofCD23orCD43.20CD11candCD25aresometimes
positive,butCD103andCD123arealmostalwaysnegative.4
Bonemarrowimmunohistochemistryanalysisreveals pos-itivityforCD45RA,CD45RB,CD19,CD20,CD79a,PAX5/BSAP, IgD, Bcl-2, DBA-44 (CD72), TRAP and CD38.5,21,22 IgM is
Figure1–Morphologicfeaturesofvillouslymphocytesin patientswithsplenicmarginalzonelymphoma.
usuallybright,butIgDisvariable.4,6 Cellsare usually
nega-tiveforCD3,CD5,CD10,CD23,CD43,cyclinD1,anexin-A1and BCL6.KI67/Mib1hasalowproliferationindexwitha charac-teristicpattern.4,6
Thespleenisfrequentlyenlarged,withamedianweightof 1750g(270–5500g)andmanygrayishnodulesthroughoutthe parenchyma.6Whitepulpisexpandedbyneoplasticcellsthat
surround and eventually substitutegerminal centers. Nod-ulesarecomposedofpathologicalcells,locatedinaninner zone ofsmall- tomedium-sizedB cellswith round nuclei, clumped chromatinand scantycytoplasm.Externally there isanouterzonewithmedium-sizedpathologicalcells,with more irregular nucleus outlines, dispersed chromatin and moderatelyclearcytoplasm.Therearescatteredcellsinthis zone resembling immunoblasts. Asthe disease progresses, the centralgerminalcenter becomeseffaced.Theredpulp isinvariablyenveloped toavaryingdegreebysmall aggre-gates oflargercells and sheetsofsmall cells,which often occupy sinuses and cords.There can beepithelioid granu-lomasandplasmacyticdifferentiation,theformerespecially whenthereisamonoclonalserumcomponent. Immunohis-tochemicalfindingsaresimilartobonemarrowfindings.5,6,9
Matutesetal.proposed minimumdiagnosticcriteriafor SMZL:
a) Whenspleenpathologyisavailable:spleenhistologyand immunophenotypewith amodifiedMatutesscoreof<3 points.19
b) Whenthepatienthasclinicalsplenomegalyand splenec-tomy is not performed, it is sufficient to make the diagnosiswithtypicalbloodandbonemarrowfindingsby morphologyandimmunophenotypewithintrasinusoidal infiltrationbyCD20+cells.
tests for hepatitis B and C and human immunodeficiency virus (HIV), renal and liver function tests, serum calcium, LDH,andb2-microglobulin.SMZLisregardedasanoneF-18 fluorodeoxyglucose-aviddisease;thus,theuseof fluorodeoxy-glucosepositronemissiontomography(FDG-PET)shouldbe discouragedinthestagingprocess.23
Differential
diagnosis
Thedifferentialdiagnosisrequiresthejointanalysisof clin-ical,morphological,immunophenotypicandgeneticdata,as wellasimmunohistochemistry.5,6
Reactive follicular hyperplasia and other small B cell lymphomas should be excluded, as the pattern of splenic micronodularinvolvementofmarginal zonedifferentiation and the villous lymphocytes in peripheral blood are not pathognomonic.4
A diagnostic test should not be performed on spleens weighinglessthan300–400gorintheabsenceofastandard monotypicpattern.8
CD43 and CD200 positivity and a high (3–5) modified Matutes score helps to differentiate between SMZL and chroniclymphocyticleukemia.19Intrasinusoidalinfiltrationis
unusualinchroniclymphocyticleukemia,butoftenseenin SMZL,inhairycellleukemiavariant(LCP-v)andsometimes inmantlecelllymphoma(MCL).5InrarecasesofSMZL,CD5+, morphology,negativityforcyclinD1andSOX11,andabsence oft(11;14)excludesMCL.
Hairycellleukemia(HCL)subtypesinvolvingthespleenare distinguishedbytheircharacteristicmorphologyand pheno-type.CD103andCD123negativityexcludeHCL.4
UnlikeSMZL,noduleshavevariablesizesandtumoralcells are seen inwhite pulp inthe caseoffollicular lymphoma (FL).CD10andBCL6expressionareusefulforthediagnosisof FL.ThemorphologicalcharacteristicsoftheMIB1tumorcell stainingpattern,residualmantlecell,IgDstainingfortumor cellsinadditiontohistologicalfindingsinbonemarrowand hilarlymphnodeshelpestablishdiagnosis.5
Differentialdiagnosisbetween SZML,splenicdiffusered pulplymphoma(SDRPL)andHCL-vcanbetrickyand some-times impossible onlyby blood or bone marrow analyses. Theseare twonewlyrecognizedentitieswith clinicopatho-logicandimmunophenotypicfeaturespartiallyoverlapping thoseofSMZL.Thediagnosisinthesecasesrequiresdetailed clinicalinformation,acomprehensivephenotypeandspleen histology,which usuallyshows a typicalpattern ofdiffuse infiltrationwithwhitepulpfolliclespreserved.8
AnimmunophenotypicprofilewiththeabsenceofCD25, CD123,interleukin-3anti-receptor,annexinA1,HC2andTRAP andresistancetoconventionalHCLtherapyisobservedfor HCL-v.6,24,25Moreover,HCL-vispositivefortheDBA-44,pan-B
cells,CD11c,surfacemonotypicIg(IgGmostoften)andCD103 FMC7.5,9
AlthoughSDRPL havecharacteristics thatoverlap classic SMZL,the expression ofIgDand the follicular micronodu-larpatternisabsentinmostcases.5Thedistinctionbetween
thosetwoentitiesmaybemerelyacademic,asthetreatment isnotdifferent.26
Lymphoplasmacyticlymphoma(LPL)may developinthe spleen,withhomogeneousinfiltrationofthewhitepulp with-outstandardmarginalzoneandmonocytoidBcells.Deletions of7q,3Tgainsandintrasinusoidalinfiltrationare character-istic ofSMZL,whiledel(6q) ismorecharacteristicofLPL.27
AnotherusefulmarkeristheMYD88L265Pmutation,thatis frequentinLPL(91–100%)andrareinSMZL(6%).28
Moreover, there are overlapping patterns of extranodal marginalzonelymphoma(EMZL)andSMZLwiththeclinical findings being crucial for differentiation. Splenic involve-mentisrareinnodalmarginalzonelymphoma(NMZL)and ImmunoglobulinSuperfamilyReceptorTranslocation Associ-ated1(IRTA1),negativein76%ofSMZL,ispositiveinNMZL.24
TwousefulfeaturestodistinguishbetweenSMZLand mucosa-associated lymphoid tissue(MALT) are the absence of the t(11;18)(q21;q21)29andthefrequentIgDexpressioninSMZL,
whichisrarelyobservedinMALTlymphoma.5,30
Prognosis
AlthoughmostofthecasesofSMZLhaveanindolentcourse withmedianoverallsurvivalofabouttenyears,18,22about30%
ofthepatientsdevelopaggressivedisease,withmedian over-all survivalofonlyfour years.10,18 There are noassociated
cytogeneticfeatures31andprognosticscoresforindolent
lym-phomas suchastheInternationalPrognosticIndex(IPI)18,32
andFollicularInternationalPrognosticIndex(FLIPI)33arenot
applicable.ThesamecanbesaidfortheAnnArborstaging system,whichisnotadequatebecauseinmostcasesthebone marrowisinvolved.34
Therearesomeclinicalfeaturesassociatedwithaworse outcome such as the development of lymphadenopathy, increasein2-microglobulin,non-hematopoieticsite involve-ment,leukocytecount>20×109/L,lymphocytosis>9×109/L, lymphopenia,anemia,thrombocytopenia,useof chemother-apy,monoclonalcomponent,performancestatus≥2, incom-pleteresponse,advancedage,diffusepatternofbonemarrow infiltrationandhistologictransformation.8,10,35–38
Many karyotypeabnormalities canbefound:trisomy 3q (85%) del or translocation of 7q32 (40%), trisomy 18, 17q isochromosome,13q14deletion,andstructuralabnormalities ofchr1.39Somemolecularaspects,suchasNOTCH2andKLF2
mutations,Iggenemutationstatus,TP53abnormalitiesand aberrantpromotermethylationseemtoberelatedtoaworse outcome.31,37,40,41 Studies from a whole exome sequencing
studyidentifiedtheMYD88L265Pmissensemutationin15% ofSMZL.42
Table1–
Hemoglobin-platelet-LDH-extra-hilar-lymphadenopathy
scoreforsplenicmarginalzonelymphomaasproposed
bytheSplenicMarginalZoneLymphomaStudyGroup.
Stratification
Riskgroup Specificeventsurvival
A Noadversefactora 95%
B 1–2adversefactorsa 87%
C 3–4adversefactorsa 68%
a Adversefactors:Hb<9.5g/dL;Platelets<80×109L;LDH>normal;
Extra-hilarlymphadenopathy.
highrisk(25%ofthecases,5-yearSESof50%).18Arecentstudy
byPerroneetal.validatedthescore.26
In 2012, the Hemoglobin-Platelet-LDH-extra-hilar-Lymphadenopathy(HPLL)score wasproposed bythe SMZL StudyGroup after a retrospective analysis of593 patients. PatientswerestratifiedinthreegroupsasshowninTable1. ThecriteriaoftheIILwereappliedtothesamepopulation butthe stratificationpowerforSESoftheHPLL scorewere better,43,44sothisseemstobethemostsuitablescoresofar.
Indication
for
treatment
Therearenostandardcriteriatoindicatetreatment.The over-allsurvivalofasymptomaticpatientscanbeashighas88%at fiveyearswithouttreatment23.
Tarellaetal.,23 proposed somecriteriatoindicate
treat-ment(Table2).
TheSMZLStudyGroup alsoconsideredlowhemoglobin levels,extranodaldiseaseandapositivityforHCVas impor-tanttoindicatetreatmenteventhoughthesefactorshavenot beenvalidatedyet.44
Arecentstudy byPerroneet al.suggestedthatpatients shouldundergoanevaluationofthetumorburdensimilarto follicularpatients,butthisawaitsfurthervalidation.26
Types
of
treatment
Asararediseasewithanindolentcourse,determiningthe standardtreatmentandmanagementisachallengeasthere havebeennorandomizedtrialsandmostreportsareof single-centerseriesofretrospectivecases;fewprospectivetrialshave beencompletedorareongoing.8Therefore,nowadaysthereis
nostandardcareforSMZL.
Table2–Criteriatoindicatetreatmentofsplenic
marginalzonelymphoma.
Progressiveorsymptomaticsplenomegaly
Cytopenias:
Hemoglobin<10g/dLor Neutrophils<1×109/L Progressivethrombocytopenia Constitutionalsymptoms Progressivenodaldisease Autoimmunehemolyticanemia
Therapeutic options for SMZL comprise splenectomy, chemotherapy and the use of the anti-CD20 mono-clonal antibody rituximab alone or in chemotherapy combinations.35,45–52
Splenectomy
Splenectomy was thetherapy ofchoicefordecades and is still frequently used, althoughthere is a tendency to pre-scriberituximabmonotherapyupfront,asmostpatientsare oldandwithco-morbidities.10,11,52–54Laparoscopyshouldbe
preferredwheneverpossibleinpatientswithadvancedageor comorbidities.8
Althoughmarrowinvolvementisnottreated,splenectomy allowsquick remission ofthe symptomsof hypersplenism andcytopenias,suchasasignificantreductionofcirculating lymphocytesin90%ofpatients.Regardingclinical improve-ment,inaseriesreport,sevenpatients(25%)hadincreases inbonemarrowinfiltrationbypathologicalcells,therewasa modificationofthepatterninfiveofthem.
Themedian overallsurvival inmostseries isabout ten yearsand70%ofthepatientscanremaintreatmentfreefor fiveyears.17,36,53Thereisnosurvivalbenefitfortheassociation
ofchemotherapywithsplenectomy,17althoughsomestudies
reportincreases inoverall responserates.47 Tables 3and4
summarize thestudies regardingdifferenttypesoftherapy forSMZL.
Pata et al. reported perioperative complications in one quarterof41patientssubmittedtosplenectomyasfirst-line treatment:eightcases(19.5%)ofpulmonarydysfunction,one case(2.4%)ofdeepveinthrombosis,onecase(2.4%)ofportal veinthrombosisandninecases(22%)ofmajorbleeding.55
Infectionscausedbyencapsulatedbacteriaarethemajor risk associated with splenectomy and vaccination against capsulatedbacteriaismandatoryatleasttwoweeksbefore electivesplenectomy.8
Splenectomy should not be performed if the patient hasnodalinvolvementoutsidethesplenichilumand, con-versely, it should not be omitted in cases with suspected transformation.8
Chemotherapy
Alkylating agents and purine analogs have been used as havemanychemotherapycombinations suchas cyclophos-phamide, vincristine and prednisone (CVP); cyclophos-phamide, doxorubicin, vincristine and prednisone (CHOP), and fludarabine and cyclophosphamide (FC).35,45–51 About
two-thirdsofpatientsdonotrespondtofirst-linetreatment withchorambucil.6
Rituximabmonotherapy
RituximabasmonotherapyiseffectiveinSMZLwithresults similartosplenectomy;ithasthepotentialtoprovidebetter responsesandhaslesstoxicitycomparedtochemotherapy.8
Table3–Splenicmarginalzonelymphomapatientstreatedwithsplenectomy.
Reference Year n ORR(%) Response Deathdueto
surgery
Duration OS
Mulliganetal. 1991 20 95 MedianDOR4years NR 1
Troussardetal. 1996 28 75 NR 71%at5years 1
Chacónetal. 2002 60a 93.3 MedianFFS40months 65%at5years NR
Thieblemontetal. 2002 48b 100 PFS48%at5years NR NR
Parry-Jonesetal. 2003 33 NR NR LSS95%at10years NR
Iannittoetal. 2004 21 91 MedianDOR4y NR NR
Tsimberidouetal. 2006 10 60 FFS80%at3years 89%at3years 0
Olszewskietal. 2012 652 NR NR 67.8%at5yearsc NR
Kalpadakisetal. 2013 27 85 PFS58%at5years 77%at5years 1
Lengletetal. 2014 100 97 PFS61%at5y 84%at5years 0
Xingetal. 2015 52d NR FFS39%at10years 61%at10years 0
Pataetal. 2015 41 90 PFS35%at5years 75%at5years 0
DOR:durationofresponse;FFS:failure-freesurvival;LSS:lymphoma-specificsurvival;NR:notreported;ORR:overallresponserate;OS:overall survival;PFS:progression-freesurvival.
a Splenectomyalonein29patients.
b Splenectomyalonein25patients.
c Survivalofentireseriesof1251patientswithnoimpactofsplenectomyonOS.
dSplenectomyalonein42patients.
Table4–Splenicmarginalzonelymphomapatientstreatedwithrituximab-basedregimens.
Reference Year Studytype Regimen Patientstatus n ORR(%) Response
Duration OS
Rituximabmonotherapy
Bennettetal. 2005 Retrospective Rmonotherapy RR 11 91% PFS60%at5years 70%at5years
Tsimberidouetal. 2006 Retrospective Rmonotherapy Firstline 25 88% FFS86%at3years 95%at3years
Kalpadakisetal. 2007 Retrospective Rmonotherapy Firstline 16 100% PFS92%at2.4years 100%at2.1years
Elseetal. 2012 Retrospective Rmonotherapy FirstlineandRR 10 100% DFS89%at3years NR
Kalpadakisetal. 2013 Retrospective Rmonotherapy Firstline 58 95% PFS73%at5years 92%at5years
Rituximab+Chemotherapy
Tsimberidouetal. 2006 Retrospective R-chemo Firstline 6 83% FFS100%at3years 100%at3years
Elseetal. 2012 Retrospective R-chemo FirstlineandRR 33 100% DFS71%at3years NR
Cervettietal. 2013 Retrospective R-2CDA FirstlineandRR 47a 87% PFS80%at5years 86%at5years
Iannittoetal. 2015 Prospective R-COMP Firstline 51 84% PFS54%at6years 72%at6years
2CDA:Cladribine;chemo:chemotherapy;DFS:disease-freesurvival;R:rituximab;COMP:non-pegylatedlyposomaldoxorubicin, cyclophos-phamide,vincristine,prednisone;RR:relapsed/refractory;NR:notreported;ORR:overallresponserate;OS:overallsurvival;PFS:progression-free survival;FFS:failure-freesurvival.
a Rituximabin32patients.
responsesarefast,withimprovementinbloodcountsinabout eightweeks.57
Some studies report inferior outcomes of rituximab monotherapy compared to splenectomy, but in non-randomized retrospective clinical trials there may be a biasofselectingyoungerandfitterpatientsforsplenectomy (Table3).8
Kalpadakis et al. reported a retrospective study of 58 patientstreated withrituximab375mg/m2 inan induction phase (weekly for six weeks) followed by a maintenance phasewithrituximabeverytwomonthsforonetotwoyears. Thecomplete response(CR) rateafter theinductionphase was45%,unconfirmedCRwas26%andpartialresponsewas 24%.The5-yearoverallsurvivalandprogression-freesurvival were92%and73%,respectively(p-value<0.001)46.Thereare
otherregimensusingrituximab;weeklyforfourweekswith orwithout maintenanceasreportedbyBennet etal.58 The
bestregimen,whethertousemaintenanceorretreatmentat relapse,isalsoareasthatneedtobeclarified.
Rituximabwithchemotherapy
The aforementioned chemotherapyoptions are used alone orwithrituximab.Purineanalogsaremoretoxicandshould be reserved for refractory or relapsed cases. Fludarabine has high response rates, with CR in 70% of cases and progression-freesurvivalof4.7years.57,58Acombinationwith
CladribineincreasedtheCRfrom21.4%to62.5%,andfour-year progression-freesurvivalfrom52.4%to83.4%.51
(* Other than spleen hilum)
Diagnosis of SMZL
Asymptomatic
Watch and wait
Symptomatic
Patients without Lymphadenopathy*
or B symptoms or inelegible for
splenectomy
Rituximab monotherapy
Patients with Lymphadenopathy * or B symptoms
Rituximab + chemotherapy
Patients refractory to rituximab, without lymphadenopathy, with
splenomegaly and low surgical risk
Splenectomy
Figure2–Suggestedapproachtotreatsplenicmarginalzonelymphoma.
(NCT01282424, NCT01732926, NCT02369016, NCT02367040, andNCT01732913)(Table4).
Treatmentofpatientswithsplenicmarginalzone
lymphomaandhepatitisC
Patients with hepatitis C who do not require an immedi-atecytoreductivetreatmentshouldreceivefirst-lineantiviral treatment with pegylated alpha-interferon and ribavirin, becauseaCRofSMZLoccursinabout75%ofthecases.39,59
Splenicirradiation
Splenicirradiationhashistoricalinterestand thereare iso-latedreportsofitsusebeforetheeraofrituximabtherapy.21,60
Treatment
considerations
Arcainiet al.proposesaconsensususingtheguidelines of boththe EuropeanSociety for MedicalOncology39 and the
SocietàItalianadiEmatologia.23 Accordingtothe European
Society,rituximabmonotherapyisareasonablefirst-line ther-apy and a less traumatic alternative to splenectomy and accordingtotheItalianSociety,rituximabisagoodoptionfor patientswithoutdisseminateddisease(nolymphadenopathy otherthanspleenhilum,noconstitutionalsymptomsorsigns ofhigh-gradetransformation)whoneedtreatmentandarenot eligibleforsplenectomy.Thegroupofpatientswith constitu-tionalsymptomsorsignsofhigh-gradetransformationmay beeligibleforrituximab-chemotherapycombinations.There isnostandardcareso far,but combinationswithCVPand chlorambucilareacceptedasfirstline.23
Figure2illustratesasuggestedalgorithmforthetreatment ofSMZLpatientsbasedontheseguidelines.
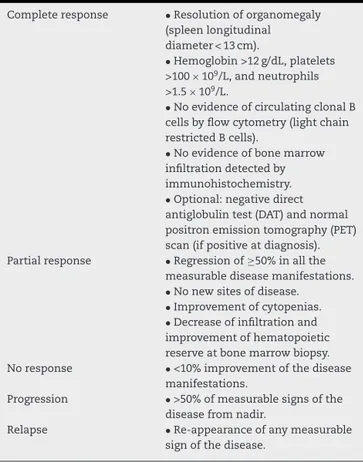
Response
evaluation
ThecriteriausedtoevaluateresponseofpatientswithSMZL totreatmentareshowninTable5.
Table5–Responsecriteriaforsplenicmarginalzone
lymphoma.
Completeresponse •Resolutionoforganomegaly
(spleenlongitudinal diameter<13cm).
•Hemoglobin>12g/dL,platelets
>100×109/L,andneutrophils
>1.5×109/L.
•NoevidenceofcirculatingclonalB cellsbyflowcytometry(lightchain restrictedBcells).
•Noevidenceofbonemarrow infiltrationdetectedby immunohistochemistry.
•Optional:negativedirect antiglobulintest(DAT)andnormal positronemissiontomography(PET) scan(ifpositiveatdiagnosis).
Partialresponse •Regressionof≥50%inallthe
measurablediseasemanifestations.
•Nonewsitesofdisease. •Improvementofcytopenias. •Decreaseofinfiltrationand
improvementofhematopoietic reserveatbonemarrowbiopsy.
Noresponse •<10%improvementofthedisease
manifestations.
Progression •>50%ofmeasurablesignsofthe
diseasefromnadir.
Relapse •Re-appearanceofanymeasurable
Follow-up
Asymptomaticpatientsshouldbeseeneverysixmonthswith nomorethanaphysicalexamination,bloodcounts,and bio-chemistry.Theintervalbetweenvisitsshouldbeshortened in cases of increasing splenomegaly or the occurrence of cytopenia.Computed tomographyandbonemarrowbiopsy are not indicated unless signs of disease progression are identified.39
Duringthefirstthreemonthsoftreatment,bloodcounts andlaboratorywork-upsshouldbeperformedeveryfourto sixweeksandeverysixmonthsthereafter.39
Final
considerations
SZMLisanindolentlymphomathatpresentsmanyunsolved questions,suchasstandardprognosticcriteriaandstandard treatment.Asitcompriseslessthan2%oflymphomas,large randomized clinical trials are not likely and review arti-cles that clarify some issues are important in the clinical practice.
Conflicts
of
interest
Theauthorsdeclarenoconflictsofinterest.
r
e
f
e
r
e
n
c
e
s
1. ZinzaniPL.Themanyfacesofmarginalzonelymphoma.ASH EducProgrB.2012;2012(1):426–32.
2. LengletJ,TraulléC,MounierN,BenetC,Munoz-BongrandN, AmorinS,etal.Long-termfollow-upanalysisof100patients withsplenicmarginalzonelymphomatreatedwith splenectomyasfirst-linetreatment.LeukLymphoma. 2013;55:1854–60.
3. SwerdlowSH,CampoE,HarrisNL,JaffeES,PileriSA,SteinH, etal.In:SwerdlowSH,CampoE,HarrisNL,JaffeES,PileriSA, SteinH,etal,editors.WorldHealthOrganization
classificationoftumoursofhaematopoieticandlymphoid tissues.4thedLyon:InternationalAgencyforResearchon Cancer(IARC);2008.InternationalAgencyforResearchon Cancer(IARC).422p.
4. BehdadA,BaileyNG.DiagnosisofsplenicB-celllymphomas inthebonemarrow:areviewofhistopathologic,
immunophenotypic,andgeneticfindings.ArchPatholLab Med.2014;138(10):1295–301.
5. MatutesE,OscierD,MontalbanC,BergerF,Callet-BauchuE, DoganA,etal.Splenicmarginalzonelymphomaproposals forarevisionofdiagnostic,stagingandtherapeuticcriteria. Leukemia.2008;22(3):487–95.
6. MendesLS,DuM-Q,MatutesE,WotherspoonA.Splenic marginalzonelymphoma:areviewoftheclinical
presentation,pathology,molecularbiology,andmanagement. BloodLymphCancer:TargetsTherapy.2014;4:29–38.
7. Traverse-GlehenA,BaseggioL,SallesG,FelmanP,BergerF. SplenicmarginalzoneB-celllymphoma:adistinct
clinicopathologicalandmolecularentity.Recentadvancesin ontogenyandclassification.CurrOpinOncol.
2011;23(5):441–8.
8.ArcainiL,RossiD,PaulliM.Splenicmarginalzonelymphoma: fromgeneticstomanagement.Blood.2016;127(17):2072–81.
9.CampoE,SwerdlowSH,HarrisNL,PileriS,SteinH,JaffeES. The2008WHOclassificationoflymphoidneoplasmsand beyond:evolvingconceptsandpracticalapplications. 2011;117(19):5019–32.
10.ChacónJI,MollejoM,Mu ˜nozE,AlgaraP,MateoM,LopezL, etal.Splenicmarginalzonelymphoma:clinical
characteristicsandprognosticfactorsinaseriesof60 patients.Blood.2002;100(5):1648–54.
11.IannittoE,AmbrosettiA,AmmatunaE,ColosioM,Florena AM,TripodoC,etal.Splenicmarginalzonelymphomawith orwithoutvillouslymphocytes.Cancer.2004;101(9):2050–7.
12.ArcainiL,MerliM,VolpettiS,RattottiS,GottiM,ZajaF. IndolentB-celllymphomasassociatedwithHCVinfection: clinicalandvirologicalfeaturesandroleofantiviraltherapy. ClinDevImmunol.2012;2012:1–10.
13.TasleemS,SoodGK.HepatitisCassociatedB-cell non-hodgkinlymphoma:clinicalfeaturesandtheroleof antiviraltherapy.JClinTranslHepatol.2015;3(2):134–9.
14.MarinhoTA.Prevalênciadainfecc¸ãopelovírusdahepatiteC emindivíduosportadoresdedoenc¸asoncohematológicasem Goiânia-GO.UniversidadeFederaldeGoiás;2013.
15.ChindamoM,SpectorN,SegadasJ,PimentaG,Vanderborght B,MoraisJ,etal.PrevalenceofhepatitisCinfectioninpatients withnon-Hodgkin’slymphomas.OncolRep.2002;9(8): 657–9.
16.BracciPM,BenaventeY,TurnerJJ,PaltielO,SlagerSL,Vajdic CM,etal.Medicalhistory,lifestyle,familyhistory,and occupationalriskfactorsformarginalzonelymphoma:the interlymphnon-Hodgkinlymphomasubtypesproject.JNCI Monogr.2014;2014(48):52–65.
17.VannataB,StathisA,ZuccaE.Managementofthemarginal zonelymphomas.In:EvensAM,BlumKA,editors.
Non-Hodgkinlymphoma.Cham:SpringerInternational Publishing;2015.p.227–49(CancerTreatmentandResearch; vol.165).
18.ArcainiL,LazzarinoM,ColomboN,BurcheriS,BoveriE,Paulli M,etal.Splenicmarginalzonelymphoma:aprognostic modelforclinicaluse.Blood.2006;107(12):4643–9.
19.MoreauEJ,MatutesE,A’HernRP,MorillaAM,MorillaRM, Owusu-AnkomahKA,etal.Improvementofthechronic lymphocyticleukemiascoringsystemwiththemonoclonal antibodySN8(CD79b).AmJClinPathol.1997;108(4):378–82.
20.EvensAM,BlumKA.Non-Hodgkinlymphoma.Switzerland: CancerTreatmentandResearch;2015,343p.
21.TroussardX,ValensiF,DuchayneE,GarandR,FelmanP, TulliezM,etal.Spleniclymphomawithvillouslymphocytes: clinicalpresentation,biologyandprognosticfactorsina seriesof100patients.BrJHaematol.1996;93(3):731–6.
22.Salomon-NguyenF,ValensiF,TroussardX,FlandrinG.The valueofthemonoclonalantibody,DBA44,inthediagnosisof B-lymphoiddisorders.LeukRes.1996;20(11/12):909–13.
23.TarellaC,ArcainiL,BaldiniL,BarosiG,BillioA,MarchettiM, etal.Italiansocietyofhematology,italiansocietyof
experimentalhematology,anditaliangroupforbonemarrow transplantationguidelinesforthemanagementofindolent, nonfollicularb-celllymphoma(marginalzone,
lymphoplasmacytic,andsmalllymphocytic).ClinLymphoma MyelomaLeuk.2015;15(2):75–85.
24.FaliniB,AgostinelliC,BigernaB,PucciariniA,PaciniR, TabarriniA,etal.IRTA1isselectivelyexpressedinnodaland extranodalmarginalzonelymphomas.Histopathology. 2012;61(5):930–41.
lymphoproliferativedisorders.AmJClinPathol. 2011;136(4):625–30.
26.PerroneS,D’EliaGM,AnnechiniG,FerrettiA,TostiME,FoàR, etal.Splenicmarginalzonelymphoma:prognosticfactors, roleofwatchandwaitpolicy,andothertherapeutic approachesintherituximabera.LeukRes.2016;44: 53–60.
27.SchopRF.Waldenstrommacroglobulinemianeoplasticcells lackimmunoglobulinheavychainlocustranslocationsbut havefrequent6qdeletions.Blood.2002;100(8):2996–3001.
28.TreonSP,HunterZR.AneweraforWaldenstrom macroglobulinemia:MYD88L265P.Blood.2013;121(22): 4434–6.
29.RemsteinED,JamesCD,KurtinPJ.Incidenceandsubtype specificityofAPI2-MALT1fusiontranslocationsinextranodal, nodal,andsplenicmarginalzonelymphomas.AmJPathol. 2000;156(4):1183–8.
30.DuMQ,PengHZ,Dogana,DissTC,LiuH,PanLX,etal. PreferentialdisseminationofB-cellgastric
mucosa-associatedlymphoidtissue(MALT)lymphomatothe splenicmarginalzone.Blood.1997;90(10):4071–7.
31.SalidoM,BaroC,OscierD,StamatopoulosK,DierlammJ, MatutesE,etal.Cytogeneticaberrationsandtheirprognostic valueinaseriesof330splenicmarginalzoneB-cell
lymphomas:amulticenterstudyoftheSplenicB-Cell LymphomaGroup.Blood.2010;116(9):1479–88.
32.FedericoM,VitoloU,ZinzaniPL,ChisesiT,ClòV,BellesiG, etal.Prognosisoffollicularlymphoma:apredictivemodel basedonaretrospectiveanalysisof987cases.Intergruppo ItalianoLinfomi.Blood.2000;95(3):783–9.
33.Solal-CelignyP,RoyP,ColombatP,WhiteJ,ArmitageJO, Arranz-SaezR,etal.Follicularlymphomainternational prognosticindex.Blood.2004;104(5):1258–65.
34.CarbonePP,KaplanHS,MusshoffK,ChairmanPP,Smithers DW,TubianaM.ReportoftheCommitteeonHodgkin’s DiseaseStagingClassificationReportoftheCommitteeon Hodgkin’sDiseaseStagingClassification.CancerRes. 1971;31(11):1860–1.
35.TsimberidouAM,CatovskyD,SchletteE,O’BrienS,Wierda WG,KantarjianH,etal.Outcomesinpatientswithsplenic marginalzonelymphomaandmarginalzonelymphoma treatedwithrituximabwithorwithoutchemotherapyor chemotherapyalone.Cancer.2006;107(1):125–35.
36.ThieblemontC,DaviF,NogueraM-E,BrièreJ,BertoniF,Zucca E,etal.Splenicmarginalzonelymphoma:currentknowledge andfuturedirections.Oncology(WillistonPark).
2012;26(2):194–202.
37.HockleySL,ElseM,MorillaA,WotherspoonA,DeardenC, CatovskyD,etal.Theprognosticimpactofclinicaland molecularfeaturesinhairycellleukaemiavariantandsplenic marginalzonelymphoma.BrJHaematol.2012;158(3):347–54.
38.MatutesE.Splenicmarginalzonelymphoma:diseasefeatures andmanagement.ExpertRevHematol.2013;6(6):735–45.
39.DreylingM,ThieblemontC,GallaminiA,ArcainiL,CampoE, HermineO,etal.Esmoconsensusconferences:guidelineson malignantlymphoma.Part2:marginalzonelymphoma, mantlecelllymphoma,peripheralT-celllymphoma.Ann Oncol.2013;24(4):857–77.
40.ParryM,Rose-ZerilliMJJ,LjungstromV,GibsonJ,WangJ, WalewskaR,etal.Geneticsandprognosticationinsplenic marginalzonelymphoma:revelationsfromdeepsequencing. ClinCancerRes.2015;21(18):4174–83.
41.Gruszka-WestwoodAM.p53abnormalitiesinsplenic lymphomawithvillouslymphocytes.Blood. 2001;97(11):3552–8.
42.Peveling-OberhagJ,WoltersF,DöringC,WalterD,SellmannL, ScholtysikR,etal.Wholeexomesequencingof
microdissectedsplenicmarginalzonelymphoma:astudyto
discovernoveltumor-specificmutations.BMCCancer. 2015;15(1):773.
43.MontalbánC,AbrairaV,ArcainiL,Domingo-DomenechE, Guisado-VascoP,IannittoE,etal.Simplificationofrisk stratificationforsplenicmarginalzonelymphoma:a point-basedscoreforpracticaluse.LeukLymphoma. 2014;55(4):929–31.
44.MontalbánC,AbrairaV,ArcainiL,Domingo-DomenechE, Guisado-VascoP,IannittoE,etal.Riskstratificationfor splenicmarginalzonelymphomabasedonhaemoglobin concentration,plateletcount,highlactatedehydrogenase levelandextrahilarlymphadenopathy:developmentand validationon593cases.BrJHaematol.2012;159(2): 164–71.
45.ThieblemontC,FelmanP,BergerF,DumontetC,ArnaudP, HequetO,etal.TreatmentofsplenicmarginalzoneB-cell lymphoma:ananalysisof81patients.ClinLymphoma. 2002;3(1):41–7.
46.KalpadakisC,PangalisGA,MariaKA,SachanasS,Kontopidou FN,YiakoumisX,etal.Treatmentofsplenicmarginalzone lymphomawithrituximabmonotherapy:progressreportand comparisonwithsplenectomy.Oncologist.2013;18(2): 190–7.
47.ElseM,Marín-NieblaA,delaCruzF,BattyP,RíosE,Dearden CE,etal.Rituximab,usedaloneorincombination,issuperior toothertreatmentmodalitiesinsplenicmarginalzone lymphoma.BrJHaematol.2012;159(3):322–8.
48.IannittoE,TripodoC.HowIdiagnoseandtreatsplenic lymphomas.Blood.2011;117(9):2585–95.
49.BennettM,SchechterGP.Treatmentofsplenicmarginalzone lymphoma:splenectomyversusrituximab.SeminHematol. 2010;47(2):143–7.
50.KalpadakisC,PangalisGA,VassilakopoulosTP,SachanasS, KyrtsonisMC,DimopoulouM,etal.Splenectomyversus Rituximabinthetreatmentofsplenicmarginalzone lymphoma.Haematologica.2009;94Suppl.2:168–9.
51.CervettiG,GalimbertiS,PelosiniM,GhioF,CecconiN,Petrini M.Significantefficacyof2-chlorodeoxyadenosine+rituximab inthetreatmentofsplenicmarginalzonelymphoma(SMZL): extendedfollow-up.AnnOncol.2013;24(9):2434–8.
52.KalpadakisC,PangalisGA,VassilakopoulosTP,SachanasS, AngelopoulouMK.Treatmentofsplenicmarginalzone lymphoma:shouldsplenectomybeabandoned?Leuk Lymphoma.2014;55(7):1463–70.
53.Parry-JonesN,MatutesE,Gruszka-WestwoodAM,Swansbury GJ,WotherspoonAC,CatovskyD.Prognosticfeaturesof spleniclymphomawithvillouslymphocytes:areporton129 patients.BrJHaematol.2003;120(5):759–64.
54.LengletJ,TraullC,MounierN,BenetC,Munoz-bongrandN, AmorinS,etal.Long-termfollow-upanalysisof100patients withsplenicmarginalzonelymphomatreatedwith splenectomyasfirst-linetreatment.LeukLymphoma. 2014;55(8):1854–60.
55.PataG,DamianiE,BartoliM,SolariS,AnastasiaA,PaganiC, etal.Peri-operativecomplicationsandhematologic
improvementafterfirst-linesplenectomyforsplenicmarginal zonelymphoma.LeukLymphoma.2016;57(6):1467–70.
56.OlszewskiAJ,AliS.Comparativeoutcomesof
rituximab-basedsystemictherapyandsplenectomyin splenicmarginalzonelymphoma.AnnHematol. 2014;93(3):449–58.
57.KalpadakisC,PangalisGA,DimopoulouMN,Vassilakopoulos TP,KyrtsonisM-C,KorkolopoulouP,etal.Rituximab monotherapyishighlyeffectiveinsplenicmarginalzone lymphoma.HematolOncol.2007;25(3):127–31.
59.ArcainiL,VallisaD,RattottiS,FerrettiVV,FerreriAJ,Bernuzzi P,etal.AntiviraltreatmentinpatientswithindolentB-cell lymphomasassociatedwithHCVinfection:astudyofthe FondazioneItalianaLinfomi.AnnOncol.2014;25(7):1404–10.