2019/2020
Catarina de Vasconcelos Peixoto Fonseca
Resultados da oclusão do apêndice auricular esquerdo com
terapia antiagregante plaquetária em pacientes com
contraindicação para hipocoagulação:
uma revisão sistemática.
Clinical outcomes of left atrial appendage closure and
post-operative antiplatelet therapy in patients with
contraindication to anticoagulation:
a systematic review
.
Mestrado Integrado em Medicina
Área: Ciências Médicas e da saúde
Tipologia: Dissertação
Trabalho efetuado sob a Orientação de:
Doutor Manuel Belchior Campelo
Trabalho organizado de acordo com as normas da revista:
Ata Médica Portuguesa
Catarina de Vasconcelos Peixoto Fonseca
Resultados da oclusão do apêndice auricular esquerdo com
terapia antiagregante plaquetária em pacientes com
contraindicação para hipocoagulação:
uma revisão sistemática.
Clinical outcomes of left atrial appendage closure and
post-operative antiplatelet therapy in patients with
contraindication to anticoagulation:
a systematic review.
Clinical outcomes of left atrial appendage closure and post-operative
antiplatelet therapy in patients with contraindication to
anticoagulation: a systematic review.
Resultados da oclusão do apêndice auricular esquerdo com terapia
antiagregante plaquetária em pacientes com contraindicação para
hipocoagulação: uma revisão sistemática.
Catarina Vasconcelos P. Fonseca
1, Luís Azevedo
2, Daniel Dias
3, Manuel Campelo
4a, b, c1 Faculty of Medicine, University of Porto, Porto, Portugal
2 Center for Research in Health Technologies and Services (CINTESIS), Porto, Portugal
3 Center for Research in Health Technologies and Services (CINTESIS), Porto, Portugal
4 a) Department of Medicine, Faculty of Medicine, University of Porto, Portugal
b) Center for Research in Health Technologies and Services (CINTESIS), Porto, Portugal
c) Department of Cardiology, Centro Hospitalar Universitário de São João, E.P.E., Porto,
Portugal
This research received no specific grant from any funding agency in the public, commercial, or
not-for-profit sectors.
Correspondence: Catarina Vasconcelos P. Fonseca, Faculdade de Medicina, Universidade do Porto,
Alameda Prof. Hernani Monteiro, 4200 Porto, Portugal.
Clinical outcomes of left atrial appendage closure and post-operative
antiplatelet therapy in patients with contraindication to anticoagulation:
a systematic review.
Abstract
Background Randomized clinical trials have been performed to analyze the efficacy and safety of left
atrial appendage closure in non-valvular atrial fibrillation versus medical management. This technique
emerged for patients with contraindication to anticoagulation therapy; nonetheless, those patients were
still medicated with anticoagulation strategies for a period of time, which gave rise to safety concerns.
Our systematic review aims to evaluate the feasibility of this intervention in patients with
contraindication to anticoagulation and post-operative antiplatelet therapy.
Methods A comprehensive search of the Medline and ISI Web of Knowledge databases was conducted
using pre-defined criteria. We included non-comparative cohort studies with at least 15 patients and a
follow-up time longer than 6 months.
Results We selected 21 eligible studies enrolling 3974 patients. The adjusted pooled incidence rate of
thromboembolic events was 1.99/100 person-years (95% CI: 1.66 to 2.38/100 person-years) and the
incidence rate of major bleeding corresponded to 2.07/100 person-years (95% CI: 1.40 to 3.06/100
person-years). These results were similar, if not better, than the outcomes of the clinical trials made so
far. The incidence rate of all-cause mortality was 6.13 per 100 person-years (95% CI: 5.18 to 7.27
person-years), with a higher proportion of mortality associated to non-cardiovascular causes. Among
patients that changed from antiplatelet therapy to anticoagulation, the incidence rate was 1.62/100
person-years (95% CI: 0.92 to 2.84 person-years).
Conclusions Our review sustains the feasibility of this technique in patients submitted to antiplatelet
therapy, on the prevention of thromboembolic and bleeding events, in comparison to anticoagulation
after procedure.
Resumo
Introdução Ensaios clínicos randomizados foram realizados para analisar a eficácia e segurança da
oclusão do apêndice auricular esquerdo na fibrilação auricular não-valvular versus terapia médica. Esta
técnica foi desenvolvida para doentes com contraindicação para anticoagulação, contudo esses pacientes
continuavam a ser hipocoagulados por um período de tempo, o que alça problemas relativos à sua
segurança. Na nossa revisão sistemática pretendemos avaliar a viabilidade desta intervenção com
terapia antiplaquetária após intervenção.
Métodos Uma pesquisa pela MedLine e ISI Web of Knowledge foi conduzida com base em critérios
pré-definidos. Nós incluímos estudos observacionais não-comparativos com pelo menos 15 pacientes e por
um período mínimo de 6 meses de seguimento.
Resultados Foram selecionados 21 estudos com 3974 pacientes na globalidade. A taxa de incidência
ajustada de eventos tromboembólicos corresponde a 1.99/ 100 pessoa-ano (95% CI: 1.66-2.38/100
pessoa-ano) e a taxa de incidência de eventos hemorrágicos major foi de 2.07/100 pessoa-ano (95% CI:
1.40-3.06/100 pessoa-ano). Os resultados foram similares, se não melhores, que os dados clínicos dos
ensaios clínicos realizados. A taxa de incidência de mortalidade corresponde a 6.13 per 100 pessoa-ano
(95% CI: 5.18-7.27 pessoa-ano), com a proporção mais elevada associada a causas não-cardiovasculares.
Uma pequena porção de pacientes mudaram a sua terapia inicial para anticoagulantes orais com uma
taxa de incidência de 1.62/100 pessoa-ano (95% CI: 0.92-2.84 pessoa-ano).
Conclusão A nossa revisão sustém a viabilidade desta técnica em pacientes submetidos a terapia
antiplaquetária após intervenção, na prevenção de eventos tromboembólicos e hemorrágicos,
comparando com a terapia anticoagulante selecionada nos ensaios clínicos.
Introduction
Atrial fibrillation (AF) is the most common sustained arrhythmia affecting
approximately 3% of the population over 20 years old, with greater prevalence in older
persons and patients with high cardiovascular risk. [1]
The left atrial appendage (LAA), an embryonic reminiscent structure of the left atrium,
is the major site of thrombogenesis in the heart, responsible for more than 90% of the
cardioembolic strokes in non-valvular AF (NVAF) patients. Indeed, 20% of ischemic
strokes among the elderly are caused by AF, emphasizing the need of stroke prevention
in these patients.[2]
While anticoagulation therapy with warfarin is effective in stroke prevention, it is
associated with an increased risk of intracerebral and extracranial bleeding and may
be contraindicated in some patients. Previous studies found a prevalence of
contraindications of around 15% in clinical AF patients, hence percutaneous LAA
occlusion devices were developed to overcome this problem.[3]
The first technology created was the Percutaneous Left Atrial Appendage Occlusion
(PLAATO) device that proved to be safe and feasible in patients with contraindication
for anticoagulation therapy.[4] Since then, other devices had emerged, such as
Watchman, Amplatzer Cardiac plug, Amplatzer Amulet and Lambre devices, all
characterized in the studies included in this review. Of the five devices mentioned, the
Watchman was the only device approved by the US Food and Drug Administration
(FDA) and with a specific indication for the prevention of stroke in atrial fibrillation.
[5]
The Watchman Left Atrial Appendage System for Embolic Protection in Patients with
AF (PROTECT AF) clinical trial showed non-inferiority of the Watchman device for the
prevention of stroke, systemic embolism and cardiovascular death when compared to
warfarin therapy and also fewer haemorrhagic strokes in non-valvular AF patients
with a CHADS2-score ≥1.[6] In the PREVAIL (Prospective Randomized Evaluation of
the WATCHMAN Device in Patients With Atrial Fibrillation Versus Long Term
Warfarin Therapy) trial, non-inferiority was also confirmed and procedural safety
improved.[7]
However, the patients in the LAA closure arm were still prescribed with warfarin
therapy for at least 6 weeks after procedure, which could be a real problem for patients
with contraindication for anticoagulation. Thus, there was
a necessity to find a
strategy that dispensed anticoagulation and reduced the bleeding risk during those
weeks; in recent years, some researchers had attempted to perform the percutaneous
occlusion of LAA, using only antiplatelet therapy.
The aim of this study was to perform a systematic review to compare clinical
outcomes, specifically adjusted rates of thromboembolic events, major bleeding,
all-cause mortality and modification of initial therapy in NVAF patients submitted to this
percutaneous procedure and then prescribed with antiplatelet therapy.
Methods
Search strategy
To identify relevant articles, the authors started with a search on Medline (PubMed)
and ISI Web of Knowledge databases during the month of September 2019. The query
used on both was “left atrial appendage closure” AND “stroke prevention” AND
“(antiplatelet therapy OR anticoagulation contraindication)”.
The search resulted in 38 articles on Medline database with the filter “clinical trial”,
“multicentre studies” and “observational studies” and 103 articles on ISI Web of
Knowledge, with the filter “clinical trial” (Figure 1).
Inclusion criteria
The studies included in this review were human studies in patients with non-valvular
atrial fibrillation submitted to left atrial appendage closure, with a CHADS
2score
or CHA
2DS
2-VASc score ≥1 and a contraindication to anticoagulation therapy.
Exclusion criteria
Follow-up studies, systematic reviews and/or meta-analysis, duplicate studies, cost
analyses or surveys, comparison between devices and studies focusing on imaging and
planning were not included in this review.
Studies with anticoagulation therapy after procedure (>5%), a number of patients
inferior to 15 and a follow-up period shorter than 6 months were excluded.
Data extraction and quality assessment of studies
Eligibility and quality of the studies were independently evaluated by two researchers.
If there was a disagreement regarding the inclusion of the articles, a consensus
between the authors was established through review and discussion. The clinical
studies were selected based on the inclusion criteria and methodological quality, by
means of the Quality Appraisal of Case Series Studies Checklist from the Institute of
Health Economics.(Supplement file 1) [8]
Outcome measures
We synthesized the clinical outcomes of the prospective studies regarding the
procedure and the antiplatelet therapy applied after intervention. The outcomes
evaluated were the incidence of thromboembolic events (stroke, TIA, systemic
embolism), major bleeding, all-cause mortality and the incidence of modification of
initial therapy, from antiplatelet therapy to anticoagulation. The reasons stated for this
alteration were varied; however, the most recurrent argument was a formation of a
device-related thrombus that required anticoagulation therapy.
Statistical Analysis
We used Poisson-normal models for the meta-analysis of incidence rates. [9] [10] [11]
These are generalized linear mixed models (GLMM) that have been shown to have
several relevant advantages when compared with the classical inverse variance weight
meta-analysis, particularly when including primary studies with sparse data as is the
case in the present meta-analysis.[10] The results are presented as expected number
of events per 100 person-years at risk. We used the log transformed incidence rate as
outcome measure and then back transformed the results by exponentiation in order
to present meta-analytical measures and construct forest plots. To calculate incidence
rates, we used the total number of events observed and the total person-years of
follow-up, based on the mean follow-up times and the total number of participants in
each study. Studies with zero events were handled by adding a 0.5 constant to the
number of events (only in studies with zero events). Heterogeneity was assessed using
the heterogeneity Chi-square test and using the I-square statistic (an I-square above
50% was considered as relevant heterogeneity). Meta-analyses was conducted with
the metafor package [11] in R, version 3.6.2. [12] We used a 5% level of significance.
Results
We selected 21
non-comparative cohort studies [4, 13-32]
from the PubMed and ISI
Web of Knowledge databases in accordance with study inclusion criteria. A total of
3974 patients were enrolled in this review. Table 1 shows the baseline characteristics
of the included studies, including the follow-up time that varied from 6 to 48 months.
The mean CHADS
2score or/and CHA
2DS
2-VASc score of each study was between
2.3-4.0 and 3.2-5.4, respectively (Table 1).
Data related to the number and proportion of clinical outcomes of each study, as
thromboembolic events, major bleeding, all-cause mortality and modification of initial
therapy, are represented on Table 2.
Among identified studies, we detected high percentages of implantation/procedure
success rates, with values that varied between 88% and 100%. Peri-procedural
complications were identified in almost all of the registries, even though in small
proportions; the causes were mainly due to cardiac tamponade, anatomic
particularities that invalidated the use of this devices and bleeding complications or
device-associated thrombus.
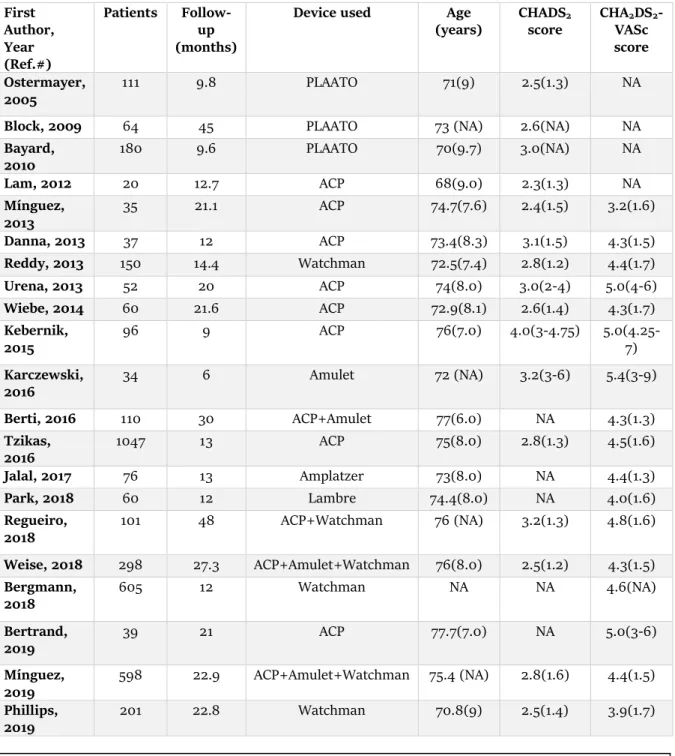
The adjusted pooled incidence rate of thromboembolic events in this analysis was
1.99/100 person-years (PY) (95% CI: 1.66 to 2.38/100 PY) (Figure 2-A), including
strokes, TIAs and systemic embolic events. The study with the highest proportion of
thromboembolic events in the review showed a proportion of 0.14%, that
corresponded to an incidence of 3.75/ 100 PY [14]. This value was attributed to an
early essay regarding the first device created, PLAATO, and therefore, one of the
earliest studies made with this new surgical technique. In the following studies, a
substantial decrease of thromboembolic events was noticeable with proportions that
varied between 0.02% and 0.05% and a value of 0.11% in one of the studies. This
improvement might be attributed to an increase of the operator experience and to the
growth of literature explaining the technicalities of percutaneous device´s
implantation.
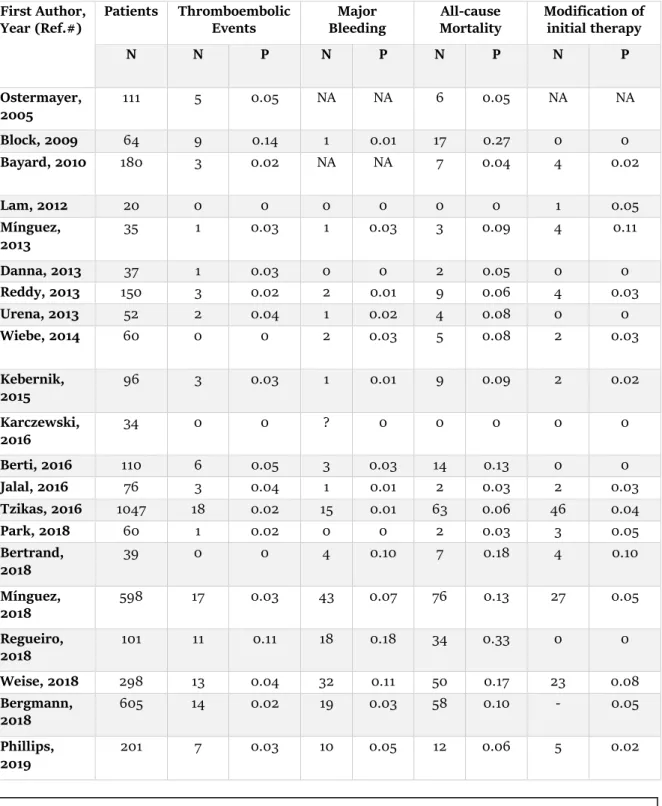
The adjusted incidence rate of major bleeding was 2.07/100 PY (95% CI: 1.40 to
3.06/100 PY) (Figure 2-B), with the highest proportion of major bleeding in the review
of 0.18%. [29]. This value was attributed mainly to gastrointestinal bleeding which
could be related to baseline comorbidities and antithrombotic therapy after procedure
in patients with high risk of hemorrhagic complications.
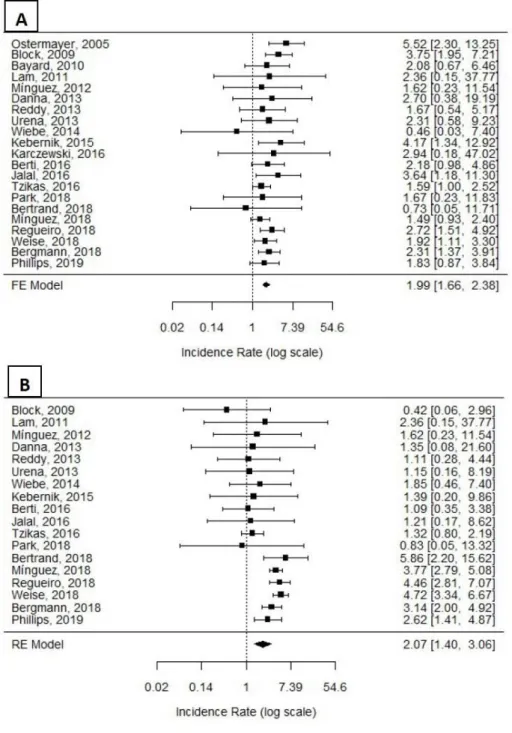
The incidence of all-cause mortality was 6.13 per 100 person-years (95% CI: 5.18 to
7.27 PY) (Figure 3-A). As explained in the studies, the major portion of deaths were
not associated to cardiovascular causes, but mainly related to the elevated rate of
comorbidities of these patients, such as hypertension, diabetes mellitus, cancer,
respiratory problems.
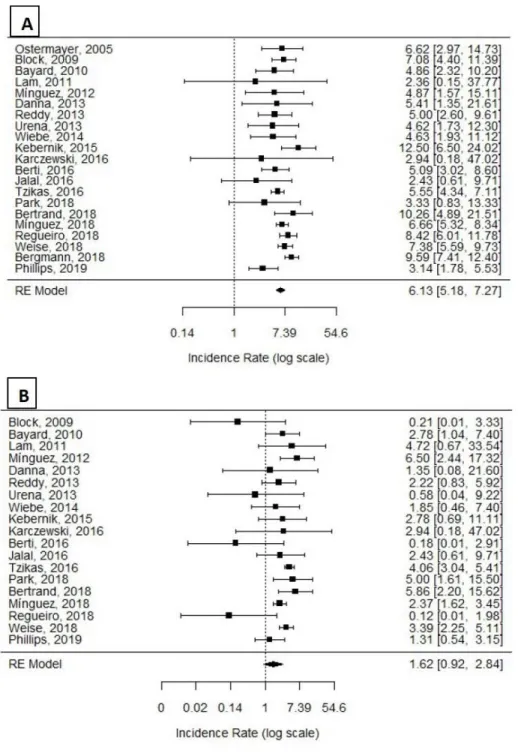
Regarding to the alteration of medication, from antiplatelet therapy to anticoagulation,
we detected an incidence rate of 1.62/100 PY (95% CI: 0.92 to 2.84 PY) (Figure 3-B).
We thought it was pertinent to include this data, since this meta-analysis focused on
patients that didn´t tolerate anticoagulation and so, were on antiplatelet therapy after
the procedure. As we could appreciate from the data, it was a small portion of patients
that changed their initial therapy.
Discussion
The emergence of percutaneous devices for occlusion of left atrial appendage, the
major site of thrombus formation, was required in NVAF patients with
contraindication for anticoagulation therapy. The WATCHMAN device is the most
methodically studied, represented in both randomized clinical trials made regarding
this matter (PROTECT-AF and PREVAIL trial [6, 7]). Nevertheless, since the devices
are fairly new, there is a scarce clinical evidence that proves their safety and efficacy.
We synthetized
non-comparative cohort studies that evaluated the clinical outcomes
of this procedure and the subsequent antiplatelet therapy used in patients with
contraindication to anticoagulation.
Regarding to stroke prophylaxis, we estimated the incidence rate of thromboembolic
events to be
1.99/100 PY (95% CI: 1.66 to 2.38/100 PY) (Figure 2-A). This value was
similar to the stroke rate of the randomized clinical trial PROTECT-AF [6] (2.2/100 PY
[95% CI: 1.2 to 3.5/100 PY]), even with the inclusion of only stroke events. In the
PREVAIL study [7], the stroke rate reported was 0.7/100 PY (95%: 0.1 to 5.1/100 PY),
lower than the incidence rate of thromboembolic events in this review, mainly because
of the disperse follow-up times of the studies included. Therefore, the antiplatelet
therapy showed a parallel prevention of stroke events, in comparison to the
anticoagulation therapy studied in the clinical trials.
The major bleeding incidence rate of
2.07/100 PY (95% CI: 1.40 to 3.06/100 PY)
(Figure 2-B) was inferior to the incidence rate of the clinical trial PROTECT-AF [6],
with a value of 2.3/100 PY (calculated based on data of the study, number of
participants and follow-up time). So, it was fair to conclude the presence of fewer
hemorrhagic complications in patients with an inherent higher risk of bleeding that
were medicated with antiplatelet therapy, in comparison to anticoagulation.
Therefore, antiplatelet therapy was a more secure treatment after procedure for
patients submitted to LAA closure.
Accordingly, the outcomes of the observational studies showed to be similar, if not
better, than the PROTECT-AF study data, indicating this procedure with post-operative
antiplatelet therapy as a feasible and secure option for stroke prophylaxis in NVAF
patients.
The PROTECT-AF study showed an incidence rate of all-cause mortality of 3.0 per 100
person-years, inferior to the rate of this review (6.13 per 100 person-years (95% CI:
5.18 to 7.27)) (Figure 2-C). In both studies, the main causes of all-cause mortality were
non-cardiovascular and so, not deemed related to the LAA closure device. Therefore,
the most logic reason for the higher incidence rate in the review could be due poorest
clinical conditions of the patients, with more co-morbidities than the patients allocated
in the clinical trial.
Furthermore, the amendment of the initial therapy was required in a minor fraction
of patients because of post-procedural complications, specifically device-associated
thrombus and thromboembolic events. Nonetheless, the change to anticoagulation
therapy was temporary with a complete resolution of the complications in most of the
cases.
Study limitations. The included studies with different baseline characteristics and the
non-uniformity of the follow-up time might introduce imprecise and disperse
outcomes, explaining the heterogeneity of the data. Because of the minor rate of
thromboembolic events after intervention, more events are more likely to appear on
studies with longer follow-up periods and larger samples, giving rise to disperse rates
of events among the studies of the review. Another limitation is related to the type of
studies inserted in this meta-analysis, linked to the inherent concerns associated to
observational cohort studies. As example, we may refer the lack of standardization of
patients and the under-reported events associated to this type of studies in comparison
to randomized clinical trials.
In spite of these limitations, this review seeks to combine and clarify the feasibility of
LAA closure for stroke prophylaxis in patients with contraindication for
anticoagulation and then submitted to antiplatelet therapy. It seems to be a different
and more secure therapeutic option that completely excludes anticoagulation therapy,
including for at least six weeks after procedure as seen in several studies.
Conclusion
In our systematic review, we included the studies available that specified the rate of
clinical outcomes in patients submitted to LAA closure and a post-operative
antiplatelet therapy. This approach may be a reasonable alternative to LAA closure
with anticoagulation after procedure for stroke prophylaxis in patients unable to
receive this therapy. Nonetheless, more randomized clinical trials are needed to
support the efficacy and safety of percutaneous left atrial appendage closure with
antiplatelet therapy.
Funding
This research received no specific grant from any funding agency in the public,
commercial, or not-for-profit sectors.
Declaration of conflicting interests
References
1.
Kirchhof, P., Bennussi, S., Kotecha, D., Ahlsson, A., Atar, D., Casadei, D., et al.,
2016 ESC Guidelines for the management of atrial fibrillation developed in
collaboration with EACTS. Eur Heart J, 2016. 37(38): p. 2893-2962.
2.
Landmesser, U. and D.R. Holmes, Jr., Left atrial appendage closure: a
percutaneous transcatheter approach for stroke prevention in atrial fibrillation.
Eur Heart J, 2012. 33(6): p. 698-704.
3.
Nieuwlaat, R., Capuci, A., Camm, J., Olsson, B., Andresen, D., Davies, D.W., et al.,
Atrial fibrillation management: a prospective survey in ESC member countries:
the Euro Heart Survey on Atrial Fibrillation. Eur Heart J, 2005. 26(22): p.
2422-34.
4.
Bayard, Y.L., Omran, H., Neuzil, P., Thuesen, L., Pichler, M., Rowland, E., et al.,
PLAATO (Percutaneous Left Atrial Appendage Transcatheter Occlusion) for
prevention of cardioembolic stroke in non-anticoagulation eligible atrial
fibrillation patients: results from the European PLAATO study. EuroIntervention,
2010. 6(2): p. 220-6.
5.
Bartus, K., Han, F.T., Bednarek, J., Myc, J., Kapelak, B., Sadowski, J., et al.,
Percutaneous left atrial appendage suture ligation using the LARIAT device in
patients with atrial fibrillation: initial clinical experience. J Am Coll Cardiol, 2013.
62(2): p. 108-118.
6.
Holmes, D.R., Reddy, V.Y., Turi, Z.G., Doshi, S.K., Sievert, H., Buchbinder, M., et
al., Percutaneous closure of the left atrial appendage versus warfarin therapy for
prevention of stroke in patients with atrial fibrillation: a randomised
non-inferiority trial. Lancet, 2009. 374(9689): p. 534-42.
7.
Holmes, D.R., Jr., Kar, S., Price, M.J., Whisenant, B., Sievert, H., Doshi, S.K., et al.,
Prospective randomized evaluation of the Watchman Left Atrial Appendage
Closure device in patients with atrial fibrillation versus long-term warfarin
therapy: the PREVAIL trial. J Am Coll Cardiol, 2014. 64(1): p. 1-12.
8.
Institute of Health Economics, Quality Appraisal of Case Series Studies Checklist.
2014: Edmonton (AB): Institute of Health Economics.
9.
Bagos Pantelis, G. and K. Nikolopoulos Georgios, Mixed-Effects Poisson
Regression Models for Meta-Analysis of Follow-Up Studies with Constant or
Varying Durations, in The International Journal of Biostatistics. 2009.
10.
Stijnen, T., T.H. Hamza, and P. Özdemir, Random effects meta-analysis of event
outcome in the framework of the generalized linear mixed model with applications
in sparse data. Statistics in Medicine, 2010. 29(29): p. 3046-3067.
11.
Viechtbauer, W., Conducting Meta-Analyses in R with the metafor Package. 2010,
2010. 36(3): p. 48.
12.
R Development Core Team, R: A language and environment for statistical
computing. 2010, R Foundation for Statistical Computing: Vienna, Austria.
P.C., et al., Percutaneous left atrial appendage transcatheter occlusion (PLAATO
system) to prevent stroke in high-risk patients with non-rheumatic atrial
fibrillation: results from the international multi-center feasibility trials. J Am Coll
Cardiol, 2005. 46(1): p. 9-14.
14.
Block, P.C., Burstein, S., Casale, P.N., Kramer, P.H., Teirstein, P., Williams, D.O.,
et al., Percutaneous left atrial appendage occlusion for patients in atrial
fibrillation suboptimal for warfarin therapy: 5-year results of the PLAATO
(Percutaneous Left Atrial Appendage Transcatheter Occlusion) Study. JACC
Cardiovasc Interv, 2009. 2(7): p. 594-600.
15.
Lam, Y.Y., Yip, G.W.K., Yu, C.M., Chan, W.W.M., Cheng, B.C.W., Yan, B.P., et al.,
Left atrial appendage closure with AMPLATZER cardiac plug for stroke prevention
in atrial fibrillation: initial Asia-Pacific experience. Catheter Cardiovasc Interv,
2012. 79(5): p. 794-800.
16.
Lopez-Minguez, J.R., Eldoayen-Gragera, J., González-Fernández, R.,
Fernández-Vegas, C., Fuentes-Cañamero, M.E., Millán-Nuñez. V., et al., Immediate and
one-year results in 35 consecutive patients after closure of left atrial appendage with
the Amplatzer cardiac plug. Rev Esp Cardiol (Engl Ed), 2013. 66(2): p. 90-7.
17.
Danna, P., Proietti, R., Sagone, A., Arensi, A., Viecca, M., Rago, A., et al., Does left
atrial appendage closure with a cardiac plug system reduce the stroke risk in
nonvalvular atrial fibrillation patients? A single-center case series. Pacing Clin
Electrophysiol, 2013. 36(3): p. 347-53.
18.
Reddy, V.Y., Möbius-Winkler, S., Miller, M.A., Neuzil, P., Schuler, G., Wiebe, J., et
al., Left atrial appendage closure with the Watchman device in patients with a
contraindication for oral anticoagulation: the ASAP study (ASA Plavix Feasibility
Study With Watchman Left Atrial Appendage Closure Technology). J Am Coll
Cardiol, 2013. 61(25): p. 2551-6.
19.
Urena, M., Rodés-Cabau, J., Freixa, X., Saw, J., Webb, J.G., Freeman, M., et al.,
Percutaneous left atrial appendage closure with the AMPLATZER cardiac plug
device in patients with nonvalvular atrial fibrillation and contraindications to
anticoagulation therapy. J Am Coll Cardiol, 2013. 62(2): p. 96-102.
20.
Wiebe, J., Bertog, S., Franke, J., Wettstein, O., Lehn, K., Hofmann, I., et al., Safety
of percutaneous left atrial appendage closure with the Amplatzer cardiac plug in
patients with atrial fibrillation and contraindications to anticoagulation. Catheter
Cardiovasc Interv, 2014. 83(5): p. 796-802.
21.
Kebernik, J., Jose, J., Abdel-Wahab, M., Stöcker, B., Geist, V., Richaardt, G., Safety
and Efficacy of Left Atrial Appendage Closure with the Amplatzer Cardiac Plug in
Very High Stroke and Bleeding Risk Patients with Non-Valvular Atrial Fibrillation.
Cardiol Ther, 2015. 4(2): p. 167-77.
22.
Karczewski, M., Wo
ź
niak, S., Skowronek, R., Burysz, M., Fischer, M.,
Anisimowicz, L., et al., Percutaneous left atrial appendage occlusion - treatment
outcomes and 6 months of follow-up - a single-center experience. Kardiochir
23.
Berti, S., Pastormerlo, L.E., Rezzaghi, M., Trianni, G., Paradossi, U., Cerone, E., et
al., Left atrial appendage occlusion in high-risk patients with non-valvular atrial
fibrillation. Heart, 2016. 102(24): p. 1969-1973.
24.
Jalal, Z., Dinet, M.L., Combes, N., Pillois, X., Renou, P., Sibon, I., et al.,
Percutaneous left atrial appendage closure followed by single antiplatelet therapy:
Short- and mid-term outcomes. Arch Cardiovasc Dis, 2016. 110(4): p. 242-249.
25.
Tzikas, A., Shakir, S., Gafoor, S., Omran, H., Berti, S., Santoro, G., et al., Left
atrial appendage occlusion for stroke prevention in atrial fibrillation: multicentre
experience with the AMPLATZER Cardiac Plug. EuroIntervention, 2016. 11(10): p.
1170-9.
26.
Park, J.W., Sievert, H., Kleinecke, C., Vaskelyte, L., Schnupp, S., Sievert, K., et al.,
Left atrial appendage occlusion with lambre in atrial fibrillation: Initial European
experience. Int J Cardiol, 2018. 265: p. 97-102.
27.
Bertrand, P.B., Habran, M., Kenis, K., Lecomte, J., Moonen, L., Strrbants, D., et
al., Dual antiplatelet therapy after percutaneous left atrial appendage occlusion:
single center experience with the Amplatzer Cardiac Plug. Acta Cardiol, 2018.
74(1): p. 74-81.
28.
Lopez-Minguez, J.R., Nogales-Asensio, J.M., Oliveira, E.I.D., Ribeiro, V.D.G,
Ruiz-Salmerón, R., Arzamendi-Aizpurua, D., et al., Long-term Event Reduction After
Left Atrial Appendage Closure. Results of the Iberian Registry II. Rev Esp Cardiol
(Engl Ed), 2018. 72(6): p. 449-455.
29.
Regueiro, A., Cruz-Gonzalez, I., Bethencourt, A., Nombela-Franco, L.,
Champagne, J., Asmarats, L., et al., Long-term outcomes following percutaneous
left atrial appendage closure in patients with atrial fibrillation and
contraindications to anticoagulation. J Interv Card Electrophysiol, 2018. 52(1): p.
53-59.
30.
Weise, F.K., Bordignon, S., Perrotta, L., Konstantinou, A., Bologna, F., Nagase, T.,
et al., Short-term dual antiplatelet therapy after interventional left atrial
appendage closure with different devices. EuroIntervention, 2018. 13(18): p.
e2138-e2146.
31.
Bergmann, M.W., Ince, H., Kische, S., Schmitz, T., Meincke, F., Schmidt, B., et al.,
Real-world safety and efficacy of WATCHMAN LAA closure at one year in patients
on dual antiplatelet therapy: results of the DAPT subgroup from the EWOLUTION
all-comers study. EuroIntervention, 2018. 13(17): p. 2003-2011.
32.
Phillips, K.P., Santoso, T., Sanders, P., Alison, J., Chan, J.L.K., Pak, H.N., et al., Left
atrial appendage closure with WATCHMAN in Asian patients: 2year outcomes
from the WASP registry. Int J Cardiol Heart Vasc, 2019. 23: p. 100358.
Table 1- Baseline characteristics of included studies
First
Author,
Year
(Ref.#)
Patients
Follow-up
(months)
Device used
Age
(years)
CHADS
2score
CHA
2DS
2-VASc
score
Ostermayer,
2005
111
9.8
PLAATO
71(9)
2.5(1.3)
NA
Block, 2009
64
45
PLAATO
73 (NA)
2.6(NA)
NA
Bayard,
2010
180
9.6
PLAATO
70(9.7)
3.0(NA)
NA
Lam, 2012
20
12.7
ACP
68(9.0)
2.3(1.3)
NA
Mínguez,
2013
35
21.1
ACP
74.7(7.6)
2.4(1.5)
3.2(1.6)
Danna, 2013
37
12
ACP
73.4(8.3)
3.1(1.5)
4.3(1.5)
Reddy, 2013
150
14.4
Watchman
72.5(7.4)
2.8(1.2)
4.4(1.7)
Urena, 2013
52
20
ACP
74(8.0)
3.0(2-4)
5.0(4-6)
Wiebe, 2014
60
21.6
ACP
72.9(8.1)
2.6(1.4)
4.3(1.7)
Kebernik,
2015
96
9
ACP
76(7.0)
4.0(3-4.75)
5.0(4.25-7)
Karczewski,
2016
34
6
Amulet
72 (NA)
3.2(3-6)
5.4(3-9)
Berti, 2016
110
30
ACP+Amulet
77(6.0)
NA
4.3(1.3)
Tzikas,
2016
1047
13
ACP
75(8.0)
2.8(1.3)
4.5(1.6)
Jalal, 2017
76
13
Amplatzer
73(8.0)
NA
4.4(1.3)
Park, 2018
60
12
Lambre
74.4(8.0)
NA
4.0(1.6)
Regueiro,
2018
101
48
ACP+Watchman
76 (NA)
3.2(1.3)
4.8(1.6)
Weise, 2018
298
27.3
ACP+Amulet+Watchman
76(8.0)
2.5(1.2)
4.3(1.5)
Bergmann,
2018
605
12
Watchman
NA
NA
4.6(NA)
Bertrand,
2019
39
21
ACP
77.7(7.0)
NA
5.0(3-6)
Mínguez,
2019
598
22.9
ACP+Amulet+Watchman
75.4 (NA)
2.8(1.6)
4.4(1.5)
Phillips,
2019
201
22.8
Watchman
70.8(9)
2.5(1.4)
3.9(1.7)
Values are n, mean, mean (SD) or mean (interquartile range). ACP= Amplatzer Cardiac Plug; CHADS2 = congestive
heart failure, hypertension, age>75, diabetes mellitus, and prior stroke or transient ischemic attack; CHA2DS2-VASc
Table 2- Clinical outcomes of included studies
First Author,
Year (Ref.#)
Patients
Thromboembolic
Events
Major
Bleeding
All-cause
Mortality
Modification of
initial therapy
N
N
P
N
P
N
P
N
P
Ostermayer,
2005
111
5
0.05
NA
NA
6
0.05
NA
NA
Block, 2009
64
9
0.14
1
0.01
17
0.27
0
0
Bayard, 2010
180
3
0.02
NA
NA
7
0.04
4
0.02
Lam, 2012
20
0
0
0
0
0
0
1
0.05
Mínguez,
2013
35
1
0.03
1
0.03
3
0.09
4
0.11
Danna, 2013
37
1
0.03
0
0
2
0.05
0
0
Reddy, 2013
150
3
0.02
2
0.01
9
0.06
4
0.03
Urena, 2013
52
2
0.04
1
0.02
4
0.08
0
0
Wiebe, 2014
60
0
0
2
0.03
5
0.08
2
0.03
Kebernik,
2015
96
3
0.03
1
0.01
9
0.09
2
0.02
Karczewski,
2016
34
0
0
?
0
0
0
0
0
Berti, 2016
110
6
0.05
3
0.03
14
0.13
0
0
Jalal, 2016
76
3
0.04
1
0.01
2
0.03
2
0.03
Tzikas, 2016
1047
18
0.02
15
0.01
63
0.06
46
0.04
Park, 2018
60
1
0.02
0
0
2
0.03
3
0.05
Bertrand,
2018
39
0
0
4
0.10
7
0.18
4
0.10
Mínguez,
2018
598
17
0.03
43
0.07
76
0.13
27
0.05
Regueiro,
2018
101
11
0.11
18
0.18
34
0.33
0
0
Weise, 2018
298
13
0.04
32
0.11
50
0.17
23
0.08
Bergmann,
2018
605
14
0.02
19
0.03
58
0.10
-
0.05
Phillips,
2019
201
7
0.03
10
0.05
12
0.06
5
0.02
Figure 1. Flow Diagram of Studies Selection
LAA= left atrial appendage.
Records identified through
database searching
(n = 38 in PubMed and 103 in ISI
Web of Knowledge
Records after duplicates removed
(n = 135)
Records screened
(n = 135)
Records excluded
(n = 79)
Study design, Reviews,
Unrelated to topic,
Surgical closure of LAA,
Studies focusing on
imaging and planning
Full-text articles assessed
for eligibility
(n =56)
Full-text articles excluded,
with reasons
(n = 35)
Follow-up studies,
Anticoagulation after
procedure (>5%),
follow-up<6 months and N<15
patients
Studies included in
qualitative synthesis
Figure 2- A and B. Forrest Plots depicting adjusted incidence rates of clinical
outcomes.
(A)Thromboembolic Event Rates. Fixed effects meta-analysis. Test for Heterogeneity with p=0.353
and I-square of 0.0%. (B)Major Bleeding Event Rates. Random effects meta-analysis. Test for Heterogeneity with p<0.001 and I-square of 67.33%.
Figure 3- A and B. Forrest Plots depicting adjusted incidence rates of clinical
outcomes.
(A)All-cause Mortality Rates. Random effects meta-analysis. Test for Heterogeneity with p=0.010
and I-square of 43.48%. (B)Modification of initial therapy Event Rates. Random effects meta-analysis. Test for Heterogeneity with p<0.001 and I-square of 82.57%.
Supplement 1
Supplementary file 1. Article Quality Assessment
Study
Ostermayer
2005
Block
2009
Bayard
2010
Lam
2011
Mínguez
2012
Study
objective
1.Was
the
hypothesis/aim of the
study clearly stated?
Yes
Yes
Yes
Yes
Yes
Study design 2. Was the study
conducted
prospectively?
Yes
Yes
Yes
Yes
Yes
3. Were the cases
collected in more
than one centre?
Yes
Yes
Yes
Yes
No
4.
Were
patients
recruited
consecutively?
Unclear
Unclear
Unclear
Yes
Unclear
Study
population
5.
Were
the
characteristics of the
patients included in
the study described?
Yes
No
Yes
Yes
Yes
6. Were the eligibility
criteria (i.e. inclusion
and
exclusion
criteria) for entry into
the
study
clearly
stated?
Yes
Yes
Yes
Yes
Partial
7. Did patients enter
the study at a similar
point in the disease?
Yes
Unclear
Yes
Yes
Unclear
Intervention
and
co-intervention
8.
Was
the
intervention
of
interest
clearly
described?
Yes
Yes
Yes
Yes
Yes
9. Were additional
interventions
(co-interventions) clearly
described?
Yes
Yes
Yes
Yes
Yes
Outcome
measures
10. Were relevant
outcome
measures
established a priori?
Yes
Yes
Yes
Yes
Yes
11. Were outcome
assessors blinded to
the intervention that
patients received?
Yes
Yes
Yes
Yes
Yes
12. Were the relevant
outcomes measured
using
appropriate
objective/subjective
methods?
Yes
Yes
Yes
Yes
Yes
outcome
measures
made before and after
the intervention?
Statistical
analysis
14.
Were
the
statistical tests used
to assess the relevant
outcomes
appropriate?
Yes
Yes
Yes
Unclear
Unclear
Results and
conclusions
15. Was follow-up
long
enough
for
important events and
outcomes to occur?
No
Yes
No
No
No
16. Were losses to
follow-up reported?
Yes
Yes
Yes
No
No
17. Did the study
provided estimates of
random variability in
the data analysis of
relevant outcomes?
Yes
No
Yes
No
Yes
18. Were the adverse
events reported?
Yes
Yes
Yes
Yes
Yes
19.
Were
the
conclusions of the
study supported by
the results?
Yes
Yes
Yes
Yes
Yes
Competing
interests
and sources
of support
20.
Were
both
competing interests
and
sources
of
support for the study
reported?
Supplementary file 1. (continued)
Study
Danna
2013
Reddy
2013
Urena
2013
Wiebe
2014
Kebernik
2015
Study
objective
1.Was
the
hypothesis/aim of the
study clearly stated?
Yes
Yes
Yes
Yes
Yes
Study design 2. Was the study
conducted
prospectively?
Unclear
Yes
Yes
No
No
3. Were the cases
collected in more
than one centre?
No
Yes
Yes
Yes
No
4.
Were
patients
recruited
consecutively?
Unclear
Unclear
Yes
Yes
Yes
Study
population
5.
Were
the
characteristics of the
patients included in
the study described?
Yes
Yes
Yes
Yes
Yes
6. Were the eligibility
criteria (i.e. inclusion
and
exclusion
criteria) for entry into
the
study
clearly
stated?
Yes
Yes
Partial
Yes
Partial
7. Did patients enter
the study at a similar
point in the disease?
Yes
Unclear
Unclear
Yes
Unclear
Intervention
and
co-intervention
8.
Was
the
intervention
of
interest
clearly
described?
Yes
Yes
Yes
Yes
Yes
9. Were additional
interventions
(co-interventions) clearly
described?
Yes
Yes
Yes
Yes
Yes
Outcome
measures
10. Were relevant
outcome
measures
established a priori?
Yes
Yes
Yes
Yes
Yes
11. Were outcome
assessors blinded to
the intervention that
patients received?
Yes
Yes
Yes
Yes
Yes
12. Were the relevant
outcomes measured
using
appropriate
objective/subjective
methods?
Yes
Yes
Yes
Yes
Yes
13. Were the relevant
outcome
measures
made before and after
the intervention?
Statistical
analysis
14.
Were
the
statistical tests used
to assess the relevant
outcomes
appropriate?
Yes
Yes
Yes
Yes
Yes
Results and
conclusions
15. Was follow-up
long
enough
for
important events and
outcomes to occur?
No
Yes
No
Yes
No
16. Were losses to
follow-up reported?
No
Yes
No
Yes
No
17. Did the study
provided estimates of
random variability in
the data analysis of
relevant outcomes?
Yes
No
Yes
Yes
Yes
18. Were the adverse
events reported?
Yes
Yes
Yes
Yes
Yes
19.
Were
the
conclusions of the
study supported by
the results?
Yes
Yes
Yes
Yes
Yes
Competing
interests
and sources
of support
20.
Were
both
competing interests
and
sources
of
support for the study
reported?
Supplementary file 1. (continued)
Study
Karczewski
2016
Berti
2016
Jalal
2016
Tzikas
2016
Park
2018
Study
objective
1.Was
the
hypothesis/aim of the
study clearly stated?
Yes
Yes
Yes
Yes
Yes
Study design 2. Was the study
conducted
prospectively?
Unclear
Yes
Yes
No
Yes
3. Were the cases
collected in more
than one centre?
Unclear
No
Yes
Yes
Yes
4.
Were
patients
recruited
consecutively?
Unclear
Yes
Yes
Yes
Unclear
Study
population
5.
Were
the
characteristics of the
patients included in
the study described?
Yes
Yes
Yes
Yes
Yes
6. Were the eligibility
criteria (i.e. inclusion
and
exclusion
criteria) for entry into
the
study
clearly
stated?
Yes
Partial
Partial
Partial
Yes
7. Did patients enter
the study at a similar
point in the disease?
Unclear
Unclear
Unclear
Unclear Unclear
Intervention
and
co-intervention
8.
Was
the
intervention
of
interest
clearly
described?
Yes
Yes
Yes
Yes
Yes
9. Were additional
interventions
(co-interventions) clearly
described?
Yes
Yes
Yes
Yes
Yes
Outcome
measures
10. Were relevant
outcome
measures
established a priori?
No
Yes
Yes
Yes
Yes
11. Were outcome
assessors blinded to
the intervention that
patients received?
Yes
Yes
Yes
Yes
Yes
12. Were the relevant
outcomes measured
using
appropriate
objective/subjective
methods?
Yes
Yes
Yes
Yes
Yes
13. Were the relevant
outcome
measures
made before and after
the intervention?
Statistical
analysis
14.
Were
the
statistical tests used
to assess the relevant
outcomes
appropriate?
Yes
Yes
Yes
Yes
Yes
Results and
conclusions
15. Was follow-up
long
enough
for
important events and
outcomes to occur?
No
Yes
Yes
Yes
Yes
16. Were losses to
follow-up reported?
No
No
No
No
Yes
17. Did the study
provided estimates of
random variability in
the data analysis of
relevant outcomes?
No
No
Yes
Yes
Yes
18. Were the adverse
events reported?
Yes
Yes
Yes
Yes
Yes
19.
Were
the
conclusions of the
study supported by
the results?
Yes
Yes
Yes
Yes
Yes
Competing
interests
and sources
of support
20.
Were
both
competing interests
and
sources
of
support for the study
reported?
Supplementary file 1. (continued)
Study
Bertrand
2018
Mínguez
2018
Regueiro
2018
Weise
2018
Bergmann
2018
Study
objective
1.Was
the
hypothesis/aim
of
the
study
clearly
stated?
Yes
Yes
Yes
Yes
Yes
Study
design
2. Was the study
conducted
prospectively?
Yes
Yes
Yes
Yes
Yes
3. Were the cases
collected in more
than one centre?
No
Yes
Yes
No
Yes
4.
Were
patients
recruited
consecutively?
Uncear
Unclear
Unclear
Unclear
Yes
Study
population
5.
Were
the
characteristics of the
patients included in
the study described?
Yes
Yes
Yes
Yes
Yes
6. Were the eligibility
criteria (i.e. inclusion
and
exclusion
criteria) for entry
into the study clearly
stated?
Partial
No
Partial
No
No
7. Did patients enter
the study at a similar
point in the disease?
Yes
Unclear
Unclear
Unclear
Unclear
Intervention
and
co-intervention
8.
Was
the
intervention
of
interest
clearly
described?
Yes
Yes
Yes
Yes
Yes
9. Were additional
interventions
(co-interventions)
clearly described?
Yes
Yes
Yes
Yes
Yes
Outcome
measures
10. Were relevant
outcome
measures
established a priori?
No
Yes
Yes
Yes
Yes
11. Were outcome
assessors blinded to
the intervention that
patients received?
Yes
Yes
Yes
Yes
Yes
12. Were the relevant
outcomes measured
using
appropriate
objective/subjective
methods?
Yes
Yes
Yes
Yes
Yes
13. Were the relevant
outcome
measures
made before and
after
the
intervention?
Statistical
analysis
14.
Were
the
statistical tests used
to assess the relevant
outcomes
appropriate?
Yes
Yes
Yes
Yes
Yes
Results and
conclusions
15. Was follow-up
long
enough
for
important events and
outcomes to occur?
No
Yes
Yes
Yes
Yes
16. Were losses to
follow-up reported?
No
No
No
No
No
17. Did the study
provided estimates of
random variability in
the data analysis of
relevant outcomes?
Yes
Yes
Yes
Yes
Yes
18. Were the adverse
events reported?
Yes
Yes
Yes
Yes
Yes
19.
Were
the
conclusions of the
study supported by
the results?
Yes
Yes
Yes
Yes
Yes
Competing
interests
and sources
of support
20.
Were
both
competing interests
and
sources
of
support for the study
reported?
Supplementary file 1. (continued)
Study
Phillips,
2019
Study
objective
1.Was
the
hypothesis/aim
of
the
study
clearly
stated?
Yes
Study
design
2. Was the study
conducted
prospectively?
Yes
3. Were the cases
collected in more
than one centre?
Yes
4.
Were
patients
recruited
consecutively?
Yes
Study
population
5.
Were
the
characteristics of the
patients included in
the study described?
Yes
6. Were the eligibility
criteria (i.e. inclusion
and
exclusion
criteria) for entry
into the study clearly
stated?
No
7. Did patients enter
the study at a similar
point in the disease?
Unclear
Intervention
and
co-intervention
8.
Was
the
intervention
of
interest
clearly
described?
Yes
9. Were additional
interventions
(co-interventions)
clearly described?
Yes
Outcome
measures
10. Were relevant
outcome
measures
established a priori?
No
11. Were outcome
assessors blinded to
the intervention that
patients received?
Yes
12. Were the relevant
outcomes measured
using
appropriate
objective/subjective
methods?
Yes
13. Were the relevant
outcome
measures
made before and
after
the
intervention?
Statistical
analysis
14.
Were
the
statistical tests used
to assess the relevant
outcomes
appropriate?
Yes
Results and
conclusions
15. Was follow-up
long
enough
for
important events and
outcomes to occur?
Yes
16. Were losses to
follow-up reported?
No
17. Did the study
provided estimates of
random variability in
the data analysis of
relevant outcomes?
Yes
18. Were the adverse
events reported?
Yes
19.
Were
the
conclusions of the
study supported by
the results?
Yes
Competing
interests
and sources
of support
20.
Were
both
competing interests
and
sources
of
support for the study
reported?
Normas de Publicação da Acta Médica Portuguesa
Acta Médica Portuguesa’s Publishing Guidelines
Conselho Editorial ACTA MÉDICA PORTUGUESA
Acta Med Port 2016, 30 dezembro 2016
NORMAS PUBLICAÇÃO
1. MISSÃO
Publicar trabalhos científicos originais e de revisão na área biomédica da mais elevada qualidade, abrangendo várias áreas do conhecimento médico, e ajudar os médicos a tomar melhores decisões.
Para atingir estes objectivos a Acta Médica Portuguesa publica artigos originais, artigos de revisão, casos clínicos, editoriais, entre outros, comentando sobre os factores clí-nicos, científicos, sociais, políticos e económicos que afec-tam a saúde. A Acta Médica Portuguesa pode considerar artigos para publicação de autores de qualquer país.
2. VALORES
Promover a qualidade científica.
Promover o conhecimento e actualidade científica. Independência e imparcialidade editorial.
Ética e respeito pela dignidade humana. Responsabilidade social.
3. VISÃO
Ser reconhecida como uma revista médica portuguesa de grande impacto internacional.
Promover a publicação científica da mais elevada quali-dade privilegiando o trabalho original de investigação (clíni-co, epidemiológi(clíni-co, multicêntri(clíni-co, ciência básica).
Constituir o fórum de publicação de normas de orienta-ção.
Ampliar a divulgação internacional.
Lema: “Primum non nocere, primeiro a Acta Médica
Portuguesa”
4. INFORMAÇÃO GERAL
A Acta Médica Portuguesa é a revista científica com revisão pelos pares (peer-review) da Ordem dos Médicos. É publicada continuamente desde 1979, estando indexa-da na PubMed / Medline desde o primeiro número. Desde 2010 tem Factor de Impacto atribuído pelo Journal Citation Reports - Thomson Reuters.
A Acta Médica Portuguesa segue a política do livre acesso. Todos os seus artigos estão disponíveis de for-ma integral, aberta e gratuita desde 1999 no seu site www.actamedicaportuguesa.com e através da Medline com interface PubMed.
A Acta Médica Portuguesa não cobra quaisquer taxas
relativamente ao processamento ou à submissão de arti-gos.
A taxa de aceitação da Acta Médica Portuguesa, em 2014, foi de aproximadamente de 20% dos mais de 700 manuscritos recebidos anualmente.
Os manuscritos devem ser submetidos online via “Submissões Online” http://www.actamedicaportuguesa.com /revista/index.php/amp/about/submissions#online Submissions.
A Acta Médica Portuguesa rege-se de acordo com as boas normas de edição biomédica do International Com-mittee of Medical Journal Editors (ICMJE), do ComCom-mittee on Publication Ethics (COPE), e do EQUATOR Network Resource Centre Guidance on Good Research Report (de-senho de estudos).
A política editorial da Revista incorpora no processo de revisão e publicação as Recomendações de Política Edi-torial (EdiEdi-torial Policy Statements) emitidas pelo Conselho de Editores Científicos (Council of Science Editors), dispo-níveis em http://www.councilscienceeditors.org/i4a/pages/ index.cfm?pageid=3331, que cobre responsabilidades e direitos dos editores das revistas com arbitragem científica. Os artigos propostos não podem ter sido objecto de qual-quer outro tipo de publicação. As opiniões expressas são da inteira responsabilidade dos autores. Os artigos publica-dos ficarão propriedade conjunta da Acta Médica Portugue-sa e dos autores.
A Acta Médica Portuguesa reserva-se o direito de co-mercialização do artigo enquanto parte integrante da revis-ta (na elaboração de separarevis-tas, por exemplo). O autor de-verá acompanhar a carta de submissão com a declaração de cedência de direitos de autor para fins comerciais. Relativamente à utilização por terceiros a Acta Médica Portuguesa rege-se pelos termos da licença Creative
Com-mons ‘Atribuição – Uso Não-Comercial – Proibição de
Rea-lização de Obras Derivadas (by-nc-nd)’.
Após publicação na Acta Médica Portuguesa, os auto-res ficam autorizados a disponibilizar os seus artigos em repositórios das suas instituições de origem, desde que mencionem sempre onde foram publicados.
5. CRITÉRIO DE AUTORIA