1M D, PhD, Division of Rheumat ology, Faculdade de M edicina da Universidade de São Paulo, São Paulo, SP, Brazil (FM USP); 2M D, PhD, Depart ment of Neurology FM USP. St udy support ed by FAPESP, # 98/16291-2.
Received 30 M arch 2004, received in f inal f orm 1 July 2004. Accept ed 10 August 2004.
Dra. Suely Kazue Nagahashi M arie - Avenida Dr. Arnaldo 453, 4º andar / sala 4110 - Faculdade de M edicina USP LIM 15 - 01246-903 - São Paulo SP Brasil. FAX: (55 11) 3062-0620
M YOSITIS IN M IXED CONNECTIVE TISSUE DISEASE
A unique syndrome charact erized by
immunohist opat hologic element s of bot h
polymyosit is and dermat omyosit is
M aria Angela A.G. Vianna
1, Claudia T.L. Borges
1, Eduardo F. Borba
1,
M aria Teresa C. Caleiro
1, Eloisa Bonf á
1, Suely K.N. M arie
2ABSTRACT - Object ive: To charact erize t he inf lammat ory cells, t he expression pat t ern of adhesion mole-cules (ICAM-1 and VCAM-1), membrane attack complex (C5b-9), and major histocompatibility complex (MHC) ant igens in muscle biopsy of mixed connect ive t issue disease (M CTD). M et hod: We st udied 14 pat ient s w it h M CTD, and compared t o 8 polimyosit is (PM ) pat ient s, 5 dermat omyosit is (DM ) and 4 dyst rophies. Inf lammat ory cells w ere examined f or CD4+, CD8+, memory and naïve T cells, nat ural killer cells, and
macrophages. Expression of M HC-I and -II, ICAM -1, VCAM -1 and C5b -9 w ere charact erized on muscle f ibers and vessels. Result s: M orphological analysis displayed a pat t ern of PM . Immunohist ochemical st udy revealed a decreased number of capillaries, predominance of CD4+and B cells in perivascular regions and
predominance of CD8+and CD45RO+in endomysial regions. The expression of M HC-I on vessels and on
degenerat ed muscle f ibers, M HC-II expression on vessels and perif ascicular muscle f ibers, and t he expres-sion of ICAM -1 / VCAM -1 on endot helial cells indicat ed bot h vascular and cellular-immune mediat ed processes causing t he muscular lesion. Conclusion: Our f indings revealed a mixed mechanism in M CTD, bot h vascular involvement as DM , and cell-mediat ed like PM .
KEY WORDS: mixed connect ive t issue disease, myosit is, major hist ocompat ibilit y complex, adhesion mo-lecules, membrane at t ack complex, lymphocyt e phenot yping.
M iosit e na doença m ist a do t ecido conect ivo: achados im unopat ológicos de polim iosit e e derm at om iosit e
RESUM O - Objet ivo: Caract erizar as células do inf ilt rado inf lamat ório, o padrão de expressão das molécu-las de adesão (ICAM -1 e VCAM -1), complexo de at aque à membrana (C5b-9) e ant ígenos de hist ocompa-t ibilidade maior (M HC) em biópsias musculares de paocompa-t ienocompa-t es com doença misocompa-t a do ocompa-t ecido conecocompa-t ivo (DM TC). M ét odo: Foram est udados14 pacient es com DM TC e comparadas com 8 pacient es com polimiosit e (PM ), 5 com dermatomiosite (DM) e 4 com distrofias. As células inflamatórias foram caracterizadas como CD4+, CD8+,
células T de memória (CD45RO+) e virgens, células “ nat ural killer” e macróf agos. As expressões de M HC-I
e –II, ICAM -1, VCAM -1 e C5b-9 f oram caract erizadas em f ibras musculares e vasos. Result ados: A análise morf ológica demonst rou um padrão t ipo PM . O est udo imuno-hist oquímico revelou diminuição do número de capilares, predomínio de células CD4+e B nas regiões perivasculares e predomínio de CD8+e CD45RO+
nas regiões endomisiais. A expressão de M HC-I nos vasos e nas f ibras degeneradas, M HC-II nos vasos e f ibras perif asciculares e expressão de ICAM -1 / VCAM -1 no endot élio indicaram uma associação de processos vas-cular e imune-celular mediando a lesão musvas-cular. Conclusão: Os achados revelaram duplo mecanismo na DM TC, imune-celular como na PM e vascular como na DM .
PALAVRAS-CHAVE: doença mist a do t ecido conect ivo, miosit e, complexo de hist ocompat ibilidade maior, moléculas de adesão, complexo de at aque à membrana, f enot ipagem linf ocit ária.
The rat her het erogeneous group of inf lamma-t ory myopalamma-t hies comprises lamma-t hree major and dislamma-t inclamma-t subset s: polymyosit is (PM ), dermat omyosit is (DM ),
other systemic diseases1. Mixed connective tissue
di-sease (M CTD) described by Sharp et al.2in 1972 is
an inf lammat ory disease w hich is clinically charac-t erized by f eacharac-t ures of syscharac-t emic lupus erycharac-t hemacharac-t o-sus, syst emic sclerosis and polymyosit is, associat ed w it h high t it ers of circulat ing ant inuclear ant ibody with specificity for nuclear ribonucleoprotein (RNP). During t he course of t his disease, t he majorit y of patients complains of myalgia, muscle tenderness or f at igue2. Clinical evidence of myosit is def ined as
symmet rical proximal w eakness, elect romyograph-ic abnormalit ies, or alt ered muscle enzymes w ere reported in almost three-fourths of 25 MCTD patients in t he original series of Sharp et al.2and in nine of
20 pat ient s described by Bennet t and O’Connell3.
Despit e t hese report s, t he pat t ern of myopat hy de-tected in MCTD is poorly characterized in the literatu-re. In cont rast , t here is a grow ing body of evidence t hat HLA class Irest rict ed cyt ot oxic T cellsmediat -ed response against surf ace ant igens express-ed by muscle f ibers is t he primary pat hogenic mechanism in PM and IBM4, w hereas DM involves
predominan-t ly an anpredominan-t ibody or immune-complex-mediapredominan-t ed res-ponse against a vascular-endot helial component5,6.
M oreover, t he st udies t hat have charact erized t he muscle involvement in M CTD2,3,7ant edat ed t he
Ka-sukawa classification criteria8actually applied for this
disease. According t o some aut hors2,3,7-9, t he
mus-cle involvement is a classic inflammatory myositis sim-ilar t o t hat det ect ed in PM w hen analyzed by his-t ology and hishis-t ochemishis-t ry. Nonehis-t heless, convenhis-t io-nal light microscopy and st aining t echniques used in t hese st udies could not dist inguish t he real sig-nif icance of cellular inf ilt rat es.
We therefore have designed the present study to characterize the infiltrating mononuclear cells, the expression of major histocompatibility complex (MHC) proteins, as well as the adhesion molecules and com-plement activation, in order to better understand the mechanism of muscle cell injury in MCTD.
M ETHOD
Pat ient s – Fourt een pat ient s w it h M CTD [12 f emales (F), 2 males (M ), age 24-54 years] prospect ively f ollow ed in our out pat ient Rheumat ology Clinic at t he Universit y of São Paulo w ere select ed f or t his st udy (Table 1). All M CTD pat ient s met t he classif icat ion crit eria of Kasuka-w a8and had clinical signs of myopat hy (w eakness or
ele-vat ed enzyme levels) at t he t ime of evaluat ion. M uscle biopsy specimens were obtained from clinically weak bra-chial biceps muscle af t er w rit t en inf ormed consent . Four MCTD patients with muscular symptoms had biopsies tak-en bef ore t reat mtak-ent in a rectak-ent diagnosis of M CTD.
The ot her t en M CTD pat ient s w ere biopsied in order t o elucidat e t he muscular sympt oms despit e t reat ment of M CTD syst emic act ivit y. Five of t hese 10 M CTD pat ient s w ere t aking prednisone f or less t han 6 mont hs and t he ot her f ive f or 6 mont hs or longer.
Eight PM pat ient s (6F, 2M , age 27-57 years) and 5 DM (3F, 2M, age 22-58 years) according to Bohan and Peter10,11
crit eria w ere select ed f or cont rol. Addit ionally, 4 pat ient s (3M , 1F, age 20-50 years) w it h non-inf lammat ory neuro-muscular diseases (NINM D) included X-linked, limb-gir-dle and f acioscapulohumeral dyst rophies w ere also in-cluded as cont rols (Table 1). All muscle biopsy of con-t rol pacon-t iencon-t s w ere perf ormed during accon-t ive disease and bef ore t reat ment , and also af t er w rit t en inf ormed con-sent . No pat ient s or cont rols had evidence of an associ-at ed malignancy.
This st udy w as approved by local et hics commit t ee.
Laborat orial dat a – Blood samples of M CTD pat ient s and cont rols w ere collect ed w it hin t w o w eeks prior t o biopsy. M uscle enzymes such as creat ine kinase (CK), lact at e dehydrogenase (LDH), aspart at e (ASP) and ala-nine (ALT) amino t ransaminases w ere analyzed by st an-dard t echniques.
Aut oant ibody assay – Serum samples w ere also t est -ed f or t he presence of ant i-nuclear and cyt oplasmic an-t ibodies by indirecan-t immunof luorescence using Hep-2 as subst rat e. Ant ibodies t o RNP and Sm w ere det ect ed by count erimmunoelect rophoresis12, and ant ibodies t o
ext ract able nuclear ant igens (ENA) w ere t it t ered by pas-sive hemaglut inat ion assay13. Crit hidia luciliae w as used
t o det ect react ivit y t o double-st randed DNA (dsDNA)14.
Ant ibodies t o Jo-1 w ere det ermined by SDS-PAGE and immunoblot t ing, as previously described15.
Hist opat hological analysis– Routine standard histolo-gical and hist ochemical t echniques w ere perf ormed on muscle biopsies. Sequent ial f rozen sect ions w ere st ained w it h hemat oxilin-eosin (H&E), modif ied Gomori t richro-me, periodic acid Schif f , cyt ochrome C oxydase (COX), NADH-t et razolium-reduct ase, succinat e dehydrogenase (SDH), adenosine t riphosphat ase (pH 4.3 and 9.4), alkali-ne and acid phosphat ases16. Each biopsy specimen w as
coded and independent ly analyzed f or inf lammat ory in-f ilt rat e (in endomysial, perimysial and perivascular sit es), necrosis and f ibrosis.
Immunohist ochemical analysis – Immunoreagent s used f or charact erizat ion of inf lammat ory inf ilt rat e and surf ace molecules expressed on muscle f iber w ere: mem-ory T cells (CD45RO), clone UCHL1, DAKO/M 0742, dilu-t ion 1:200; naïve T cells (CD45RA), 4KB5, DAKO/M 0754, 1:200; CD4+T cells, OPD4, DAKO/M834, 1:150; CD8+T cells,
Table 1. Dat a of cases included in t he st udy concerning age, gender, vascular densit y, expression of M HC-I, M HC-II, ICAM -1, VCAM -1 and C5b-9 at immunohist ochemical preparat ions of f rozen muscle biopsies.
Case Gender Age Vascular M HC-I M HC-II ICAM -1 VCAM -1 C5b-9
(years) densit y if s if s v if v if v if
M CTD
1 F 42 0.23 + – + + + + + – + –
2 F 32 0.30 – – + + + – + – + –
3 M 25 0.83 – – + + + + + – ++ –
4 F 54 0.21 – – + + + + + – + –
5 F 24 0.34 ++ +++ – – + – + – ++ –
6 F 27 0.27 – – – – + – + + ++ –
7 F 31 0.38 – – – – + + + – + +
8 F 49 0.33 – – – – + – + – ++ +
9 F 39 0.45 + + + + + + + – ++ +
10 F 48 0.17 + – – – + – + – + –
11 F 29 0.41 – – – – + – + – + –
12 F 38 0.22 – – – – + – + – + +
13 F 26 0.53 – – – – + – + – + –
14 M 34 0.40 – – – – + – + – + –
PM
15 F 47 0.82 +++ +++ – – + – + – +++ +++
16 F 35 0.64 + + + – + – – – + –
17 M 27 0.61 +++ +++ + – ++ + ++ + ++ ++
18 F 57 0.46 +++ +++ +++ – + – + – ++ –
19 F 32 0.43 – – + – + – + – + ++
20 F 40 0.54 ++ ++ – – – – – – + –
21 M 50 1.08 +++ +++ ++ ++ + – + – + –
22 F 50 0.29 – – + – + – + – + +
DM
23 F 22 0.26 +++ +++ – – +++ – ++ + ++ +
24 F 44 0.18 ++ +++ + – + ++ + + ++ ++
25 F 24 0.19 ++ ++ ++ ++ +++ +++ ++ ++ ++ +++
26 M 58 0.33 ++ +++ ++ + ++ ++ + – + +
27 M 35 0.24 ++ ++ ++ ++ ++ ++ + – ++ +
NINM D
28 M 50 0.38 – – – – + – – – + –
29 M 27 0.16 – – – – + – – – – –
30 M 40 0.87 – + – – + – + – + –
31 F 20 0.38 – – – – + – – – – –
M CTD, M ixed connect ive t issue disease; PM , polimyosit is; DM , dermat omyosit is; NINM D, non-inf lammat ory muscle disease; vascular densit y calculat ed as number of vessels/number of f ibers; v, vessels; if , invaded f iber; S, sarcolemmal membrane; – no expression in st ruct ures analyzed: + expression observed in < 25% , ++ < 50% , +++ < 75% of st ruct ures analyzed.
DAKO/M 1014, 1:100; B cells (CD20), L26, DAKO/M 755, 1:200; MHC class I, W6/32, DAKO/M0736, 1:100; MHC class II, CD3/43, DAKO/M 0775, 1:100; ICAM -1 (CD54), 6.5B5, D A K O / M 7 0 6 3 , 1 : 2 0 0 ; V CA M - 1 ( CD 1 0 6 ) , 1 . 4 C3 , DAKO/M 7106, 1:100; C5b-9, aE11, DAKO/M 0777, 1:200. Immunohist ochemical procedure consist ed by serial 4µf rozen sect ions w hich w ere f ixed f or 10 minut es in acet one at 4º C and t hen w ashed in dist illed w at er f ollo-w ed by phosphat e-buf f ered-saline (PBS 0.01M , pH 7.4) f or 5 minut es. Endogenous peroxidase w as blocked w it h H2O23% in absolute methanol four times, 5 minutes each.
After a rinse in distilled water followed by PBS for 5 minu-t es, minu-t he primary anminu-t ibody w as added diluminu-t ed in bovine serum albumine (BSA) 1% , sodium azide 0.1% and PBS. Sequent ial incubat ion in a w et chamber at 37º C f or 30 minut es w as f ollow ed by an overnight incubat ion at 4º C. The secondary ant ibodies w ere applied af t er t hree t i-mes washed by 5 minutes in PBS by 3 different techniques:
LSAB+procedure (f or CD45RO, CD45RA, NK, CD20,
K0690) was applied for 30 minutes at 37º C. Subsequently, it w as incubat ed w it h a chromogenic subst rat e solu-t ion f or peroxidase 3,3’-diaminobenzidine solu-t esolu-t rahydro-chloride (DAB). After a final rinse, haematoxylin counters-t aining w as perf ormed. The slides w ere mouncounters-t ed and coverslipped w it h a permanent medium (Ent erllan).
St rept ABComplex/HRP procedure (f or M HC-I, -II, ICAM -1, VCAM -1, C5b-9)) – The prepared secondary bio-t inylabio-t ed goabio-t and rabbibio-t immunoglobulin (Sbio-t rep-t ABComplex/HRP-Duerep-t , mouse/rabbirep-t , DAKO, S/A Den-mark; code K0492) w as applied f or 30 minut es at 37º C and rinsed in PBS. Subsequent ly, it w as incubat ed w it h a chromogenic subst rat e solut ion f or peroxidase 3,3’-dia-minobenzidine t et rahydrochloride (DAB). Af t er a f inal rinse, haemat oxylin count erst aining w as perf ormed. The slides were mounted and coverslipped with Enterllan.
EnVision-AP procedure (f or CD4+, CD8+) – A
conjuga-t ed polymer w iconjuga-t h alkaline phosphaconjuga-t ase w as applied f or 30 minut es (DAKO, A/S Denmark; code K4016). Af t er a f urt her rinse w it h PBS, f ast -red subst rat e-chromogen w as applied f or 10 min, f ollow ed by haemat oxylin coun-t erscoun-t aining. The slides w ere mouncoun-t ed and coverslipped w it h a permanent medium.
For cont rol purposes addit ional sect ions w ere not in-cubat ed w it h primary ant ibody and lymphoid t issue w as used as posit ive cont rol f or lymphocyt e subt ype ant ibody react ions.
Quantitative analysis – Each biopsy specimen was cod-ed and analyzcod-ed “ blind” by t he t w o invest igat ors (M AAGV and SKNM ). Ten random analyzed f ields of 400x represent ed t he ent ire area of t he specimen.
Analysis of inf lammat ory cells – CD4+, CD8+, CD45RO,
CD45RA, NK, CD20, and CD68 w ere count ed on t he
pe-rivascular and endomysial regions separat ely. The cells w ere count ed using t he program “ CELL” w hich w as de-veloped in order t o process images obt ained by a digi-tal camera attached to a microscope (“ CELL” , MEVIS – M e-dical Informatic Consulting, São Paulo, Brazil) and accepts images in TIF or JPEG f ormat . This program allow s organ-izing, rendering and calibrat ing a set of images, and is also able t o ident if y and count up t hree dif f erent t ypes of cells, in an aut omat ic or semi-aut omat ic w ay. All ima-ges are t ranslat ed int o RGB w it h 8 bit s per channel (24 bit s per pixel). The segment at ion process is based in col-or masks, w it h adjust able range f col-or each primary colcol-or. Reject ion algorit hms w ere int roduced based in cell area and f orm f act or crit eria. Cell borders could be draw n by t he user, if necessary. The result s present at ion could be in t he f orm of hist ograms and t ables, including diame-t er, area, f orm f acdiame-t or and number of each diame-t ype of cells.
Analysis of M HC-I, M HC-II, ICAM -1, Vcam-1 and C5b-9 – Expression of t hose ant igens w ere assessed by a semiquant it at ive met hod w here: (-) = no expression in structures analyzed; (+) = expression observed in less than 25% of st ruct ures; (++) = in less t han 50% ; (+++) = in less t han 75% ; (++++) = in 76 t o 100% of st ruct ures analyzed.
St at ist ical analysis– Cont inuous dat a w ere expressed as mean and st andard deviat ion or in median w it h min-imum and maxmin-imum values. Comparison among groups and M CTD regarding cont inuous variables w ere perf or-med using Kruskal-Wallis t est . In case of st at ist ical signi-f icance, mult iple comparisons bet w een groups w ere done using Wilcoxon rank sum t est . Pearson’s Chi-square t est w as used t o compare groups regarding t he propor-t ion of papropor-t ienpropor-t s w ipropor-t h 0/+ and ++/+++ propor-t o immunohispropor-t oche-mical paramet ers. Level of signif icance w as set at p<0.05, and t he SPSS®st at ist ical syst em w as used f or all st at
is-t ical calculais-t ions.
Table 2. M ean values of t he myosit is-associat ed enzymes in M CTD, PM , DM , and NINM D groups.
Paramet er M CTD PM DM NINM D p
(n = 14) (n = 8) (n = 5) (n = 4)
CK* 523 1654 2781 1556 0.075
(33-5030) (68-6219) (971-15000) (1184-1929)
LDH 746 492 1173 395 0.31
(275-2052) (239-1311) (324-2355) (271-521)
AST 39 102 101 49 0.55
(11-291) (31-482) (25-495) (44-98)
ALT 31 78 82 69 0.26
(9-147) (37-263) (2-283) (44-94)
RESULTS
Laborat orial dat a
M CTD pat ient s had a signif icant ly low er medi-an CK level compared to DM (p=0.0142), but not sig-nif icant ly diff erent f rom PM (p=0.34) nor NINM M (p=0.17). Similarly, no diff erence w as det ect ed f or LDH, AST and ALT levels among groups (Table 2).
Aut oant ibody assay
All M CTD sera present ed an unif ormly posit ive speckled ANA w it h high t it er ant RNP and negat i-ve ant i-Sm, ant i-dsDNA and ant i-Jo1 ant ibodies. All except one of DM patients presented a positive spe-ckled ANA. On t he ot her hand, 4 of 8 PM pat ient s present ed a posit ive speckled ANA and 2 of t hem
also present ed ant i-Jo1 ant ibodies. In cont rast , t he NINM D group w as invariably negat ive f or t he presence of all aut oant ibodies t est ed.
Hist ological and hist ochemical f indings
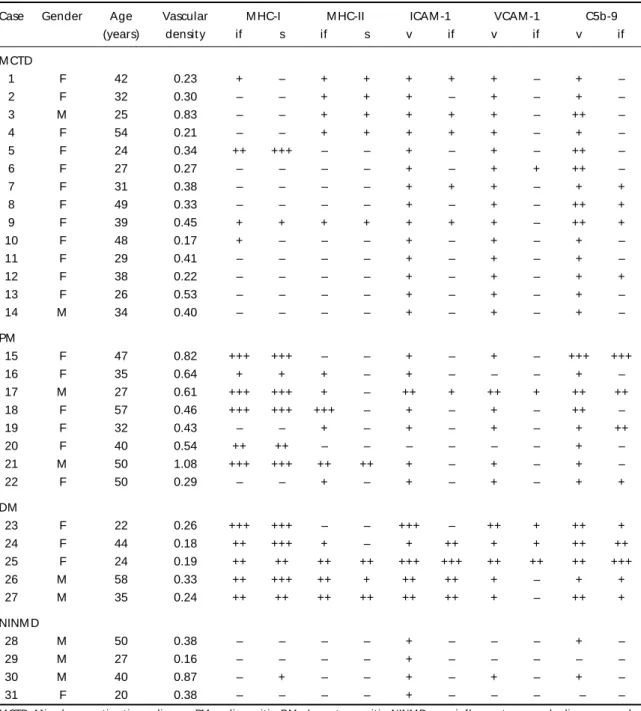
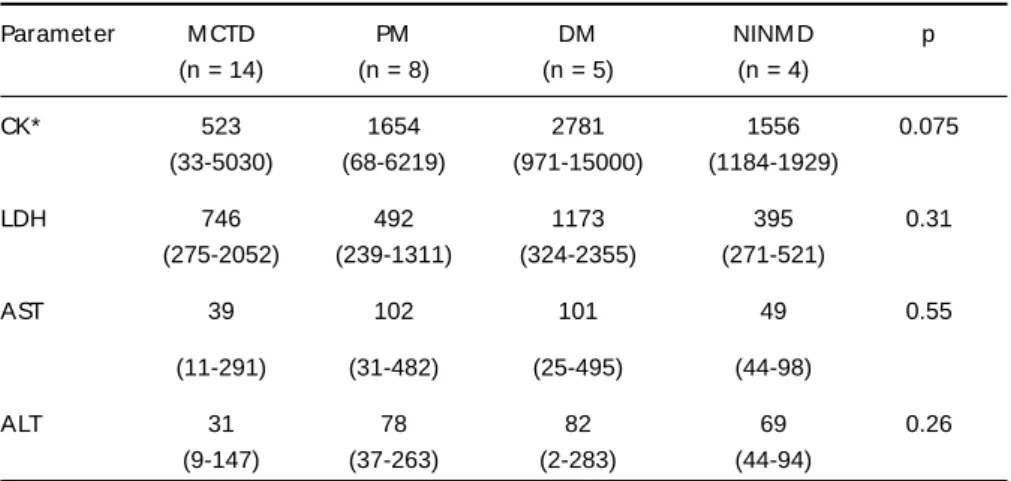
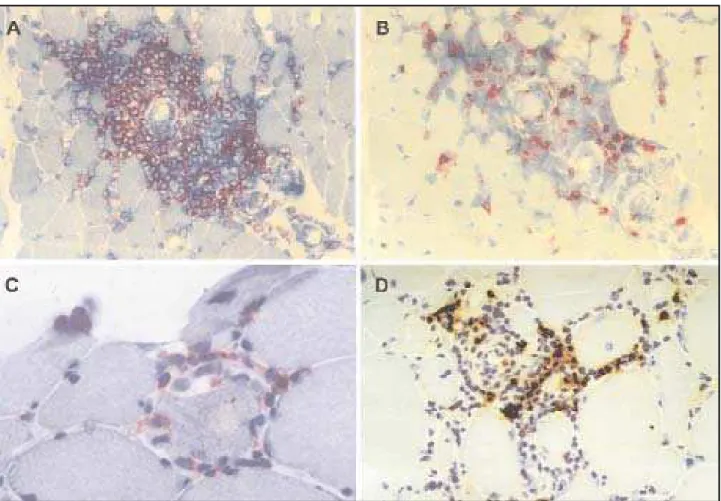
All MCTD muscle biopsies revealed an inflamma-t ory cell inf ilinflamma-t rainflamma-t e displaying a heinflamma-t erogeneous dis-t ribudis-t ion, predominadis-t ing in dis-t he perivascular, and endomysial regions (Fig 1A). M oreover, some per-imysial vessels present ed slight w all t hickness increase (Figs 1B, 1C). Some necrot ic and nonnecrot -ic muscle f ibers w ere surrounded by inf lammat o-ry cells, w hich w ere sparsely dist ribut ed among t he f ascicles (Fig 1A), and show ed posit ivit y on acid phosphatase reaction. In addition, the affected
cle f ibers demonst rat ed int ermiof ibrilar disorgan-izat ion on oxidat ive react ions (NADH, SDH, and COX). Slight endomysial and perimysial connect i-ve t issue prolif erat ion w as obseri-ved in f ew (3/14) M CTD muscle biopsies.
In cont rast , PM muscle specimens displayed an endomysium and perivascular inflammatory
infiltra-t e in a moderainfiltra-t e ininfiltra-t ensiinfiltra-t y, w iinfiltra-t h some of infiltra-t hose cells surrounding and invading single nonnecrot -ic muscle f ibers. The necrot -ic and ghost f ibers w ere also present .
No prolif erat ion of connect ive t issue w as observed on DM and PM samples.
As expect ed, prolif erat ion of connect ive t issue w as t he main f inding on t he NINM D group, w hich also present ed some degree of necrot ic muscle f i-bers and a sparse inf lammat ory inf ilt rat e, w it h ex-cept ion of t he f ascioscapulohumeral dyst rophy ca-se (Caca-se 30) w hich had an import ant inf lammat o-ry inf ilt rat e.
Immunohist ochemical result s
Expression of M HC class I and class II
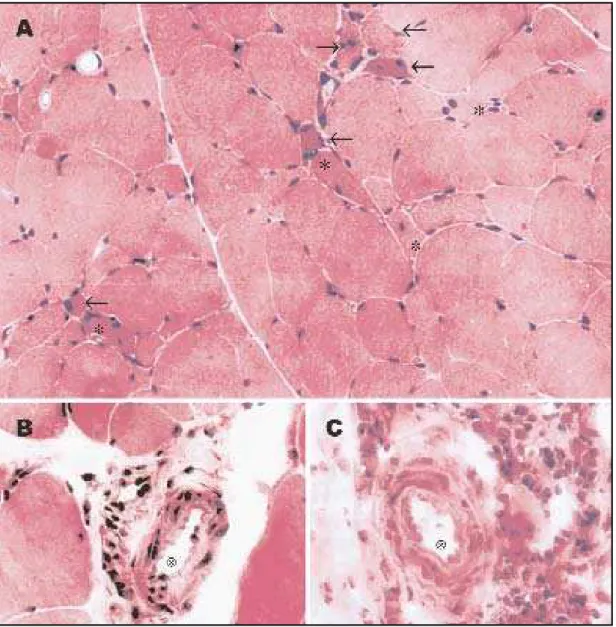
The expression of M HC-I w as present bot h on in-vaded f ibers and sarcolemmal membrane in M CTD (Fig 2A). It s posit ivit y w as unif ormly scarce, and sig-nif icant ly lesser t han PM and DM (p<0.001) (Tables 1 and 3). Import ant ly, degenerat ed and normal fibers surrounded by inflammatory cells in MCTD (Fig 2A* ) and PM expressed M HC-I, as necrot ic and degenerat ed f ibers mainly in perif ascicular region.
The expression of M HC-II w as on invaded f ibers, on f ibers present ing int ermyof ibrillar abnormali-t ies, and on f ibers w iabnormali-t h any degree of necrosis. The reactivity of MHC-II on invaded fibers was significan-tly slighter on MCTD (p=0.012) compared to controls. Of not e, sarcolemmal membrane M HC-II expres-sion w as posit ive in M CTD on t he perif ascicular re-gion, as in DM (Table 1 and 3) (Figs 2B and 2F).
tive on vessels among or near inflammatory infiltra-t e (Tables 1 and 3) (Fig 2D).
The apparent low er expression of C5b-9, mem-brane at t ack complex (M AC), on vessels in M CTD (Fig 2E) compared t o cont rols did not reach st at is-t ical signif icance (p=0.18). In addiis-t ion, is-t he M AC ex-pressed in t he membrane w as scarce in t he major-it y of all groups st udied (Tables 1 and 3).
Phenot yping of inf ilt rat ing mononuclear cells
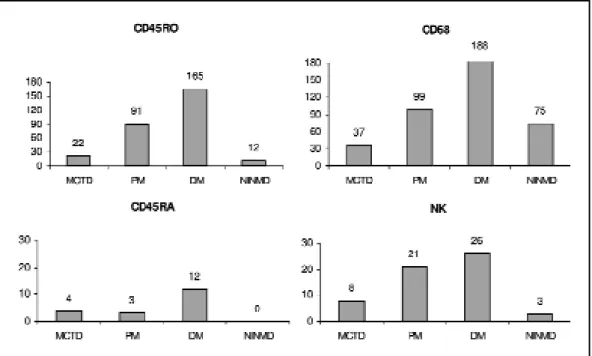
M acrophages (CD68) w ere one of t he predomi-nant cell of the inflammatory infiltrate in all groups, and it w as st at ist ically low er in M CTD t han PM (p=0.024) and DM (p=0.035) (Graphic 1).
Similarly, the CD45RO+(memory T cells) were
al-so predominant in the inflammatory infiltrate and they were more frequently found on PM and DM, compared to MCTD and NINMD (p=0.005). On the ot-her hand, CD45RA (virgin T cells) and NK cells (natu-ral killer) were rarely seen in the infiltrate in all gro-ups and no significant difference was detected among them (p=0.07, and p=0.4, respectively) (Graphic 1).
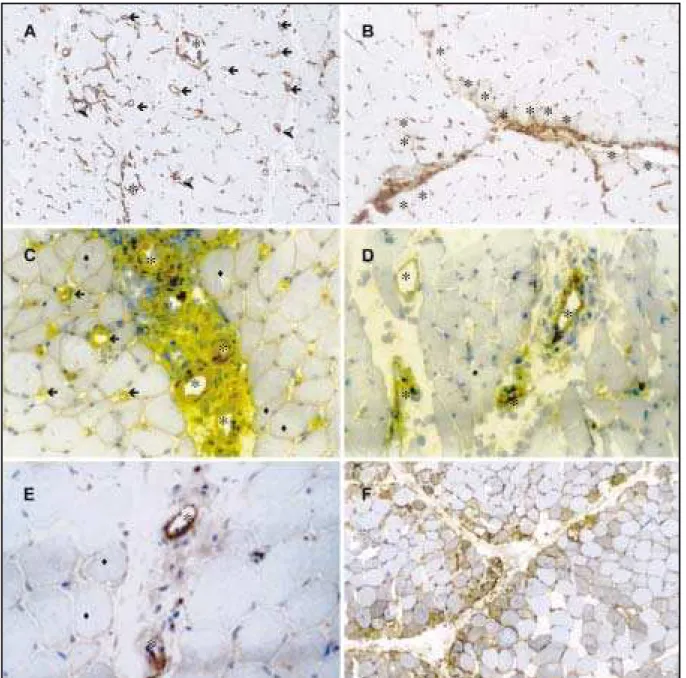
In M CTD myosit is t he CD4+T cells predominat e
at perivascular t han at endomysial regions, but t o one f iber w as considered as normal. This
deplt ion w as more evidendeplt in DM , as expecdeplt ed. How e-ver, interestingly, it was greater in MCTD than in PM.
Expression of adhesion molecules (ICAM -1, VCAM -1), and C5b-9
The intensity of expression of ICAM-1, a constitu-t ive endoconstitu-t helial adhesion molecule, on vessel w all w as signif icant ly diff erent among groups (p=0.012) and revealed a w eak pat t ern in t he M CTD w hich w as similar t o PM , but dif f erent f rom DM (Tables 1 and 3). In MCTD, ICAM-1 expression was observed on vessels surrounded by inf lammat ory cells (Fig 2C). Similarly, ICAM -1 expressed on invaded mus-cle f ibers w as posit ive in 80% of DM cases. On t he ot her hand, t he expression on t he membrane of muscle f ibers w as similarly slight among t he f our groups (p=0.26).
In cont rast , t here w as no dif f erence in t he ex-pression of VCAM -1, an inducible adhesion mole-cule, on vessels among all groups st udied (p=0.06). Some PM (1 out of 8) DM (2 out of 5) present ed +2 react ion, in cont rast t o +1 expression in M CTD (14 out of 14). Similarly, in MCTD, VCAM-1 was
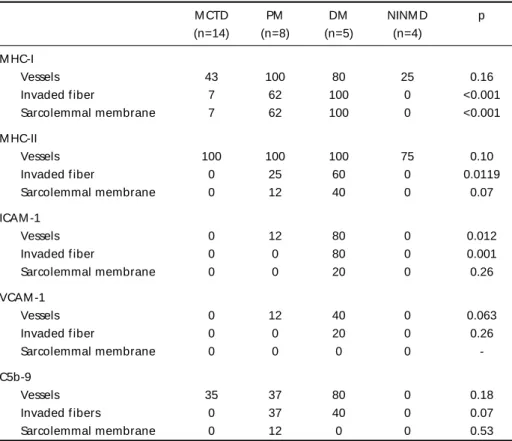
posi-Table 3. Expressions of M HC-I, M HC-II, ICAM -1, VCAM -1, and C5b-9 on vessels and muscle f ibers of M CTD, PM , DM , and NINM D groups.
M CTD PM DM NINM D p
(n=14) (n=8) (n=5) (n=4) M HC-I
Vessels 43 100 80 25 0.16
Invaded f iber 7 62 100 0 <0.001
Sarcolemmal membrane 7 62 100 0 <0.001
M HC-II
Vessels 100 100 100 75 0.10
Invaded f iber 0 25 60 0 0.0119
Sarcolemmal membrane 0 12 40 0 0.07
ICAM -1
Vessels 0 12 80 0 0.012
Invaded f iber 0 0 80 0 0.001
Sarcolemmal membrane 0 0 20 0 0.26
VCAM -1
Vessels 0 12 40 0 0.063
Invaded f iber 0 0 20 0 0.26
Sarcolemmal membrane 0 0 0 0
-C5b-9
Vessels 35 37 80 0 0.18
Invaded f ibers 0 37 40 0 0.07
Sarcolemmal membrane 0 12 0 0 0.53
Graphic 1. M edian of t he absolut e number of CD45RO (memory T cells), CD45RA (naive T cells), NK (nat u-ral killer cells) and CD68 (macrophages) count ed in all specimens (10 f ields in 400 x), in M CTD, PM . DM and NINM D biopsies and a comparison among groups.
despit e t he dif f erence, it did not reach st at ist ical significance (p=0.057) (Fig 3A). The CD8+T cells were
present more of t en in endomysial region t han at perivascular region (p=0.007) (Graphic 2, Figs 3B, 3C). Similarly t o helper T cells, B cells (CD20) w ere more abundant at perivascular t han at endomysial sit es (p<0.001) (Graphic 2, Fig 3D). Consequent ly, t he CD4/CD8 rat io w as highest at perivascular t han at endomysial sit e (p=0.017) (Graphic 2).
In DM , t here w ere a prevalence of CD4 T cells at perivascular region, and B cells (CD20) dist ribu-t ion on perivascular and endomysial region. The-refore, there was higher CD4/CD8 ratio at perivascu-lar t han at endomysial sit e (Graphic 2).
On PM , t he CD4+T cells at perivascular region
w ere quit e similar t o endomysial region w it h a predominance of CD8+T cells at endomysial region,
and B cells (CD20) at perivascular sit e. Theref ore, t he CD4/CD8 rat io at perivascular and endomysial w as 3:1 w it h no st at ist ical dif f erence (Graphic 2). In NINM D cont rol group, B cells w ere slight ly more common in perivascular region w it h no diff e-rence bet w een t he CD8 at endomysial and perivas-cular regions, and a slight predominance of CD4 at endomysial region t han at perivascular sit e. Con-sequent ly, t here w ere a higher CD4/CD8 rat io en-domysially t han at perivascular sit e (Graphic2).
In summary, the percentage of lymphocyte T hel-per CD4+w ere more f requent ly f ound in t he
peri-vascular t han endomysial region of M CTD and DM but not in PM . The percent age of lymphocyt es T cy-totoxic CD8+, were frequently detected in the
endo-mysial t han perivascular region in all groups, except in NINM D, mainly in PM but w it hout st at ist ical dif -f erence among groups. The percent age o-f B cells (CD20) w as similar in all groups st udied (Graphic 2).
DISCUSSION
This is t he f irst immunohist ochemical st udy t o provide clues t o t he pat hogenesis of M CTD myosi-t is. This smyosi-t udy suggesmyosi-t s myosi-t hamyosi-t myosi-t he myosimyosi-t is in M CTD may represent a unique syndrome, w it h element s of PM and DM , charact erized by relat ively low CKs, abnormally decreased vessel densit y, low ex-pression of M HC-I, ICAM -1, and VCAM -1, w it h pre-dominance of CD45RO cells and macrophages at inf lammat ory inf ilt rat e, and CD4 and B cells main-ly dist ribut ed on perivascular sit e.
Ot her invest igat ors have described M CTD myo-pat hy w it h PM myo-pat t ern2,3,8, w hen analyzed by
clini-cal and hist ologiclini-cal f eat ures. On t he ot her hand, Dalakas1,17 and ot hers18 had described t hat t he
myopat hy in M CTD resembled more t o DM , based on clinical observat ion.
Hist ochemical react ions of all M CTD cases in t his series revealed t he presence of non-necrot ic invaded muscle f ibers associat ed w it h predomi-nant endomysial and perivascular involvement which confers a pattern similar to PM in accordance t o t he previous descript ions on t he lit erat ure2,3,19.
How ever, t he present result s also demonst rat -ed a remarkable decrease in t he number of ves-sels observed in the quantitative analysis and a pres-ence of endot helial t hickness associat ed t o a pe-rivascular inf lammat ory inf ilt rat ion, w hich indica-t e a role of vascular paindica-t hology in M CTD myosiindica-t is. The deplet ion of blood vessels has already been described on initial phase of DM20, which is also
prceded by M AC deposit ion on blood vessels. Int e-rest ingly, vascular involvement associat ed t o immu-noglobulin deposit ion has been report ed in a f ew M CTD pat ient s more t han 20 years ago7. In t his
re-gard, prolif erat ive vascular lesions w hich are a consequence of int imal and medial t hickening of blood vessel w all due t o inf lammat ory cell inf ilt ra-t ion w ere observed in ra-t he M CTD muscle of aura-t op-sy cases by Singsen et al.9. Alt hough t his t ype of
vascular lesion is t he charact erist ic injury of sclero-derma, t he f indings of perivascular inf lammat ory infiltration, absence of fibrosis and no fibrinoid vas-cular change observed in t his st udy support t he hy-pot hesis t hat t he vascular involvement in M CTD muscle dif f ers f rom t hose of scleroderma.
On t he ot her hand, det ect ion of M AC on mus-cle vessels of t hese pat ient s also raises t he ques-t ion w heques-t her ques-t he complemenques-t sysques-t em may conques-t ri-but e t o t he pat hogenic mechanism in M CTD myosi-t is. A grow ing body of evidence demonsmyosi-t ramyosi-t es myosi-t he important role of capillary injury by MAC in the ear-ly phased of muscle involvement of DM20,21.
The expression of ICAM -1, a const it ut ive adhe-sion molecule, on vessels w as observed in our M CTD cases. Alt hough it s react ivit y w as det ect ed in f ew vessels, it w as posit ive in all cases, w hich also emphasizes t he import ance of t he vascular compo-nent in t he immunopat hogenesis of M CTD mus-cle injury. It may be relevant t hat pref erent ial ICAM -1 upregulat ion on endot helial cells of per-i m ysper-i al vessel s an d cap per-i l l ar per-i es w as p r evper-i o u sl y described in DM1,22. The react ivit y observed at
recent ly demonst rat ed t hat aut oant ibodies against U1-ribonucleoprot ein (U1-RNP), a biological mark-er of M CTD, can up-regulat e ICAM -1 expression on endot helial cells of pulmonary art ery, suggest ing t hat t his aut oant ibody may play an import ant role in t he immunopat hological processes leading t o a prolif erat ive vasculopat hy.
Similarly, t he expression of t he VCAM -1 w as no-t ed on endono-t helial cells of some arno-t erioles sur-rounded by inf lammat ory cells. A w idespread ex-pression of VCAM -1 has been described in rheuma-t oid arrheuma-t hrirheuma-t is, AIDS encephalirheuma-t is, and orheuma-t her condi-t ions22. It s expression is not st riking in DM and PM ,
suggest ing t hat t his inducible endot helial adhesion molecule does not play an import ant role in t he pat hogenesis of t hose inf lammat ory myopat hies. Further studies will be necessary to determine more det ails about t heir part icipat ion in M CTD myosit is.
In addit ion, t he M HC-II expression on degener-at ed f ibers, remarkably on t hose w it h a ret icular int racyt oplasmic pat t ern on perif ascicular region, also show s t he import ance of t he vascular compo-nent in t he pat hogenesis of M CTD muscle injury, as already described in DM1.
M HC-I posit ive muscle f ibers in our M CTD pa-t ienpa-t s w ere respa-t ricpa-t ed pa-t o endomysial non-necropa-t ic invaded f ibers t hat w ere surrounded and invaded by CD8+cyt ot oxic T cell, prevailing over CD4+on
t he endomysial region. In f act , Engel and Araha-t a24,25described a PM pat t ern w hich is charact
eri-zed by a progressive posit ive gradient f ro CD8+T
cells f rom perivascular t o endomysial region t hat w as f urt her conf irmed by ot her invest igat ors26-28.
In agreement w it h t hese dat a, our observat ions also indicat e t he exist ence of a cyt ot oxic cell me-diat ed injury. How ever, t hese f indings w ere obser-ved in scat t ered muscle f ibers suggest ing t hat t he cellular immune response alt hough present may not be the major mechanism involved in MCTD. Con-versely, the prevalence of CD4+over CD8+T cell
asso-ciat ed t o t he presence of B cells on perivascular region advocat es f or a vascular involvement and a probable humoral part icipat ion in M CTD myosit is.
The inf lammat ory cell inf ilt rat e of M CTD mus-cle observed herein has a dual int erest ing charact e-rist ic: one t hat resembles PM in t he endomysial region, and t he ot her t hat resembles DM in per-imysial region. How ever, a signif icant ly f ew er t ot al number of T cells is observed in M CTD compared t o t hat observed in PM and DM w hich is also
as-sociat ed t o a smaller number of macrophages, re-f lect s a low er degree ore-f t he inre-f lammat ory process in M CTD in relat ion t o bot h t he ot her inf lamma-t ory muscle diseases.
In summary, our findings support the notion that muscle involvement in M CTD is a preponderant ves-sel t arget ed disease w it h a probable humoral role, and a concomitant minor cytotoxic immune respon-se. Further studies to evaluate the role of pro-inflam-mat ory cyt okines and U1-RPN ant ibodies in per-pet uat ing muscle injury are required f or a bet t er understanding of the immunopathogenesis of MCTD.
Acknow legem ent s – We are indebt ed t o Frederick
W M iller, M D, PhD, f or his caref ul review of t he manu-script. We also thank Mariko Yokoo, and Alda Wakamatsu f or t echnical help.
REFERENCES
1. Dalakas MC. Immunopathogenesis of inflamatory myopathies. Ann Neurol 1995;37 (S1):S74-S86.
2. Sharp GC, Irwin WS, Tan EM, et al. Mixed connective tissue disease: an apparently distinct rheumatic disease syndrome associated with a specific antibody to an extractable nuclear antigen (ENA). Am J Med 1972;52:148-158.
3. Bennett RM, O’Connell, DJ. Mixed connective tissue disease: a clinico-pathologic study of 20 cases. Sem Arthritis Rheum1980;10:25-51. 4. Karpati G, Pouliot Y, Carpenter S. Expression of immunoreactive major
histocompatibility complex products in human skeletal muscles. Ann Neurol 1988;23:64-72.
5. Engel AG, Arahata K. Mononuclear cells in myopathies: quantification of functionally distinct subsets, recognition of antigen-specific cell-mediated cytotoxicity in some diseases, and implications for the patho-genesis of the different inflammatory myopathies. Human Pathol 1986;17:704-721.
6. Kissel JT, Mendell JR, Rammohan KW. Microvascular deposition of com-plement membrane attack complex in dermatomyositis. N Engl J Med 1986;314:329-334.
7. Oxenhandler R, Hart M, Corman L, Sharp G, Adelstein E. Pathology of skeletal muscle in mixed connective tissue disease. Arthritis Rheum 1977;20:985-988.
8. Burdt MA, Hoffman RW, Deutscher SL, Wang GS, Johnson JC, Sharp GC. Long-term outcome in mixed connective tissue disease. longitu-dinal clinical and serologic findings. Arthritis Rheum 1999;42:899-909. 9. Singsen BH, Swanson VL, Bernstein BH, Heuser ET, Hanson V, Landing BH. A histologic evaluation of mixed connective tissue disease in child-hood. Am J Med 1980;68:710-717.
10. Bohan A, Peter JB. Polymyositis and dermatomyositis. N Engl J Med 1975;292:344-347.
11. Bohan A, Peter JB. Polymyositis and dermatomyositis. N Engl J Med 1975;292:403–407.
12. Kurata N, Tan EM. Identification of antibodies to nuclear acidic anti-gen by counterimmunoeletrophoresis. Arthritis Rheum 1976;19:574-580. 13. Tan EM. Antinuclear antibodies: diagnostic markers for autoimmune
diseases and probes for cell biology. Adv Immunol 1989;44:93-151. 14. Laemmli UK. Cleavage of structural proteins during the assembly of
the head of bacteriophage T4. Nature 1970;227:680-685.
15. Towbin H, Staehelin T, Gordon J. Electrophoretic transfer to proteins from polyacrylamide gels to nityrocellulose sheets procedure and some applications. Proc Nat Acad Sci USA 1979;76:4350-4354.
17. Dalakas MC. Polymyositis, dermatomyositis and inclusion body myosi-tis. N Engl J Med 1991;325:1487-1498.
18. Kotajima L, Aotsuka S, Sumiya M, Yokohari R, Tojo T, Kasukawa R. Clinical features of patients with juvenile onset mixed connective tis-sue disease: analysis of data collected in a nationwide collaborative study in Japan. J Rheumatol 1996;23:1088-1094.
19. Isenberg D. Myositis in other connective tissue disorders. Clin Rheum Dis 1984;10:151-174.
20. Mendell JR, Garcha TS, Kissel JT. The immunopathogenic role of com-plement in human muscle disease. Curr Opin Neurol 1996;9:226-234. 21. Crowson NA, Magro CM. The role of microvascular injury in the pathoge-nesis of cutaneous lesions of dermatomyositis. Hum Pathol 1996;27:15-19. 22. Bleecker JL, Engel AG. Expression of cell adhesion molecules in inflam-matory myopathies and Duchenne dystrophy. J Neuropatol Exp Neurol 1994;53:369-376.
23. Okawa-Takatsuji M, Aotsuka S, Fujinami M, Uwatoko S, Kinishita M, Sumiya M. Up-regulation of intercellular adhesion molecule-1
(ICAM-1), endothelial leukocyte adhesion molecule-1 (ELAM-1) and class II MHC molecules on pulmonary artery endothelial cells by antibodies against U1-ribonucleoprotein. Clin Exp Immunol 1999;116:174-180. 24. Arahata K, Engel AG. Monoclonal antibody analysis of mononuclear
cells in myopathies: V. identification and quantitation of T8+ cytotox-ic suppressor cells. Ann Neurol 1988;23:493-499.
25. Arahata K, Engel AG. Monoclonal antibody analysis of mononuclear cells in myopathies I: quantitation of subsets according to diagnosis and sites of accumulation and demonstration and counts of muscle fibers invaded by T cells. Ann Neurol 1984;16:193-208.
26. Lemoine NR, Ryan JF, Cox EL, Mayston V, Revell PA, Swash. Immunohistochemical analysis of mononuclear cell subsets in inflamma-tory and non-inflammainflamma-tory myopathies. J Clin Pathol 1986;39:271-274. 27. Botet JCP, Grau JM, Casademont J, Urbano-Márquez A, Rozman C.
Characterization of mononuclear exudates in idiopathic inflammato-ry myopathies. Virchows Archiv Pathol Anat 1988;412:371-374. 28. Lindberg C, Oldfors A, Tarkowski. A local T-cell proliferation and