www.bjorl.org
Brazilian
Journal
of
OTORHINOLARYNGOLOGY
ORIGINAL
ARTICLE
Translation
into
Portuguese
and
validation
of
the
Rhinitis
Control
Assessment
Test
(RCAT)
questionnaire
夽
Pedro
Henrique
Fernandes,
Fausto
Matsumoto,
Dirceu
Solé,
Gustavo
Falbo
Wandalsen
∗UniversidadeFederaldeSãoPaulo(UNIFESP),EscolaPaulistadeMedicina,DepartamentodePediatria, DisciplinadeAlergia,SãoPaulo,SP,Brazil
Received26May2015;accepted6December2015 Availableonline16April2016
KEYWORDS
Allergicrhinitis; Questionnaires; Control;
Validationstudies
Abstract
Introduction:TheRhinitisControlAssessmentTest(RCAT)isasimpleself-administered ques-tionnairedevelopedtoassesscontrolofrhinitis.
Objectives:TranslateintoBrazilianPortugueseandvalidatetheRCAT.
Methods:TheRCATwastranslatedintoPortuguesebytwotranslatorsandsubsequently back-translatedintoEnglish.Itwasthenappliedto141adolescentswithallergicrhinitis.
Results:TheinternalconsistencyoftheRCATwas0.73.Thequestionnairescoresshowed sig-nificantcorrelationwithtotalnasalandextra-nasalsymptomscoresandnasalpeakinspiratory flow(r:−0.73,−0.58and0.52,respectively;p<0.001)andweresignificantlydifferentwhen
dividedbyphysicianglobalassessmentandtotalnasalsymptomscoreseverity.Cutoffpoints between22and24hadthehigherareasundertheROCcurvetoidentifypatientswithrhinitis control.Totalnasalandextra-nasalsymptomscoresweresignificantlydifferentwhenacutoff pointof22wasused(median:4.0vs.8.0and2.0vs.5.0;p<0.001).
Conclusions:TheBrazilianPortugueseversionoftheRCATwasshowntobeavalidand discrim-inanttooltoidentifypatientswithcontrolledanduncontrolledallergicrhinitis.
© 2016 Associac¸˜ao Brasileira de Otorrinolaringologia e Cirurgia C´ervico-Facial. Published by Elsevier Editora Ltda. This is an open access article under the CC BY license (http:// creativecommons.org/licenses/by/4.0/).
PALAVRAS-CHAVE
Rinitealérgica; Questionários; Controle;
Estudosdevalidac¸ão
Traduc¸ãoparaoportuguêsevalidac¸ãodoquestionáriodecontroledariniteRhinitis ControlAssessmentTest(RCAT)
Resumo
Introduc¸ão:O Rhinitis Control Assessment Test (RCAT) é um questionário simples e autoaplicáveldesenvolvidoparaavaliarocontroledarinite.
夽 Pleasecitethisarticleas:FernandesPH,MatsumotoF,SoléD,WandalsenGF.TranslationintoPortugueseandvalidationoftheRhinitis ControlAssessmentTest(RCAT)questionnaire.BrazJOtorhinolaryngol.2016;82:674---9.
∗Correspondingauthor.
E-mail:gfwandalsen@uol.com.br(G.F.Wandalsen).
http://dx.doi.org/10.1016/j.bjorl.2015.12.011
Objetivos: TraduzirparaoPortuguêsvalidaroRCAT.
Método: Atraduc¸ãodoRCATfoifeitapordoistradutorescomposteriorversãoparaaLíngua Inglesa.ORCATfoientãoaplicadodeformatransversala141adolescentescomrinitealérgica. Resultados: AconsistênciainternadoRCATfoi0,73.Asnotastotaisdoquestionáriose correla-cionaramsignificantementecomosescoresdesintomasnasaiseextra-nasaisepicodefluxo inspiratórionasal(r:-0,73,-0,58e0,52,respectivamente;p<0,001)eforamsignificantemente diferentesquandoseparadaspelaopiniãomédicasobreocontroledariniteepelagravidade dossintomasnasais.Ospontosdecorteentre22e24foramoscommaioresáreassobacurva ROCparadefinic¸ãodocontroledarinite.Osescoresdesintomasnasaiseextra-nasaisforam significantementediferentesquandoospacientesforamseparadospelopontodecortede22 (medianasde4,0vs.8,0e2,0vs.5,0;p<0,001).
Conclusões: A versãoem PortuguêsdoRCATsemostrou umaferramenta válida ecombom poderdiscriminativoparasepararpacientescomrinitealérgicacontroladaenãocontrolada. © 2016 Associac¸˜ao Brasileira de Otorrinolaringologia e Cirurgia C´ervico-Facial. Publicado por Elsevier Editora Ltda. Este ´e um artigo Open Access sob uma licenc¸a CC BY (http:// creativecommons.org/licenses/by/4.0/).
Introduction
Allergic rhinitis is the most prevalent allergic disease in Brazil. Brazilian data, obtained through the International Study of Asthma and Allergiesin Childhood (ISAAC) study showedthattheprevalenceofrhinitisinchildrenand ado-lescentsrangesfrom10%to47%,dependingonthedefinition usedandtheagegroupstudied.1Traditionally,allergic
rhini-tis wasconsidered a disease of minor importance due to its low morbidity and mortality. In the last decade, the importanceof allergic rhinitis hasbeen increasingly high-lighted, mainly because of its complications, high cost, negativeimpactonqualityoflife,andassociationwithother diseases.2,3
Currently, the recommendations for the drug manage-mentofallergicrhinitisarebasedontheclassificationofthe diseaseseverityandpersistenceofsymptoms.2These
rec-ommendationsareeasily applicable topatients notunder current treatment at the beginning of follow-up, but are less applicable toevaluatechanges over timeand cannot measure response totreatment. Managingthe diseaseby controllingthemagnitudeofitssymptomshasbeen demon-stratedtobeusefulandpracticalinasthma,andiscurrently therecommendedformofmanagement.4
Afewyearsago,aquestionnairewasdevelopedtoassess the levelof rhinitis controlcalled Rhinitis Control Assess-ment Test (RCAT).5,6 This questionnaire, developed in the
Englishlanguage,consistsofsixquestionswithfiveanswer levelsthat comprise atotal score.The questionsrefer to thepreviousweekandaddresssymptoms(nasalcongestion, sneezing,andeyetearing),interferencewithsleepanddaily activities,andtheindividual’spersonalopinionabout symp-tomcontrol.5,6
EvaluationoftheoriginalversionofRCATshowedthatthe questionnaireisvalidandreliableandcanbeusedforrapid screeningofpatientswithdifficult-to-controlrhinitis symp-tomsandasanadditionaltoolintheclinicalmanagement ofrhinitis.6
ThisstudyaimedtotranslateandadapttheRCAT ques-tionnaireintoPortugueselanguage(Brazilianculture),and tovalidatethattranslatedversion.
Methods
The RCAT questionnaire translation into Portuguese was performedby twodifferent translatorswithasubsequent back-translationtoEnglishandfinalconciliationofthe ver-sions.Theresultingversionwasthenappliedtotenpatients withallergicrhinitis(olderthan11yearsofage)toevaluate itsintellection.
The validation of the translated version of the ques-tionnairewascarriedoutasan observational,descriptive, analytical, and cross-sectional assessment, applied to patientsinareferralallergyoutpatientclinic.Adolescents (12---18years,bothgenders),withdocumenteddiagnosisof allergicrhinitisforatleastsixmonthswereinvitedto par-ticipateinthequestionnairevalidation.Onlypatientswith provenallergicsensitizationtoatleastoneinhaledallergen, demonstratedbyskinpricktestorspecificserumIgEtests performedinthelasttwoyearsparticipatedinthestudy.
Thosewithahistoryofsymptomsconsistentwith infec-tiousdiseasesoftheupperairwaysinthelast15days,aswell asthosewithneuropathy,cognitivedeficits,andstructural changesoftheupperairways(clinicalassessment)werenot invitedtoparticipateinthestudy.
The patients answered the translatedversion of RCAT, whichconsistedofsixquestionsrelatedtosymptoms expe-riencedinthepreviousweek.Eachquestionreceivedscores ranging from1 to 5 pointsaccording to the frequency of reporting,withscore5for ‘‘never,’’4for ‘‘rarely,’’ 3for ‘‘sometimes,’’2for‘‘often,’’and1for‘‘veryoften’’.The finalscore(RCATT),givenbythesumofallquestions,could rangefromsixto30 points.6 According tothetotalscore
obtained, patients were divided into two groups accord-ingtotheoriginalquestionnairevalidation:controlled(≥22
points)anduncontrolled(<22points).6
classifiedaccordingtotheNSS,beingconsideredmild(NSS 0---5), moderate (NSS 6---10), or severe (NSS 11---15).6 The
ENSS,alsorelatedtotheweekpriortotheassessment,was measuredbyaddressingthefollowingsymptoms:eye tear-ing,eyeitching,pharyngealpruritus,andocularhyperemia, whichwerescoredaccordingtotheNSS(maximum12).
Anobjectiveassessmentofnasalfunctionwasperformed bymeasuringthePeak NasalInspiratoryFlow(PNIF) using specific equipment(In-Check®; ClementClarke, England).
Duringtheprocedure,patientswereinstructedtoperform deepinspirationsuptototallungcapacity,keepingthelips tightlyclosed.Themaximumflowratewasreadbythe cur-sorinlitersperminute.Atleastthreemeasurementswere performedandthebestmeasurementamongthethreewas recorded,withavariationoflessthan10%.7
All patients underwent medicalassessment beforethe questionnaireswereapplied,inwhichanymedicationsbeing usedandassociatedcomorbiditieswererecorded.Medical opinion was requested about the patients’ nasal symp-tom control, and the physician’s subjective classification notedascontrolled,partially controlled,or uncontrolled. Similarly,thephysicianreportedhis/heropinionregarding adherence or not to the proposed treatment in previous consultations,accordingtodataobtainedattheinterview. ThestudywasapprovedbytheResearchEthics Commit-teeoftheinstitution(protocoln.282867)andaninformed consentformwasobtainedfromallparticipantsandtheir parents/guardians.
Sample size calculation was performed based on the correlation coefficients found between the scores of the originalquestionnaireandtheclinical scoresranging from 0.3to0.6.6Thus,estimatingaminimumrof0.3,atleast116
patientswouldbenecessarytoensuresignificantcorrelation withapowerof95%andp=0.05.
Theconstructive validationof thetranslatedversionof RCATwasperformedbycomparingthescoresobtainedwith theNSS,ENSS,andPNIF,usingSpearman’scorrelationtest. Thediscriminatorycapacitywasassessedbycomparingthe RCATscoresaccordingtothemedicalclassificationofrhinitis controlandseverityofnasalsymptomsusingnon-parametric tests(Mann---WhitneyandKruskal---Wallis).Theinternal con-sistencyofRCATwasassessedbyCronbach’salpha.Receiver operatingcharacteristiccurveswereconstructedto estab-lishcutoffscores, accordingtothemedicalopinion about diseasecontrol (controlledrhinitis vs.partiallycontrolled anduncontrolledrhinitis).
Results
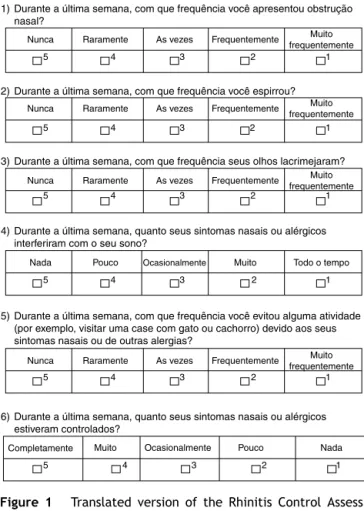
The RCAT version that was translated into Portuguese (Brazilian culture) is shown in Fig. 1. There were no significant differences between the two translations into Portuguese, and therefore the final consolidation of the translatedRCATandtheEnglishversiondidnotresultinany changestotheoriginaltool.Attheinitialassessment,the questionnairewaseasilyunderstoodbytheadolescentsand itwasquicklycompleted.
Atotalof141adolescentsagedbetween12and18years (median 13 years), participated in the validation phase, of whom the majority was male (74%) and white (47%). The median of years with nasal symptoms was 8 years
Durante a última semana, com que frequência você apresentou obstrução nasal?
1)
2)
3)
4)
5)
6) Nunca
5 4 3 2 1
5 4 3 2 1
5 4 3 2 1
5 4 3 2 1
5 4 3 2 1
5 4 3 2 1
Raramente As vezes Frequentemente frequentementeMuito
Nunca Raramente As vezes Frequentemente frequentementeMuito
Nunca Raramente As vezes Frequentemente frequentementeMuito
Nunca
Completamente
Raramente As vezes Frequentemente frequentementeMuito Nada
Nada Pouco
Pouco Ocasionalmente
Ocasionalmente Muito
Muito
Todo o tempo
Durante a última semana, com que frequência você espirrou?
Durante a última semana, com que frequência seus olhos lacrimejaram?
Durante a última semana, quanto seus sintomas nasais ou alérgicos interferiram com o seu sono?
Durante a última semana, com que frequência você evitou alguma atividade (por exemplo, visitar uma case com gato ou cachorro) devido aos seus sintomas nasais ou de outras alergias?
Durante a última semana, quanto seus sintomas nasais ou alérgicos estiveram controlados?
Figure 1 Translated version ofthe Rhinitis Control Assess-ment Test (RCAT) into the Portuguese language (Brazilian culture).
(interquartile range [IQR]: 5---11 years). All patients attendedschoolandwereliterate.Theinternalconsistency ofthequestionnairemeasuredbyCronbach’s˛was0.73.
Regarding the medical opinion about the control of patients’nasalsymptoms,53(38%)wereclassifiedas con-trolled, 51 (36%) aspartially controlled, and 37 (26%) as uncontrolled. Accordingtothemedicalopinion, 106(75%) patientswereconsideredadherentand35(25%)were non-adherenttotreatment.
The NSS median was 5 (IQR: 3---8), the ENSS median was 3 (IQR: 1---6), and PNIF median was 130L/min (IQR: 100---150L/min).AccordingtotheNSSscore,mildsymptoms wereobservedin78patients(55%),moderatein50(36%), andseverein13(9%).TheRCATscoresrangedbetween10 and30,withamedianof22(IQR:19---26).
The correlation coefficients of RCAT with the NSS, ENSS and PNIFwere respectively −0.73; −0.58; and 0.52
(p<0.001).IndividualvaluesareshowninFig.2.
TheRCAT scores weresignificantly different(p<0.001) whenseparatedbythemedicalopinionaboutnasalsymptom control(Fig.3)andnasalsymptomseverity(Fig.4).
16 14 12 10 8 6 4 2 0
0 50 100 150 200 250 300
14 12 10 8 6 4 2 0 5 10 15 20 25 30 35
5 10 15 20 25 30 35
RCATT RCATT
NSS
PFIN
ENSS
RCATT r: 0.52; p < 0.001
r: –0.73; p < 0.001 r: –0.58; p < 0.001
5 10 15 20 25 30 35
Figure2 Correlationbetween totalRhinitis Control AssessmentTest (RCAT)scores (RCATT) andNasal SymptomScore (NSS), Extra-NasalSymptomScore(ENSS),andPeakNasalInspiratoryFlow(PNIF)values.
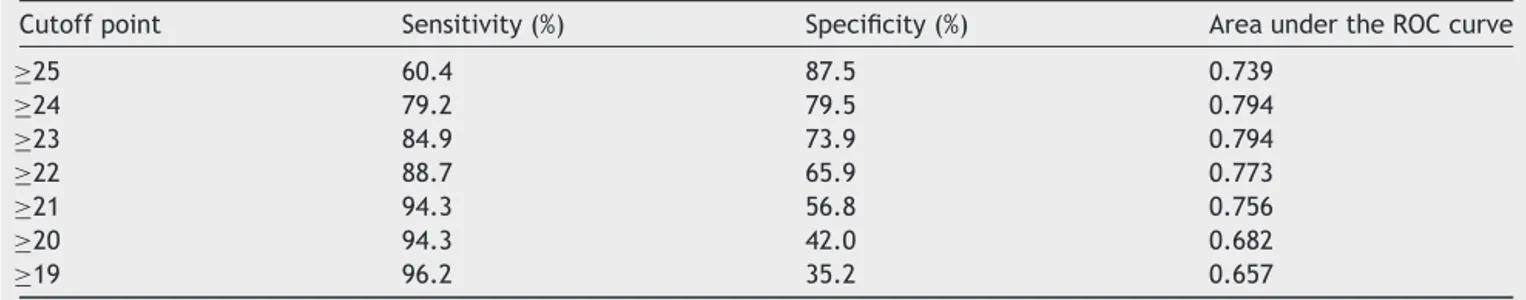
Table1 Sensitivity,specificity,andareaunderthereceiveroperatingcharacteristic(ROC)curveofdifferentRhinitisControl AssessmentTest(RCAT)cutoffstodefinerhinitiscontrol.
Cutoffpoint Sensitivity(%) Specificity(%) AreaundertheROCcurve
≥25 60.4 87.5 0.739
≥24 79.2 79.5 0.794
≥23 84.9 73.9 0.794
≥22 88.7 65.9 0.773
≥21 94.3 56.8 0.756
≥20 94.3 42.0 0.682
≥19 96.2 35.2 0.657
30
25
20
RCA
TT
15
10
Figure 3 Total score values ofRhinitis Control Assessment Test (RCAT) discriminated by the medical opinion about the controlofnasalsymptomsascontrolled(white),partially con-trolled(lightgray),anduncontrolled(darkgray).
30
RCA
TT
25
20
15
10
15
12
NSS
PFIN
ENSS
12
10
8
6
4
2
0 9
6
3
0
250
200
150
100
50
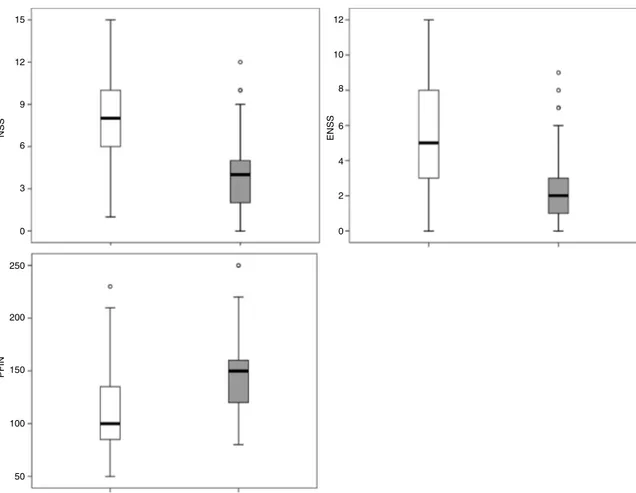
Figure5 ScorevaluesofNasalSymptomScore(NSS),Extra-NasalSymptomScore(ENSS)andPeakNasalInspiratoryFlow(PNIF) valuesofpatientsclassifiedascontrolled(gray;totalscorevaluesofRhinitisControlAssessmentTest(RCAT)≥22),oruncontrolled
(white;RCATT<22).
ApplyingtheRCATcutoffscoreof22points,77patients weredefinedascontrolledand64asuncontrolled.TheNSS, ENSS,andPNIFweresignificantlydifferentbetweenthese twogroups(p<0.001)withmediansof,respectively:4.0vs. 8.0;2.0vs.5.0;and150L/minvs.100L/min(Fig.5).
Discussion
Toolsor scoreshave frequentlybeen usedinthe manage-mentofseveralchronicdiseases,suchasasthmaandchronic hives.8,9 These tools can have many applications and are
usedbothtoscreenpatientsinprimarycaresettingsandto assistinmedicalmanagementbyspecialists.
Severalquestionnairesareavailablefor theassessment ofpatientswithnasaldiseases.Amongthese,those devel-opedtoassessthe qualityoflife inpatients withspecific diseases,suchasallergicrhinoconjunctivitis10 andchronic
rhinosinusitis/nasal polyposis (SNOT-22),11 translated and
validatedintothePortugueselanguage(Brazilianculture), are noteworthy.12,13 Other questionnaires assess certain
symptoms,suchasnasalobstruction(NOSE),14 ora
combi-nationofdiseases, suchasrhinitisandasthma.15,16 Tothe
bestofthe present authors’knowledge and thatof other authors,5 the RCAT was the first tool of this type to be
developedfortheassessmentofallergicrhinitiscontrol. TheRCATwasdevelopedasasimple,concise,and self-administeredtoolto assessrhinitis control.5 The items in
thequestionnairewereselectedwiththehelpofgroupsof patients and physicians. Initially, 26 questionswere iden-tified,separatedintofiveareas:symptoms,interferencein activities,limitations,rhinitiscontrol,andmedicationuse.5
After application toa large group of patients, this initial version of the questionnaire went through an assessment processinwhichthemostrelevantquestionswereidentified through logistic regression analyses, and the final version comprisedsixquestions.5
Inthisstudy,someimportantpropertiesofthetranslated versionofRCATwereassessed.Thereliabilitywasevaluated byitsinternalconsistency,withanacceptablevalue(>0.7) asdemonstratedbyCronbach’s˛coefficient.
Intheconstructivevalidation,theoverallquestionnaire scores (RCAT) showed a strong correlation with the nasal symptom score (NSS). The correlation coefficient found (−0.73) washigher than that observed when the original
version of RCAT was validated (−0.57).6 Unlike the NSS,
theRCATaddressesadditionalaspectsbesidesrhinitis symp-toms,suchastheinterferenceofrhinitiswithsleepanddaily activitiesand,thus,somedegreeofdisagreementbetween thesetoolswasexpected.Differencesinthetoolsaremore marked in relation to the ENSS and the objective mea-surementofnasalfunction(PNIF).Nevertheless,moderate correlationswereobservedbetweenthem(Fig.2).
finalscoresoftheRCATareclearlydifferentwhenpatients areseparatedbythemedicalopinionaboutdiseasecontrol ortheseverityoftheNSS.
The identification of the best RCAT cutoff to discrimi-natecontrolledpatientsfromthosewithproblemsinallergic rhinitiscontrolmayvaryaccordingtothepurposesandaims ofitsapplication,anditispossibletochoosecutoffpoints withgreatersensitivity or greaterspecificity. Inthe origi-nalvalidationofRCAT,thecutoffpointwiththelargestarea undertheROCcurvewas22points(AUC=0.689).Thiscutoff pointalsoshowedalargeareaundertheROCcurveinthe validationofthetranslatedversion(AUC=0.773;Table1). Whenthiscutoffpointwasappliedtothegroupofstudied patients,significantdifferenceswereobservedfortheNSS, ENSS,andPNIFvalues(Fig.5).Amongthepresentpatients, however,thecutoffpointsof23and24showedlargerareas undertheROCcurve,particularlyduetothebiggestgainin specificitywithoutsignificantlossesinsensitivity(Table1). The group of patients evaluated in our study differed in some aspects fromthe group evaluatedin the original validation of RCAT; the most relevant aspects were the age group and the diagnosis. In the original validationof RCAT, only patients aged 18 years or older could partici-pate,whereasthepresent studyincludedonlyadolescents aged 12---18 years. The present patients had persistent allergicrhinitis,exclusively, unlikethe originalvalidation, comprising patientswithperennial allergic,seasonal, and non-allergicrhinitis.6
Furtherstudiesarenecessarytoevaluateother proper-ties of the translated version of RCAT, already studied in theoriginalversionofthequestionnaire,suchasits repro-ducibilityandtheclinicallyrelevantminimumdifference.
Conclusions
In conclusion, thisstudy presented a versionof the RCAT rhinitiscontrolquestionnairetranslatedintothePortuguese language(Brazilianculture).Itwasobservedthatthis ver-sionofthequestionnaireiseasilyunderstoodbyadolescents withallergicrhinitis,anditisavalidtoolwithgood discrimi-natorypowertodifferentiatecontrolledpatientsfromthose non-controlled.
Conflicts
of
interest
Theauthorsdeclarenoconflictsofinterest.
References
1.SoléD,Camelo-NunesI,WandalsenG,RosárioFilhoN,Naspitz C, Brazilian ISAAC’s Group. Prevalence of rhinitis among Brazilian schoolchildren: ISAAC phase 3 results. Rhinology. 2007;45:122---87.
2.BousquetJ,KhaltaevN,CruzA,DenburgJ,FokkensW,TogiasA. AllergicRhinitisanditsImpactonAsthma(ARIA)2008.Allergy. 2008;63Suppl.86:8---160.
3.Meltzer E, Nathan R, Derebery J, Dalal A, Stanford R, Cor-rao M, et al.Sleep, quality of life, and productivityimpact of nasal symptoms in the United States: findings from the Burden of Rhinitis in America survey. Allergy Asthma Proc. 2009;30:244---54.
4.GINA. Global Initiative for Asthma; 2015. Available from: www.ginasthma.org
5.Schatz M, Meltzer E, Nathan R, DereberyJ, Mintz M, Stan-fordR, etal.Psychometric validationoftheRhinitisControl AssessmentTest:abriefpatient-completedinstrumentfor eval-uatingrhinitissymptomcontrol.AnnAllergyAsthmaImmunol. 2010;104:118---24.
6.MeltzerE,SchatzM,NathanR,GarrisC,StanfordR,KosinskiM. Reliability,validity,andresponsivenessoftheRhinitisControl AssessmentTestinpatientswithrhinitis.JAllergyClinImmunol. 2013;131:379---86.
7.NathanRA,EcclesR,HowarthPH,SteinsvågSK,TogiasA. Objec-tivemonitoringofnasalpatencyandnasalphysiologyinrhinitis. JAllergyClinImmunol.2005;115:S442---59.
8.SchatzM,SorknessC,LiJ,MarcusP,MurrayJ,NathanR,etal. TheAsthmaControl Test:reliability,validity,and responsive-nessinpatientsnotpreviouslyfollowedbyasthmaspecialists. JAllergyClinImmunol.2006;117:549---56.
9.Weller K, Groffik A, Church M, Hawro T, Krause K, Metz M, et al. Development and validation of the Urticaria Control Test: a patient-reported outcome instrument for assessingurticariacontrol.JAllergyClinImmunol.2014;133: 1365---72.
10.JuniperE,GuyattG,DolovichJ.Assessmentofqualityoflife inadolescentswithallergicrhinoconjunctivitis:development andtestingofaquestionnaireforclinicaltrials.JAllergyClin Immunol.1994;93:413---23.
11.Hopkins C, Gillett S, Slack R, Lund V, Browne J. Psycho-metric validity of the 22-item Sinonasal Outcome Test. Clin Otolaryngol.2009;34:447---54.
12.Nascimento S, Naspitz C, Solé D. Evaluation of quality of life in children and teenagers with allergic rhinitis: adap-tation and validation of the Rhinoconjunctivitis Quality of Life Questionnaire (RQLQ). Allergol Immunopathol. 2001;29: 111---8.
13.Kosugi E, Chen V, Fonseca V, Cursino M, Mendes Neto J, GregórioL.Translation,cross-culturaladaptationandvalidation ofSinoNasalOutcomeTest(SNOT)---22toBrazilianPortuguese. BrazJOtorhinolaryngol.2011;77:663---9.
14.StewartM,WitsellD,SmithT,WeaverE,YuehB,HannleyM. Development and validation of the NasalObstruction Symp-tom Evaluation (NOSE) scale. Otolaryngol Head Neck Surg. 2004;130:157---63.
15.FonsecaJ,Nogueira-SilvaL,Morais-AlmeidaM,AzevedoL, Sá-SousaA,Branco-FerreiraM,etal.Validationofaquestionnaire (CARAT10)toassessrhinitisandasthmainpatientswithasthma. Allergy.2010;65:1042---8.