UNIVERSIDADE DE LISBOA FACULDADE DE CIÊNCIAS
DEPARTAMENTO DE BIOLOGIA VEGETAL
Centrosome biogenesis and number:
mechanisms of control
- Determination of SAK/PLK4 interactors
FRANCISCO DUQUE PROJECTO AVÓ FREIXOMESTRADO EM BIOLOGIA MOLECULAR HUMANA 2009
UNIVERSIDADE DE LISBOA FACULDADE DE CIÊNCIAS
DEPARTAMENTO DE BIOLOGIA VEGETAL
Centrosome biogenesis and number:
mechanisms of control
- Determination of SAK/PLK4 interactors
FRANCISCO DUQUE PROJECTO AVÓ FREIXOTese orientada pelos Prof.es Doutores
Mónica Bettencourt Dias
GRUPO DE CELL CYCLE REGULATION INSTITUTO GULBENKIAN DE CIÊNCIA
e Rui Gomes
DEPARTAMENTO DE BIOLOGIA VEGETAL
FACULDADE DE CIÊNCIAS DA UNIVERSIDADE DE LISBOA
MESTRADO EM BIOLOGIA MOLECULAR HUMANA 2009
Abstract
The centrosome is the major microtubule organizing center in animal cells and regulates cell adhesion, migration and polarity in interphase and the formation of the mitotic spindle. It is formed by a dense protein lattice that surrounds two centrioles, which grant the centrosome its ability to duplicate. Centriole duplication occurs only once per cell cycle in order to avoid multipolar spindle formation which can lead to genomic instability or loss of asymmetric cell division. In fact, centrosome amplification is often associated with early stages of tumorigenesis. In Drosophila melanogaster, centrosome duplication is triggered by a kinase from the Polo family, SAK/Plk4, and regulated by other proteins such as SAS-6, SAS-4 and Bdl10, that also play a role in centriole assembly. It is known that SAK/Plk4 depletion leads to centrosome loss both in Drosophila and humans, and that overexpression causes centriole overduplication in a single cell cycle. In cells that don’t have centrioles, SAK/Plk4 overexpression causes de novo formation of multiple centrosomes, by a process that is not yet fully understood. Other than its degradation by the SCF/Slimb complex, there are no other known SAK interactors or substrates.
Here we show the result of an RNAi screen for novel SAK substrates and interactors whose depletion could affect centrosome duplication. The candidates were chosen coupling previous immunoprecipitation results with bioinformatic analysis. Minispindles (Msps) and Yps depletion lead to an increase in cells with 0 centrosomes when compared to the negative control. The depletion of these genes does not seem to affect SAK localization. Msps can act as microtubule (MT) polymerase. The centrosome number and microtubule defect phenotypes generated by its depletion are not rescued by co-depletion of MT depolymerases. This suggests that centrosome loss may be related to a MT network disruption in interphase, possibly due to impairment of centrosomal proteins transport. Yps localizes to the cytoplasm and nucleus, which is consistent with its transcription factor and RNA binding activities. It may be involved in regulation of gene expression, for genes whose product is required for centrosome duplication or stability. Its human homologue, YB-1, has tubulin binding activity, suggesting that it may also exist a similar function for Yps. Together, these results bring a new perspective on how centrosome duplication and stability can be regulated. This suggests the existence of one or several novel protein interactions and pathways through which SAK is playing its role.
Summary (Portuguese)
O centrossoma é, em células animais, o principal centro organizador de microtúbulos (MTOC). Em interfase, regula processos importantes como a adesão, mobilidade, e polaridade celulares, enquanto que em mitose participa na formação e organização do fuso mitótico. Cada centrossoma é composto por dois centríolos, organelos cilíndricos constituídos por microtúbulos, envolvidos numa densa matriz proteica denominada material pericentriolar (PCM). Os centríolos podem também converter-se converter-se em corpos basais para formação de cílios e flagelos, estruturas importantes a diversos níveis, como a regulação fisiológica, desenvolvimento embrionário e mobilidade do espermatozóide. A simetria do centríolo é conservadam apesar de a estrutura centriolar variar ligeiramente de espécie para espécie, sendo que em vertebrados o centríolo é composto por 9 tripletos de microtúbulos dispostos em torno de uma estrutura central (a cartwheel), enquanto que em células somáticas de Drosophila melanogaster existem 9 dupletos e em Caenorhabditis elegans 9 singletos. Um dos centríolos do par é denominado centríolo-mãe, que possui apêndices subdistais e distais, estruturas proteicas onde se dá ancoragem de microtúbulos e que permitem ligação do centríolo à membrana na formação do corpo basal, respectivamente. O outro centríolo, em posição ortogonal em relação ao primeiro, é o centríolo-filho. O PCM, por sua vez, é constituído por mais de 300 tipos de proteínas como domínios de coiled-coils, da família das pericentrinas e AKAP450, envolvidas na formação do corpo basal, na nucleação e ancoragem de microtúbulos, e na duplicação dos centríolos. O ciclo de duplicação centriolar ocorre de forma simultânea ao ciclo de replicação do DNA, e envolve duas regras principais, que ditam que em cada ciclo celular cada centríolo deve duplicar apenas uma vez, e que cada centríolo-mãe deverá formar apenas um centríolo-filho. O cumprimento destas regras é essencial para evitar a formação de fusos mitóticos anormais e existência de excesso de centríolos na célula, que podem levar a anomalias na segregação do material genético e a instabilidade genómica. Anomalias no número de centrossomas podem também conduzir a perda de divisão celular assimétrica, e estão associadas tanto a fases precoces do processo de tumorigénese, como a diversos tipos de cancro.
O ciclo canónico de duplicação dos centrossomas é activado, tanto em humanos como em Drosophila, por uma cinase designada SAK/Plk4 (polo-like kinase 4). Os níveis de SAK são mais elevados no centrossoma em mitose, onde se crê que esta cinase actue num processo de activação da duplicação centriolar. Após separação dos centriolos em anafase, esta activação levaria à formação de um
procentríolo em posição ortogonal a cada centríolo-mãe, na fase S. Este processo requer proteínas estruturais como SAS-6 e Bld10, que formam um precursor central que dita a simetria do centríolo, e SAS-4, que está envolvida no recrutamento/nucleação dos microtúbulos centriolares. Durante a fase G2, o procentríolo sofre elongação até atingir o seu comprimento máximo na entrada em mitose. Nesta fase, o centrossoma passa por um processo de maturação, no qual recruta mais proteínas para o PCM, que serão necessárias para nuclear microtúbulos em mitose. Na entrada em mitose, os centrossomas separam-se e migram para cada um dos pólos do fuso mitótico, sendo depois segregados cada um para cada célula-filha. É sabido que a ausência de SAK leva a perda de centríolos, enquanto que a sua sobre-expressão causa sobre-duplicação de centrossomas, com formação de múltiplos centríolos filhos por cada centríolo-mãe. Em células sem centríolos, a sobre-expressão de SAK consegue causar a formação de centríolos de novo, sem controlo sobre o número de centríolos gerado. Desta forma, é fácil compreender que os níveis de actividade desta cinase têm de ser bem regulados dentro da célula, para que as duas regras mencionadas anteriormente sejam cumpridas. Para além da degradação da SAK pelo complexo SCF/Slimb em Drosophila, não são conhecidos outros interactuantes ou substratos desta cinase.
No trabalho descrito nesta tese foi efectuado um rastreio (screen) por RNAi em células S2 (Drosophila) de candidatos a interactuantes/substratos da SAK/Plk4, tendo como base resultados imunoprecipitações (pulldowns) e experiências Two-Hybrid disponíveis, ambos importados para uma base de dados. Esta base de dados permitiu escolher os candidatos com base em diversos critérios, como fortes interactuantes da SAK/Plk4 em diversos pulldowns, interactuantes de outras proteínas centrossomais, localização no centrossoma, entre outros. Após realizar duas experiências de RNAi para cada candidato e fazer imunofluorescência das células com D-PLP (pericentrin-like protein, marcador do centrossoma), foi contado o número de centrossomas por célula (n=250). A análise de citometria de fluxo com marcação de DNA foi também realizada, para apurar se a depleção dos candidatos tem um efeito no ciclo celular. Dos quatro candidatos significativamente diferentes do controlo negativo (células com GFP dsRNA) em ambas as experiências, dois foram escolhidos para uma caracterização mais detalhada do fenótipo. Estes dois candidatos, Minispindles (Msps) e Ypsilon Schachtel (Yps), originaram após depleção um fenótipo de aumento de células com 0 centrossomas, sendo que as células RNAi para a Yps também revelam um ligeiro aumento na população com mais de 4 centrossomas por célula. Msps é uma proteína do grupo XMAP215/chTOG, com actividade de polimerase de microtúbulos nas extremidades
positivas. Regula a instabilidade dinâmica da rede de microtúbulos em interfase, e a sua depleção origina defeitos na organização do citoesqueleto microtubular. Yps é uma proteína da família das Y-Box, sendo que esta família de proteínas tem uma actividade pleiotrópica, desde ligação ao DNA, actuando como factores de transcrição, a ligação a mRNA, regulando a sua tradução. O homólogo humano da Yps, YB-1, tem ainda actividade de ligação a tubulina, sugerindo que Yps também possa ter uma função semelhante. Numa experiência de imunofluorescência para detecção da SAK/Plk4, não foram observadas diferenças de localização da SAK/Plk4 no centrossoma em prometafase e em metáfase, aquando da depleção de Yps e Msps em comparação com o controlo negativo. Isto torna mais provável a actuação destas proteínas a jusante (downstream) da SAK/Plk4 na regulação da duplicação dos centríolos.
Verificou-se que o fenótipo de depleção da Msps não é contrabalançado pela co-depleção de cinesinas com actividade de despolimerização de microtúbulos, tanto em termos de defeitos no citoesqueleto como de número de centrossomas. Este resultado sugere que perturbações na rede de microtúbulos em interfase poderá interferir no transporte de proteínas essenciais para a duplicação/estabilidade dos centrossomas, e estar na base do fenótipo. Outra possibilidade é que a Msps poderá actuar na polimerização dos microtúbulos centriolares.
A clonagem de Yps em vectores de expressão permitiu fundir esta proteína, a N ou a C-terminal, com GFP ou com Proteína A de S. aureus. A fusão com GFP permitiu observar, em linhas celulares estáveis transfectadas com estes vectores, que Yps localiza no núcleo e citoplasma, mas não no centrossoma. Isto indica que pode estar envolvida na transcrição ou estabilização de RNA para proteínas importantes na duplicação/estabilidade dos centrossomas. Existe ainda a possibilidade de Yps estar a interagir com os microtúbulos, dado que o seu homólogo humano, YB-1, tem esta função. As fusões com Proteína A foram utilizadas para criar linhas celulares para futuros pulldowns desta proteína, e em conjunto com myc-SAK, para futuras experiências de co-imunoprecipitação.
Em conjunto, estes resultados trazem novas perspectivas acerca da regulação da duplicação dos centrossomas e sugerem a existência de novas vias pelas quais a SAK/Plk4 poderá actuar neste processo. O facto de os homólogos humanos da Msps e Yps, chTOG e YB-1, respectivamente, se encontrarem sobre-expressos em diversos tipos de cancro, abre a possibilidade de estudar uma possível interacção com a SAK/Plk4 neste contexto.
Palavras-chave: Centrosoma, SAK/PLK4, Duplicação do centrossoma, Minispindles, Yps
Acknowledgements
Quero agradecer em primeiro lugar à minha família, ao meu pai, mãe e irmã, por todo o apoio e por estarem sempre comigo.
A todos os membros do Cell Cycle Regulation Lab pela ajuda sem a qual este trabalho não teria sido possível, e pela amizade e carinho com que aqui fui recebido e que ao longo deste ano fomos construindo. À Dra. Mónica Dias, por me ter aceite no grupo, por me ajudar a crescer como pessoa e como cientista, por ter sempre tempo para orientar, falar e ensinar, por conseguir que este seja muito mais que um grupo de trabalho. À Inês Ferreira, por ter sido a minha primeira professora, mais-que-mãe das minhas experiências, mais-que-colega de trabalho, uma amiga. À Inês Bento, por me ajudar a pensar de forma crítica nos resultados, pelas bolachas, pelos contagiantes humor e gosto por animais. À Ana, pela paciência para as perguntas, pela companhia de ginásio. À Zita, a prova viva que as pessoas pequenas chegam onde chegam as grandes (mas cantam muito melhor), por me ensinar a “clonar”. Ao Pedro “MacShade”, por partilhar comigo durante o ano a dura tarefa de representar à altura a componente masculina do laboratório, gargalhadas à parte. À Daniela, pelos ensinamentos importantes e boas conversas de bancada. Ao Filipe, por partilhar a experiência de manter a sanidade mental em condições extremas.
Ao Computational Genomics Lab, nomeadamente o Dr. José Pereira Leal, por todas as sugestões e discussões de trabalho. Ao Renato e ao Filipe, pela ajuda com SQL e bioinformática em geral. Ao Jorge Beira por ter criado a CCRDB e me ensinar a usar o seu potencial. Ao Nuno Sepúlveda a sua preciosa ajuda e dedicação, na arte de analisar e representar resultados, e de encontrar um lugar significativamente melhor no meio de uma população de fãs do “metal”! A toda a Ala Zheng Ho e ao Paulo, técnico da ala, pelas discussões, ajuda, e bom ambiente de trabalho. Aos outros grupos do IGC pela ajuda prestada e reagentes partilhados. Ao Prof. António Coutinho, Director do IGC , pela possibilidade de realizar o meu trabalho nestas instalações. Ao professor Rui Gomes, por ter aceite ser meu orientador e pela prontidão com que me ajudou sempre que necessário.
Aos meus amigos próximos e de sempre, por todos os momentos ao longo deste ano e não só. Fábio, obrigado por me levantares sempre a moral. À Rita E. e à Ana, obrigado pela hospitalidade em terras de sua majestade e em Amsterdão. Ritinha, obrigado por todo o apoio (às vezes diário), pela força, e pelo sorriso.
Last but not least, I am also grateful to Dr. Juliette Azimzadeh and to Dr. Gohta Goshima for useful work discussions.
List of Abbreviations
% - Percentageµg - micrograms µl - microliters µm - micrometers
APC/C – Anaphase promoting complex/ cyclosome ATP – Adenosin triphosphate
Asl - Asterless
BLAST – Basic local alignment search tool Bld – Bald
Bp – base pairs
Bsg25D – Blastoderm specific gene 25D CAK – Cdk Activating Kinase
CCRDB – Cell Cycle Regulation Lab Database Cdc – Cell division cycle
Cdk – Cyclin dependent kinase cDNA – Complementary DNA Cep – Centrosomal protein
chTOG – Colon and hepatic tumor overexpressed gene CKI – CDK Inhibitor
CKIIα – Casein kinase II α subunit CNN - Centrosomin
CP – Centrosomal protein Cp1 – Cystein proteinase 1
CPAP – Centrosomal protein 4.1-associated protein Dmel – Drosophila melanogaster
DNA – Deoxyribonucleic acid
D-PLP – Drosophila pericentrin-like protein dsRNA – Double strand RNA
D-TACC – Drosophila transforming acidic coiled coil γ-TuRC – γ-Tubulin ring complex
γ-TuSC - γ-Tubulin small complex GDP – Guanosine diphosphate GFP – Green fluorescent protein GTP - Guanosine triphosphate
Kin – Kinesin
Klp – Kinesin-like protein M – Molar
MAP – Microtubule associated protein
MCAK - Mitotic centromere-associated kinesin ml – milliliters
mM - milimolar Msps – Minispindles MT - Microtubule
MTOC – Microtubule organizing center
NACα - Nascent polypeptide associated complex protein α subunit Nek2 – NIMA related kinase 2
ng - nanograms
NIMA – Never in mitosis A nm - nanometers
PAK – p21 activated kinase PCM – Pericentriolar material PD - Pulldown
PLK – Polo-like kinase pmol - picomol
ProtA – Protein A RNA – Ribonucleic acid RNAi – RNA interference
RT-PCR – Reverse transcriptase polymerase chain reaction SAK – Snk/Plk-akin kinase
SAS – Spindle assembly SCF – Skp1, cullin, F-box SPB – Spindle pole body SPD-2 – Spindle defective 2 TAP- Tandem affinity purification
XKCM1 – Xenopus kinesin catastrophe modulator 1 XMAP215 – Xenopus microtubule associated protein 215 YB-1 – Y-Box binding protein 1
Yps – Ypsilon schachtel ZYG-1 – Zygote defective 1
Index
1. Introduction ... 1
1.1. The Cell cycle ... 1
1.1.1. Phases and structure ... 1
1.1.2. The Cell cycle control machinery ... 2
1.2. Cytoskeleton ... 3
1.2.1. Microtubules ... 3
1.3. Centrosome ... 4
1.3.1. Structure and Composition... 4
1.3.2. Centrosome and human disease ... 6
1.3.3. Centrosome cycle ... 6
1.3.4. Centriole assembly module - canonical and de novo biogenesis .. 7
1.4. Drosophila as a model organism ... 9
1.5. Objectives ... 10
2. Materials and Methods ... 10
2.1. Bioinformatics ... 10
2.2. Tissue culture ... 11
2.2.1. Transient plasmid transfections and Stable cell lines ... 11
2.2.2. RNAi in S2 cells ... 11
2.3. Cytology and Microscopy ... 12
2.3.1. Immunostainings of S2 cells ... 12
2.3.2. Image acquisition and centrosome scoring ... 12
2.3.3. Flow cytometry ... 12
2.4. Molecular Biology... 13
2.4.1. Standard procedures ... 13
2.4.2. Agarose gel electrophoresis ... 13
2.4.3. Restriction analysis ... 13
2.4.4. Plasmid DNA extraction ... 13
2.4.5. RNA extraction and RT-PCR ... 13
2.4.6. DNA extraction from agarose gel ... 13
2.4.7. Cloning of Yps using the Gateway system ... 14
2.5. Biochemistry ... 14
2.5.1. Western Blot ... 14
2.5.2. Co-Immunoprecipitation ... 14
3. Results ... 15
3.1. The RNAi Screen ... 15
3.2. Msps phenotype and MT-depolymerases ... 23
3.3 Cloning Msps and Yps ... 24
4. Discussion ... 27
5. Conclusion and future perspectives ... 30
6. Bibliography ... 31
7. Annex ... 38
7.1. Methods ... 38
List of Figures
Figure 1.1. Centriole duplication cycle and cell cycle………..…...8
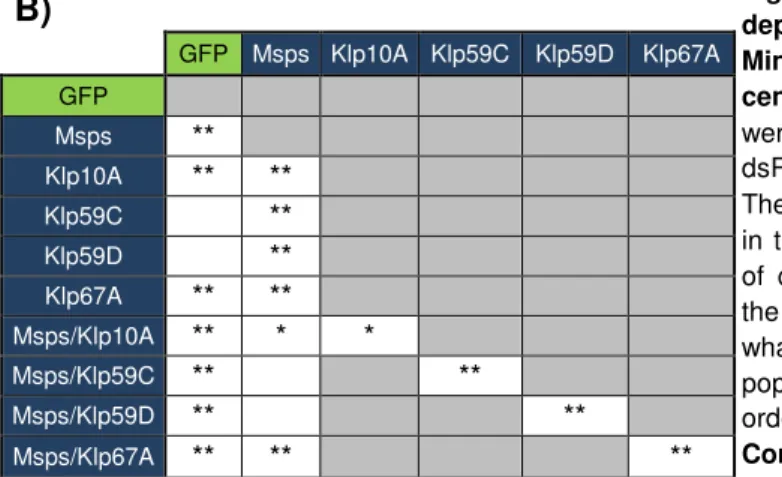
Figure 3.1. DSAS-6, Msps and Yps depletion give rise to centrosome number abnormalities in S2 cells, while depletion of GFP and Centrobin do not………...19
Figure 3.2. RNAi screen transfection II centrosome count - Msps and Yps show an
increase in cells with 0 centrosomes……….…...20
Figure 3.3. Flow cytometry results - Msps, but not Yps, has a phenotype in cell cycle profile……….…….…..21
Figure 3.4. Msps depletion is not rescued by co-depletion of MT depolymerizing kinesins ……….……..23
Figure 3.5. Yps expression vectors produce proteins with the expected size in S2 cells transient transfections………..25
Figure 3.6. GFP-Yps and Yps-GFP localize to the cytoplasm and interphasic nucleus, but are negative at the centrosome……….……25
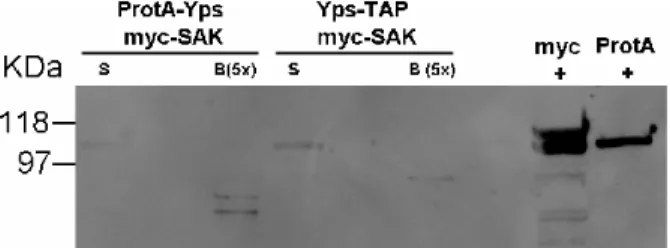
Figure 3.7. Myc-SAK is detected as a faint band in the supernatant but not on the beads samples for Yps-Protein A and Protein A-Yps with myc-SAK cell lines. .…….26
Annex
Figure 7.1. SAK and centrosomal proteins interaction network……….……....40
Figure 7.2. SAK and candidates interaction network……….……….….40
Figure 7.3. Pentry-Yps and P-entryYpsSTOP schematic view……….…...41
Figure 7.4. Pentry-Yps and P-entryYpsSTOP restriction analysis reveals clones with the expected band sizes……….………...…41
Figure 7.5. pMT-GFP-Yps and pMT-Yps-GFP schematic view………....….42
Figure 7.6. pMT-GFP-Yps and pMT-Yps-GFP restriction analysis reveals clones with the expected band sizes……….………...…42
List of Tables
Table 2.1. Immunostaining primary antibodies……….…………12
Table 3.1. The chosen candidates for the RNAi screen……….….16
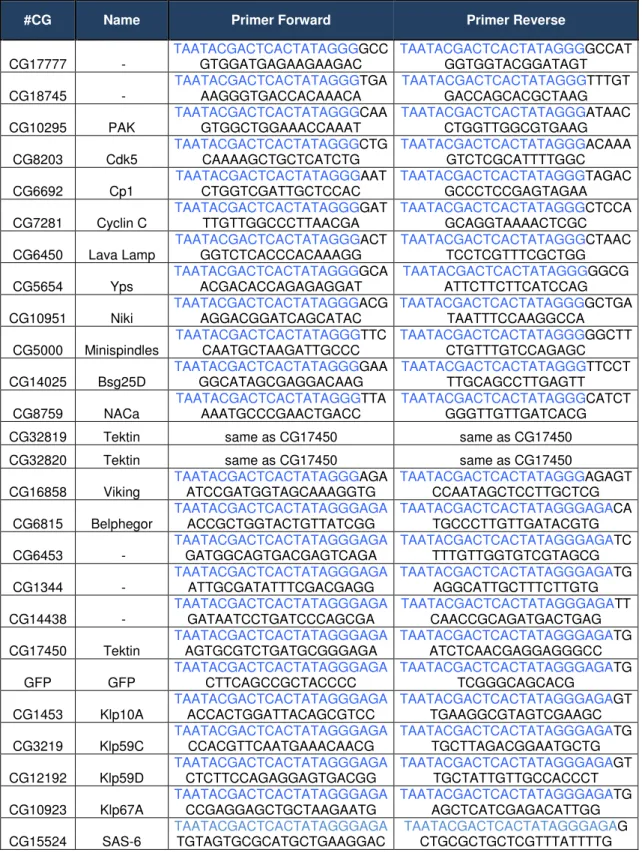
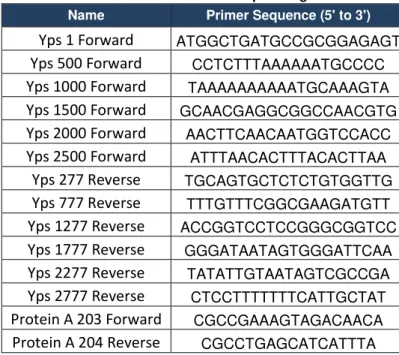
Table 7.1. Primers for producing dsRNA……….………..38
Table 7.2. Primers to confirm knock down……….………39
Table 7.3. Primers for cloning……….……….39
1
1. Introduction
1.1. The Cell cycle
The eukaryotic cell division is a process in which two daughter cells normally receive all the genetic information from their predecessor. In order for this to occur, the DNA must be duplicated before it is equally segregated between the two daughter cells. These two processes take place in a sequence of four ordered phases called the eukaryotic cell cycle.
1.1.1. Phases and structure
In the beginning of a new cell cycle, a gap-phase called G1-phase is associated with cell growth. At this stage, cells become committed to either proceed through the cell cycle or to become quiescent, in a non-dividing state, called G0. In
S-phase, DNA is replicated, so that each chromosome will have two equal sister-chromatids. A licensing mechanism guarantees that the DNA is replicated only once per cell cycle. After S-phase and before the onset of M-phase there is another gap-phase named G2-gap-phase, which comprises additional cell growth and synthesis of regulatory proteins [2].
When cells enter M-phase, great structural and organizational changes take place, which lead to the division of this phase into six subphases: prophase, prometaphase, metaphase, anaphase, telophase, and cytokinesis. In prophase, chromosomes condense and the cytoskeleton reorganizes, the mitotic spindle begins to assemble as its two future poles begin to move apart from each other, close to the nuclear envelope. In prometaphase microtubules (MTs) emanating from each spindle pole are captured and attach to the sister kinetochore facing that pole, a protein-based structure that localizes at the centromeric region of each sister-chromatid. When a chromosome is stably attached to these MTs, it goes to the middle of the spindle, and cells enter metaphase. All chromosomes converge on the metaphase plate, oscillating at midway between the two poles. Anaphase is triggered by loss of cohesion between sister-chromatids, which leads to their segregation towards the poles (anaphase A), which then move apart (anaphase B). In Telophase sister-chromatids cluster near the spindle poles, nuclear envelope re-forms, and there is constriction of a cleavage furrow. Cytokinesis is the cytoplasmatic division, when animal cells are separated by a contractile ring, which forms the cleavage furrow and causes cell abscission. Altogether, the G1-, S- and G2-phases compose a period
2 generally called interphase, while the M-phase is generally called mitosis or meiosis
[2-4].
The cell cycle structure can vary: a human cell cycle takes about 18 hours, while Drosophila melanogaster early embryos have rapid cell cycles (8 minutes) with no gap-phases nor cytokinesis in the first 13 rounds of nuclear division. Multiple rounds of S phase or M-phase in only one cell cycle can occur in specialized cell types. The meiotic cell cycle includes two rounds of chromosome segregation after only one round of DNA replication, giving rise to haploid cells, withonly one homolog from each pair [4].
1.1.2. The Cell cycle control machinery
In order for the cell cycle to be unidirectional, it needs to be tightly controlled and regulated. The central controllers of the cell cycle engine are a family of serine/threonine kinases called the cyclin-dependent kinases (Cdks). Cdks phosphorylate several proteins involved in the control of cell cycle events, changing their state of activation. Cdk activity is regulated by binding of different types of cyclins, whose levels change in each cell cycle phase, causing abrupt oscillations of Cdk activity [2, 5]. Different cyclin-Cdk complexes promote the events that happen in
each cell cycle phase, in an ordered way. Some Cdks can be further regulated by CAKs (Cdk-activating kinases), by CKIs (Cdk inhibitors), or by inhibitory phosphorylations [2].
The central cell cycle functions are regulated by two Cdks, called Cdk1 and Cdk2. Cdk2 forms a complex with cyclin E that regulates the G1/S transition. In S-phase, Cdk2 associates with cyclin E and later with cyclin A, and phosphorylates proteins required to initiate and perpetrate DNA replication [2]. Cdk1-cyclin B is the
complex that primarily regulates G2/M transition in animal cells. Its activation allows a sudden and irreversible entry into mitosis as it promotes the condensation of chromatin, and it can phosphorylate its own regulators [4, 6], creating a positive
feedback loop for its own activity.
Other Cdks complexes in animal cells, such as Cdk4 and Cdk6 with Cyclin D, function mainly in G1 and help to coordinate cell growth and entry into a new cycle, [7].
The activation/inactivation of cyclin-Cdk complexes is also achieved through control of gene expression [2] and through controlled proteolitic degradation in the 26S
proteasome, via addition of a polyubiquitin chain to cyclins. The ubiquitination machinery includes the SCF complex (Skp1, Cullin, F-box), which functions mostly in G1/S and S phases and recognizes specific phosphorylated motifs on target proteins
3 and the APC/C – Anaphase Promoting Complex/Cyclosome. The APC/C is controlled by different activator subunits at different stages of the cell cycle [8-10]. The first of APC/C activators is Cdc20 (Fizzy in Drosophila), that functions in the metaphase to anphase transition. The second activator, Cdh1 (Fizzy-related in Drosophila), binds to the APC/C in mitotic exit and early G1 [2, 10, 11].
Although Cdks are the master regulators of cell cycle, other protein kinases such as the Plk (Polo-like-kinase) family and Aurora family also regulate cell cycle events. Plks are serine/threonine kinases with a Polo-Box Domain (PBD), which usually recognizes motifs phosphorylated by other kinases,. Phosphorylation of the T-loop of Plks enhances their activity, although the upstream activating kinases are still largely unknown [12]. Plk1 is activated early in mitosis, and in animals it is required for
the assembly of a bipolar spindle, kinetochore function, cytokinesis, and centrosome separation. In mammals, Plk2 localizes near the centrosomes and seems to function in S-phase, while Plk3 localizes to the nucleolus. The known functions of Plk4 will be addressed below, and further in the Results and Discussion sections of my thesis [12-16].
Aurora kinase family includes Aurora A and B (and C, in mammals). Aurora A regulates bipolar spindle assembly and stability, and centrosome separation after the mitotic spindle has been formed [17, 18]. Aurora B helps controlling sister-chromatid
structure and segregation, and it is also known to be involved in cytokinesis [16, 19, 20]
Many other kinases [21] and regulatory proteins cooperate to build the cell cycle engine.
1.2. Cytoskeleton
The eukaryotic cytoskeleton comprises three types of protein filaments with different properties – actin microfilaments, intermediate filaments, and MTs. Altogether, these filaments are essential for cell shape, migration and motility, as well as for the maintenance of internal processes such as vesicle trafficking and organelle positioning. Since the work of my thesis is related with MTs, I will address MT cytoskeleton with more detail.
1.2.1. Microtubules
MTs are hollow cylinders formed by 13 polarized protofilaments of α-tubulin and β-tubulin heterodimers [22, 23]. MTs minus ends have the α-tubulin molecules
exposed and grow slower, while the plus ends terminate in β-tubulin and grow faster
4 nucleation process is dependent on another tubulin isoform, γ-tubulin, which exists in the γ-tubulin Small Complex (γ-TuSC). This complex contains two γ-tubulin molecules and one molecule each of DGrip84 and Dgrip91 in Drosophila. About four other proteins form a ring that holds γ-TuSCs together, forming the γ-tubulin ring complex (γ-TuRC) [24-26].
MT growth is dependent on GTP hydrolysis by tubulin. GTP-tubulin addition occurs at the MT ends, forming a GTP-cap, while GTP hydrolysis occurs mostly in GTP-tubulin trapped in the MT. Variations in the ratio of these two processes give rise to alternating periods of MT growth, also known as rescue, and MT shrinkage, also called catastrophe [23, 27, 28]. This behavior known as dynamic instability is essential for
MT cytoskeleton remodeling throughout the cell cycle. In interphase, MTs are long and form a complex network that spreads throughout the cytoplasm. In M-phase there is more nucleation at the centrosome, and MTs become shorter and highly dynamic
[22].
MT dynamics are also regulated by the activity of MT-associated proteins (MAPs). These include MT severing proteins, end-binding proteins, motor proteins that transport cargo along the MTs (kinesins, dyneins), and mostly MT stability regulators, such as tektins and the Tau family [29]. The Dis1/TOG protein family -
XMAP215 in Xenopus, Minispindles (Msps) in Drosophila melanogaster, TOGp in humans - associates with growing MT plus ends, and these proteins seem to act both as MT stabilizers and destabilizers. In Drosophila, Msps has polymerase activity at the plus ends of interphase MTs [30, 31]. Other MAPs act as MT destabilizers,
promoting catastrophe or actively removing tubulin dimmers from the ends. The latter activity is associated with the family 13 of the kinesin-related proteins [32].
1.3. Centrosome
Spontaneous MT nucleation in the cellular context is highly unfavorable. In a cell, MTs are generally nucleated and organized from a primary MT-organizing centre (MTOC)[33], such as the spindle pole body (SPB) in budding yeast, or the centrosome
(first described in the late 19th century by Boveri and Van Beneden) in animal cells. In
interphase centrosomes localize close to the nucleus, being associated with cell motility, adhesion, and polarity, while in mitosis, they organize the spindle poles [34].
1.3.1. Structure and Composition
The centrosome is composed of a pair of centrioles embedded in a dense protein matrix called pericentriolar material (PCM). Centrioles are MT-based,
barrel-5 shaped organelles, approximately 200nm in diameter and 500nm in length [35]. The
centriole proximal end (with MT plus-ends) has 9 MT triplets, disposed in a radial symmetry, where each triplet is formed by a complete A-tubule and two partial tubules, B and C. The distal end lacks the C tubule and has MT doublets [34].
The two centrioles are generally orthogonal to each other, where one is the mother centriole, with distal and subdistal appendages, while the younger one is called the daughter centriole. The mother centriole appendages can dock this centriole to the cytoplasmic membrane, forming a basal body [36]. Basal bodies can
template the growth of axonemes of cilia and flagella, organelles that are involved in development, reception of sensory stimuli, and cell motility. The basal body structure is very similar to the one of the centrosomal centriole, and the first studies on its architecture were performed in basal bodies of Tetrahymena, Chlamydomonas, Paramecium, and Trypanosoma [37, 38].
Some of these studies revealed the existence a cartwheel structure at the proximal end of the basal body [39, 40].The cartwheel was later identified in Drosophila
and mammalian ciliated cells, at the base from which the new centriole elongates. It is constituted by a central hub and nine radial spokes that connect to the A-tubule of the triplets, imposing the nine-fold symmetry [41-43] Although the nine-fold symmetry is
conserved, centriole architecture can vary in the eukaryotic tree of life - Drosophila melanogaster has MT doublets instead of triplets (except in the sperm) and Caenorhabditis elegans has MT singlets [34]. Centriole components include polyglutamylated α and β-tubulin, tektins [44, 45], and the centriole lumen contains
γ-tubulin and several isotypes of centrins, which are also present in the intercentriolar links [46].
The PCM is composed of more than 300 types of proteins, mostly coiled-coiled, from the pericentrin/kendrin and AKAP450/350 families [47, 48]. Many of these
proteins are involved in centriole duplication or MT nucleation through recruitment of γ-TuRCs. Nucleated MTs can anchor to the subdistal appendages, through ninein and centriolin, or to the PCM, through dynein-dynactin complexes [16, 34, 49].
The centrosome is also involved in cell cycle regulation, anchoring a great number of regulatory proteins. Plk1 localizes at the centrosome, and is required for recruitment of γ-tubulin complexes to increase MT nucleation in mitotic centrosomes
[16]. Aurora A and B also localize at the centrosome [50-52]. Centrosomes themselves
are not essential for G1/S transition in human cells [53, 54] neither in Drosophila[55, 56],
and although Cdk1-cyclin B localize to the centrosome, the absence of centrosomes does not impair entry into mitosis [55, 57, 58].
6
1.3.2. Centrosome and human disease
The majority of cells have either one or two centrosomes, depending on the cell cycle phase. This seems to be dependent on mechanisms that ensure that the centrosome duplicates only once per cell cycle and that only one daughter-centriole can be formed per mother-centriole [34]. The fulfillment of these two rules is crucial in
order to avoid excess of centrosomes, which can lead to genomic instability, aneuploidy, and misregulation of asymmetrical cell division. Increased centrosome numbers have been detected in early stages of tumorigenesis in several human cancers [34, 59]. Although in many cases it is not clear whether this is a cause or a
consequence of the neoplasic condition, numerical, structural and positional centrosome abnormalities have been detected in breast cancer [60] and others [59].
Misregulation of kinases such as Plk1, Nek2 and Aurora A are often linked to such anomalies in cancer [61, 62]. Other centrosome-related diseases such as mycrocephaly
and dwarfism have been associated with mutations in pericentrin and other centrosomal proteins [63]. Defects in ciliogenesis can also lead to many diseases such
as retinal degeneration, polycystic kidney disease, and infertility [64, 65]. It is therefore
very important to understand the mechanisms governing centriole/centrosome biogenesis and duplication.
1.3.3. Centrosome cycle
In most animal species, upon fertilization, the sperm brings one or two centrioles which combine with maternal proteins to form the first centrosome. This centrosome must duplicate so that in the first embryonic division each daughter cell inherits one centrosome. Centrioles confer to the centrosome its ability to duplicate, through a process called canonical centriole duplication cycle, which occurs in the presence of a mother centriole [66, 67].
At the end of mitosis/beginning of G1, centrioles disengage and lose their orthogonal position. This disengagement depends on separase and may be involved in exposing sites where a new centriole can form [68]. In S phase, a procentriole
(daughter centriole) starts to appear at right angles to each mother. Elongation of these procentrioles occurs during S and G2 phases, until the onset of mitosis. In late G2, centrosomes maturate by recruiting additional PCM proteins, and in the G2/M transition the two centrosomes separate and start to migrate to opposite cell poles, so that at the end of mitosis each daughter cell receives one centrosome [34, 67, 69].
The coordination of DNA replication and centrosome duplication cycles was thought to occur via Cdk2-cyclin E. However it was shown that centrioles can
7 duplicate, but not re-duplicate, in the absence of Cdk2 [70]. Cdk1, geminin, and the
SCF complex prevent DNA re-replication and centrosome overduplication, coupling the regulation of both cycles [34, 71, 72]. Downregulation of Cdk1-cyclin B in Drosophila
somatic cells was shown to promote the formation of several daughter centrioles near the same mother-centriole [73]. Besides these major cell cycle regulators, there are
other proteins required for the canonical centriole formation, which I will address in the next section.
1.3.4. Centriole assembly module - canonical and de novo biogenesis
A variety of proteins were discovered in several studies performed in C. elegans embryos [74]. A kinase named ZYG-1 (zygote defective 1) was shown to trigger the formation of the procentriole by recruiting a complex of two coiled-coil proteins, SAS-6 (spindle assembly 6) and SAS-5 (spindle assembly 5). These proteins are required for the formation and elongation of a central tube, while SAS-4 (spindle assembly 4) is recruited to assemble the nine MT singlets around that tube
[75, 76]. SPD-2 (spindle defective 2) is the first protein of this assembly module [77]. It is
phosphorylated by Cdk2 and it recruits ZYG-1 to the sperm centrioles upon fertilization [78].
Homologues of these molecules (except for SAS-5) have also been found in Drosophila and humans. In Drosophila, one of the master regulators of centriole duplication is SAK/Plk4 [55, 79], the functional homologue of ZYG-1. This kinase
belongs to the Plk family, and it is known that in Drosophila Plk1 and Plk4 are both required for centrosome integrity [21]. SAK localizes at the centrosome, and it was
shown that both in a Drosophila hypomorphic SAK/Plk4 mutant, as well as in SAK-depleted S2 cells, there is loss of centrioles [55]. In agreement, depletion of SAK/Plk4
in human tissue culture cells also impaired centriole duplication [79]. SAK recruits
DSAS-6, which is required for centriole duplication, cohesion, and for the establishment of the nine-fold symmetry, through formation of the cartwheel-like structure[80]. In Chlamydomonas reinhardtii, the human Cep135 homolog Bld10
localizes to the cartwheel in early stages of basal body assembly [81]. In Drosophila,
DBld10 is a centriolar protein that also localizes to the spermatid basal body, and mutants that lack this protein have shorter centrioles and basal bodies. [82]. DSAS-4 is
required for centriole duplication, as null mutants of DSAS-4 lack centrioles [56],and its
human homolog, CPAP/CENPJ is also necessary for centrioles to duplicate [83].
DSPD-2 is only required for PCM recruitment in centrosome maturation, and for basal body duplication [84, 85].
Other PCM and centriolar proteins,
Cep152) were shown to play early roles in PCM recruitment and centriole duplication
[86-88]. A Cdk2 substrate, CP110
regulating the length of the new centriole
identified in two screens as being required for centriole duplication in tissue culture cells [92, 93].
Centriole biogenesis and
cycle. However, centrioles can also assemble through a novo centriole biogenesis.
generated centrioles, and is inhibited by
some insect species that have parthenogenic development, in vertebrate somatic cells [94], and also in HeLa cells wher
novo formation of a new centriole starts with small centrin agglomerates in S that give rise to a complete centriole
In Drosophila, accumulation of biogenesis in unfertilized eggs
overduplication in embryos, leading to an excess of structurally normal centrosomes that caused developmental arrest
to be tightly regulated, since its absence leads to a failure in centriole duplication,
will only occur in the next S-phase. The time of activity of differ
replication occurs in S-phase, and chromosomes condense upon entry into mitosis, being aligned in the center of the spindle in metaphase. Completion of mitosis generates both centrosome and chromosomes segregatio between the two daughter cells. Adapted from
PCM and centriolar proteins, γ-tubulin and Asterless (
Cep152) were shown to play early roles in PCM recruitment and centriole duplication A Cdk2 substrate, CP110 [89], is essential for capping the centriole and
regulating the length of the new centriole [90, 91]. Ana-1, Ana-2 and Ana
screens as being required for centriole duplication in
biogenesis and duplication are usually occurring in
cycle. However, centrioles can also assemble through another pathway called This pathway does not impose a control on the number of , and is inhibited by a pre-existing centriole [34, 53]
some insect species that have parthenogenic development, in vertebrate somatic nd also in HeLa cells where centrosomes were laser-ablated
formation of a new centriole starts with small centrin agglomerates in S that give rise to a complete centriole before M-phase, which can recruit the PCM
accumulation of SAK/Plk4 was able to induce
zed eggs. When overexpressed, SAK induced centrosome n in embryos, leading to an excess of structurally normal centrosomes that caused developmental arrest[95, 96]. Therefore, SAK/Plk4 levels and activity need
to be tightly regulated, since its absence leads to a failure in centriole duplication,
phase. The time of activity of different Cdk complexes is also shown. Chromosome phase, and chromosomes condense upon entry into mitosis, being aligned in the center of the spindle in metaphase. Completion of mitosis generates both centrosome and chromosomes segregatio between the two daughter cells. Adapted from [1].
8 Asterless (Asl; human Cep152) were shown to play early roles in PCM recruitment and centriole duplication , is essential for capping the centriole and 2 and Ana-3 were screens as being required for centriole duplication in Drosophila
are usually occurring in the canonical pathway called de impose a control on the number of
[34, 53]. It occurs in
some insect species that have parthenogenic development, in vertebrate somatic ablated [53]. The de
formation of a new centriole starts with small centrin agglomerates in S-phase, phase, which can recruit the PCM[53].
4 was able to induce de novo . When overexpressed, SAK induced centrosome n in embryos, leading to an excess of structurally normal centrosomes 4 levels and activity need to be tightly regulated, since its absence leads to a failure in centriole duplication,
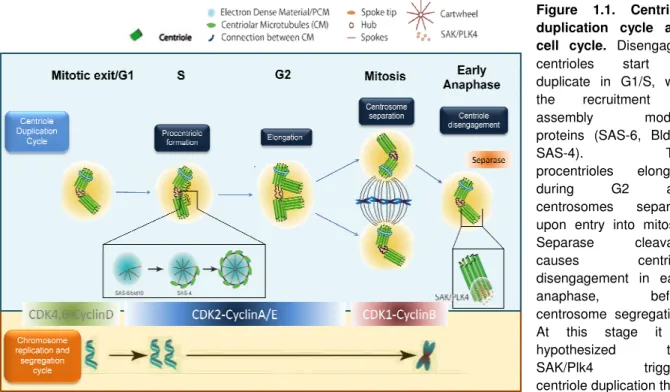
Figure 1.1. Centriole duplication cycle and cell cycle. Disengaged
centrioles start to duplicate in G1/S, with the recruitment of assembly module proteins (SAS-6, Bld10, SAS-4). The procentrioles elongate during G2 and centrosomes separate
upon entry into mitosis.
Separase cleavage causes centriole disengagement in early anaphase, before centrosome segregation. At this stage it is hypothesized that SAK/Plk4 triggers
centriole duplication that
ent Cdk complexes is also shown. Chromosome phase, and chromosomes condense upon entry into mitosis, being aligned in the center of the spindle in metaphase. Completion of mitosis generates both centrosome and chromosomes segregation
9 while its overexpression leads to centriole overduplication. This regulation seems to be cell-type dependent, as SAK levels are higher in epithelial multiciliated cells where multiple centrioles are formed next to each mother-centriole (originating flower-like structures) [97]. Moreover, Plk4 mRNA levels seem to be cell-cycle-regulated. Indeed,
in mouse cells, Plk4 mRNA levels start to increase in S-phase and peak in M-phase, decreasing to a minimum in G1 [98]. If protein levels follow mRNA levels, then in
M-phase this would allow SAK to activate molecules that would only be required for centriole duplication in the next S-phase. It would also avoid early duplication in G1, when SAK is not present. In fact, it has been suggested that SAK is degraded in interphase by the SCF/Slimb complex, being detected at the centrosomes only in mitosis [99, 100]. Drosophila tissue culture cells expressing a non-degradable form of
SAK, or depleted of Slimb show the previously mentioned flower-like structures and centriole amplification [100].
In summary, misregulation of SAK/Plk4 levels and activity is often associated with tumorigenesis and found in different cancer tissues, correlating with abnormalities in centrosome number and structure [101]. This makes it extremely
important to know which proteins are regulating SAK activity, and which proteins are regulated by SAK. This knowledge can contribute to the development of new forms of diagnosis, prognosis, therapeutical molecules, and new targets for the therapy of cancers and other centrosome and SAK-related diseases.
1.4. Drosophila as a model organism
All eukaryotes employ similar machinery to regulate cell division. Drosophila was among the first organisms used as model for genetic analysis. Today it is one of the most widely-used and well characterized organisms, and its genome has been sequenced and published in 2000 [102].
The advantages of D. melanogaster include the low cost, little space, equipment, and maintenance that its cultures require, its short generation time and high fecundity, amongst others. Additionally, it offers a wide stock of mutants that can be crossed thanks to the use of chromosome balancers that carry phenotypic markers [103, 104]. The use of D. melanogaster in cell cycle research is well known,
since the original alleles of Polo and Aurora were identified in this organism [105, 106].
The most common developmental stages used in centrosome studies include early embryogenesis, spermatogenesis, and oogenesis. Many kinds of studies and screens are also conducted in cultured cell lines, such as Schneider (S2) cells derived from late stage embryos [107]. Because these cells easily incorporate dsRNA, they have
10 been widely used in RNAi screens, spanning mitotic spindle assembly [92], centriole
duplication [93], cell cycle kinases [21]. Therefore, it is possible to study protein(s)
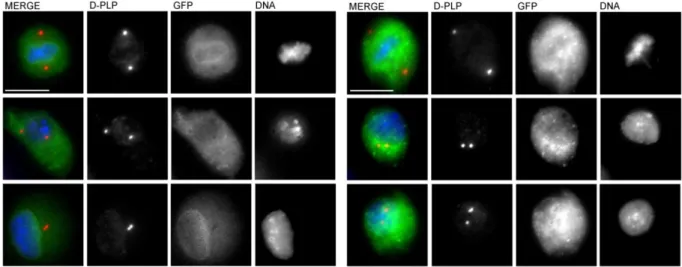
depletion effect on the centrosome, by coupling RNAi with immunofluorescence against a centrosomal marker, such as Pericentrin-Like-Protein (D-PLP).
1.5. Objectives
The objective of the work described in my thesis was to gain a better understanding of the cellular mechanisms that control centriole biogenesis and number. In this context, this work comprised the identification of novel SAK interactors/regulators and characterization of their role in centriole/centrosome duplication. There was data already available from immunoprecipitation assays (pulldowns) done in our lab (and others), as well as other published data, such as two-hybrid experiments, from where I could start to choose candidates for SAK interactors. To identify SAK interactors that participate in centriole duplication, the candidates were further screened through RNAi to check whether their depletion altered centrosome number.
2. Materials and Methods
2.1. Bioinformatics
The Cell Cycle Regulation Lab Database (CCRDB) was created by Jorge Beira, a student that worked at the CCR Lab. This database has information on cell cycle and centrosome-related genes, from D. melanogaster, Homo sapiens, Tetrahymena termophila, and other species.
The information is stored in specific tables: Homologs table, Localizations table, Interactions table, and other tables with the data from the pulldown assays. The Interactions table information sources include pulldowns from our lab and others, two-hybrid experiments, and online databases (such as BioGRID). After importing new data into the CCRDB in order to update it, I performed queries for genes that fulfilled certain criteria:
- SAK interactors that interacted at least with one of these proteins: DSAS-6, DSAS-4, Asterless, Slimb, DBld10, DSPD-2, Ana1, Ana2, γ-tubulin 23C, PAK, Cyclin C, Cdk5, Tektins A, C and others (CG17450, CG32819, CG32820), CG17777 and CG18745. This short list includes centrosome-related proteins,
11 cell cycle related proteins, and the latter five (referred as their #CG) were interactors of both SAK and some of the previously mentioned proteins;
- SAK interactors that also localized to the centrosome or basal body; - Appearing in several SAK pull downs with high scores (>100);
With the results from the queries I built a network with these interactors (see
Annex - figures 7.1 and 7.2), by importing this data into Cytoscape®, a program which allows the user to create interaction networks.
2.2. Tissue culture
2.2.1. Transient plasmid transfections and Stable cell lines
Drosophila Schneider 2 (S2) cells were cultured in Exp.5 Serum Free Media (Gibco) supplemented with Penicillin/Streptomycin/Glutamine (1/10) (Invitrogen) at 25°C. Plasmid transfection into the cells was done with 15µl of FuGENE HD (Roche) and 3µg of plasmid DNA + 0.3µg of Picoblast plasmid, in 3x106 cells, in 6-well plates.
Transient transfections were induced with 200µM CuSO4 in the next day and cells
were harvested 24 hours after either for immunostaining or for protein extraction. Cells were selected for stable cell lines with Blasticidin (Invitrogen) at 30µg/ml in the medium in the first 15 days and 20µg/ml after. For the immunostainings of GFP-Yps and Yps-GFP stable cell lines, 3x106 cells were induced with 200µM CuSO
4
for 15 hours. For the co-immunoprecipitation, 1x108 cells were induced with 200µM
CuSO4 for 15 hours.
2.2.2. RNAi in S2 cells
RNAi in S2 cells and production of dsRNA was performed as described in [108].
The primers were designed using SNAPDRAGON, in order to obtain gene-specific primers, targeting exons, and with the lowest off-target effect possible (see primer list in Annex - tables 7.1 to 7.4.). dsRNA fragments were between 100 and 500bp, and for each transfection I used 40µg of dsRNA in 2x106 cells. For double transfections I
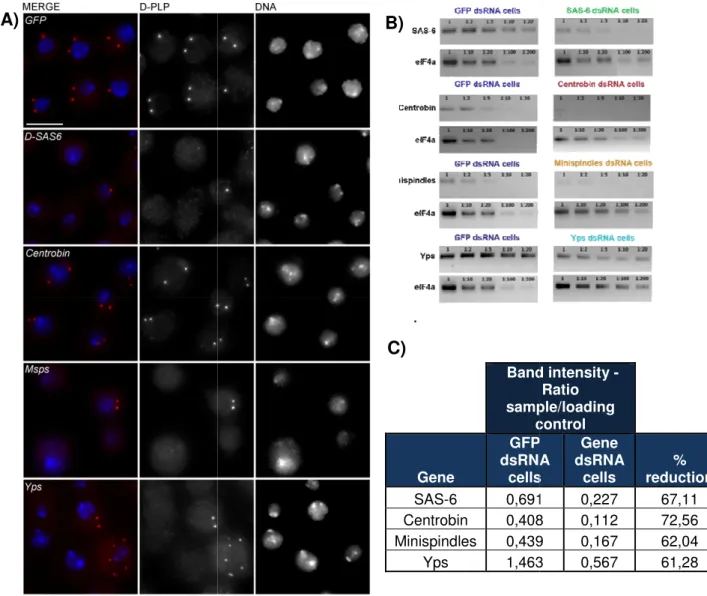
used 30ug of each dsRNA, with 30µg of GFP dsRNA in the single transfections. Each RNAi experiment included a negative control (dsRNA for GFP) and a positive control (dsRNA for DSAS-6). Retransfections were performed 3 days after the first transfection, using 1ml of cells. 3 days later, cells were harvested and fixed to perform immunostainings, and the centrosome number was scored.
12
2.3. Cytology and Microscopy
2.3.1. Immunostainings of S2 cells
Immunostaining of Drosophila tissue culture cells was performed as described in [21]. Cells were left to attach to coverslips for 1 hour and then fixed in 4 %
Formaldehyde with 0.06 M PIPES (pH 6.8), 0.03 M HEPES (pH 7), 0.01 M EGTA (pH 6.8) and 4 mM MgSO4 for 10 minutes at room temperature. After two washes with
Phosphate-buffered saline (PBS) (10 mM Na2HPO4, 1.8 mM KH2PO4, 137 mM, NaCl,
2.7 mM KCl (pH 7.4)) cells were permeabilised and blocked in for 1 hour in PBS with 0,1% Triton X and 1% BSA (PBSTB).
Primary antibodies were diluted in PBSTB and cells were incubated overnight at 4 ºC in the dark in a humid container. After washing the cells twice in PBSTB, they were incubated for 2 hours with secondary antibodies diluted in PBSTB at room temperature, in the dark in a humid container. Cells were washed in PBSTB and then in PBS, and the coverslips were mounted in slides with Vectashield mounting medium with DAPI. The list of primary antibodies is displayed below:
Primary antibody Dilution Supplier/Reference
Chicken anti-D-PLP 1:1000 Bettencourt-Dias et al., 2005
Rat anti-α-tubulin (YL1/2) 1:50 Oxford Biosciences
Rabbit anti-SAK (1050) 1:10 Rodrigues-Martins, 2009
Secondary antibodies were conjugated with Rhodamine Redex (Jackson Immunochemicals), FITC (Sigma), or Horse-Radish-Peroxidase (Jackson Immunochemicals). A TSA (Tyramide Signal Amplification) kit (Molecular probes) was used to amplify the signal from SAK antibody.
2.3.2. Image acquisition and centrosome scoring
Cells were observed and centrosomes scored by using a Leica DMRA2 microscope. Images were acquired with a Photometrics Cool SNAP HQ camera and analysed on Metamorph®. Panels were assembled with Adobe Photoshop CS2 ®.
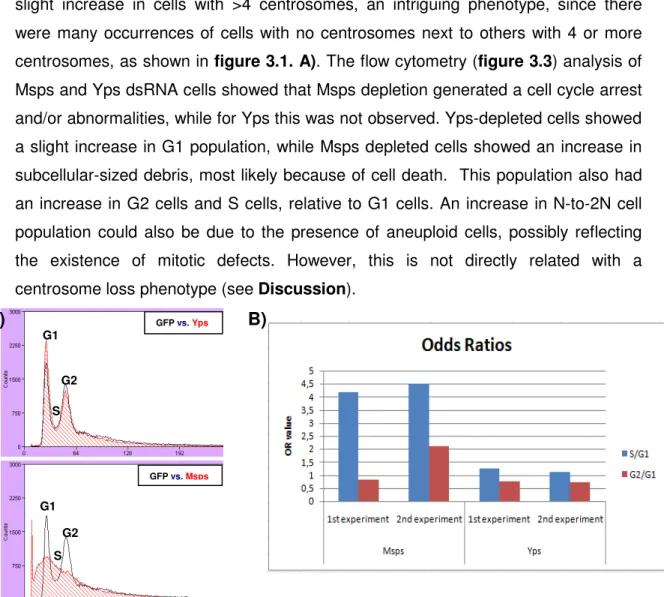
2.3.3. Flow cytometry
I used 1ml of dsRNA-treated cells for flow cytometry. These cells were centrifuged at 1000rpm for 5 min., ressuspended in PBS (200µl) and 2ml of ice-cold 70% EtOH were added drop-by-drop while vortexing the cells. After a 30 min. incubation on ice and new centrifugation, the cells were washed twice in PBS, and
13 incubated for 30 minutes at 37ºC, in a PBS solution with 1% Propidium Iodide and 0,1% RNase A. The samples DNA content was acquired in a FACSCalibur (Becton Dickinson) cytometer, using the CellQuest software. This data was then analysed with Dako Summit v4.3® software.
2.4. Molecular Biology
2.4.1. Standard procedures
The Polymerase-Chain Reaction (PCR), agarose gel electrophoresis, restriction enzyme analysis and bacteria transformation were performed as described in [109]. Kits and other products were used according to the supplier’s protocols and
indications.
2.4.2. Agarose gel electrophoresis
DNA or RNA samples were loaded onto 1% agarose gels, and ran in Tris-acetate-EDTA (TAE): 40 mM Tris-acetate, 1 mM EDTA buffer. DNA or RNA band size was estimated using 100bp, 1kb (Promega) or low mass ladder (Invitrogen).
2.4.3. Restriction analysis
The restriction enzymes – EcoRI (BioLabs), XmaI, PstI, SmaI (Fermentas) – were used according to the supplier’s instructions for optimal activity, for no longer than 2 hours, with 1µg of DNA.
2.4.4. Plasmid DNA extraction
Plasmid DNA was extracted from bacteria either using the Wizard plus SV Miniprep Kit (Promega) for small scale or the HiSpeed Plasmid Midi kit (Qiagen) for large scale.
2.4.5. RNA extraction and RT-PCR
RNA was extracted from 1ml of dsRNA-treated cells using RNeasy Mini Kit (Qiagen) and quantitated. 1µg of each sample was used for cDNA synthesis by Reverse-Transcriptase PCR (RT-PCR) using Transcriptor First Strand cDNA Synthesis kit (Roche), with the oligo(dT)18 primers.This cDNA was used in a range of
five dilutions, as template for PCR to confirm the knock-down of a specific gene. The primers used for these PCRs are described in Annex – table 7.2..
2.4.6. DNA extraction from agarose gel
DNA bands were excised from 1% agarose gels and DNA was purified using QIAquick gel extraction (Qiagen) kit.
14
2.4.7. Cloning of Yps using the Gateway system
The expression vectors were produced using the Gateway system (Invitrogen). The gateway technology is based on the lambda phage site-specific system that allows highly efficient recombination reactions (see detail in Annex –
figures 7.3 to 7.6.)
2.4.8. Sequencing
Each sequencing reaction had 32pmol of the primer, 200ng of double-stranded DNA, and sequencing buffer and Terminator Ready Reaction Mix (Applied Biosystems), 2µl each. After the thermal cycling, samples were precipitated overnight with Sodium Acetate 3M pH 4,6 and 95% EtOH, and then centrifuged and washed in 70% EtOH on the next day. The pellets were drought and then sent for sequencing.
2.5. Biochemistry
2.5.1. Western Blot
Protein extracts from Drosophila tissue cultured cells were prepared by homogenizing the tissues in Sample Laemli buffer 1X in PBS, boiling for 10 minutes and spinning at 14.000rpm to clear the lysate. Proteins were separated using sodium dodecyl sulphate-polyacrylamide gel electrophoresis (SDS-PAGE), transferred to nitrocellulose membrane by semi-dry blotting using the Trans-Blot Semi-Dry Transfer Cell (Bio-Rad). Blocking with Tris-buffered saline (TBS) (25 mM Tris-HCl (pH 7.4), 137 mM NaCl, 2.7 mM) containing 5 % dry milk and 0.1 % Tween-20 (TBSTA) was performed for 1 hour at room temperature with shaking. Membranes were incubated with primary antibodies diluted in TBSTA (with 1% milk) for 1 hour at room temperature with shaking. After primary antibody incubation membranes were washed three times in TBSTA, for 15 minutes each, and incubated with an appropriate peroxidase-conjugated secondary antibody diluted in TBSTM for 1 hour at room temperature with shaking. Membranes were then washed in TBS and incubated with 1 mL of Enhanced ChemiLuminescence (ECL) (Amersham) reagent for 5 minutes to allow protein visualization.
2.5.2. Co-Immunoprecipitation
The Dyna beads (Dynal-Invitrogen) were conjugated with a rabbit IgG antibody for protein A fusion proteins affinity purification. After the process pMT-ProtA-Yps + Act5-myc-SAK and pMT-Yps-TAP + Act5-myc-SAK cells induction, cells were centrifuged and lysed. Cells lysis and binding to IgG coated beads was performed with EB buffer (50 mM Hepes pH 7.5, 100 mM NaAc, 50mM KCl, 2mM MgCl2, 0,1%
15 NP-40, 5mM DTT, 2mM EGTA, 5% Glycerol, Complete Protease Inhibitors EDTA-free). Antibody coated beads were incubated with lysates for 4 hours, and washed 5 times with 1 ml EB buffer (using an EB buffer with with no glycerol in the final wash). The beads protein extract was then prepared by adding Sample Buffer (1X) to the Beads, boiling for 5’ at 100ºC, and centrifuging the samples. After immunoprecipitation, protein complexes were resolved by SDSPage Polyacrilamide Gels, followed by western blot analysis. The first analysis to detect ProtA-Yps and Yps-TAP in the supernatants, pellets, washes, and beads was performed with a Horseradish Peroxidase ChromPure rabbit IgG (Jackson Immunoresearch), at 1:10000 dilution. The detection of myc-SAK in the supernatant and in the beads was made with mouse Myc antibody (Sigma) at 1:2000 dilution and mouse Horseradish Peroxidase antibody (Jackson Immunoreseach) at 1:10000 dilution.
3. Results
3.1. The RNAi Screen
In order to identify and study novel SAK interactors that could have a role in centriole duplication I conducted an RNAi screen on 24 candidates, in S2 cells. The choice of the candidates in table 3.1, amongst all that satisfied the criteria (see
Methods), was made by giving priority to the strongest SAK pulldown interactors and to cell cycle, cytoskeleton, and centriole/centrosome-related proteins. None of the screened candidates was previously shown to be involved in centriole duplication in Drosophila.
The candidates included several kinases - Cdk5, Cdk4, CG1344, Niki, PAK, CKIIα - (see table 3.1.) because SAK degradation is known to be regulated through phosphorylation [99, 100]. The phosphorylating kinases are still unknown, although PAK
(p21 activated kinase) seemed a good candidate because it is a serine/threonine kinase that is known to phosphorylate Plk1 in HeLa cells [110]. The three tektins
(CG17450, CG32819, CG32820) could be involved in centriole assembly and stability, since it is known that this type of proteins associate with centriolar/basal body MTs in several organisms [111]. CG17777 and CG18745 are interactors of at
least one of these tektins, and CG18745 is also a SAK interactor (see table 3.1). Although their functions are unknown, they could be acting as intermediates, downstream of SAK and upstream of these structural proteins, or as structural proteins themselves. Cytoskeleton-related proteins such as Minispindles, Viking, and Lava Lamp were also promising candidates, since the centrosome is the primary MTOC in the cell and centriole duplication could be related with cytoskeleton
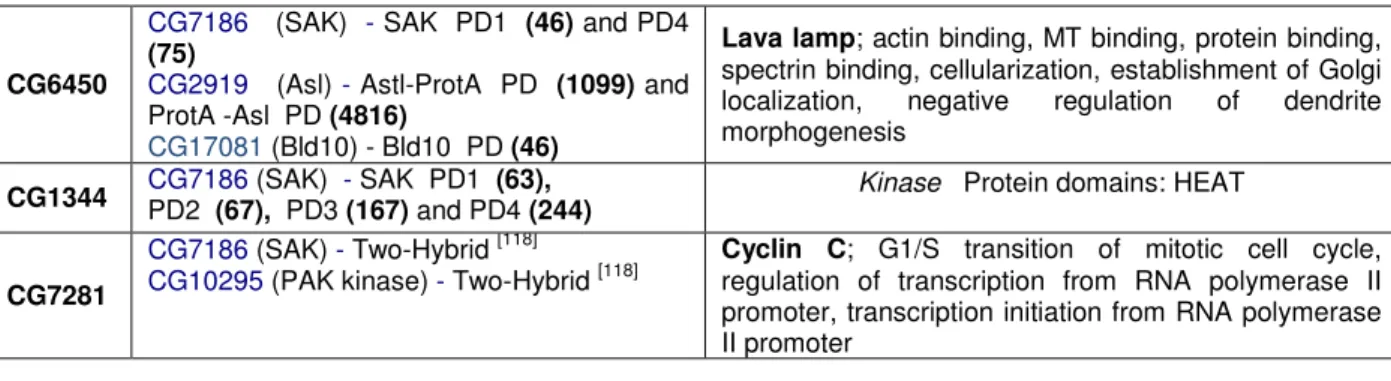
16 organization and dynamics. NACα and Furry are interacting with SAK at least in one pulldown (except for NACα, which came out only on SAS-6 pulldown). CG6450 was a strong candidate from the start, since its human homolog, Centrobin/NIP2, was shown to be required for centriole duplication and MT stability, and interacts with Nek2, a centrosomal kinase. In human cells, Centrobin localizes to the daughter centriole, and its depletion through RNAi lead to centriole loss [112, 113]. It was also
shown to be required for spermatogenesis in rat [114]. Therefore, and even though its
interaction with SAK didn’t seem very strong (table 3.1), it was included in the screen. Yps, HERC2, Cp1, Bsg25D, and Belphegor were included in the candidate list because they showed a very strong interaction with SAK in several pulldowns (table
3.1.). Bsg25D seems to be required in early stages of development, since it is expressed in the embryonic blastoderm [115]. Finally, the “nameless” genes: CG6453,
CG9795 and CG14438. The first two genes from this group were added to the list for the same reasons as the previous group, i.e., their strong interaction with SAK in several pulldowns. The last candidate, CG14438, has its mRNA localized to the centrosome [116], and its protein product is an interactor of the previously mentioned
tektins (see table 3.1.). The fact that its transcript localizes at the centrosome raises the possibility that it could be a centrosomal protein, and since it interacts with the screened tektins, it could as well be involved in centriole biogenesis and stability.
The following table summarizes some of the known functions of each candidate, as well as their interactions from CCRDB.
Gene (ACC)
Interactions from CCRDB Interactor - Source (pulldown score)
Information (from Flybase Indiana University, and other references)
CG5690 CG7186 (SAK) - SAK PD2 (43)
Homologue of human Centrobin/NIP2; In human cell lines (epithelial and cancer), shown to be required for centriole duplication and cytokinesis [113]
CG17520
CG7186 (SAK) - SAK PD1 (258) and PD2
(66)
CG2919 (Asl) - Asl-ProtA PD (49) and ProtA-Asl PD (99)
CG3412 (Slimb) - Slimb pulldown (243)
Casein Kinase II alpha subunit (CKIIα); protein serine/threonine kinase activity, bristle development, circadian rhythm, compound eye development, locomotor rhythm, Wnt receptor signaling pathway. Localizes at centrosome [117]
CG32045 CG7186 (SAK) - Two-Hybrid
[118]
Human homolog (KIAA0826 furry-homolog-like) interacts with human Plk4 (O00444) [119]
Furry; Wnt signaling pathway, antennal morphogenesis, bristle morphogenesis, imaginal disc-derived wing morphogenesis, non-sensory hair organization and biogenesis, positive regulation of protein kinase activity, regulation of dendrite
morphogenesis and development, rhabdomere
development
CG11734 CG7186 (94) (SAK) - SAK PD1 (39) and PD2 HERC2; heme binding, transition metal ion binding, ubiquitin-guanyl-nucleotide exchange factor activity, protein ligase activity.
CG17450 CG32819 CG32820
CG7186 (SAK) - SAK PD1 (only 1st) (44)
CG18745 - Two-Hybrid [120]
CG15524 (SAS-6) - Two-Hybrid [120]
Tektins; MT cytoskeleton organization and biogenesis; Protein domains: tektin
These three genes show a great similarity when