w ww.e l s e v i e r . c o m / l o c a t e / b j p
Original
Article
UPLC–QTOF–MS
and
NMR
analyses
of
graviola
(
Annona
muricata
)
leaves
Ingrid
Vieira
Machado
de
Moraes
a,
Paulo
Riceli
Vasconcelos
Ribeiro
a,
Flávio
Luis
Schmidt
b,
Kirley
Marques
Canuto
a,
Guilherme
Julião
Zocolo
a,
Edy
Sousa
de
Brito
a,
Rensheng
Luo
c,
Kristy
M.
Richards
d,
Kevin
Tran
d,
Robert
E.
Smith
d,∗aEmbrapaAgroindústriaTropical,Fortaleza,CE,Brazil
bDepartamentodeTecnologiadeAlimentos,FaculdadedeEngenhariadeAlimentos,UniversidadedeCampinas,Campinas,SP,Brazil
cUniversityofMissouri,St.Louis,MO,USA
dFoodandDrugAdminsitration,Lenexa,KS,USA
a
r
t
i
c
l
e
i
n
f
o
Articlehistory:
Received6April2015 Accepted7December2015 Availableonline6February2016
Keywords:
Graviola
Annonamuricata
UPLC–QTOF–MS Parkinson’sdisease NMR
a
b
s
t
r
a
c
t
Graviolaleaves(AnnonamuricataL.,Annonaceae)areusedbysomepeopletotrytotreatoreven curecancer,eventhoughover-consumptionofthefruit,whichcontainstheneurotoxinsannonacin andsquamocinhascausedanatypicalformofParkinson’sdisease.Inpreviousanalyses,thefruitswere extractedwithmethanolunderambientconditionsbeforeanalyses.Inthepresentstudy,UPLC–QTOF–MS andNMRwereusedtoanalyzefreeze-driedgraviolaleavesthatwereextractedusingdrymethanoland ethanolat100◦Cand10MPa(100atm)pressureinasealedcontainer.Methanolsolubilized33%ofthe metabolitesinthelyophilizedleaves.Ethanolsolubilized41%ofmetabolitesinthelyophilizedleaves.The concentrationsoftotalphenoliccompoundswere100.3±2.8and93.2±2.0mggallicacidequivalentsper gofsample,forthemethanolicandethanolicextracts,respectively.Moreover,thetoxicophore (unsat-urated␥-lactone)thatispresentinneurotoxicacetogeninswasfoundinthelipophilicportionofthis extract.TheconcentrationsoftheneurotoxinsannonacinandsquamocinwerefoundbyUPLC–QTOF–MS tobe305.6±28.3and17.4±0.89g/g-dw,respectively,inthedriedleaves.Pressurizedmethanol solubi-lizedmoreannonacinandsquamocinthanethanol.Ontheotherhand,ahot,aqueousinfusionsolubilized only0.213%oftheannonacinandtoolittleofthesquamocintobedetected.So,graviolaleavescontain significantamountsoftheneurotoxinsannonacinandsquamocin,aswellassomepotentiallyhealthy phenoliccompounds.Finally,thepotentialneurotoxicityofwholeleavesindietarysupplementscould bemuchhigherthanthatofatea(hotaqueousinfusion)thatismadefromthem.
©2015SociedadeBrasileiradeFarmacognosia.PublishedbyElsevierEditoraLtda.Allrightsreserved.
Introduction
Graviola (Annona muricata L.) is a tropical fruit in the AnnonaceaefamilythatisgrowninAsia,SouthAmericaandmany tropicalislands(Lannuzeland Michel,2009;Gajalakshmiet al., 2012).Theleavescanbeusedtomakeatea(Port’setal.,2013; Hansraetal.,2014)orconsumedwholeasdietarysupplementin capsulesthatmayhavesomehealtheffects(Torresetal.,2012). However,over-consumptionofgraviolaandproductsmadefrom itmayhavecausedanatypicalformofParkinson’sdiseasesonthe FrenchCaribbeanislandofGuadeloupeandthePacificislandof
∗ Correspondingauthor.
E-mail:Robert.smith@fda.hhs.gov(R.E.Smith).
Guam(Caparros-LefebvreandElbaz,1999;Champyetal.,2005; BadrieandSchauss,2009).Thisisduetothepresenceof neuro-toxicacetogenins,suchasannonacin(1)(C35H64O7,MW596.88)
andsquamocin(2)(C37H66O7,MW622.92).Likeotherneurotoxic
acetogenins,theycontainanunsaturated␥-lactonegroupthatis thetoxicophore(Smithetal.,2014).However,theneurotoxicity couldbestronglydependentonthedose.Thatis,thefirstruleof toxicologyisthatthedoseisthepoison(Smith,2014).Itwas over-consumption,notconsumptionofgraviolathatcausedParkinson’s disease.Thedoseofneurotoxicacetogeninsthatoneconsumesis verydependentontheformofgraviolathatisingested.For exam-ple,onestudyusedateamadefrom10to12dryleavesthatwere boiledin8oz(about237ml)waterfor5–7min(Hansraetal.,2014). Sinceacetogeninshaveverylowsolubilitiesinwater(Höllerhage etal.,2009),thiswouldhavebeenarelativelylowdose.Thedose
http://dx.doi.org/10.1016/j.bjp.2015.12.001
canbeestimatedfromapreviousstudythatfoundabout56mg of annonacinperkgof leaveswhen about2.5g ofleaveswere boiledfor10mininwater(Champyetal.,2005).Ontheotherhand, muchhigherdoseswouldbeconsumedifoneweretoingestthe entireleavesinadietarysupplement.Thatis,onegroupreported thatthe“therapeuticdose”ofgraviolaleavesis2–3g,taken3or4 timesdaily(Sunetal.,2014).Ifwholeleavesareconsumed,the expecteddosewould behigher than thatin a tea.To estimate theconcentration,100gofdriedleaveswereextractedwith1lof methanol(Champyetal.,2005).Thisrecoveredalmostsixtimes asmuch annonacin(about 300mg/kg)as didtheboilingwater. However,thisanalysiswasdonewithmatrixassistedlaser desorp-tionandionizationcoupledtotimeofflightmassspectrometryor MALDI–TOFMS(Champyetal.,2005),whichseparatesanalytes basedontheirmolecularweights.So,annonacinwillnotbe sepa-ratedfromitsisomers.Thiscouldcauseanover-estimationofthe concentrationofannonacin,sinceitsisomerswillalsobedetected by theMALDI–TOF MS. It was observed,boiling methanolwas notabletosolubilizealltheannonacin(1)orsquamocin(2)from anotherfruitintheAnnonaceaefamily,theNorthAmerican paw-paw(Asiminatriloba;Pottsetal.,2012).Thatis,themethodusedto extractannonacinandsquamocincanaffecttheresultsofthe analy-sis.Boilingwaterwillnotsolubilizealltheannonacinorsquamocin. Instead,pressurizedliquidextractionusingdrymethanolat100◦C and10MPa(100atm)pressurecansolubilizeoverseventimesas muchannonacinandevensomesquamocinfromthefruits(Potts etal.,2012).So,itisimportanttousehot,drypressurizedmethanol toextractalltheannonacinandsquamocin.Itisalsoimportantto useliquidchromatographycoupledtohighresolutionmass spec-trometry(LC-HRMS)toseparateanddetectisomersofannonacin andsquamocin(Levineetal.,2015).
LC-HRMSisespeciallyusefulwhentryingtoquantifytrace com-ponents,like acetogenins.TheMS canbetunedsothat it isas sensitiveaspossiblefortheanalytesofinterest(Smith,2014). How-ever,thesensitivitiesforotheranalytesthatarepresentinasample arequitedifferent.So,standardsarerequiredwhenquantifying analytesbyMS.Ontheotherhand,1H NMRhasthesame
sen-sitivityfor allthehydrogens(1Hisotope)inthesample(Smith,
2014).So,itcanbeusedwithoutstandardstodeterminetherelative concentrationsofdifferenttypesofhydrogensinasample(Smith, 2014).Moreover,13CNMRcanbeusedtodeterminehowmany
differentkindsofcarbonsareinasampleandtoidentifythetypes ofcompoundsthatarepresent(Smith,2014).Therefore,NMRcan beveryusefulinanalyzingrelativelyunknownsamples.NMRwas usedtoanalyzeextractsofA.muricataforthepresenceofthe␣, -unsaturated-␥-lactonetoxicophorethatis presentinneurotoxic acetogenins(Machadoetal.,2015).
Even though graviola leaves have been reported to contain about300mg/kgannonacin(Höllerhageetal.,2009),32mg/kgtotal phenolicsand5.6mg/kgtotalflavonoids(Port’setal.,2013),little isknownaboutitsmajorchemicalconstituents.Eventhe concen-trationoftotalsolublesubstancesisnotwellknown.Thatis,the concentrationdependsonthemethodusedtoextractthesoluble substances.Forexample,whendriedleavesweresoakedin dis-tilledwater for48h,about 5%ofthesolids dissolved(Florence et al.,2014).Another obtainedonly a 3.62% yield when leaves wereextractedtwicewithdistilled water atroomtemperature (AdewoleandAjewole,2009).Whensoakedfor48hin80%ethanol, about10.55%ofthecompoundsintheleavesdissolved(Foongand Hamid,2012;Hamizahetal.,2012).Adifferentstudypercolateddry leaveswith95%ethanoltogeta19.3%yieldofsolublesubstances (Singletonetal.,1999).
So, the aims of this work were characterizing the extracts obtainedbythistechniqueandseeifmethanolandethanolpresent differentresults,aswellasanalyzeannonacinandsquamocinby UPLC–QTOF–MS.
Materialsandmethods
Chemicalsandgraviolaleaves
Methanol(CH3OH),chloroform(CHCl3), acetonitrile(CH3CN)
andethanolwerefromHoneywellBurdick&Jackson(Muskegon, MI, USA). Deuterated chloroform (CDCl3), deuteratedmethanol
(CD3OD)anddeuteratedwater(D2O)werefromSigmaAldrich(St.
Louis,MO).Leucine-enkephalinwaspurchasedfromWaters(North Kingstown,RI,USA).Lyophilizedgraviolaleaveswereobtainedby Ingrid de Moraes froma commercialcultivationlocated in the cityofTrairi(Ceará,Brazil),latitude3◦22′15.98′′Sand longitude 39◦17′34.46′′W.VoucherspecimensarekeptatEmbrapain For-taleza.Thevouchernumberis49002.Afterbeingharvested,the leaveswerewashed,sanitizedwithasodiumhypochlorite solu-tion(100ll-1or100ppm)andthenlyophilizedinamodelLP510
lyophilizer(Liobrás,SãoCarlos,SP-Brazil),atEmbrapaTropical AgroindustrylocatedinFortaleza,Ceará,Brazil.
Extractions
About10goflyophilizedleavesweremixed withenoughof HydroMatrixTM (SigmaAldrich, St. Louis, MO) to fill the 100ml
stainlesssteelsamplecellusedinanAcceleratedSolvent Extrac-tor(ASE, ThermoFisher Scientific, Sunnyvale, CA).Then, CH3OH
orethanolwasaddedwhilethetemperatureandpressurewere increasedto100◦Cand10.3MPa(100atm)overa3mintime(static time).Next,thesolventwaspurgedintoacollectionvessel.Atotal offourcycleswereruntostaticallyextractthesample,resulting inatotalvolumeofabout160ml.Thesolventwasevaporatedoff and theoily residuesremaining afterextractionwitheach sol-vent wereweighed.Aportionoftheresidueobtainedfromthe methanolextractionwasdissolvedin99.99%CD3ODandanalyzed
byNMR.Portionsofthemethanolicandethanolicresidueswere analyzedfortotalphenolics,asdescribedbelow.Then,portionsof theresiduesobtainedfromthemethanolicandethanolic extrac-tionwerepartitionedbetweenCHCl3andwater.TheCHCl3phase
wascollected,thesolventevaporatedoff,theresidueredissolved inCDCl3andNMRspectraacquired.Finally,a10gportionofdried
leaveswereextractedbyultrasonication.Itwasdonewith200ml of50%ethanolplus50%waterat40◦Cfor10min,usingan Ultra-celaner1450ultrasonicatorfromUnique(Indaia,SP,Brazil).Itwas doneatafrequencyof20kHzandwith800Wpower.Thisextract wasalsoanalyzedfortotalphenolics.
Also,threeportionsofabout2.5gandoneportionofabout5.0g oflyophilizedleaveswereaddedto237ml(onecup)ofwaterat 90◦Cfor10min,afterwhichthesolutionswerefilteredand ana-lyzedbyLC–MS/MS.
NMRanalyses
1Hand13C{1H}-NMRspectrawereobtainedusinganAgilent
DD2600MHzNMR (SantaClara,CA).A30◦ pulsewidthand 1s pulsedelaywereusedforthe1HNMR,whilea30◦pulsewidthand 2spulsedelaywereusedforthe13CNMRspectra.Chemicalshifts
werereferencedtoeithertheCD3ODpeaksat3.35and4.78(for1H)
and49.3ppm(for13C)ortheCDCl
3peaksat7.27and77.23ppm
for1Hand13C,respectively.
Analysisfortotalphenoliccompounds
previously(Singletonetal.,1999).Resultswereexpressedasmg gallicacidequivalentspergofsample,ormgGAE/g-dw.
UPLC–QTOF–MSanalysis
First,about10mgofeachoftheresiduesremainingafter evapo-ratingthesolventoffofthehot,pressurizedextractswasdissolved in 5ml of ethanol:H2O (1:1, v/v) and passed through a LC-18
Supelcoclean 6ml (0.5g) solid phase extraction (SPE)cartridge (Supelco,Bellefonte,PA,USA)thatwaspreconditionedby wash-ingitthreetimeswithCH3OHandthenequilibratedwith3mlof
deionizedwater.Theacetogeninswereelutedwith6mlofCH3CN.
TheCH3CNwasevaporatedoffandtheresidueredissolvedin1ml
ofCH3CN:H2O(15:7,v/v).Theresiduesfromeachsamplewere
ana-lyzedbypositiveionUPLC–MSanalysisusingaXevoUPLC-QTOF fromWaters(Milford,MA)andanAcquityUPLCBEHC18column, 1.7m,2.1mm×100mm.Agradientelutionwasusedwitha mix-tureofH2OandCH3CNand0.1%formicacid,flowingat400l/min.
Thatis,eventhoughtheconcentrationsofH2OandCH3CNvaried,
theconcentrationofformicaciddidnot.Itwasheldconstantat 0.1%.Itstartedwith35:65H2O/CH3CN(v/v)forthefirst25min,
thenincreasedlinearlyto1:9H2O/CH3CN(v/v)from25to30min.It
washeldat1:9H2O/CH3CN(v/v)from30to35min,thendecreased
linearlyto35:65H2O/CH3CN(v/v)from35to36minandheldat
thisfrom36to40min.TheMSspectrawereacquiredinpositiveion modewithanelectrosprayionization(ESI)source.TheMS param-eterswereasfollows:desolvationtemperature350◦C, capillary voltage3.2kV,samplingconevoltage32V,extractioncone volt-age1.0V,sourcetemperature120◦C, conegas2l/h,desolvation gasflow350l/h,purgegasflow350l/h,collisionenergy5.0V. Cen-troiddatawerecollectedforeachsampleinarange110–800Da, andthem/zvalueofallacquiredspectrawasautomatically cor-rectedduringacquisitionbasedonlockmassbyinfusing200pg/lof leucine-enkephalinthoroughLocksprayataflowrateof20l/min. Thecalibrationwasperformedtoachieveanacceptableaccuracy errorof±5ppm.Theaccuratemassandmolecularformula denom-inationwereacquiredwiththeMassLynx4.1software(WatersMS Technologies).
Annonacinand squamocinstandards fromPierreChampy at theUniversity of Paris were used toidentify and quantify the peaksduetoannonacin(1)andsquamocin(2).Theyelutedat14.1 and15.6min,respectively.Ionswithm/z=597.47and623.48Da were used to quantify annonacin and squamocin, respectively. Calibrationcurveswereobtainedusing10–200ng/mlannonacin and squamocin, assuming that the standards were 100% pure. Sampleswereinjected intriplicatewithresultsreportedasthe average±standarddeviation.
Resultsanddiscussion
Extraction
Drymethanolat100◦Cand10MPa(100atm)pressure solubi-lized33%oftheleaves,whileethanolunderthesameconditions solubilized 41%. So, hot, pressurized methanol extracted much morematerialthanboiling(orpercolating)underambient pres-sure,which solubilized19.3% of theleavesin a previousstudy (Port’setal.,2013).Theconcentrationoftotalphenoliccompounds thatwerefoundusinghot,pressurizedmethanolextractionwas 100.3±2.8mgGAE/g.Thiscomparesto93.2±2.0mgGAE/ginthe hot,pressurizedethanolextract,54.6mgGAE/gintheultrasonic extractusing50%ethanolandthevalueof33mgGAE/gthatwas foundbyotherswhenleaveswereextractedwithboilingwater. So,extractionwithdrymethanolat100◦Cand10MPa(100atm)
CD3OD CD3OD 2
4
1
3
5
9 8 7 6 5 4 3 2 1 ppm
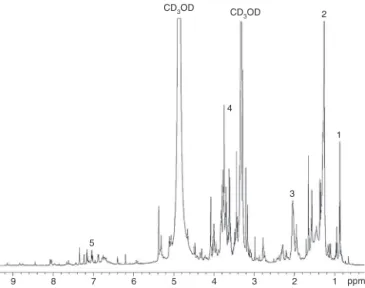
Fig.1.1H-NMRspectrumofmethanolicextract(100◦C,100MPascal)oflyophilized
graviolaleaves,inCD3OD.Peaks:1:–CH3;2:–(CH2)n;3:H2C–CH CH–;4:HCOof
sugars5:phenylsand/orphenolics.
pressurecanextractmorephenoliccompoundsthanboilingwater underambientpressure.
NMRanalysis
The1Hand13C{1H}-NMRspectraofthemethanolicextractof
lyophilizedgraviolaleavesareshowninFigs.1and2.Theyboth containsignals(chemicalshifts)duetotheCH3–(CH2)nCH2COOR
of esters (1H: 0.66–1.0 and 1.1–1.4ppm; 13C: 14.6, 16.3–39.3
and 175–177ppm), the –HCOH and –H2COH of
carbohy-drates (1H: 3.0–5.4ppm; 13C: 62.2–108.8ppm) and the
HC C of aromatic hydrogens in phenolic compounds (1H:
6.4–8.1ppm;13C:125.5–166.2ppm).Therearealsosignalsdueto
–CH2–HC CH–portionof unsaturatedfattyacyls (1H:6.4ppm; 13C: 108.9–119.5ppm). The relative contributions to the total
peakareainthe1Hspectrumwere24%alkyl(CH
3–(CH2)n),46%
carbohydrate,and 4.2%phenyl.Therewerenopeaksdueto di-ortriglycerides.Thealkyl,carbohydrateandcarbonylcarbonsall producedchemicalshiftsthatwereconsistentwiththepresence of esters of fatty acyls and sugars. These are more commonly calledfattyacidglycosides.Theyhavebeenfoundinthetropical fruitcallednoni(Morindacitrifolia)andhaveanticancerproperties
CD3OD
CH2
CH3 CHO of glycosides
Aromatic carbons
C=O
180 160 140 120 100 80 60 40 20 ppm
Fig.2.13C{1H}-NMRspectrumofmethanolicextract(100◦C,100MPascal)of
CHCl3 2
1
3
4 5 6
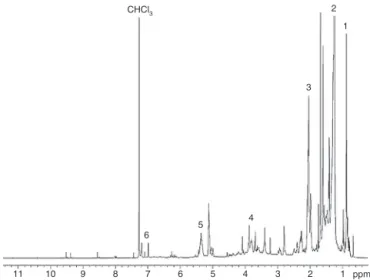
11 10 9 8 7 6 5 4 3 2 1 ppm
Fig.3. 1HNMRspectrumoftheportionofthemethanolicextract(100◦C,10MPa)of
ovendriedgraviolaleavesthatpartitionedintoCHCl3,redissolvedinCDCl3.Peaks:
1:–CH3;2:–(CH2)n;3:H2C–CH CH–;4:HCOofsugars;5:HC CH;6:HC Cin
theunsaturated␥-lactonetoxicophoreinacetogenins;0.1%CHCl3in99.9%CDCl3
solvent.
(Smithetal.,2014;Akihisaetal.,2007;Kim,2010).Itshouldalso benotedthateventhoughthemethanolicextractwasgreendue tothepresenceofchlorophyll,thechlorophyllwaspresentattoo lowofaconcentrationtoproducepeaksintheNMRspectra.There mayalsobesomesignals due toterpenesthatmay beslightly solubleinmethanol,eventhoughtheyareusuallyextractedusing hexane.
O
O
O
O
O O
O
OH
OH OH
1
2
OH OH
OH OH
OH
About37.5%ofthemethanolicextractpartitionedintoCHCl3.
The1Hand13C{1H}-NMRspectraofthislipophilicportionofthe
methanolicextractareshowninFigs.3and4.Therearechemical shiftsatorabout7.0and7.2ppmduetotheunsaturated␥-lactone toxicophorethatis inneurotoxicacetogenins(Luoetal.,2013), andverysmallsignalsdue tofourdifferentHC C hydrogensin chlorophyllat8.00,8.55,9.29and9.52ppm.
Incomparison,the1Hand13C{1H}-NMRspectraoftheportion
oftheethanolicextractthatpartitionedintoCDCl3areshownin
theSupplementaryMaterial.Thechemicalshiftsduetothe unsat-urated␥-lactonetoxicophorearealsopresent.
LC–MSanalysis
Seven point calibration curves each for annonacin (1) and squamocin(2)showedadequatelinearcorrelationbetween con-centration and area (R2>0.99), the linear range of standard
compounds was between10 and 200ng/ml for standard com-pounds. Standardsand samples wereinjected in triplicate.The
CDCl3 (CH2)n
CHO of glycosides
Aromatic carbons
Ester C=O
180 160 140 120 100 80 60 40 20 ppm
CH3
Fig.4. 13C{1H}-NMRoftheportionofthemethanolicextract(100◦C,10MPa)of
ovendriedgraviolaleavesthatpartitionedintoCHCl3,redissolvedinCDCl3.
injections showed that theresults are highly reproducible and repetitive. The LC–MS chromatograms of 200ng/ml annonacin andsquamocinstandardsandthemethanolicextractofgraviola leaves are shown in Fig. 5. The concentrations of the neuro-toxins annonacin and squamocinwere found tobe 1.68±0.08 and0.023±0.001mg/g-dw,respectivelyinthedriedleaves. Pres-surized methanol solubilized more annonacin and squamocin thanethanol.Thatis,305.6±28.3g/g-dwannonacinwasfound
in the methanolic extract and 226.0±0.07g/g-dw was found in the ethanolic extract. On the other hand, 17.4±0.89 g/g-dw squamocin was found in the methanolic extract, and 4.75±0.41mg/g-dwsquamocinwasfoundintheethanolicextract. Ontheotherhand,ahot,aqueousinfusion(tea)solubilizedonly 0.213%(0.65±0.07g/g-dw)oftheannonacinfrom2.5gofdry leavesandtoolittleofthesquamocintobedetected.When5.0gof leaveswereusedtoprepareatea,only0.108%(0.33g/g-dw)ofthe leaves.So,teabagscontainingabout5gofdrygraviolaleaveswill notsolubilizemuchmoreannonacinthanteabagscontaining2.5g ofleaves.Thus,wholegraviolaleavescontainsignificantamounts oftheneurotoxinsannonacinandsquamocin, aswellas pheno-liccompounds.Moreover,thepotentialneurotoxicitiesofwhole leavesindietarysupplementscouldbemuchhigherthanthatofa teathatismadefromthem.
100
Total Ion chromatogram
%
0
4.00 6.00
1 - Annonacin standard
[M+H]+ 597.47
8.00 10.00 12.00 14.00 16.00 18.00 20.00 22.00 24.00
100
%
0
4.00 6.00 8.00 10.00 12.00 1
2
2 1
14.00 16.00 18.00 20.00 22.00 24.00
100
Extracted Ion chromatogram
2 - Squamocin standard
[M+H]+ 623.48
Extracted Ion chromatogram
[M+H]+ 623.48 [M+H]+ 597.47 %
0
4.00 6.00 8.00 10.00 12.00 14.00 16.00 18.00 20.00 22.00 24.00
100
%
0
4.00 6.00 8.00 10.00 12.00 14.00 16.00 18.00 20.00 22.00 24.00
100
%
0
4.00 6.00 8.00 10.00 12.00 14.00 16.00 18.00 20.00 22.00 24.00 Time
A
B
C
D
E
Fig.5.UPLC–QTOF–MSchromatogramofthehot,pressurizedmethanolicextract(100◦C,10MPa)oflyophilizedgraviolaleaves–totalionchromatogramofthesample
(A);annonacinstandard–200ng/ml(Rt=14.1min)(B);allpeaksofions[M+H]+=597.47extractedionchromatogramofthesample(C);squamocinstandard–200ng/ml
(Rt=15.6min)(D);allpeaksofions[M+H]+=623.48extractedofthesample(E).
TheNMRspectraweredominatedbypeaksduetofattyacid gly-cosides,whichmayhaveanticancerandotherhealthbenefits(Kim etal.,1998;Akihisaetal.,2007;Smith,2014).Theyarealso surfac-tants,sotheywillprobablyincreasethesolubilitiesofacetogenins inhotwater.TheNMRspectraalsocontainedchemicalshiftsdue totheunsaturated␥-lactonetoxicophorethatisinannonacinand squamocin(Machadoetal.,2015).Theyarealsosurfactants,sothey willprobablyincreasethesolubilitiesofacetogeninsinhotwater. Finally,thetotalphenolicsinthehot,pressurizedmethanolicand ethanolicextractswere83and64mfGAE/g,respectively.
Moreover,methanolat100◦Cand10MPa(100atm)pressure solubilized33% ofthelyophilizedgraviolaleaves. Ethanol solu-bilized41%ofthelyophilizedleaves.Theconcentrationsoftotal phenoliccompoundswere83and64mgGAE/gforthe methano-licandethanolicextracts,respectively.Moreover,thetoxicophore (unsaturated␥-lactone)thatispresentinneurotoxicacetogenins wasfoundinthelipophilicportionoftheseextracts.The concen-trationsoftheneurotoxinsannonacin(1)andsquamocin(2)were foundbyUPLC–TOF–MStobe305.6±28.3and17.4±0.89mg/g, respectivelyinthedriedleaves.Pressurizedmethanolsolubilized
more annonacin than ethanol, while pressurized ethanol solu-bilized more squamocin than methanol. On the other hand, a hot, aqueous infusion (tea)made from 2.5g of dried leaves in 237ml(onecup)ofwatersolubilizedonly0.213%(0.65±0.07 g/g-dw) of the annonacin and too little of the squamocin to be detected.
ThisworkshouldnotbetakenasreflectingFDApolicyor regu-lations.
Authors’contributions
IVMM,PRVR,FLS,KMC,GJZ,ESBandRESconceivedanddesigned theexperiments.IVMM,PRVR,FLS,KMC,GJZandESBdidtheLC-MS andtotalphenolicsanalysesaswellasdatainterpretation.RLdid theNMRanalyses.IVMM,KMRandKTdidthepressurizedliquid extractions.RESwrotethearticle.Everyoneread thearticleand suggestedsomechanges.
Conflictsofinterest
Theauthorsdeclarenoconflictsofinterest.
AppendixA. Supplementarydata
Supplementarydataassociatedwiththisarticlecanbefound,in theonlineversion,atdoi:10.1016/j.bjp.2015.12.001.
References
Adewole,S.O.,Ajewole,J.A.O.,2009.ProtectiveeffectsofAnnonamuricataLinn. (Annonaceae)leafaqueousextractonserumlipidprofilesandoxidativestress inhepatocytesofstreptozotocin-treateddiabeticrats.Afr.J.Compl.Alt.Med.6, 30–41.
Akihisa,T.,Matsumoto,K.,Tokuda,H.,Yasukawa,K.,Seine,K.,Nakamoto,K., Kun-inaga,H.,Suzuki,T.,Kimura,Y.,2007.Anti-inflammatoryandpotentialcancer chemopreventiveconstituentsofthefruitsofMorindacitrifolia(Noni).J.Nat. Prod.70,754–757.
Badrie,N.,Schauss,A.G.,2009.Soursop(AnnonamuricataL.):composition, nutri-tionalvalue,medicinaluses,andtoxicology.In:Watson,R.R.,Preddy,V.R.(Eds.), BioactiveFoodsinPromotingHealth.FruitsandVegetables.AcademicPress, Oxford,pp.621–643.
Caparros-Lefebvre,D.,Elbaz,A.,1999.Possiblerelationofatypicalparkinsonism intheFrenchWestIndieswithconsumptionoftropicalplants:acase–control study.Lancet354,281–285.
Champy,P.,Melot,A.,Guerineau,V.,Gleye,C.,Fall,D.,Höglinger,G.U.,Ruberg,M., Lannuzel,A.,Laprevote,O.,Laurens,A.,Hocquemiller,R.,2005.Quantificationof acetogeninsinAnnonamuricatalinkedtoatypicalparkinsonisminGuadeloupe. MovementDisorders20,1629–1633.
Florence, N.T., Benoit,M.Z., Jonas, K., Alexandra,T., Desire, D.D.P., Pierre,K., Theophile,D., 2014. Antidiabeticand antioxidanteffects ofAnnona muri-cata(Annonaceae),aqueousextractonstreptozotocin-induceddiabeticrats.J. Ethnopharmacol.151,784–790.
Foong,C.P.,Hamid,R.A.,2012.Evaluationofanti-inflammatoryactivitiesofethanolic extractofAnnonamuricataleaves.Rev.Bras.Farmacogn.22,1301–1307.
Gajalakshmi,S.,Vijayalakshmi,S.,Devi,R.V.,2012.Phytochemicaland pharmaco-logicalpropertiesofAnnonamuricata:areview.Intl.J.Pharm.Pharmaceut.Sci. 4,3–6.
Hamizah,S.,Roslida,A.H.,Fezah,O.,Tan,K.L.,Tor,Y.S.,Tan,C.I.,2012. Chemo-preventivepotentialofAnnonaMuricataLleavesonchemically-inducedskin papillomagenesisinmice.AsianPacificJ.CancerPrevention13,2533–2539.
Hansra,D.M.,Silva,O.,Mehta,A.,Ahn,E.,2014.Patientwithmetastaticbreastcancer achievesstablediseasefor5yearsongraviolaandXelodaafterprogressingon multiplelinesoftherapy.Adv.BreastCancerRes.3,84–87.
Höllerhage,M.,Matusch,A.,Champy,P., Lombes,A.,Ruberg,M.,Oertel,W.H., Höglinger,G.U.,2009.NaturallipophilicinhibitorsofmitochondrialcomplexI arecandidatetoxinsforsporadicneurodegenerativetaupathologies.Exp. Neu-rol.220,133–142.
Kim,H.-K.,2010.Identificationofnovelfattyacidglucosidesfromthetropicalfruit
MorindacitrifoliaL.Phytochem.Lett.3,238–241.
Kim,G.-S.,Alali,F.,Rogers,L.L.,Wu,F.-E.,Sastrodihardjo,S.,McLaughlin,J.L.,1998.
MuricoreacinandmurihexocinC,mono-tetrahydrofuranacetogeninsfromthe leavesofAnnonamuricata.Phytochemistry49,565–571.
Luo,R.,Maia,J.G.,deMoraes,M.R.,Godoy,H.T.,Sabaa-Srur,A.U.O.,Tran,K.,Richards, K.M.,Monroe,D.M.,Vocque,R.H.,Smith,R.E.,2013.NMRanalysisofpotentially neurotoxicannonaceousfruits.Nat.Prod.J.3,230–241.
Lannuzel,A.,Michel,P.P.,2009.AtypicalparkinsonismintheFrenchWestIndies: theplanttoxinannonacinasapotentialetiologicalfactor.In:Tseng,K.-Y.(Ed.), Cortico-SubcorticalDynamicsinParkinson’sDisease.HumanaPress,NewYork, pp.283–290.
Levine,R.A.,Richards,K.M.,Tran,K.,Luo,R.,Thomas,A.L.,Smith,R.E.,2015. Determi-nationofneurotoxicacetogeninsinpawpaw(Asiminatriloba)fruitbyLC-HRMS. J.Agric.FoodChem.63,1053–1056.
Machado,A.R.T.,Lage,G.A.,Medeiros,F.S.,Filho,J.D.S.,Pimenta,L.P.S.,2015.Total ␣,-unsaturated-␥-lactoneacetogeninsinAnnonamuricatabyprotonNMR spectroscopy.Appl.Mag.Res.46,153–160.
Port’s,P.S.,Chisté,R.C.,Godoy,H.T.,Prado,M.A.,2013.Thephenoliccompounds andtheantioxidantpotentialofinfusionofherbsfromtheBrazilianAmazonian region.FoodRes.Intl.53,875–883.
Potts,L.F.,Luzzio,F.A.,Smith,S.C.,Hetman,M.,Champy,P.,Litvan,I.,2012.Annonacin inAsiminatrilobafruit:Implicationforneurotoxicity.NeuroToxicol.33,53–58.
Singleton,V.L.,Orthofer,R.,Lamuela-Raventos,R.M.,1999.Analysisoftotalphenols andothersoxidationsubstratesandantioxidantsbyFolin–Ciocauteaureagent. MethodsEnzymol.299,152.
Smith, R.E.,2014. Medicinal Chemistry – Fusion of Traditional and Western Medicine,SecondEdition.BenthamScience,Sharjah,U.A.E,pp.316–326.
Smith,R.E.,Tran,K.,Richards,K.M.,2014.BioactiveAnnonaceousAcetogenins, Chap-ter4,inStudiesinNaturalProductsChemistry,vol.41.Elsevier,NewYork,pp. 93–117.
Sun,S.,Liu,J.,Kadouh,H.,Sun,X.,Zhou,K.,2014.Threenewanti-proliferative Annonaceousacetogeninswithmono-tetrahydrofuranringfromgraviolafruit (Annonamuricata).Bioorg.Med.ChemLett.24,2773–2776.
![Fig. 5. UPLC–QTOF–MS chromatogram of the hot, pressurized methanolic extract (100 ◦ C, 10 MPa) of lyophilized graviola leaves – total ion chromatogram of the sample (A); annonacin standard – 200 ng/ml (Rt = 14.1 min) (B); all peaks of ions [M+H] + = 597.47](https://thumb-eu.123doks.com/thumbv2/123dok_br/14931136.501450/5.918.196.705.83.730/chromatogram-pressurized-methanolic-lyophilized-graviola-chromatogram-annonacin-standard.webp)