* Corresponding author:
E-mail: zangguogang@163.com
Received: February 12, 2018
Approved: August 3, 2018
How to cite: Meiqing J, Jing H, Yinghua Y, Guodong
H, Guogang Z. Effects of
simulated nitrogen deposition and precipitation manipulation on soil microorganisms in the
desert steppe of Northern
China. Rev Bras Cienc Solo. 2019;43:e0180031.
https://doi.org/10.1590/18069657rbcs20180031
Copyright: This is an open-access article distributed under the
terms of the Creative Commons
Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided that the original author and source are credited.
Effects of Simulated Nitrogen
Deposition and Precipitation
Manipulation on Soil
Microorganisms in the Desert
Steppe of Northern China
Jia Meiqing(1), Huang Jing(2), Yang Yinghua(2), Han Guodong(3) and Zhang Guogang(2)*
(1) Tianjin Normal University, Key Laboratory of Water Resource and Environment, Tianjin, China
(2) Tianjin Normal University, College of Life Sciences, Tianjin Key Laboratory of Animal and Plant Resistance, Tianjin, China.
(3) Inner Mongolia Agricultural University, College of Ecology and Environmental Science, Key Laboratory of Grassland Resources, Ministry of Education, Hottot, China.
ABSTRACT: Soil microorganisms are influenced by climate change. However, the effect of climate change on soil cultivable bacteria are unclear. In this study, the composition and diversity of the soil cultivable bacterial community were explored by a dilution–plate method, PCR, and 16S rRNA sequencing in a desert steppe of northern China after repeated NH4NO3 amendments and precipitation manipulation for seven years. The experimental treatments were as follows: control (CK), N addition (+N), N addition plus water addition (+N+W), and N addition plus water reduction (+N-W). Among the treatment groups, 11 genera and 17 bacterial species were isolated. Nitrogen addition and precipitation manipulation significantly increased the number of cultivable bacteria in the 0.00-0.30 m layer compared to CK. Compared to +N treatment, the +N+W and +N–W treatments had no significant impact on the number of cultivable bacteria. Compared to the CK community, bacterial communities exposed to the other three treatments did not show shifts in the relative abundance of dominant genera and other cultivable bacteria, except for Pontibacter and Staphylococcus. The treatments +N+W and +N-W significantly
modified the relative abundance of Pontibacter and Staphylococcus compared to the +N
treatment. Available potassium and phosphorus, and moisture content contributed to
the change in the composition of the cultivable bacterial community (p>0.05). Nitrogen addition and precipitation manipulation significantly decreased species richness in the 0.00-0.02 m layer, but they did not affect evenness and the Shannon-Wiener Index in the
0.00-0.30 m layer. This study provides insights into how the composition and diversity
of the bacterial community is affected by climate change scenarios. Keywords: soil microorganisms, climate change, 16S rRNA.
INTRODUCTION
Due to increased fossil fuel combustion and excessive application of fertilizer, reactive nitrogen (N) deposition has nearly quintupled over the last century (IPCC, 2014). In China, atmospheric N deposition has increased by 8 kg ha-1
N since the 1980s
(Liu et al., 2013). Meanwhile, changes in precipitation patterns have been observed
in terrestrial ecosystems, with increasing precipitation in arid regions of central Asia (IPCC, 2007).
Climate changes affecting atmospheric N deposition and precipitation patterns have resulted in broad awareness of profound effects on ecosystem functioning and structure in grasslands, thus potentially affecting the biogeochemical cycle on a regional to global scale (Ladwig et al., 2012; Liu et al., 2013). Soil microorganisms are recognized as key players in sustaining ecosystem functions and services. Long˗term N deposition has various effects on microbial community structure and diversity (Balser, 2001; Frey et al., 2004; Zeng et al., 2016; Yuan et al., 2016), life–history traits via changes in pH and
Al3+ content in soil (Vitousek et al., 1997), nutrient mineralization, nitrogen availability (Balser, 2001; Aber and Magill, 2004; Compton et al., 2004), soil enzymatic activities
(Frey et al., 2004; Hu et al., 2013), and microbial biomass and activities (Boxman et al.,
1998; Aber and Magill, 2004; Gerdol et al., 2006). In addition, seasonal variations in
microbial abundance and biomass were confirmed by Chen et al. (2010). The effects of N deposition on microorganisms have been context dependent, and biomass responses vary across experiments (Allen and Schlesinger, 2004; Compton et al., 2004; Frey et al.,
2004), and they might be determined by the ecosystem.
Simultaneous changes in precipitation and soil hydrology have multiple effects, including
changes in plant physiology and growth (Xu et al., 2010), plant community composition,
diversity, and yield (Knapp et al., 2002; Suttle et al., 2007). Previous studies suggested that changes in precipitation can influence soil microbial community composition (Lindberg et al., 2002; Williams and Rice, 2007; Hawkes et al., 2011), increase the relative abundances of soil fungi and gram-negative bacteria (Bell et al., 2010), and result in greater fungi/bacteria ratios (Williams and Rice, 2007). However, some studies
indicated water addition had no impacts on microbial community composition and on
fungal to bacterial phospholipid fatty acid ratio (PLFA) (Bi et al., 2012; Sun et al., 2014;
Huang et al., 2015).
Water and nitrogen are two coupling factors, as well as primary limiting factors, in semiarid grassland ecosystems (Hooper and Johnson, 1999; Niu et al.,2009). Both water and N addition increased soil inorganic N availability, but had no impact on soil organic C, total C, and N storage (Lü et al., 2011; Wang et al., 2015). Soil dehydrogenase and acid and alkaline phosphomonoesterase enzyme activities decreased with N addition; however, water addition alone caused these enzyme activities to increase (Wang et al., 2014). It has been well documented that net primary production (Niu et al.,2009; Lü et al.,
2012) and microbial C utilization potentials (Bi et al., 2012) tend to be enhanced when
water and N availability are sufficient. Bi et al. (2012) reported the interactive effects of water and N addition on gram negative/gram positive bacteria and the ratio of fungal to bacterial PLFA in a semiarid steppe. In addition, the response of microbial communities to precipitation changes and N deposition may depend on the inter-annual variation of
the climate (Sun et al., 2014), variation in inter-annual precipitation (Sun et al., 2017),
and vegetation type (Khalili et al., 2016).
Previous studies suggested above-ground plant communities were sensitive to climate change and N deposition in grasslands of northern China (Bai et al., 2010; Yang et al., 2011). Therefore, it is necessary to evaluate how the soil microbial community and its diversity are affected by precipitation changes and N deposition to predict grassland
Desert steppe is the desert/grassland biome transition zone, which makes up 10.7 % of
the grasslands in the Inner Mongolia Autonomous Region in China (Liao and Jia, 1996).
The fragile desert steppe is more sensitive to climatic changes and human disturbances (like overgrazing) than other grassland patterns (Li, 1990). Soil bacteria play key roles in the soil food web and ecosystem carbon and nitrogen cycling. Despite the widely acknowledged importance of soil bacteria, how cultivable bacteria respond to long-term repeated N addition and precipitation manipulation is not well understood.
To examine how the composition and diversity of the cultivable bacterial community are affected by N deposition and precipitation manipulation, we conducted a manipulative experiment including water addition (or water reduction) of approximately 30 % annual precipitation and N addition in a soil of the Inner Mongolia desert steppe of northern China. The present study especially addressed the specific questions of: 1) How the cultivable bacterial community and its diversity (based on genera) are affected by N deposition and precipitation manipulation? and 2) Which soil factors are responsible for this variation in the soil bacterial community composition in the desert steppe of Inner Mongolia?
MATERIALS AND METHODS
Study siteThe study was conducted in a desert steppe located in Siziwang Banner near the Grassland
Ecosystem Research Station (41° 46’ 43.6” N, 111° 53’ 41.7” E; 1,456 m a.s.l.) in Inner Mongolia of Northern China. The steppe is in a continental temperate climate. Vegetation is
dominated by Stipa breviflora Griseb., Artemisia frigida Willd., and Cleistogenes songorica
Roshev. The 50 years mean annual precipitation is 248 mm, with approximately 70 % of the total precipitation occurring from June to September (Lin et al., 2010). Mean annual
evaporation is 2,947 mm and average annual air temperature is 3.4 °C. The monthly
mean air temperature was highest from June to August with mean temperatures of 21.5, 24.0, and 23.5 ℃, respectively, and lowest in January. The soil of the study site is categorized as Chestnut soil, a Haplic Calcisols (IUSS Working Group WRB, 2015), with a loamy-sand texture. The study site had not been subjected to prior fertilizer application
or hydrological manipulation.
Experimental design
In this study, the experiments involved a completely randomized block design with six treatments: control (CK), N addition (+N), water addition (+W), water reduction (–W), N addition plus water addition (+N+W), and N addition plus water reduction (+N–W). We arranged plots 6 × 15 m in size and applied a split-plot design with N addition as the main plot effect and precipitation manipulation as the subplot effect. Each subplot was 3 × 3 m in size. Main plots and subplots were separated by 2 m walkways and physical isolation, respectively. Nitrogen and precipitation were regulated continuously starting in spring 2006. In nitrogen addition plots, NH4NO3 was applied at an annual rate of 10 (g of N) m-2
yr-1
, before the first rain event at the end of April or at the beginning of May. Precipitation was manipulated using an artificial drought shed (Figure 1). Six 12 × 4 m artificial drought sheds were randomly constructed on the 12 main plots, with the top of the shed having a rain shelter with 100 % transmittance. Each artificial drought shed can eliminate 30 % of natural precipitation as a water reduction treatment. The rainfall from the water reduction treatment was used to irrigate “water addition” subplots with 30 % addition to natural precipitation after each rainfall. Each treatment had six replications, this sampling was randomly performed on triplicate replications. Here, CK, +N, +N+W, and +N-W treatments were studied; the results of the other two treatments (+W and -W) have
Soil sampling and measurement of soil physicochemical indices
Soil samples were collected on August 10, 2012. In each plot, four cores were randomly taken with an auger (0.05 m diameter) from the 0.00-0.30 m layer. Every soil core was divided into five layers according to preliminary results (Jia et al., 2017), namely,
0.00-0.02, 0.02-0.05, 0.05-0.10, 0.10-0.20, and 0.20-0.30 m. Soil samples at the same
layer from each plot were mixed evenly in a plastic bag to prepare a composite sample. Then, each sample was divided into two sub-samples and transported to the lab at 4 ℃; one part was used for soil physicochemical measurements, and the other was stored at -20 ℃ for soil microbial analysis.
The physical and chemical properties of soil in this study were analysed using standard
methods (Lu, 2000). Soil pH(H2O) was determined at a ratio of 1:2.5 v/v with a pH meter (Sartorius PB–10). Ammonia nitrogen (NH4+-N) and nitrate nitrogen (NO3
--N ) were extracted with KCl 1 mol L-1
from the fresh soil samples and measured using a continuous flow
analyzer AA3 (SEAL, Germany). Soil moisture was determined by oven drying a 20 g
subsample of soil at 105 ℃ until constant weight. Soil organic matter was quantified by dichromate oxidation. Available P in the soil was extracted with sodium bicarbonate and available K, with ammonium acetate, and they were quantified by the molybdenum-blue
W–CK: Precipitation-control
– W: 30 % precipitation reduction
+ W: 30 % precipitation addition
2 m
INNER MONGOLIA
BEIJING
W E
N S
6 m
2 m
15 m
colorimetric method and atomic absorption spectrometry (ICE 3500, Thermo Electron
Corporation, USA), respectively. Isolation of cultivable bacteria
The total numbers of cultivable bacteria were examined as colony-forming units (CFUs) by the dilution-plate method (Xu and Zheng, 1986). One gram of each fresh soil sample was well mixed with 99 mL of sterilized water and shaken in an oscillator at 150 rpm for 20 min, then serially diluted. Triplicate 0.2 mL samples of the diluted suspension were spread on 90 mm plates containing the beef extract-peptone medium and cultivated at 28 ℃ for 4 days in an artificial climate chamber. The plates that contained between 30 and 300 bacterial colonies were counted after the cultivation period. Colonies that represented treatment groups were picked and identified, and then used for taxonomic identification of the bacteria.
Molecular identification and diversity analysis of the cultivable bacteria Genomic DNA extraction and PCR amplification of 16S rRNA of cultivable bacteria
Bacterial genomic DNA was extracted using a SANGON Soil Bacterial Genomic DNA Extraction Kit (Sangon Biotech Co., Ltd. China). The 16S rRNA genes were amplified from bacterial genomic DNA using bacterial universal primers: the forward primer was
27F (5’-AGA GTT TGA TCC TGG CTC AG-3’, 20 bp) and the reverse primer was 1492R
(5’-TAC GGC TAC CTT GTT ACG ACT T-3’, 22 bp). The PCR reaction was conducted on a Blue Marlin TC-960F PCR cycler (Switzerland). The PCR was carried out in a 50 μL reaction containing 5 μL of Ex buffer (10 ×, including Mg2+), 4 μL of dNTP (2.5 mmol L-1
each),
2 μL of each primer (10 μmol L-1
each), and 5 U of Tap Ex DNA polymerase. Finally, 1 μL of template DNA was combined with double-distilled H2O for a total reaction volume of 50 μL. We used the following cycling conditions: initial denaturation at 94 ℃ for 5 min; 30 cycles of denaturation (1 min at 94 ℃), annealing (45 s at 55 ℃), and extension (45 s at 72 ℃); and a final extension at 72 ℃ for 10 min. The PCR products were verified by electrophoresis in 1.5 % agarose gel.
Nucleotide sequencing of PCR products
The DNA fragments with correct size from cultivable bacteria were commercially (BGI) sequenced to obtain 16S rRNA gene sequences. Identification was performed by FASTA search of the NCBI database, and phylogenetic analysis involved alignment with type strains of the nearest neighbors. Isolates were assigned to a species when sequence similarity with the species type strain was at least 97 % (Suzuki et al., 1997).
Analysis of the cultivable bacteria diversity
For this purpose, community parameters were estimated by means of the Shannon-Wiener diversity index (H’), evenness (E), and richness (S) (Liu et al., 2010) according to the
equations 1, 2, and 3:
Relative abundance = (number of microbes in a genus/total number of microbes in a sample) × 100 %:
S = Ni Eq. 1
H' = –
Σ
si=1pi In pi Eq. 2
E = H' H'
Hmax In s
in which: Niis the total number of taxa in each treatment group; and Pi is the proportion
of the number of species i in a sample.
Statistical analysis
All the data were formatted as graphs in Sigmaplot 8.0, Microsoft Excel software, and statistics were calculated using SPSS 13.0 with one-way ANOVA followed by least significant difference (LSD) multiple-comparison tests of significance. The data were considered significant at p<0.05. The relationship between the relative abundance of cultivable bacteria and soil factors was analyzed using redundancy analysis (RDA) of the CANOCO 5.0, in which the relative abundance of cultivable bacteria was used as dependent variables, and soil factors in the 0.00-0.30 m soil layer were explanatory variables. A Monte Carlo permutation test (999 permutations) was used to test the soil factors affecting bacterial community structure.
RESULTS AND DISCUSSION
The effect of N addition and precipitation manipulation on the number of cultivable bacteria
Our results showed that the number of cultivable bacteria in treatment groups ranged from 37.33 × 104
to 48.94 × 104
CFU·g-1
in the 0.00-0.30 m soil layer (Figure 2), in agreement
with the results of another study (Kurapova et al., 2012). The quantity of cultivable bacteria peaked in the layer of 0.00-0.10 m, and was lowest in the layer of 0.20-0.30 m; this is consistent with the results of the study on microbes by Liu (2014) and Jia et al. (2016,
2017) in the same plot. This phenomenon may strongly be associated with the shallow
and dense root systems of the above-ground community and the high content of soil physical and chemical factors in the 0.00-0.10 m layer, as detailed in Jia et al. (2017).
As shown in Jia et al. (2017), soil pH(H2O) values were appreciably lower in groups +N, +N+W, and +N-W, at the 0.00-0.02 m layer, than in the CK treatment group (7.85). In contrast, no differences in soil pH were observed among the treatment groups at other soil layers. The amount of NH4+-N was higher in the +N+W treatment group at the 0.00-0.02 and 0.02-0.05 m layers. Soil nitrate content in the +N-W treatment group was significantly higher than that in other treatments in the 0.02-0.05, 0.05-0.10, 0.10-0.20, and 0.20-0.30 m layers (uniformed p<0.05). Moisture content was significantly reduced by the addition of N plus removal of precipitation (p<0.05). The results revealed that the concentrations of soil moisture, organic matter, available K, and available P decreased with depth. However, no apparent differences in organic matter and in available P content were observed among the treatment groups, and available K was higher in the +N-W
treatment group than in the others.
Studies have shown that there are no consistent responses to N addition or to changes in precipitation for number of bacteria or fungi. Sarathchandra et al. (2001)used two
decrease in the number of cultivable bacteria in the 0.00-0.02 and 0.05-0.10 m layers (p<0.05), and the +N-W treatment resulted in a significant decrease in cultivable bacteria in the 0.05-0.10 and 0.10-0.20 m layers (p<0.05). Compared to the +N treatment, +N-W did not result in changes in cultivable bacteria. Nitrogen and water are limiting factors in desert steppe ecosystems (Wang, 2014). Nitrogen availability depended on water availability in the soil. Water deficit decreased N availability in our study area. Therefore, the +N and +N-W treatments did not impact the number of cultivable bacteria. Compared to the +N treatment, the +N+W treatment did not significantly change the number of cultivable bacteria at any soil layer (p>0.05), which is consistent with the results of the study on microbes in a meadow steppe by Li et al. (2016). Average annual rainfall was approximately 248 mm in our experimental zone, and evaporation was about 10 times greater than rainfall. Thus, an increase of 30 % in rainfall was too small and too quickly
evaporated to induce changes in cultivable bacteria, which is consistent with the results
of soil physicochemical factors in the +N and +N+W treatments. In addition, there is keen competition for water and N among above-ground plants and soil microbes over the growing season in N- and water-limited environments. These results likely indicate limited effects from water and N on cultivable bacteria and plants in experimental plots,
especially in the 0.00-0.02 and 0.05-0.10 m layers.
Effects of simulated nitrogen deposition and precipitation manipulation on the community structure and diversity of cultivable bacteria
Across treatments, 11 genera and 1 unclassified species (XJ25) were identified, which belong to Firmicutes, α-Proteobacteria, β-Proteobacteria, γ-Proteobacteria, Bacteroidetes, and Actinobacteria (Table 1), thus confirming what was observed in soil bacteria in the desert steppe of Inner Mongolia (Wu et al., 2010).
Among the treatment groups, Firmicutes included eight species which belonged to
four genera (Table 1): five species of Bacillus (XJ2, XJ40, XJ43, XJ44 and XJ45), one
species of Staphylococcus (XJ21), one species of Trichococcus sp. (XJ19), and one
species of Paenibacillus (XJ11). The XJ14 and XJ15 were classified into Microvirga of
Soil layers
0.00-0.02 m
Cultivable bacteria (10
4 CFU per g of soil)
0 20 40 60 80 100
CK +N +N+W +N-W
a a
ab
b a
ab ab
b
a
b b
b a
ab b
a a a a a
b b b ab
0.00-0.30 m 0.20-0.30 m
0.10-0.20 m 0.05-0.10 m
0.02-0.05 m
Figure 2. The number of cultivable bacteria at each soil layer in different treatment groups. Note:
Alphaproteobacteria and Pseudomonas of Gammaproteobacteria, respectively. One
species (XJ32) is affiliated with Duganella of β-Proteobacteria. Two species (XJ9 and
XJ5) of Chryseobacterium and one species (XJ13) of Pontibacter were classified as
Bacteroidetes. Only two species, XJ10 and XJ17, were classified into Clavibacter and
Streptomyces, respectively, of Actinobacteria, in addition, an unclassified species
(XJ25) was found.
Among the species examined, XJ40 and XJ19 were primarily identified as potential novel species of the genus, based on 97 % similarity with their closest neighbors.
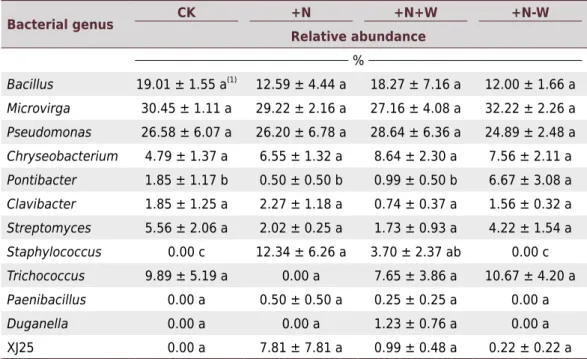
Pseudomonas, Microvirga, and Bacillus were the dominant genera in each treatment
group. The relative abundance of Microvirga (29.22, 32.22, 27.1, and 30.45 % in the
+N, +N+W, +N-W, and CK treatment groups, respectively), Pseudomonas (26.2, 24.89,
28.64, and 26.58 % in the +N, +N+W, +N-W, and CK treatment groups, respectively)and
Bacillus (12.59, 18.27, 12, and 19 % in the +N, +N+W, +N-W, and CK treatment groups,
respectively)were not significantly different among the treatment groups (Table 2).
The relative abundance of Pontibacter decreased in the +N (0.5 %) and +N+W treatments
(0.99 %), compared to the CK group (1.85 %). However, the relative abundance of
Pontibacter increased in the +N–W treatment. We suspect that the effects of nitrogen
addition – via limitation of bacterial growth and reproduction – may contribute to the responses of Pontibacter, whereas more precipitation during N deposition may release
them from suppression. In contrast, compared to CK, the +N and +N+W treatments significantly increased the relative abundance of Staphylococcus (p<0.05). The +N–W
treatment significantly increased the relative abundance of Pontibacter (p<0.05) and
decreased the relative abundance of Staphylococcus (p<0.05) compared to the +N
treatment.We found experimental treatment groups did not significantly change the
relative abundance of other genera (p<0.05).
Table 1. Phylogenetic similarity between the cultivable bacteria isolated and their closest type strains
Groups No. of isolates Closest type strain (accession number) Similarity
%
Firmicutes
XJ2 Bacillus sp. CRRI-93_SWAzo (KJ847762) 99
XJ40 Bacillus subtilis strain CYBS-19 (JQ361068) 97
XJ43 Bacillus sp. KT15 (KJ734005) 99
XJ44 Bacillus sp.W30 (KC527674) 99
XJ45 Bacillus sp. JZDN43 (DQ659022) 99
XJ11 Paenibacillus castaneae (JF496373) 99
XJ19 Uncultured Trichococcus sp. QRSYY9 (EU919224) 97
XJ21 Staphylococcus sp. AJAR-J1 (HM103367) 99
Bacteroidetes
XJ5 Chryseobacterium sp. (F022) 99
XJ9 Chryseobacterium sp. S63.04.P.COS.HB.H.Ulcer.D.M
(JX287786) 100
XJ13 Pontibacter sp. Al-Dhabi-10 (KJ406573) 98
Alphaproteobacteria XJ14 Microvirga aerilata (KF580850) 99
Gammaproteobacteria XJ15 Pseudomonas sp. R-45822 (FR775122) 99
Actinobacteria XJ10 uncultured Clavibacter sp. (EF554974) 100
XJ17 Streptomyces sampsonii (HQ439407) 98
Betaproteobacteria XJ32 Duganella sp. 7A-619 (KF441649) 98
Li (2016) indicated that N addition for five consecutive years at the rate of 50 kg ha-1
yr-1 N
had little effect onsoil bacterial community composition in a meadow steppe of Northern China. Zeng et al. (2016) reported N addition at rates of 60 kg ha-1
yr-1 N or 120 kg ha-1 yr-1 N
for six years did not result in a significant response in soil bacterial community composition in a typical steppe of northern China. In the present study, nitrogen addition (10 g m-2
yr-1 N), precipitation manipulation, and the treatment with their interaction had no notable effect on dominant genera and other genera, except for Pontibacter and Staphylococcus in the bacterial community. Interactions among plants, microorganisms, and soil play a
key role in nutrient cycling (Miki et al., 2010). In our study zone, soil fertility was poor with low N content (1.27 g kg-1
). Moisture and N content are believed to be the limiting factors in this region (Wang et al., 2014). The magnitude of N addition, coupled with low soil moisture content in our study, may not fulfill all the needs of vegetation and microorganisms (Zechmeister-Boltenstern et al., 2011). Plants may have consumed more N for growth if precipitation had not been limited. Therefore, considering the influence of above-ground vegetation, the response mechanism of N addition and precipitation manipulation on the microbial community requires further comprehensive research. In addition, further research on the historical strategy of bacterial life and the ecological amplitude of Pontibacter and Staphylococcus are needed to gain clearer insights into
their sensitivity to the addition of N, to changes in precipitation, and to the combination between these factors.
Redundancy analysis (RDA) showed that the 1st (RD1) and 2nd (RD2) principal components
explained 47.7 and 20.1 % of the total variance of bacterial groups, respectively (Figure 3). A Monte Carlo permutation test revealed that available potassium was significant in explaining the variability of the bacterial community structure among treatments (p<0.05). Available potassium (28.5 % of the variability), available phosphorus (16.9 % of the variability), and moisture content (15.9 % of the variability) were the main factors influencing the variance of the cultivable bacteria community among treatments, followed by NO3
--N content (12.7 % of the variability), organic matter (12.5 % of the variability), soil NH4+-N content (7.68 % of the variability), and pH (5.30 %). Nielsen et al. (2010) reported that dissimilarity in bacterial communities were positively related to differences in soil pH between habitats.
Table 2. Composition of the community and relative abundance of cultivable bacteria at the genus
level in different treatment groups
Bacterial genus CK +N +N+W +N-W
Relative abundance %
Bacillus 19.01 ± 1.55 a(1) 12.59 ± 4.44 a 18.27 ± 7.16 a 12.00 ± 1.66 a
Microvirga 30.45 ± 1.11 a 29.22 ± 2.16 a 27.16 ± 4.08 a 32.22 ± 2.26 a
Pseudomonas 26.58 ± 6.07 a 26.20 ± 6.78 a 28.64 ± 6.36 a 24.89 ± 2.48 a
Chryseobacterium 4.79 ± 1.37 a 6.55 ± 1.32 a 8.64 ± 2.30 a 7.56 ± 2.11 a
Pontibacter 1.85 ± 1.17 b 0.50 ± 0.50 b 0.99 ± 0.50 b 6.67 ± 3.08 a
Clavibacter 1.85 ± 1.25 a 2.27 ± 1.18 a 0.74 ± 0.37 a 1.56 ± 0.32 a
Streptomyces 5.56 ± 2.06 a 2.02 ± 0.25 a 1.73 ± 0.93 a 4.22 ± 1.54 a
Staphylococcus 0.00 c 12.34 ± 6.26 a 3.70 ± 2.37 ab 0.00 c
Trichococcus 9.89 ± 5.19 a 0.00 a 7.65 ± 3.86 a 10.67 ± 4.20 a
Paenibacillus 0.00 a 0.50 ± 0.50 a 0.25 ± 0.25 a 0.00 a
Duganella 0.00 a 0.00 a 1.23 ± 0.76 a 0.00 a
XJ25 0.00 a 7.81 ± 7.81 a 0.99 ± 0.48 a 0.22 ± 0.22 a
Moreover, Griffiths et al. (2011) provided large-scale confirmation of the role of pH in structuring bacterial taxa. Yuan et al. (2016) also showed that alterations in soil pH and plant composition indirectly affect bacterial community composition. Ramirez et al. (2010) suggested responses in microbial respiration to N addition resulted from direct effects of N availability. A recent study found the change in bacterial structure was caused by nitrogen addition (Zhang and Han, 2012). Our results were inconsistent with the results of the above study. Our data supported that the assemblage of the microbial community driven by
arid climates may adapt to desiccation stress and become less sensitive to precipitation
changes (Sun et al., 2014; Yao et al., 2017) and low N addition (Sun et al., 2014) in such
arid steppe conditions. Due to high evaporation and transpiration with little precipitation,
N availability was limited, and N addition plus precipitation manipulation did not result in significant variation in the cultivable bacterial community structure.
Soil microorganisms perform a key function in ecosystem sustainability. Soil microbial diversity is a sensitive bioindicator for evaluation of ecosystem stability and for monitoring of soil quality in grasslands. Loss of biodiversity is considered a major threat to ecosystem services. The +N treatment had a significantly negative impact on the species richness of cultivable bacteria compared to CK in the 0.00-0.02 m layer (p<0.01) (Table 3), which is consistent with the findings of Zeng et al. (2016) and Ling et al. (2017). Zeng et al. (2016) reported ammonium availability directly affects soil bacterial diversity. However, in this study, +N did not influence bacterial evenness, contrary to previous studies showing a
RAD Axis 1
– 1.0 1.0
Bacillus
– 1.
01
.0
RAD Axis 2
Microvirga
Streptomyces
Duganella
pH
Clavibacter
Pseudomonas
Paenibacillus
Moisture content
XJ25
Chyseobacterium Staphylococcus
Organic matter
Pontibacter
NO3
--N Available phosphorus
Available potassium
Trichococcus
NH4 +
-N
Figure 3. Redundancy discriminate analysis (RDA) plots of the relative abundance of bacterial
genera and soil properties. Soil properties included available potassium, available phosphorus,
moisture content, NO3
--N, organic matter, NH4+-N, and pH (dashed arrows). Bacterial genera included
decline in bacterial evenness in grassland soils after fertilization (McCaig et al., 2001). Additionally, compared to CK, the +N+W and +N-W treatments also had a negative
impact on species richness in the 0.00-0.02 m soil layer (Table 3). As shown in Jia et al.
(2017), this finding may be supported by related changes in soil moisture and nitrogen content, and the maladjustment of bacteria to a reduction in pH in the 0.00-0.02 m layer. Compared to the +N treatment group, the +N+W and +N-W treatment groups had no
impact on bacterial diversity in the 0.00-0.02 m layer, consistent with previous studies showing little impact on bacterial diversity (Li, 2016). Zeng et al. (2016) showed that
the content of NH4+-N and NO3
--N negatively correlated with bacterial diversity. In the present study, soil NH4+-N content played an important role in structuring the bacterial community. Indeed, compared to N addition, N addition plus precipitation manipulation
did not cause a change in bacterial diversity, indicating that bacteria may be insensitive
to precipitation addition or reduction, as Cruz-Martínez et al. (2009) found. Our data demonstrated that there may be some kind of bacterial adaption mechanism to limited water and N to maintain relative stability in such desert steppe conditions. We did not observe significant effects of treatments on microbial diversity at other soil layers, except for the 0.00-0.02 m.
CONCLUSIONS
Seven years of simulated nitrogen deposition or the combination of nitrogen deposition and precipitation manipulation significantly decreased the number of cultivable soil bacteria. Precipitation manipulation based on N addition did not impact the number of cultivable soil bacteria. Nitrogen addition and precipitation manipulation modified the bacterial communities at the genus level by shifting the relative abundance of the minority
Table 3. Diversity of cultivable bacteria at each soil layer in different treatment groups
Diversity Index Soil layer CK +N +N+W +N–W
m
Species richness (S)
0.00-0.02 10.0 ± 1.73 A(1) 5.33 ± 1.15 B 5.00 ± 1.00 B 6.00 ± 0.00 B
0.02-0.05 6.67 ± 1.53 a 6.00 ± 0.00 a 7.00 ± 1.00 a 7.33 ± 0.58 a
0.05-0.10 6.33 ± 1.53 a 6.00 ± 2.00 a 6.33 ± 1.53 a 6.67 ± 0.58 a
0.10-0.20 5.67 ± 0.58 a 4.67 ± 0.58 b 5.00 ± 1.00 b 3.67 ± 1.53 b
0.20-0.30 3.33 ± 0.58 a 3.33 ± 1.15 a 3.33 ± 1.15 a 3.67 ± 0.58 a
0.00-0.30 11.00 ± 1.00 a 9.33 ± 0.58 a 11.33 ± 1.15 a 10.00 ± 1.73 a
Shannon-Weiner Index (H’)
0.00-0.02 1.67 ± 0.24 a 1.24 ± 0.17 a 1.21 ± 0.38 a 1.53 ± 0.23 a
0.02-0.05 1.56 ± 0.25 a 1.50 ± 0.17 a 1.55 ± 0.17 a 1.68 ± 0.08 a
0.05-0.10 1.02 ± 0.19 a 1.34 ± 0.31 a 1.48 ± 0.13 a 1.46 ± 0.20 a
0.10-0.20 1.45 ± 0.05 a 1.42 ± 0.14 a 1.34 ± 0.10 a 1.03 ± 0.43 a
0.20-0.30 0.91 ± 0.16 a 0.98 ± 0.30 a 0.91 ± 0.27 a 1.06 ± 0.07 a
0.00-0.30 1.74 ± 0.15 a 1.70 ± 0.14 a 1.81 ± 0.11 a 1.85 ± 0.03 a
Evenness (E)
0.00-0.02 0.84 ± 0.03 a 0.79 ± 0.19 a 0.75 ± 0.16 a 0.85 ± 0.13 a
0.02-0.05 0.83 ± 0.06 a 0.86 ± 0.09 a 0.80 ± 0.14 a 0.84 ± 0.02 a
0.05-0.10 0.56 ± 0.05 a 0.76 ± 0.06 a 0.81 ± 0.05 a 0.77 ± 0.08 a
0.10-0.20 0.84 ± 0.02 a 0.92 ± 0.04 a 0.84 ± 0.05 a 0.85 ± 0.14 a
0.20-0.30 0.77 ± 0.03 a 0.86 ± 0.07 a 0.82 ± 0.20 a 0.83 ± 0.06 a
0.00-0.30 0.75 ± 0.05 a 0.77 ± 0.06 a 0.75 ± 0.05 a 0.81 ± 0.04 a
genus of bacteria in the 0.00-0.30 m layer and resulted in the loss of species richness in the 0.00-0.02 m layer, although the present levels of N addition and precipitation manipulation in our study could not significantly alter the bacterial Shannon-Wiener and evenness indexes in the 0.00-0.30 m layer. In general, it is necessary to take above-ground plants, inter-annual precipitation, increased level of nitrogen addition and precipitation manipulation, and the duration of treatments into consideration when we study how the composition and diversity of the microbial community in the soil are affected by climate
change (nitrogen deposition and precipitation manipulation).
ACKNOWLEDGMENTS
We thank Guodong Han, Guogang Zhang, and many others for setting up the experiment. Research was funded by the National Natural Science Foundation of China (31500365, 31770499, 31560140, 31100330, 31270502, 41403082), National Key Research and Development Program of China (2016YFC0500504) and the Key Technology Research and Development Program of Tianjin (15ZCZDSF00410).
REFERENCES
Aber JD, Magill AH. Chronic nitrogen addition at the Harvard Forest (USA): the first 15 years of a nitrogen saturation experiment. Forest Ecol Manag. 2004;196:1-5. https://doi.org/10.1016/j.foreco.2004.03.009
Allen AS, Schlesinger WH. Nutrient limitations to soil microbial biomass and activity in loblolly pine forests. Soil Biol Biochem. 2004;36:581-9. https://doi.org/10.1016/j.soilbio.2003.12.002 Bai Y, Wu J, Clark CM, Naeem S, Pan Q, Huang J, Zhang L, Han X. Tradeoffs and
thresholds in the effects of nitrogen addition on biodiversity and ecosystem functioning: evidence from inner Mongolia Grasslands. Glob Change Biol. 2010;16:358-72.
https://doi.org/10.1111/j.1365-2486.2009.01950.x
Balser TC. The impact of long-term nitrogen addition on microbial community
composition in three Hawaiian forest soils. TheScientificWorldJOURNAL. 2001;1:500-4. https://doi.org/10.1100/tsw.2001.450
Bell C, Acosta-Martinez V, McIntyre NE, Cox SB, TissueD T, Zak J. Long-term responses of soil microbial community structure and function to changes in precipitation in a Chihuahuan Desert grassland: impacts towards understanding soil responses to global climate change. In: 95th ESA Annual Convention; 2010 Aug; Pittsburgh, Pennsylvania. Washington, DC: Ecological Society of America; 2010.
Bi J, Zhang N, Liang Y, Yang H, Ma K. Interactive effects of water and nitrogen addition on soil microbial communities in a semiarid steppe. J Plant Ecol. 2012;5:320-9. https://doi.org/10.1093/jpe/rtr046
Boxman AW, Blanck K, Brandrud T-E, Emmett BA, Gundersen P, Hogervorst RF, Kjønaas OJ, Persson H, Timmermann V. Vegetation and soil biota response to experimentally changed nitrogen inputs in coniferous forest ecosystems of the NITREX project. Forest Ecol Manag. 1998;101:65-79. https://doi.org/10.1016/S0378-1127(97)00126-6
Chen Q, Wang Q, Han X, Wan S, Li L. Temporal and spatial variability and controls of soil respiration in a temperate steppe in northern China. Global Biogeochem Cy. 2010;24:GB2010. https://doi.org/10.1029/2009GB003538
Compton JE, Watrud LS, Porteous LA, DeGrood S. Response of soil microbial biomass and community composition to chronic nitrogen additions at Harvard forest. Forest Ecol Manag. 2004;196:143-58. https://doi.org/10.1016/j.foreco.2004.03.017
Frey SD, Knorr M, Parrent JL, Simpson RT. Chronic nitrogen enrichment affects the structure and function of the soil microbial community in temperate hardwood and pine forests. Forest Ecol Manag. 2004;196:159-71. https://doi.org/10.1016/j.foreco.2004.03.018
Gerdol R, Bragazza L, Brancaleoni L. Microbial nitrogen cycling interacts with exogenous nitrogen supply in affecting growth of Sphagnum papillosum. Environ Exp Bot. 2006;57:1-8.
https://doi.org/10.1016/j.envexpbot.2005.03.005
Griffiths RI, Thomson BC, James P, Bell T, Bailey M, Whiteley AS. The bacterial biogeography of British soils. Environ Microbiol. 2011;13:1642-54. https://doi.org/10.1111/j.1462-2920.2011.02480.x
Hawkes CV, Kivlin SN, Rocca JD, Huguet V, Thomsen MA, Suttle KB. Fungal community responses to precipitation. Glob Change Biol. 2011;17:1637-45. https://doi.org/10.1111/j.1365-2486.2010.02327.x
Hooper DU, Johnson L. Nitrogen limitation in dryland ecosystems: responses to
geographical and temporal variation in precipitation. Biogeochemistry. 1999;46:247-93. https://doi.org/10.1007/BF01007582
Hu Y-L, Jung K, Zeng D-H, Chang SX. Nitrogen- and sulfur-deposition-altered soil microbial community functions and enzyme activities in a boreal mixedwood forest in western Canada. Can J For Res. 2013;43:777-84. https://doi.org/10.1139/cjfr-2013-0049
Huang G, Li Y, Su YG. Effects of increasing precipitation on soil microbial community
composition and soil respiration in a temperate desert, Northwestern China. Soil Biol Biochem. 2015;83:52-6. https://doi.org/10.1016/j.soilbio.2015.01.007
Intergovernmental Panel on Climate Change - IPCC. Climate change 2013: the physical science basis. Contribution of Working Group I to the fifth assessment report of the intergovernmental panel on climate change. Cambridge: Cambridge University Press; 2014.
Intergovernmental Panel on Climate Change - IPCC. Climatic Change 2007: the physical science basis. Contribution of Working Group I to the fourth assessment report of the intergovernmental panel on climate change. Cambridge: Cambridge University Press; 2007.
IUSS Working Group WRB. World reference base for soil resources 2014, update 2015: International soil classification system for naming soils and creating legends for soil maps. Rome: Food and Agriculture Organization of the United Nations; 2015. (World Soil Resources Reports, 106).
Jia M, Liu C, Li Y, Xü S, Han G, Huang J, Jin B, Zou Y, Zhang G. Response of fungal composition and diversity to simulated nitrogen deposition and manipulation of precipitation in soils of an Inner Mongolia desert steppe of Northern China. Can J Soil Sci. 2017;97:613-25. https://doi.org/10.1139/cjss-2016-0135
Jia MQ, Liu CB, Xü S, Li Y, Han GD, Jin BH, Zou Y, Zhang GG. Spatial distribution of culturable actinomycete in the desert steppe of Inner Mongolia, China. Journal of Tianjin Normal University. 2016;36:50-4.
Johnson D, Leake JR, Lee JA, Campbell CD. Changes in soil microbial biomass and microbial activities in response to 7 years simulated pollutant nitrogen deposition on a heathland and two grasslands. Environ Pollut. 1998;103:239-50. https://doi.org/10.1016/S0269-7491(98)00115-8
Khalili B, Ogunseitan OA, Goulden ML, Allison SD. Interactive effects of precipitation manipulation and nitrogen addition on soil properties in California grassland and shrubland. Appl Soil Ecol. 2016;107:144-53. https://doi.org/10.1016/j.apsoil.2016.05.018
Knapp AK, Fay PA, Blair JM, Collins SL, Smith MD, Carlisle JD, Harper CW, Danner BT, Lett MS McCarron JK. Rainfall variability, carbon cycling, and plant species diversity in a mesic grassland. Science. 2002;298:2202-5. https://doi.org/10.1126/science.1076347
Kurapova AI, Zenova GM, Sudnitsyn II, Kizilova AK, Manucharova NA, Norovsuren Zh,
Ladwig LM, Collins SL, Swann AL, Xia Y, Allen MF, Allen EB. Above- and belowground responses to nitrogen addition in a Chihuahuan Desert grassland. Oecologia. 2012;169:177-85.
https://doi.org/10.1007/s00442-011-2173-z
Li D. Dynamics and ecological stability of form stipa breviflora desert steppe. Grassland of China. 1990;4:1-5.
Li WJ. The influence of nitrogen deposition and increased precipitation on soil microbial
composition and diversity dynamic in a Stipa baicalensis meadow steppe [thesis]. Changchun:
Northeast Normal University; 2016.
Liao G, Jia Y. Grassland Resource of China. Beijing: Chinese Science and Technology Press; 1996. Lin D, Xia J, Wan S. Climate warming and biomass accumulation of terrestrial plants: a meta-analysis. New Phytol. 2010;188:187-98. https://doi.org/10.1111/j.1469-8137.2010.03347.x Lindberg N, Engtsson JB, Persson T. Effects of experimental irrigation and drought on the composition and diversity of soil fauna in a coniferous stand. J Appl Ecol. 2002;39:924-36. https://doi.org/10.1046/j.1365-2664.2002.00769.x
Ling N, Chen D, Guo H, Wei J, Bai Y, Shen Q, Hu S. Differential responses of soil bacterial communities to long-term N and P inputs in a semi-arid steppe. Geoderma. 2017;292:25-33. https://doi.org/10.1016/j.geoderma.2017.01.013
Liu L. Effect of warmer and drier climate on the soil microorganism composition and diversity of inner Mongolia desert steppe soil [thesis]. Tianjin: Tianjin Normal University; 2014.
Liu X, Zhang Y, Han W, Tang A, Shen J, Cui Z, Vitousek P, Erisman JW, Goulding K, Christie P, Fangmeier A, Zhang F. Enhanced nitrogen deposition over China. Nature. 2013;494:459-62. https://doi.org/10.1038/nature11917
Liu Z, Fu B, Zheng X, Liu G. Plant biomass, soil water content and soil N:P ratio regulating soil microbial functional diversity in a temperate steppe: a regional scale study. Soil Biol Biochem. 2010;42:445-50. https://doi.org/10.1016/j.soilbio.2009.11.027
Lü F-M, Lü X-T, Liu W, Han X, Zhang G-M, Kong D-L, Han X-G. Carbon and nitrogen storage in plant and soil as related to nitrogen and water amendment in a temperate steppe of northern China. Biol Fert Soils. 2011;47:187-96. https://doi.org/10.1007/s00374-010-0522-4
Lu RK. Methods of soil and agrochemistry analysis. Beijing: Chinese Agricultural Press; 2000. Lü X-T, Kong D-L, Pan Q-M, Simmons ME, Han X-G. Nitrogen and water availability interact to affect leaf stoichiometry in a semi-arid grassland. Oecologia. 2012;168:301-10. https://doi.org/10.1007/s00442-011-2097-7
Ma H-k, Bai G-y, Sun Y, Kostenko O, Zhu X, Lin S, Ruan W-b, Zhao N-x, Bezemer TM. Opposing effects of nitrogen and water addition on soil bacterial and fungal communities in the Inner Mongolia steppe: a field experiment. Appl Soil Ecol. 2016;108:128-35. https://doi.org/10.1016/j.apsoil.2016.08.008
McCaig AE, Glover LA, Prosser JI. Numerical analysis of grassland bacteria community structure under different land management regimens by using 16S ribosomal DNA sequence data and denaturing gradient gel electrophoresis banding patterns. Appl Environ Microb. 2001;67:4554-9. https://doi.org/10.1128/AEM.67.10.4554-4559.2001
Miki T, Ushio M, Fukui S, Kondoh M. Functional diversity of microbial decomposers facilitates plant coexistence in a plant-microbe-soil feedback model. PNAS. 2010;107:14251-6. https://doi.org/10.1073/pnas.0914281107
Nielsen UN, Osler GHR, Campbell CD, Burslem DFRP, van der Wal R. The influence of vegetation type, soil properties and precipitation on the composition of soil mite and microbial communities at the landscape scale. J Biogeogr. 2010;37:1317-28. https://doi.org/10.1111/j.1365-2699.2010.02281.x
Ramirez KS, Craine JM, Fierer N. Nitrogen fertilization inhibits soil microbial respiration regardless of the form of nitrogen applied. Soil Biol Biochem. 2010;42:2336-8. https://doi.org/10.1016/j.soilbio.2010.08.032
Sarathchandra SU, Ghani A, Yeates GW, Burch G, Cox NR. Effect of nitrogen and phosphate fertilisers on microbial and nematode diversity in pasture soils. Soil Biol Biochem. 2001;33:953-64. https://doi.org/10.1016/S0038-0717(00)00245-5
Shi Y, Wang ZQ, Zhang XY, Sun XM, Liu XY, He NP, Yu Q. Effects of nitrogen and phosphorus addition on soil microbial community composition in temperate typical grassland in Inner Mongolia. Acta Ecologica Sinica. 2014;34:4943-9. https://doi.org/10.5846/stxb201306081430 Sun L-j, Qi Y-c, Dong Y-s, He Y-t, Peng Q, Liu X-c, Jia J-q, Guo S-f, Cao C-c. Interactions of water and nitrogen addition on soil microbial community composition and functional diversity depending on the inter-annual precipitation in a Chinese steppe. J Integr Agr. 2015;14:788-99. https://doi.org/10.1016/S2095-3119(14)60773-5
Sun S, Zhao H, Xing F, Bai Z, Gao Y, Dong Y, Zhou J, Wu Y, Yang Y. Response of soil microbial community structure to increased precipitation and nitrogen addition in a semiarid meadow steppe. Eur J Soil Sci. 2017;68:524-36. https://doi.org/10.1111/ejss.12441
Suttle KB, Thomsen MA, Power ME. Species interactions reverse grassland responses to changing climate. Science. 2007;315:640-2. https://doi.org/10.1126/science.1136401
Suzuki MT, Rappé MS, Haimberger ZW, Winfield H, Adair N, Ströbel J, Giovannoni S. Bacterial diversity among small-subunit rRNA gene clones and cellular isolates from the same seawater sample. Appl Environ Microb. 1997;63:983-9.
Vitousek PM, Aber JD, Howarth RW, Likens GE, Matson PA, Schindler DW, Schlesinger WH, Tilman DG. Human alteration of the global nitrogen cycle: sources and consequences. Ecol Appl. 1997;7:737-50. https://doi.org/10.1890/1051-0761(1997)007[0737:HAOTGN]2.0.CO;2 Wang R, Dungait JAJ, Creamer CA, Cai J, Li B, Xu Z, Zhang Y, Ma Y, Jiang Y. Carbon and nitrogen dynamics in soil aggregates under long-term nitrogen and water addition in a temperate steppe. Soil Biol Biochem. 2015;79:527-35. https://doi.org/10.2136/sssaj2014.09.0351
Wang R, Filley TR, Xu Z, Wang X, Li M-H, Zhang Y, Luo W, Jiang Y. Coupled response of soil carbon and nitrogen pools and enzyme activities to nitrogen and water addition in a semi-arid grassland of Inner Mongolia. Plant Soil. 2014;381:323-36. https://doi.org/10.1007/s11104-014-2129-2
Wang CC. Influences of warming and nitrogen addition on soil respiration and plant community in inner Mongolia desert setppe [thesis]. Huhhot: Inner Mongolia Agricultural University; 2014. Williams MA, Rice CW. Seven years of enhanced water availability influences the physiological, structural, and functional attributes of a soil microbial community. Appl Soil Ecol. 2007;35:535-45. https://doi.org/10.1016/j.apsoil.2006.09.014
Wu Y, Ma W, Li H, Lu P, Lü G. Characteristics of bacterial community structure in degraded desert steppe of Inner Mongolia. Acta Ecologica Sinica. 2010;30:6355-62.
Xu GH, Zheng HY. Handbook of analysis methods of soil microbiology. Beijing: Agricultural Press; 1986.
Xu Z, Wan S, Zhu G, Ren H, Han X. The influence of historical land use and water availability on grassland restoration. Restor Ecol. 2010;18:217-25. https://doi.org/10.1111/j.1526-100X.2009.00595.x
Yang H, Li Y, Wu M, Zhang Z, Li L, Wan S. Plant community responses to nitrogen addition and increased precipitation: the importance of water availability and species traits. Glob Change Biol. 2011;17:2936-44. https://doi.org/10.1111/j.1365-2486.2011.02423.x
Yao M, Rui J, Niu H, Heděnec P, Li J, He Z, Wang J, Cao W, Li X. The differentiation of soil bacterial communities along a precipitation and temperature gradient in the eastern Inner Mongolia steppe. Catena. 2017;152:47-56. https://doi.org/10.1016/j.catena.2017.01.007
Zechmeister-Boltenstern S, Michel K, Pfeffer M. Soil microbial community in European forests in relation to forest type and atmospheric nitrogen deposition. Plant Soil. 2011;343:37-50. https://doi.org/10.1007/s11104-010-0528-6
Zeng J, Liu X, Song L, Lin X, Zhang H, Shen C, Chu H. Nitrogen fertilization directly affects soil bacterial diversity and indirectly affects bacterial community composition. Soil Biol Biochem. 2016;92:41-9. https://doi.org/10.1016/j.soilbio.2015.09.018