92 (2013) 1908–1914
ContentslistsavailableatSciVerseScienceDirect
Carbohydrate
Polymers
j o ur na l h o me p ag e : w w w . e l s e v i e r . c o m / l o c a t e / c a r b p o l
Sulfonation
and
anticoagulant
activity
of
fungal
exocellular

-(1
→
6)-
d
-glucan
(lasiodiplodan)
Ana
Flora
D.
Vasconcelos
a,
Robert
F.H.
Dekker
b,
Aneli
M.
Barbosa
b,
Elaine
R.
Carbonero
c,
Joana
L.M.
Silveira
c,
Bianca
Glauser
d,
Mariana
Sá
Pereira
d,
Maria
de
Lourdes
Corradi
da
Silva
a,∗aDeptodeFísica,QuímicaeBiologia,UniversidadeEstadualPaulista–UNESP,CEP19060-900,PresidentePrudente,SãoPaulo,Brazil bBiorefiningResearchInstitute,LakeheadUniversity,ThunderBay,Ontario,CanadaP7B5E1
cDeptodeBioquímicaeBiologiaMolecular,UniversidadeFederaldoParaná,CEP81531-980,Curitiba,Paraná,Brazil
dLaboratóriodeTecidoConjuntivo,HospitalUniversitárioClementinoFragaFilho–UniversidadeFederaldoRiodeJaneiro,CEP21941-590,RiodeJaneiro,RJ,Brazil
a
r
t
i
c
l
e
i
n
f
o
Articlehistory: Received11June2012
Receivedinrevisedform31August2012 Accepted11October2012
Available online 14 November 2012
Keywords: -(1→6)-d-Glucan Lasiodiplodiatheobromae Lasiodiplodan Sulfonation Anticoagulantactivity
a
b
s
t
r
a
c
t
An exocellular-(1→6)-d-glucan(lasiodiplodan) producedbya strainof Lasiodiplodiatheobromae
(MMLR)grownonsucrosewasderivatizedbysulfonationtopromoteanticoagulantactivity.The struc-turalfeaturesofthesulfonated-(1→6)-d-glucanwereinvestigatedbyUV–vis,FT-IRand13CNMR
spectroscopy,andtheanticoagulantactivitywasinvestigatedbytheclassicalcoagulationassaysAPTT, PTandTTusingheparinasstandard.Thecontentofsulfuranddegreeofsubstitutionofthesulfonated glucanwas11.73%and0.95,respectively.UVspectroscopyshowedabandat261nmduetothe unsat-uratedbondformedinthesulfonationreaction.ResultsofFT-IRand13CNMRindicatedthatsulfonyl groupswereinsertedonthepolysaccharide.Thesulfonated-(1→6)-d-glucanpresentedanticoagulant
activityasdemonstratedbytheincreaseindosedependenceofAPTTandTT,andtheseactionsmost likelyoccurredbecauseoftheinsertedsulfonategroupsonthepolysaccharide.Thelasiodiplodandidnot inhibitthecoagulationtests.
© 2012 Elsevier Ltd.
1. Introduction
-Glucansarefoundmainlyinthecellwallofyeastsand fil-amentousfungi,asminorityconstituentsinthecytosol,andcan alsobesecretedasexo-biopolymerstotheenvironment(Williams, 1997).Theyhaveemergedasanimportantclassofbioactive prod-uctswithbiologicalresponsemodifying(BRM)activities(Bohn& BeMiller,1995).Besidesimmunoprotectiveactivity, exopolysac-charidesofthe-glucantypehavebeenexaminedinrelationto theantithrombotic,antioxidant,antiviral,anti-inflammatory, anti-coagulantactions,andantiproliferativeactivityonbreastcancer cells (Brandi etal., 2011;Cunha et al., 2012;Kato et al.,2010; Martinichen-Herrero,Carbonero,Gorin,&Iacomini,2005;Wang etal.,2010).
Thebiologicalactivitiescanbepresentedbythemoleculesin natura,orthroughchemicalmodification.Thechemical derivati-zationofglycansoffersanopportunitytoenhancetheiraction,
and even develop activity in non-bioactive molecules, which
can be used as newpharmacological agents (Mantovani et al.,
2008; Vetvicka, Vetvickova, Frank, & Yvin,2008).Furthermore,
∗Correspondingauthor.Tel.:+551832295743;fax:+551832215682. E-mailaddress:corradi@fct.unesp.br(M.d.L.CorradidaSilva).
the inclusion of sulfonate groups onglycans enablesthe
gen-eration of water-soluble molecules, and this is important for
anticoagulant and other biological activities (Mendes et al.,
2009).
Glucans of the -(1→3)- and -(1→3;1→6)-types are the mostdescribed inscientific articlesand patents.Theyhave lin-ear,branchedorcyclic(Laroche&Michaud,2007)structures,and aretargetsforresearchfortheirpharmacologicaland immunolog-icaleffects(Vetvickaetal.,2008).-(1→6)-d-Glucansarewidely
knownaspustulanproducedbylichensofUmbilicariaceaespecies (Narui,Sawada,Culberson,Culberson,&Shibata, 1999), andare commonlypresentasconstituentsofthefungalandyeastcellwall (Klis,Mol,Hellingwerf,&Brul,2002).Asexocellularbiopolymers, -(1→6)-d-glucansareuncommon,ifnotrare,andareknownto
beproducedbyonlysomefungi(Cunhaetal.,2012;Vasconcelos etal.,2008).
Thebiological activityofthe-glucansfromfungiincluding mushrooms thatcompriseboth -(1→3)-and (1→6)-d-glucans
areconsideredthemosteffectiveimmunestimulatoryagents(Rop, Mlcek&Jurikova,2009),andthepresenceofbranchesatC-6onthe -(1→3)-glucanchainaswellasatriplehelixconformationare
importantstructuralfeaturesdeterminingBRMactivityofthese
polysaccharides(Bohn&BeMiller,1995;Leung,Liu,Koon,&Fung, 2006).
0144-8617© 2012 Elsevier Ltd.
http://dx.doi.org/10.1016/j.carbpol.2012.10.034
Open access under the Elsevier OA license.
92 (2013) 1908–1914 1909
Substanceswithanticoagulantpropertieshavebeenusedboth
in therapeutic processes and in vitro to treat medical
condi-tions,andnaturalheparinismostwidelyused(Wang,Li,Zheng, Normakhamatov, & Guo, 2007). Unfortunately, heparin shows
somecontra-indicationssuchasbleeding,andmoreover,because
heparinisextractedfromanimaltissues,ittendstocausearisk
ofcontaminationbyanimal-derivedpathogens.Developmentof
alternativestoheparinthereforeisanimportantfieldofresearch. Nomammaliansourceofheparinoritsderivativesareconsidered tobeidealchoices,andconsequently,polysaccharidesulfonates (naturalorchemicallyderivatized)areofspecialinterest(Glauser etal.,2009;Pomin,2012).
Anabundantsourceofanticoagulantpolysaccharidesismarine algaethatcontainavarietyofnaturalsulfatedgalactansand sul-fatedfucans(Melo,Pereira,Foguel,&Mourão,2004).Othersulfated
polysaccharideswithanticoagulantactivityarefoundin marine
invertebrates (Pomin,2012).Chemically sulfonated
polysaccha-rides, which present anticoagulant and antithrombotic activity,
have been obtainedfrom various polysaccharidetypes suchas
microbial -glucans (Brandi et al., 2011; Mendes et al., 2009)
anddextrans,andplant-derivedgalactoglucomannansand
galac-tomannans(Martinichen-Herreroetal.,2005;Yang,Du,Wen,Li,& Hu,2003).
Inthisworkwedescribeforthefirsttimethesulfonationofan exocellular -(1→6)-d-glucan(lasiodiplodan) fromLasiodiplodia
theobromaeMMLRgrownonsucroseascarbonsource(Vasconcelos
et al., 2008), and the effect of sulfonation on promoting the
solubilityofthispolysaccharideinaqueoussolutions,andthe phys-iologicalactivityasananticoagulantasalternativetoheparin.
2. Experimental
2.1. Materials
Sodium heparin (5000UI/mL) was purchased from Akzo
Organon (São Paulo, Brazil). Human plasma was obtained by
centrifugation (450×g/15min at25◦C)of citrated blood. Blood
coagulationtimereagents:activatedpartialthromboplastintime
(APTT), thrombin time (TT) and prothrombin time (PT), were
acquired from In-Vitro Diagnóstica S/A (Itabira, MG, Brazil).
Thrombin and antithrombin were obtainedfrom Haematologic
Technologies,USA,andchromogenicsubstrateS-2238from
Chro-mogenix,Sweden. ChlorosulfonicacidwasobtainedfromVetec
(RiodeJaneiro,RJ,Brazil).
2.2. Productionandpreparationofˇ-(1→6)-d-glucan
(lasiodiplodan)
-(1→6)-d-Glucan(lasiodiplodan)wasproducedbyL.
theobro-maeMMLRandgrownonnutrientmediumcontainingsucroseas
previouslydescribed(Vasconcelosetal.,2008).Cell-freeculture fluidwasobtainedafterremovalofthemyceliumbycentrifugation (5500×g/20min)at4◦C.Thesupernatantwastreatedwith3
vol-umesofabsoluteethanol,theprecipitatedmaterialrecoveredand dissolvedindistilledwater,followedbyextensivedialysisagainst frequentchangesofdistilledwaterover48h,andthenfreeze-dried.
2.3. Analyticaltechniques
Carbohydrate was determined by the phenol–sulfuric acid
method(Dubois,Gilles,Hamilton,&Rebers,1956)withd-glucose
as standard. Protein was measured by the Bradford method
(Bradford,1976)usingbovineserumalbuminasstandard.
2.4. Sulfonation
Sulfonationof-(1→6)-d-glucanwasperformedaccordingto O’Neill (1955) with some modifications: lasiodiplodan powder (50.0mg)wassolubilizedindryformamide(10.0mL)with vigor-ousstirringfor24hatroomtemperature.Then10.0mLofpyridine
wasaddedtothemixturefollowed bycontinuationofvigorous
stirringforanother30hatroomtemperature.Chlorosulfonicacid
(4.0mL)wasnextaddeddrop-wisetothemixtureinanice-bath
overanintervalof2h,andthenleftat4◦Cfor12h.Thereaction
wasterminatedbyaddingice-water,andneutralizedbyaddinga
solutionof10%(w/v)NaHCO3untilallCO2evolutionceased.The
reactionmixturewasthendialyzedexhaustivelyagainstdistilled waterfor6dayswithseveralchangesofwater,andthedialysate concentratedunderreducedpressure(<39◦C)andlyophilized.The
productobtainedwasreferredtoassulfonated-(1→6)-d-glucan.
Thesulfonationreactionwasrepeatedtwofurthertimesuntila
degreeofsubstitution(DS)ofgreaterthan0.80wasobtained.
2.5. Determinationofthedegreeofsubstitution(DS)
Samples of sulfonated -(1→6)-d-glucan (1.0mg) were
hydrolyzedusing1.0MHCl(1.0mL)for5hat100◦C.Todetermine
the DS, 0.2mLof hydrolyzedthe sulfonated -(1→6)-d-glucan
samplewasreactedwith3.8mLof3%(w/v)trichloroaceticacid
(TCA)inaglasstube,andthen1.0mLofprotectorsolution(6.0g NaCl,0.5mLc.HCl,2.5mLof0.1%(w/v)gelatinand47.0mLdistilled
water) and 0.03gBaCl2 wereadded. Thecontents were stirred
for 1minand left for 15min.The resulting BaSO4 formed was
measuredturbidimetricallyat360nm.TheDS,whichdesignates
the average number of sulfonyl groups oneach sugar-residue,
wasestablishedfromthesulfurcontentaccordingtoWhistlerand Spencer(1964),inwhichS=%sulfur:
S(%)= (BaSO4,g)×0.1374×100
Sample,g
DS= 162×S
3200−102×S
2.6. Homogeneityoftheˇ-(1→6)-d-glucanandsulfonated ˇ-(1→6)-d-glucan
Thehomogeneityofthe-(1→6)-d-glucans(naturaland
sul-fonated) was determined by gel permeation chromatography.
Onemilligramofeachofthe2polysaccharidesampleswas
dis-solvedinwater(1.5mL)andappliedtoaSepharoseCL-4Bcolumn (1.5cm×30cm),andelutedwithdistilledwaterataflowrateof 0.5mL/min.Fractions(1.5mL)werecollectedandanalyzedfor
car-bohydrate(490nm).Thevoidvolume(19.5mL)wasdetermined
usingbluedextran.
2.7. Spectroscopyanalysis
Fourier-transform infra-red (FT-IR) spectra of the
exopolysaccharides samples (-(1→6)-d-glucan and sulfonated
-(1→6)-d-glucan, 1mg) were recorded using KBr pellets
(250.0mg) on a Bruker Vector 22 Model spectrometer. The
ultraviolet–visible(UV–vis)absorptionspectrafordiluteaqueous solutions (1.0mg/mL) of -(1→6)-d-glucan and sulfonated 
-(1→6)-d-glucanweredeterminedusingaShimadzu1601UV-Vis
spectrophotometer. Nuclear magnetic resonance spectroscopy
ofcarbon thirteen(13CNMR)analysisof-(1→6)-d-glucanand
sulfonated -(1→6)-d-glucanwerecarriedoutusinga400MHz
Bruker model DRX Avance spectrometer incorporating Fourier
1910 92 (2013) 1908–1914
examinedat50or70◦C.Chemicalshiftsareexpressedinppm(ı)
relativetoresonanceofMe2SO-d6at39.70forsamplesexamined
inthissolvent.
2.8. Bloodclottingassays
Theanticoagulantactivityofthesamples(-(1→6)-d-glucan
andsulfonated-(1→6)-d-glucan;0–200g/mL)wasdetermined
bymeasuringthe clottingtimes (inseconds)of humanplasma
using the reagents prothrombin time (PT), thrombin time (TT)
and activatedpartial thromboplastin time (APTT) according to
the manufacturer’s instructions. Each assay was performed at
37◦C.Heparin(0–30g/mL)wasusedasstandard.Normalhuman
plasma (900L) wasmixed with 100L of exopolysaccharides
solution (-(1→6)-d-glucan and sulfonated -(1→6)-d-glucan),
orheparindissolvedinisotonicsaline,withintheconcentration rangesindicated above.Isotonicsaline(100L)wasusedinthe controlgroup.Ineachofthebloodclottingassays,thereagents(PT, TTandAPTT)andallglasstubeswerepre-heatedat37◦Cbeforeto
use.Theassayswereperformedintriplicateandtheresults repre-sentthemeans±SD.
2.9. Inhibitionofthrombinbyantithrombininthepresenceof
ˇ-(1→6)-d-glucan,sulfonatedˇ-(1→6)-d-glucanandheparin
The inhibition of thrombin by antithrombin wasperformed
according to Melo et al. (2004) with the following
modifica-tions:incubationswereperformedin disposablemicrocuvettes.
Thereactionmixturecontained25Lofhumanplasmaorpurified
antithrombin (40nM), and 25L of exopolymer solution (
-(1→6)-d-glucanorsulfonated-(1→6)-d-glucan;0–400g/mL),
orheparin(0–1g/mL)inTS/PEGbuffer(0.02MTris/HCl,0.15M NaCl,and1.0mg/mLpolyethyleneglycol,pH7.4).Thrombin(10L, 20nM)wasnextaddedtothemixturetoinitiatethereaction,and after60sincubation(roomtemperature),500LofTS/PEGbuffer
containing25MchromogenicsubstrateS-2238forthrombinwas
added,andtheabsorbanceat405nmrecordedfor300s.No
inhi-bitionoccurredinthecontrolexperimentinwhichthrombinwas
incubatedwithantithrombinintheabsenceofsulfated
polysac-charides.
3. Resultsanddiscussion
3.1. Sulfonationandstructuralcharacterization
Theexocellular-(1→6)-d-glucanwasselectedforthiswork
asitpresentsimportantcharacteristicsforbiologicalactivitywith regardstopurity,uniformity,homogeneity(Fig.1)andtriplehelix
conformational structure at previously described (Vasconcelos
etal.,2008).Ashydratedthebiopolymerformedaviscous solu-tion,andthederivatizationbysulfonationwasawaytoimprove itssolubilityinaqueoussolutions,andtopromoteanticoagulant activity.Itiswellknownthattheintroductionofchargedgroups
onaneutralpolysaccharidechainimproveswatersolubilityand
canenhanceitsbiologicalactivitiessuchasanticoagulation(Brandi etal.,2011;Jung,Bae,Lee,&Lee,2011).
Inthis work,sulfonation(threecycles) wasperformedusing
formamideassolvent,pyridineascatalyticreagentand chlorosul-fonicacidasthehydroxylgroupdonor.Theeffectivenessofeach
cycleof thesulfonationreactionwasmonitoredbyUV–vis and
FT-IRanalysis.Theintegrityofsulfonatedmaterialwasperformed
bygelpermeationchromatographyonSepharoseCL-4B(Fig.1),
andpresentedasinglecarbohydratepeak.Followingsulfonation,
UV–visspectroscopyshowedanewbandat261nm(Fig.2)that
canbe attributabletothen→* transition ofsulfonate orthe
Fig.1. Gelpermeationchromatographyprofileofthe-(1→6)-d-glucan(---)and
sulfonated-(1→6)-d-glucan(—)onacolumnofSepharoseCL-4B.Thecolumn (1.5cm×30cm)waselutedwithwaterataflowrateof0.5mL/min.
unsaturatedbondformedinthesulfonationprocess(Brandietal., 2011;Yangetal.,2003).
Thesuccessofthereactionwasaccompaniedbytheappearance oftwocharacteristicsabsorptionbandsonFT-IRspectraofthe sul-fonated-(1→6)-d-glucan(Fig.2):oneat1258cm−1describingan
asymmetricalS Ostretchingvibration(Yang,Du,Huang,Wan,&Li, 2002;Zhang,Zhang,Zhou,Chen,&Zeng,2000),whereastheband at810cm−1 representingasymmetricalC O Svibration
associ-atedwithaC O SO3group(Brandietal.,2011;Nie,Shi,Ding,&
Tao,2006)demonstratedthatlasiodiplodanwassuccessfully sul-fonated.Additionally,anewbandat1631cm−1couldberelatedto
theunsaturatedbondformedduetothesulfonationprocess(Yang etal.,2003).
Thesulfurcontentofthesulfonatedpolysaccharidewas
deter-mined by calculating the DS, and in this case was 0.95. In
studiesrelatedtoanticoagulantactivitiesofchemicallymodified -glucans,theDSwasaround1.95(Martinichen-Herreroetal.,2005), 1.74(Nieetal.,2006)and1.54(Brandietal.,2011).Accordingto publishedreports,DSvaluesequalorhigherthan0.80arerequired foranticoagulantactivity(Han,Yao,Yang,Liu,&Gao,2005).The contentof sulfurobtainedfor thesulfonated-(1→6)-d-glucan
was11.73%,andwithintherangeofvaluesnecessaryfor antico-agulantactivity.
Thepositionsofthesulfonylgroupsinpolysaccharidescanbe determinedby13CNMR(Hanetal.,2005;Zhangetal.,2008),and
theliteratureshowsthatthemainpositionfortheentryof sul-fonylgroupsin-(1→3)-glucansistheprimarycarbon,i.e.atC-6, followedbyC-2andC-4(Tellesetal.,2011;Zhangetal.,2000).
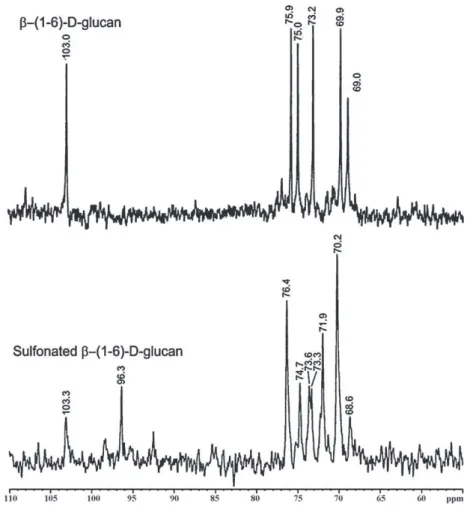
The13CNMRspectraofnative-(1→6)-d-glucanandits
sul-fonatedderivativearepresentedinFig.3.Thechemical shiftat 103.0ppmof-(1→6)-d-glucanwasattributedtotheanomeric
carbonandthatat69.0ppmtosubstitutedC-6.Thesignalsat75.9,
75.0,73.2and69.9ppmwereattributedtoC-3,C-5,C-2and
C-4,respectively (Naruiet al.,1999;Sassakiet al.,2002).The13C
NMRspectrumofsulfonatedglucanswasmorecomplicatedwith
broader signals, resulting fromthe sulfonation of thehydroxyl
groups (Brandi et al., 2011). Usuallyfollowing sulfonation, the
spectrabecame morecomplicated becausethecarbons directly
attachedtotheelectronegativesulfonatedestergroupsshiftdown field,whilethecarbonsindirectlyattached(neighborhood)tothe sulfonylgroupshifttoanupfieldposition(Perlin&Casu,1982; Tellesetal.,2011).
Thechemicalshiftsinthe13CNMRspectrumofthesulfonated
-(1→6)-d-glucanpresentedasmallvariation(+0.3ppm),in
92 (2013) 1908–1914 1911
Fig.2.FT-IRandUV–vis(inset)spectraof-(1→6)-d-glucanbefore(---)andaftersulfonation(—).
attributedtoC-1asthesignalofC-1splitswhenan OHgroupon C-2issubstitutedwithasulfonylgroup.Inaddition,thechemical
shiftat73.2ppmassignedasC-2alsosplitandmoveddownfield
(73.6ppm),suggestingthatpartofC-2wassulfonated.Theintense
signalat70.2ppmcorrespondedprobablytothesubstitutedC-6
andfreeC-4,whilethatat71.9ppmcouldbeassignedtoC-4 sub-stitutedbysulfonylgroups(␣shift).Thesmallsignalat68.6ppm (shift)canberelatedtocarbonindirectlyattachedtosulfonyl
groups.Probablypartof C-3wasalsosubstituted,however,the
attributionisdifficultbecausethechemicalshiftsmaycorrespond tomorethanonecarbon.Fromtheresultsweconcludedthat non-selectivesulfonationof-(1→6)-d-glucanhasoccurred,asC-2and
C-4weresplitindicatingpartialsubstitution.
Afterthesulfonationreaction,thesolutions ofsulfonated 
-(1→6)-d-glucan became less viscous and more water soluble,
whichfacilitatesthebioassaystodetermineanticoagulant activ-itythroughtraditionaltestsofbloodcoagulationwithheparinas thereference.
3.2. Anticoagulantactivity
3.2.1. APTT,TTandPTclottingtimes
Toinvestigatetheanticoagulantpropertyof-(1→6)-d-glucan
andsulfonated -(1→6)-d-glucan,APTT,PTandTTassays were
conductedusingnormalhumanplasmaandtheeffectsofthe
-glucansontheclottingtimesmeasured.Theresultswerecompared withthoseforheparinasthereferencestandard.
TheAPTTtestisrelatedtothephaseofintrinsiccoagulationin plasmaandmeasuresthefunctionofbloodcoagulationfactorsXII, XI,IXandVIII.PTisrelatedtotheextrinsicphase,whichdepends
uponthetissuefactoroftheactivationprocessandmeasuresthe integrityofthecommonphaseofcoagulation.TheTTassay evalu-atestheconversionofplasmaticfibrinogentofibrininthepresence ofexogenousthrombin.Thecoagulationtimeinthelaststageof thecoagulationcascadeofeventsistheconversionoffibrinogento fibrinbythrombin(Beutler,Coller,Lichtman,&Kipps,2001;Melo etal.,2004;Mendesetal.,2009).
TheresultsfortheAPPT,TTandPTassaysareshowninTable1.
ThesulfonatedpolysaccharidewasabletoprolongtheAPPTand
TTtimes ina concentration-dependentmanner.Withregard to
theAPTTandTTresults,theanticoagulanteffectofsulfonated -(1→6)-d-glucanat 30and 40g/mLconcentrationwas∼5and ∼7 times greater, respectively, than the control. These results demonstratedanimportantinvitroanticoagulantactivityforthe sulfonated -(1→6)-d-glucan asdemonstrated by theincreases
inthedose-dependenceofAPTTandTT,andthisactioncouldbe
attributabletothedegreeofsulfonation(DS0.95).
TheAPTTprolongationtimesuggestsinhibitionoftheintrinsic
coagulationpathway,whereasprolongationofTTtimeindicates
inhibition of thrombin-mediated fibrin formation (Wanget al.,
2007).NoprolongationofPTdemonstratedtherewasnoinhibition oftheextrinsicpathwayofcoagulation(Maoetal.,2009).Sincethe anticoagulanteffectofheparinisnotmediatedbymodulationof theextrinsicsystem,thesulfonated-(1→6)-d-glucanisapoor
inhibitoroftheextrinsicpathway.TheAPTTandTTvalueswere
compared withheparin,and a concentration∼10times greater
ofthesulfonated-(1→6)-d-glucanwasnecessarytoachievethe
sameeffectasexhibitedbyheparin.Thesulfonated-(1→3;1→
6)-d-glucan (named botryosphaeran) from Botryosphaeria rhodina
1912 92 (2013) 1908–1914
Fig.3.13CNMRspectraofexocellular-(1
→6)-d-glucanandsulfonated-(1→6)-d-glucan.
(Brandi et al., 2011), showed similar results as anticoagulants. AccordingtoMartinichen-Herreroetal.(2005),sulfated
polysac-charideswithaloweranticoagulantactivitythanheparin could
exhibit a potent antithrombotic effect with less hemorrhagic
risk.
Aswithheparin, theweakest effect wasobservedin thePT
assayfor thesulfonated -(1→6)-d-glucan. Therelative lackof
effectofsulfonated-(1→6)-d-glucanonthePTisconsistentwith
theobservationthatthistestisalsonotsensitivetoheparin,and severalothersulfatedpolysaccharides(Martinichen-Herreroetal.,
Table1
Anticoagulantactivityofnormalhumanplasmainthepresenceof-(1→6)-d-glucan,sulfonated-(1→6)-d-glucanandheparinasmeasuredbytheactivatedpartial
thromboplastintime(APTT),thrombintime(TT)andprothrombintime(PT).
Polysaccharide Amount(g/mL) Clottingtimes(s)
APTT TT PT
-(1→6)-d-Glucan Control(0) 46.65±0.8 19.45±0.4 12.87±0.8
1 39.45±0.2 15.92±0.4 16.04±0.1
5 39.94±0.6 16.56±0.5 14.87±0.1
10 42.30±0.1 14.24±0.3 15.77±0.1
15 41.07±0.7 14.53±0.2 14.27±0.2
20 41,09±0.2 14.43±0.4 16.87±0.1
Sulfonated-(1→6)-d-glucan Control(0) 46.65±0.8 19.45±0.4 12.87±0.8
1 46.79±0.2 15.67±0.3 15.12±0.0
5 58.27±0.1 26.75±1.6 17.56±0.1
10 70.55±3.6 50.87±1.1 17.52±0.4
15 96.97±1.3 70.06±0.6 18.65±0.2
20 105.69±0.1 187.54±1.5 18.61±0.4
30 228.49±3.7 227.06±2.2 17.45±0.4
40 298.13±1.0 249.45±0.6 17.17±0.7
Heparina Control(0) 46.65±0.8 19.45±0.4 12.87±0.8
1 55.60±0.6 95.00±0.7 16.56±0.2
2 76.19±1.0 275.98±1.3 17.93±0.6
3 106.08±0.3 592.70±2.9 19.20±0.2
92 (2013) 1908–1914 1913
Fig.4.Concentrationdependenceof-(1→6)-d-glucan,sulfonated-(1→6)-d -glucanandheparinontheinactivationofthrombinby(a)antithrombinofhuman plasmaand(b)apurifiedantithrombinpreparation.
2005).The-(1→6)-d-glucan(lasiodiplodan)didnotinhibitthe
APTT,TTandPTassays,anddemonstratedthatthepresenceof sul-fonylgroupswasanessentialrequirementfortheseanticoagulant activities.
3.2.2. Inhibitionofthrombinbyantithrombininthepresenceof
ˇ-(1→6)-d-glucan,sulfonatedˇ-(1→6)-d-glucanandheparin
Thebiologicalmechanismofsulfonatedpolysaccharidesoccurs bythepotentiationofplasmaticcofactors,whichare physiologi-calinhibitorsofthecoagulationcascadeasantithrombinthatacts byinhibitingthrombinandfactorsXa,XIIa,XIaandIX,andmay haveitsactionstrengthenedbythepresenceofheparin(Meloetal., 2004; Mendes etal., 2009).Toelucidate the inhibitory mecha-nismoftheanticoagulantactivityofsulfonated-(1→6)-d-glucan,
theeffectsonthrombinactivitywerestudiedusingchromogenic
substratesinthepresenceofplasmaasasourceofphysiological inhibitors(antithrombinandheparincofactorII),andalsopurified antithrombin.
Fig.4(a)and(b)showsthatthesulfonated-(1→6)-d-glucan
wasabletopotentiatethrombininhibitioninamannersimilarto thatofheparin.However,comparingthevaluesofIC50
(concentra-tionofthesulfonated-(1→6)-d-glucannecessarytoobtain50%
inhibitionofthrombinactivity)obtainedforbothsetsofassays, theactivityofthesulfonated-(1→6)-d-glucanwasapproximately
180-foldlowerthanthatofheparininexperimentsusingthe
puri-fiedantithrombin,and∼38-foldlowerwithhumanplasma.These
resultssuggestthatbesidestheactivationofantithrombin,the sul-fonated-(1→6)-d-glucancouldpossiblycontributetoanincrease
intheactionofanotherphysiologicalinhibitorofthrombin (hep-arincofactorII)absentintheassayswithpurifiedantithrombin. HeparincofactorIIisaninhibitorofserineproteaseandthrombin.
Antithrombininhibitsallintrinsicpathwaycoagulationenzymes
(Maoetal.,2009).
Therefore,theresultsoftheanticoagulanttestsdescribedinthis workdemonstratedthatthesulfonated-(1→6)-d-glucan
exhib-itedanticoagulantactivity,andwasmostlikelyinvolvedwiththe
intrinsic pathway. The lasiodiplodan didnot present inhibiting
activityatanyoftheconcentrationsexamined,demonstratingthat thepresenceofsulfonylgroupsinthis-(1→6)-d-glucanwasan
importantcharacteristicofanticoagulantaction.
4. Conclusions
Anexocellular-(1-6)-d-glucan(lasiodiplodan)wassulfonated
(three cycles)using formamide assolvent,pyridine ascatalytic reagentandchlorosulfonicacidasthehydroxylgroupdonor.The
effectiveness of each sulfonation reaction cyclewas monitored
byUV–visandFT-IRanalysis,andonlythesulfonated-(1-6)-d
-glucanshowedsulfonateortheunsaturatedbondformedinthe
sulfonation process. The content of sulfur and DS obtainedfor
the sulfonated -(1→6)-d-glucanwas 11.73% and0.95,
respec-tively,whichindicatedthattherewasapproximatelyonesulfonyl groupperresidueofglucose.Thepositionsofthesulfonylgroups introducedinlasiodiplodanweredeterminedby13CNMR,andfrom
theresultsitwasconcludedthatnon-selectivesulfonationofthe -(1→6)-d-glucanhadoccurred,withC-2andC-4beingpartially
substituted.ItispossiblethatC-3alsoreceivedasulfonylgroup.The prolongationofAPTTinthepresenceofthesulfonated-(1→6)-d
-glucansuggestedinhibitionoftheintrinsicpathwayofcoagulation, whileanextensionoftheTTtimeprobablyindicatedinhibitionof thereactionresultingintheconversionoffibrinogenintofibrin. Thus,thisworkdemonstratedthatthechemicalderivatizationof exocellular-(1→6)-d-glucanbysulfonationproducedamodified
polysaccharideresultinginanticoagulationactivity.
Acknowledgements
AFDVasconcelosthanksCAPES(Brazil)foradoctoral scholar-ship.TheauthorsaregratefultoAnaMariaTovarandPauloA.de SouzaMourão(Lab.Tec.Conjuntivo,H.U.ClementinoFraga Filho-UniversidadeFederaldoRiodeJaneiro)forscientificassistanceand discussions.
References
Beutler,E.,Coller,B.S.,Lichtman,M.A.,&Kipps,T.J.(2001).Williamshematology (6thed.).NewYork:MacGraw-Hill.
Bohn,J.A.,&BeMiller,J.N.(1995).(1→3)--d-Glucansasbiologicalresponse modifiers:Areviewofstructure–functionalactivityrelationships.Carbohydrate Polymers,28,3–14.
Bradford,M.M.A.(1976).Arapidandsensitivemethodforthequantitationof microgramsquantitiesofproteinutilizingtheprincipleorprotein-dyebinding. AnalyticalBiochemistry,72,248–254.
Brandi, J., Oliveira,E. C.,Monteiro, N.K.,Vasconcelos, A.F. D., Dekker,R. F. H.,Barbosa,A.M.,etal.(2011).Chemicalmodificationofbotryosphaeran: Structuralcharacterizationandanticoagulantactivityofawater-soluble sul-fonated(1→3)(1→6)--d-glucan.JournalofMicrobiologyandBiotechnology,21, 1036–1042.
Cunha,M.A.A.,Turmina,J.A.,Ivanov,R.C.,Barroso,R.R.,Marques,P.T.,Fonseca,E.A. I.,etal.(2012).Lasiodiplodan,anexocellular(1(6)--d-glucanfromLasiodiplodia theobromaeMMPI:Productiononglucose,fermentationkinetics,rheologyand anti-proliferativeactivity.JournalofIndustrialMicrobiologyandBiotechnology, 39,1–10.
Dubois,N.,Gilles,K.A.,Hamilton,J.K.,&Rebers,P.A.(1956).Colorimetricmethod fordeterminationofsugarandrelatedsubstances.AnalyticalChemistry,28, 350–356.
1914 92 (2013) 1908–1914
Serpin-independenteffectandspecificinteractionwithfactorXa.Thrombosis andHaemostasis,102,1183–1193.
Han, F.,Yao, W., Yang, X., Liu, X., & Gao, X.(2005). Experimentalstudy on anticoagulantandantiplateletaggregationactivityofachemicallysulfated marinepolysaccharideYCP.InternationalJournalofBiologicalMacromolecules, 36,201–207.
Jung,H.J.,Bae,J.Y.,Lee,S.,&Lee,H.G.(2011).Effectofthedegreeofsulfationonthe physicochemicalandbiologicalpropertiesofPleurotuseryngiipolysaccharides. FoodHydrocolloids,25,1291–1295.
Kato,D.,Era,S.,Watanabe,I.,Arihara,M.,Sugiura,N.,Kimata,K.,etal.(2010). Antivi-ralactivityofchondroitinsulphateEtargetingdenguevirusenvelopeprotein. AntiviralResearch,88,236–243.
Klis,P.,Mol,K.,Hellingwerf,L.,&Brul,S.(2002).Dynamicsofcellwallstructurein Saccharomycescerevisiae.MicrobiologyReviews,26,239–256.
Laroche,C.,&Michaud,P.(2007).Newdevelopmentsandprospectiveapplications for-(1→3)-d-glucans.RecentPatentsonBiotechnology,1,59–73.
Leung,M.Y.K.,Liu,C.,Koon,J.C.M.,&Fung,K.P.(2006).Polysaccharidebiological responsemodifiers.ImmunologyLetters,105,101–114.
Mantovani,M.S.,Bellini,M.F.,Angeli,J.P.F.,Oliveira,R.J.,Silva,A.F.,&Ribeiro, L.R.(2008).-Glucansinpromotinghealth:Preventionagainstmutationand cancer.MutationResearch,658,154–161.
Mao,W.,Li,H.,Zhang,H.,Qi,X.,Sun,H.,Chen,Y.,etal.(2009).Chemical charac-teristicandanticoagulantactivityofthesulfatedpolysaccharideisolatedfrom Monostromalatissimum(Chlorophyta).InternationalJournalofBiological Macro-molecules,44,70–74.
Martinichen-Herrero,J.C.,Carbonero,E.R.,Gorin,P.A.J.,&Iacomini,M.(2005). Anticoagulantandantithromboticactivityofasulfateobtainedfromaglucan componentofthelichenParmotremamantiqueirenseHale.Carbohydrate Poly-mers,60,7–13.
Melo,F.R.,Pereira,M.S.,Foguel,D.,&Mourão,P.A.S.(2004).Antithrombin-mediated anticoagulantactivityofsulfatedpolysaccharidesdifferentmechanismsfor heparin and sulfated galactans. The Journal of Biological Chemistry, 279, 20824–20835.
Mendes,S.F.,Santos,O.,Jr.,Barbosa,A.M.,Vasconcelos,A.F.D.,Aranda-Selverio, G.,Monteiro,N.K.,etal.(2009).Sulfonationand anticoagulantactivityof botryosphaeranfromBotryosphaeriarhodinaMAMB-05grownonfructose. Inter-nationalJournalofBiologicalMacromolecules,45,305–309.
Narui,T.,Sawada,K.,Culberson,C.F.,Culberson,W.L.,&Shibata,S.(1999). Pustulan-typepolysaccharidesasaconstantcharacteroftheUmbilicariaceae(Lichenized Ascomycotina).TheBryologist,102,80–85.
Nie,X.,Shi,B.,Ding,Y.,&Tao,W.(2006).Preparationofachemicallysulfated polysaccharidederivedfromGrifolafrondosaanditspotentialbiological activi-ties.InternationalJournalofBiologicalMacromolecules,39,228–233.
O’Neill,A.N.(1955).Sulphatedderivativesoflaminarin.CanadianJournalChemistry, 33,1097–1101.
Perlin,A.S.,&Casu,B.(1982).Spectroscopicmethods.InG.O.Aspinall(Ed.),The polysaccharides(pp.133–189).NewYork:AcademicPress.
Pomin, V. H. (2012). Structure–function relationship of anticoagulant and antithrombotic well-defined sulfated polysaccharides from marine invertebrates.AdvancesinFoodandNutritionResearch,65,196–207.
Rop,O.,Mlcek,J.,&Jurikova,T.(2009).Beta-glucansinhigherfungiandtheirhealth effects.NutritionReviews,67,624–631.
Sassaki,G.L.,Ferreira,J.C.,Glienke-Blanco,C.,Torri,G.,Toni,F.D.,Gorin,P.A.J.,etal. (2002).Pustulanandbranched-galactofurananfromthephytopathogenic fun-gusGuignardiacitricarpa,excretedfrommediacontainingglucoseandsucrose. CarbohydratePolymers,48,385–389.
Telles,C.B.S.,Sabry,D.A.,Almeida-Lima,J.,Costa,M.S.S.P.,Melo-Silveira,R. F.,Trindade,E.S.,etal.(2011).Sulfationoftheextracellularpolysaccharide producedbytheediblemushroomPleurotussajor-cajualtersitsantioxidant, anticoagulantandantiproliferativepropertiesinvitro.CarbohydratePolymers, 85,514–521.
Vasconcelos,A.F.D.,Monteiro,N.K.,Dekker,R.F.H.,Barbosa,A.M.,Carbonero,E.R., Silveira,J.L.M.,etal.(2008).Threeexopolysaccharidesofthe-(1→6)-d-glucan typeanda-(1→3;1→6)-d-glucanproducedbystrainsofBotryosphaeria rhod-inaisolatedfromrottingtropicalfruit.CarbohydrateResearch,14,2481–2485. Vetvicka,V.,Vetvickova,J.,Frank,J.,&Yvin,J-C.(2008).Enhancingeffectsofnew
biologicalresponsemodifier-1,3glucansulfatePS3onimmunereactions. BiomedicineandPharmacotherapy,62,283–288.
Wang,J.,Guo,H.,Zhang,J.,Wang,X.,Zhao,B.,Yao,J.,etal.(2010).Sulfated mod-ification,characterizationandstructure–antioxidantrelationshipsofArtemisia sphaerocephalapolysaccharides.CarbohydratePolymers,81,897–905. Wang,Z.M.,Li,L.,Zheng,B.S.,Normakhamatov,N.,&Guo,S.Y.(2007).Preparation
andanticoagulationactivityofsodiumcellulosesulfate.InternationalJournalof BiologicalMacromolecules,41,41376–41382.
Whistler,R.L.,&Spencer,W.W.(1964).Sulfation.MethodsinCarbohydrateChemistry, 4,235–275.
Williams,D.L.(1997).Overviewof(1→3)--d-glucanimmunobiology.Mediatorsof Inflammation,6,247–250.
Yang,J.,Du,Y.,Huang,R.,Wan,Y.,&Li,T.(2002).Chemicalmodification, charac-terizationandstructure–anticoagulantactivityrelationshipsofChineselacquer polysaccharides.InternationalJournalofBiologicalMacromolecules,31,55–62. Yang,J.,Du,Y.,Wen,Y.,Li,T.,&Hu,L.(2003).SulfationofChineselacquer
polysac-charidesindifferentsolvents.CarbohydratePolymers,52,397–403.
Zhang,L.,Zhang,M.,Zhou,Q.,Chen,J.,&Zeng,F.(2000).Solutionpropertiesof antitumorsulfatedderivativeof␣-(1→3)-d-glucanfromGanodermalucidum. BiosciencesofBiotechnologyandBiochemistry,64,2172–2178.