MicrobiologicalResearch168 (2013) 50–55
ContentslistsavailableatSciVerseScienceDirect
Microbiological
Research
j o ur n a l ho me p ag e :w w w . e l s e v i e r . c o m / l o c a t e / m i c r e s
Synthesis,
characterization
and
antifungal
activity
of
quaternary
derivatives
of
chitosan
on
Aspergillus
flavus
Rafael
de
Oliveira
Pedro
a,
Mirelle
Takaki
a,
Teresa
Cristina
Castilho
Gorayeb
b,
Vanildo
Luiz
Del
Bianchi
b,
João
Cláudio
Thomeo
b,
Marcio
José
Tiera
a,
Vera
Aparecida
de
Oliveira
Tiera
a,∗aDepartmentofChemistryandEnvironmentalSciences,InstituteofBiosciences,HumanitiesandExactSciences–IBILCE,SãoPauloStateUniversity–UNESP,SãoJosédoRioPreto,
SãoPaulo,Brazil
bDepartmentofTechnologyandFoodEngineering,InstituteofBiosciences,HumanitiesandExactSciences–IBILCE,SãoPauloStateUniversity–UNESP,SãoJosédoRioPreto,São
Paulo,Brazil
a
r
t
i
c
l
e
i
n
f
o
Articlehistory:
Received29February2012
Receivedinrevisedform16June2012 Accepted23June2012
Keywords: Chitosan Derivatives Aspergillusflavus Antifungalactivity Mycotoxins
a
b
s
t
r
a
c
t
Twoseriesofnewchitosanderivativesweresynthesizedbyreactionofdeacetylatedchitosan(CH)with propyl(CH-Propyl)andpentyl(CH-Pentyl)trimethylammoniumbromidestoobtainderivativeswith increasingdegreesofsubstitution(DS).Thederivativeswerecharacterizedby1HNMRand
potentiomet-rictitrationtechniquesandtheirantifungalactivitiesonthemycelialgrowthofAspergillusflavuswere investigatedinvitro.TheantifungalactivitiesincreasewithDSandthemoresubstitutedderivativesof bothseries,CH-PropylandCH-Pentyl,exhibitedantifungalactivitiesrespectivelythreeandsixtimes higherthanthoseobtainedwithcommercialanddeacetylatedchitosan.Theminimuminhibitory con-centrations(MIC)wereevaluatedat24,48and72hbyvaryingthepolymerconcentrationfrom0.5to 16g/Landtheresultsshowedthatthequaternaryderivativesinhibitedthefungusgrowthatpolymer concentrationsfourtimeslowerthanthatobtainedwithdeacetylatedchitosan(CH).Thechitosans mod-ifiedwithpentyltrimethylammoniumbromideexhibitedhigheractivityandresultsarediscussedtaking intoaccountthedegreeofsubstitution(DS).
© 2012 Elsevier GmbH. All rights reserved.
1. Introduction
Chitosanisapolysaccharideusuallyobtainedfrom deacetyla-tionofchitin,whichaftercelluloseisthesecondmostabundant natural biopolymer foundin nature. It may be extracted from various sources, particularly from exoskeletons of arthropods ofcrustaceans, fungi,insects, annelids, mollusks and coelenter-ata. The structures of chitin and chitosan correspond to those of poly[(1→4)-2-acetamide-2-deoxy-d-glucopyranose] and
poly[(1→4)-2-amino-2-deoxy-d-glucopyranose], respectively
(Ravi Kumar 2000). The homopolymer is a weak base with a pKa valueofthed-glucosamineresidue ofabout6.2–7.0and is
therefore insolubleat neutral and alkaline pH values. In acidic mediums,theaminegroupswillbepositivelycharged,conferring tothepolysaccharidea highcharge density. Duetoits polyca-tionic nature, chitosan, after being dissolved in aqueous acid solutions,canbeeasilymoldedandusedasmembranes,beads, microparticlesandgels(Rinaudo 2006;RaviKumar2000).Also, itsfunctionalpropertiessuchasbiodegradabilityandlowtoxicity (Domard2011;KeanandThanou2010)havedriventheresearch
∗Correspondingauthor.Tel.:+551732212358;fax:+551732212356.
E-mailaddress:verapoli@ibilce.unesp.br(V.A.deOliveiraTiera).
andapplicationsofchitosantomedicine(Rinaudo2006;Shietal. 2006;Jayakumar etal.2010), foodsadditivesand preservatives (Shahidietal.1999)aswellasinthepaperindustryandforthe treatmentofindustrialwastewater(NgahandTeong2011).
Oneofthemostattractivefeaturesofchitosanisitsantibacterial, antiviralandantifungalactivity(Zhangetal.2011;Jayakumaretal. 2011).Recentlytheutilizationofchitosanasafoodpreservative oradjuvantinagriculturetoprotectorstimulatethedefenseof differentcropshasincreased(Zhangetal.2011;Jayakumaretal. 2011).Itiswellknownthatpesticideresiduesaretoxicforhumans andanimalsandmanyofthemarenotbiodegradable,whatcan causeseriousenvironmentalproblemssuchascontaminationof thewaterandsoil(Satpathyetal.2011).Chitosananditsoligomers haveemergedasapromisingsourceformanyapplicationssinceit canbeusedtoproducebiodegradablefungicidestoregulatethe growingofplantsandtoprotectseeds(Alburquenqueetal.2010). Theantifungal activityof chitosan is believedto occurfrom the interaction between the cationic chain and the negatively chargedresiduesofmacromolecules exposedonthefungalcell surface,leadingtoleakageofintracellularelectrolytesandother constituents(Muzzarellietal.2001).Itisbelievedthatchitosan mayaffectthemorphogenisisofthecellwallinterferingdirectly ontheactivityofenzymesresponsibleforthegrowingofthefungi (ElGhaouthetal.1992).RecentlyLietal.,basedonconfocallaser
subsequentaflatoxin productionbyinducing susceptibletissues toproducemorephenoliccompounds.Recently,Zhangetal. pre-paredchitosan-basedblendfilmsusingchitosan,soybeantrypsin inhibitorextractsandglycerolsolutions(Zhangetal.,2009).The authors showed that the germination and growth of A. flavus
werestronglyinhibitedbyfilmspreparedfromsoybeantrypsin inhibitorextract(STI)/wildsoybeantrypsininhibitorextract(WTI) andglycerol(Gly)solutions(chitosan-STI/WTI-Gly),indicatingthat thefilmscouldbeusefulaspotentialbio-controlpackagingagainst
A.flavusduringthepeanut’sandothercereal’sstorage.Thestudy ofnewbiomoleculeshavingantifungalactivitieshasemergedasa newsubjectandthemodificationofthechitosanstructureaiming toimproveitsactivityisapromisingwaytoachieveeffective bio-fungicides(Kenawyetal.2005;Mássonetal.2008)andbactericides (Xuetal.2011).
Theaimofthepaperistoreportasimpleandreliablemethodto preparechitosansderivativeswithimprovedcapabilitiesof inhib-itingthegrowthofA.flavus.Thesynthesis,characterizationand antifungal activityof chitosan derivativesagainst thefungus A. flavusaredescribed.Aseriesofchitosanderivativeswasobtained bythereactionoftheaminogroupsofchitosanwithpropyland pentyltrimethylammoniumbromides.Theresultsoftheantifungal activityofthesederivativeswerepresentedanddiscussed,taking intoaccountthedegreeofsubstitutionofthesubstituted deriva-tives.
2. Materialsandmethods
2.1. Materials
Chitosan(degree of deacetylation (DD) 85%) was purchased fromPolymarCo., Brazil,(3-bromopropyl) trimethylammonium bromide,(5-bromopentyl)trimethylammoniumbromide,sodium hydroxide,sodiumacetate,andaceticacidwerepurchasedfrom SigmaAldrichChemicalCo.,Brazil.Spectra/poremembranes (Spec-trum)were employedfor dialysis.All solventswere ofreagent gradeandusedasreceived.WaterwasdeionizedusingaGehaka waterpurificationsystem.
2.2. Instrumentation
1HNMRspectrawererecordedonaBrukerARX-500500MHz
spectrometer.UV/VisspectraweremeasuredwithaCary100 spec-trophotometerequippedwithaPeltiersystem.pHvaluesofthe solutionsweredeterminedusinganDigimedpH-meter.
2.3. Preparationofthealkyltrimethylammonium-modified chitosans
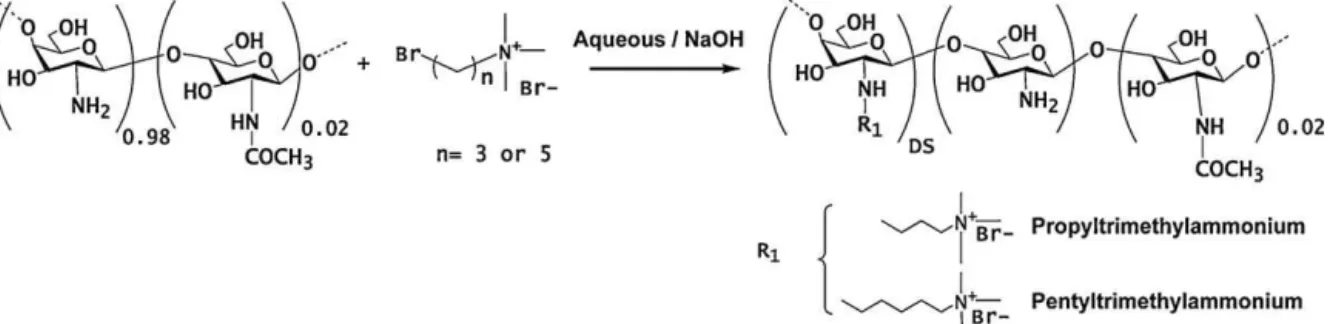
Chitosan was deacetylated as described earlier (Tiera et al. 2006).Theresultingpolymerwaspurifiedbydialysisagainstwater for3daysandisolatedbylyophilization.Next,chitosanswith vary-ingamountofgraftedalkyltrimethylammoniumbromideswere preparedinaqueousNaOHsolutionsandtheprocedureisdescribed below(Fig.1).Thedegreeofsubstitutionwasvariedbysettingthe
for2days,thenagainstaqueousNaOH(0.05M)for1day,andfinally againstwaterfor2days.Theproductwasisolatedbylyophilization andcharacterizedby1HNMRandpotentiometry.
2.4. Viscositymeasurements
Viscosity measurements were carried out in water ther-mostatedbathwithacapillarycalibratedviscosimeterfordilution Cannon-Ubbelohde9722M-50(CannonInstr.Co.)atpH4.5acetic acid(0.3M)/sodiumacetate(0.2M)buffer.Theviscosimeterwas immersed in a thermostatic bath at 298.15±0.05◦C and the
samples were allowed to equilibrate for 10min in the bath beforemeasurements.Measurementsateachconcentrationwere repeated and the reproducibility was better than ±0.01s. The
resultsoftheviscositymeasurementswereexpressedasreduced viscosityvaluescalculatedfrom
=(t−to)/to
c
wheretisthemeasuredeffluxtimeofthepolymersolution,toisthe
effluxtimeofthepuresolvent,andcisthepolymerconcentration (g/L).Themeanviscosimetricmolecularweightofchitosanandits derivativesweredeterminedbyusingtheMark–Houwink equa-tion,withconstantsa=0.796,K=0.079mLg−1(RobertsandWang 1996).
2.5. Pathogenandcultures
The microorganism chosen totest theantifungal activity of chitosanand itsderivativeswasA.flavus.Thestrainwaskindly providedbyBrazilianCollectionofMicroorganismsfromthe Envi-ronmentandIndustry–CBMAI,Campinas–SãoPaulo,Brazil,and it wasmaintained onpotato dextroseagar (PDA) (potato infu-sionfrom200g/L,20g/Ldextrose,and15g/Lagar)inthedarkat 25±2◦C.
2.6. Antifungalassays
Theantifungalactivityofdeacetylatedandcommercialchitosan wascompared tothoseofthealkyltrimethylammonium deriva-tivesmodifiedwithadegreeofsubstitutionvaryingfrom0.5to65%. ThepolymerssolutionwerepreparedatpH5.5inaceticacidand addedatconcentrationsof0.0(controlplate)0.1,0.5and1.0g/L tothemelted culturemedium, whichcontained10.0mLof10% potatodextrosebrothandthentransferredtoPetridishes.After solidification,themixtureswereinoculatedwitha1mmin diame-termyceliumfungusA.flavusatthecenterofPetridishesandthese wereincubatedinanovenfor7daysat25±2◦C.Inhibitionindexof
thefungusbythepolymerswasdeterminedbytheradialgrowthof thecolonywithacaliperonthe3rd,5thand7thdaysofcultivation, withtheresultofthe7thdayusedforcomparativepurposes.
Theantifungalindexwascalculatedasfollows:
Antifungalindex (%)=1−Da
Db
52 168 (2013) 50–55
Fig.1.Schematicrepresentationofthesynthesisofthequaternaryderivativesofchitosan.
whereDaisadiameterofthegrowthzoneinthetestplatesandDb
isgrowthzoneinthecontrolplate,accordingtoGuoetal.(2006). Eachexperimentwasperformedinquadruplicate,andthedata wereaveraged.ThestudentT-testandtheKruskal–Wallistestwith Dunn’smultiplecomparisonwereusedtoevaluatethedifferences inantifungalindexinantifungaltests.ResultswithP<0.05were consideredstatisticallysignificant.
2.7. Minimuminhibitoryconcentrations
The minimum inhibitory concentrations (MICs) were deter-minedusingthemicrodilutionassayinpotatodextroseagar(PDA), followingthestandardmethodappliedtofilamentousfungi(CLSI 2008).Thechitosanderivativesweredissolvedinaqueousacetic acidatpH5.5toobtainstocksolutionsof16.0g/L.Thesesolutions weresubsequentlydilutedtoobtaindecreasingpolymer concen-trations(16.0,8.0,4.0,2.0,1.0,0.5,0.25g/L).Thereafter100Lof thepolymersolutionswastransferredon96-wellplatesand2L ofthefungussuspensionatadensityof106conidia/mLwere
inoc-ulatedateachwell.Theexperimentswerecarriedoutintriplicate andtheMICsweredeterminedbyvisualinspectionofthewells. Thelowestconcentrationthatgavecompletegrowthinhibitionin allthreereplicateswasrecordedastheminimuminhibitory con-centration(MIC)after24,48and72h.
3. Resultsanddiscussion
3.1. Preparationoftheantifungalderivatives
Toachieve thefunctionalizationofchitosan withquaternary aminogroups,weusednucleophilicsubstitutionoftheC-2amine groups, thus leaving intact all thehydroxyl groups which play an important partin the biological activity of chitosan deriva-tives(Fig.1).Thesyntheticstrategywassuccessivelycarriedout byusingthealquiltrimethylammonium bromides.The transfor-mation was carried out starting from a deacetylated chitosan, obtainedviadeacetylationofacommercialchitosansamplewitha
nominaldegreeofdeacetylation(DD)of85%,followinga previ-ouslydescribedprocedure(Tieraetal. 2006).Thedeacetylation wascarriedoutundercontinuousbubblingofnitrogeninorderto preventdegradationofthepolymer.Thedegreeofdeacetylation, expressedin NH2mol%,determinedfromthe1HNMRspectraof
thestartingmaterialandthedeacetylatedsample,were86.1and 98.7mol%,respectively.Thesevalueswereobtainedfromtheareas ofthedoubletat5.5ppm,duetotheresonanceoftheanomeric pro-ton(H1)andofthesingletat2.7ppmattributedtotheacetamido methylprotons(Fig.2a).Potentiometrictitrationconductedona solutionofCHconfirmedthatthedeacetylationoccurredwithhigh efficiency,yieldingaDDvalueof98.5.
Thedegreeofsubstitutionwasvariedbysettingtheinitialmolar ratioofN-alkyltrimethylammoniumtoglucosamineunitsto val-uesrangingfrom1.2to8.0.The1HNMRspectraofthechitosan
derivativeobtainedfromreactionwith(3-bromopropyl) trimethy-lammoniumbromideexhibitedasingletat3.67ppm,whichwas attributedtotheresonanceofthetrimethylammoniumprotons of theN-alkyltrimethylammonium moiety and alsoexhibited a signalatı2.81ppmcorrespondingtotheresonanceofthe methy-lene protons CH2CH2CH2N+(CH3)3 (Fig. 2a). The attachment of
alkyltrimethylammoniumgroupstothechitosanframeworkbrings furtherchangestothe1HNMRspectrumofchitosan,mostnotably
intheresonancesoftheprotonsincloseproximitytothe substitu-tionsite:theanomericprotonH-1andtheprotonH-2linkedtothe C2oftheglucosamineunit.Theanomericprotonsignalundergoes adownfieldshift,fromı5.40ppmtoı5.56ppm,whilethepeakat
ı3.67ppmshiftsto3.85ppm.Thesamepatternwasalsoobserved inthe1HNMRspectraofthepentyltrimethylammonium
deriva-tives,howeverthepentyltrimethylammoniumbromideexhibited atripletfrom2.00to2.50ppmattributedtothemethyleneprotons frompentylhydrocarbonchain.SimilardeshieldingofH-1andH-2 uponC-2derivatisationofchitosanhasbeenreportedinthecase ofchitosancarryingoligosaccharidebranches(Tømmeraasetal. 2002).
Thedegreeofsubstitution(DS)wasdeterminedfromtheareas ofthesignalatı2.81ppm,whichcorrespondstotheresonanceof
Table1
PropertiesandDS(degreeofsubstitution)ofthechitosanderivatives.
Derivative Molarratioa
R/NH2
DS(potentiometry) DS(1HNMR) Mv(kDa)
CH-C – 86.1% 85.9% 32.8
CHb – 98.5% 98.7% 24.4
CH-Propyl-10 1.2 11.2% 10.7% 30.3
CH-Propyl-15 1.5 14.3% 14.7% 11.9
CH-Propyl-20 2.3 23.4% 18.1% 18.8
CH-Propyl-40 3.8 39.1% 38.4% –
CH-Pentyl-25 1.5 – 25.1% 17.4
CH-Pentyl-30 2.5 – 30.0% –
CH-Pentyl-65 8.0 – 64.9% 20.1
aMolarratioofalkyltrimethylammoniumbromide(R)toaminogroups( NH
Fig.2. 1HNMRspectraofdeacetylatedchitosanandtheir(a) propyltrimethylam-moniumand(b)pentyltrimethylammoniumderivativesofchitosan.Theresonance ofthemethyleneprotons(greencolor)equidistantfromthenitrogenatomswas usedtodetermineDS.(Forinterpretationofthereferencestocolorinthisfigure legend,thereaderisreferredtothewebversionofthearticle.)
themethyleneprotonsCH2CH2CH2N+(CH3)3(Fig.2a)andfromthe
signalsduetotheanomericprotonsofpropyltrimethylammonium substitutedandunsubstitutedglucosamineresidues,H1sandH1,
respectively(Fig.2a)usingEq.(1):
DS= (1/2)ICH2
IH1+IH1s
(1)
ThesameequationwasusedtodetermineDSforthederivatives obtainedwithpentyltrimethylammonium,howeverthesignalatı
2.05ppm,whichcorrespondstotheresonanceofthemethylene protonsCH2CH2CH2CH2 CH2N+(CH3)3,wasutilizedfor this
pur-pose(Fig.2b).Alldegreesofsubstitution(DS)areshowninTable1.
3.2. Antifungalactivityofcommercialchitosanandits deacetylatedandquaternaryderivatives
TheantifungalactivityagainstA.flavuswaspreviouslytested forcommercial(CH-C)anddeacetylated(CH)chitosans(Table1). Theconcentrationrangewasvariedfrom0.1to1.0g/Land inhi-bitionpercentageswerecalculated asdescribedin theprevious section(Section2.6).Theinhibition decreaseswithtime, which isdue tothe adaptationofthe fungustoitsnew environment, forinstancetheinhibitionindexobtainedwithdeacetylated chi-tosanis8.1,6.5and5.1inthe3rd,5thand7thdayrespectively
Fig.3.InhibitionpercentagesforCH-CandCHatdifferentpolymerconcentrations. ThedifferencesbetweenthegroupswerenotstatisticallysignificantwithP<0.05.
andthesamepatternwasobservedfortheotherderivatives.As canbeseenfromFig.3theinhibitionpercentageobtainedfor CH-CandCHremainedaround5%andincreasedslightlyfrom0.1to 1.0g/Lforthederivativesubstitutedwith10%ofpropyltrimethyl ammoniumgroups(CH-Propyl-10),which isduetothelowthe degreeofsubstitution.ThisresultissimilartothatobservedbyLi etal.investigatingtheantifungalactivitiesofchitosansonF.fulva. Theseauthorsshowedthatinhibitionpercentagesoflow molec-ularweightchitosans,measuredby themycelialradial growth, exhibitedmodestincreasesintheconcentrationrangefrom0.1to 1.0g/L(Lietal.2011).Althoughunexpected,inthisworkthefungal adaptationtothenewenvironmentwasrapidandasthetimeof incubationwas7daystheeffectofconcentrationwasnotclearly observed.However,thedeterminationoftheminimuminhibitory concentrations(MICs),discussedaheadinthepaper,makesclear thatderivativeshavingthehigherdegreesofsubstitutionexhibita significantinhibitionincrease.
Althoughincreasingconcentrations ofchitosanmayimprove theinhibitionpercentageonmanypathogensthistrenddepends onseveralfactorssuchasmolecularweightofchitosan,targeted pathogenandpH(Yangetal.2005).Theactivityofchitosanhas beenexplainedasbeingbasedontheelectrostaticinteractionof thechargedaminogroupsofchitosanwithnegativelychargedcell wallsurfaceofthetargetedmicroorganisms,whichcanleadtothe disruptionofthecellwallandthereforetothedeathofthecell. Anotherpossibilityforantifungalactivityofchitosanaccountsfor itschainstocrossthecellmembraneinhibitingthecellgrowing frominside(Guoetal.2007).However,fordeacetylatedchitosan (CH)andthederivativeshavinglow degreesofsubstitutionthe mechanismonA.flavusandtheinteractionwithcellsurfacemay formanimpermeablelayeraroundthecell,thusblockingthe trans-portofessentialsolutesintothecell(Eatonetal.2008).
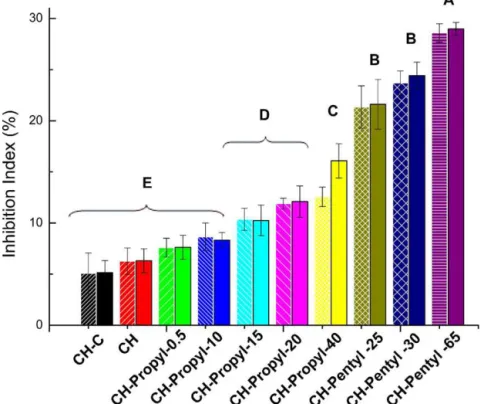
Inthiswork,wehypothesizedthat,themodificationofchitosan by introducing permanently charged quaternary groups could improvetheantifungalactivityofchitosan.Theadditionof qua-ternizedchitosantotheBDAmediuminhibitedmycelialgrowth ofA.flavussignificantlyatallconcentrationstested.A representa-tiveexperimentcomparingtheeffectofCHandCH-Propyl-40is showninFig.4.AsshowninFig.5theresultsobtainedin microbi-ologicalassaysshowedthatthecapabilitytoinhibitfungusgrowth
54 168 (2013) 50–55
Fig.4. Comparativeeffectofthequaternizationofchitosanontheinhibitionindexat0.5g/Lafter3daysofincubation:fromlefttorightBDA;CHandCH-Propyl-40.
significanceof0.05aimingtoverifytheexistenceofsignificant dif-ferencesbetweenresultsobtainedfortheinhibitionindex with 0.1and0.5g/L.Thedescriptivestatisticsshowedthatno signifi-cantdifferenceswereobservedregardingthe0.1and0.5g/L,since theobtainedPvalueswerealwayshigherthan0.5,whichconfirm that,inthisconcentrationrange,theinhibitiondoesnotchange. Acomparisonbetweentheinhibitionindexesofthedifferent chi-tosanderivativeswasalsoperformed.TheKruskal–Wallistestwith Dunn’smultiplecomparisonwasutilizedtothelevelofsignificance 0.05andthedescriptivestatisticsshowedthatPvaluesfoundfor 0.1g/Lweresmallerthan0.001,indicatingthatstructuralchanges onthechitosanchainsignificantlyincreasestheantifungalactivity. The most substituted derivative from CH-Propyl series (DS=38.5%)showedaninhibitionindexthreetimeshigherthan thatobservedwithdeaceylatedchitosan(CH).
However,thehighest inhibition indexwas obtainedfor CH-PentylderivativehavingwithDS65%,whichexhibitedaninhibition indexalmostsixtimeshigherthanCH.Asshownbyotherauthors, thisactivitycanbeattributedtoahighernumberofcationicgroups availableonthepolymerchain,whichallowsastronger interac-tionbetweenthepolycationchainsandthecellwall,increasing
the antifungal activity. However by comparingthe efficiencies of both series it becomes clear that the less substituted CH-Pentylderivative(DS=25%)exhibitedahigherinhibitionthanthat obtainedusingthemore substitutedderivativefromCH-Propyl series(DS=38.5%).Since boththeseriespresent thesame qua-ternary group,this higher activity for CH-Pentyl seriesmay be attributedtothelongerhydrocarbonchainofthegraftedpentyl groups,whichcouldfavorahydrophobicandstrongerinteraction withthecellmembrane.
3.3. Minimuminhibitoryconcentrations
TheresultsobtainedfromMICsdeterminationconfirmedthat thecationicderivativessynthesizedexhibitincreasingefficiencies withthedegreeofsubstitutionbythecationicgroups,withMICsin therangeof1.0–4.0g/L.AscanbeseenfromTable2inthefirst48h, thederivativesCH-Pentyl-30,CH-Propyl-40,CH-Pentyl-25and CH-Pentyl-65inhibitedcompletelythegrowthofthefungusat1.0g/L,
i.e.,aconcentrationfourtimessmallerthanthoseneededfor com-mercial(CH-C)anddeacetylatedchitosan(CH)(Table2).Moreover, after72hnogrowthwasobservedinthepresenceofCH-Pentyl-65
CH-Pentyl-25 0.5 1.0 2.0
CH-Pentyl-30 0.5 1.0 2.0
CH-Pentyl-65 0.5 1.0 1.0
atconcentrationof2.0g/L.TheMICsforCH-CandCHwas4.0g/L andthisvalueiswithinoftherangereportedintheliterature,which variesfrom10to5000ppmdependingonmolecularweight,degree ofacetylationandtypeoffungi(Sajomsangetal.2012).Similar responseswereobservedbyotherauthorsandtheMICsof quater-nizedchitosansdecreasewithdegreeofsubstitution,whichcanbe explainedbasedontheinteractionofthesegroupswithnegatively chargedcellsurfacemembrane,preventingnutrientsfromentering thecell(Guoetal.2007;Yangetal.2005).
4. Conclusions
Thesynthesisandcharacterizationofnewquaternary deriva-tivesofchitosanwerecarriedoutandtheirantifungalactivities againstA.flavusweretested.Theresultshaveshownthatthe anti-fungalactivityofchitosancanbeimprovedbyincreasingthedegree of substitution(DS) of alkyltrimethylammoniumgroups onthe polymerchain.Theinhibitionindexesforbothsynthesizedseries (propyland pentyltrimethylammonium) increasedwith DS and themostsubstitutedderivatives(CH-Propyl-40andCH-Pentyl-65) exhibitedinhibitionvaluesthreeandsixtimesrespectivelyhigher thanthoseobtainedwithchitosan.Thehigheractivityexhibited byCH-Pentylderivativescanbeattributedtothelonger hydrocar-bonchainofthissubstituent,whichallowsastrongerinteraction withthe cellmembrane. Thesynthesis and characterization of newderivatives,havinglongerhydrocarbonchains,areongoingin ourlaboratoryaimingtoobtainderivativeswithhigherantifungal activities.
Acknowledgements
FinancialsupportbyFAPESP(GrantNo.2012/03619-9), FUN-DUNESP (Grant 430/09). R.O.P and M.T. thank FAPESP and PET-CAPESforundergraduatefellowship.
References
AlburquenqueC,BucareySA,Neira-CarrilloA,UrzúaB,HermosillaG,TapiaCV. Antifungalactivityoflowmolecularweightchitosanagainstclinicalisolates ofCandidaspp.MedMycol2010;48(8):1018–23.
BinderEM,TanLM,ChinLJ,HandlJ,RichardJ.Worldwideoccurrenceof myco-toxinsincommodities,feedsandfeedingredients. AnimFeed SciTechnol 2007;137(3–4):265–82.
ductionbyAspergillusflavus.FoodBiotechnol1994;8(2–3):191–211. GuoZY,ChenR,XingR,LiuS,YuH,WangP,etal.Novelderivativesofchitosanand
theirantifungalactivitiesinvitro.CarbohydrRes2006;341(3):351–4. GuoZY,XingR,LiuS,ZhongZ,JiX,WangL,etal.Theinfluenceofthecationicof
qua-ternizedchitosanonantifungalactivity.IntJFoodMicrobiol2007;118(2):214–7. JayakumarR,PrabaharanM,SudheeshKumarPT,NairSV,TamuraH.Biomaterials basedonchitinandchitosaninwounddressingapplications.BiotechnolAdv 2011;29(3):322–37.
JayakumarR,PrabaharanM,NairSV,TokuraS,TamuraH,SelvamuruganN.Novel carboxymethylderivativesofchitinandchitosanmaterialsandtheirbiomedical applications.ProgMaterSci2010;55(7):675–709.
KeanT,ThanouM.Biodegradation,biodistributionandtoxicityofchitosan.AdvDrug DeliverRev2010;62(1):3–11.
KenawyEl-R,Abdel-HayFI,El-MagdAA,MahmoudY.Biologicallyactivepolymers: modificationandanti-microbialactivityofchitosanderivatives.JBioactCompat Polym2005;20:95–111.
LiMQ,ChenXG,LiuJM,ZhangWF,TangXX.Molecularweight-dependentantifungal activityandactionmodeofchitosanagainstFulviafulva(Cooke)Ciffrri.JAppl PolymSci2011;119(6):3127–35.
MássonM,HolappaJ,HjálmarsdóttirM,RúnarssonÖV,NevalainenT,JärvinenT. Antimicrobialactivityofpiperazinederivativesofchitosan.CarbohydrPolym 2008;74(3):566–71.
MuzzarelliRAA,MuzzarelliC,TarsiR,MilianiM,GabbanelliF,CartolariM.Fungistatic activityofmodifiedchitosansagainstSaprolegniaparasitica.Biomacromolecules 2001;2(1):165–9.
NgahWSW,TeongLC.HanafiahMAKMadsorptionofdyesandheavymetalionsby chitosancomposites:areview.CarbohydrPolym2011;83(4):1446–56. RaviKumarMNV.Areviewofchitinandchitosanapplications.ReactFunctPolym
2000;46(1):1–27.
RobertsGAF,WangW.EvaluationofMark–Houwinkviscosimetricconstantsfor chitosan.In:DomardJ,MuzzarelliR,editors.Advancesinchitinscience.Lyon: JacquesAndre;1996.p.279–84.
RinaudoM.Chitin andchitosan: properties and applications.Prog PolymSci 2006;31(7):603–32.
SajomsangW,GonilP,SaesooS,OvatlarnpornC.Antifungalpropertyofquaternized chitosananditsderivatives.IntJBiolMacromol2012;50(1):263–9.
SatpathyG,TyagiYK,GuptaRK.Anoveloptimisedandvalidatedmethodfor analysisofmulti-residuesofpesticidesinfruitsandvegetablesby microwave-assistedextraction(MAE)–dispersivesolid-phaseextraction(d-SPE)–retention timelocked(RTL)–gaschromatography–massspectrometrywithdeconvolution reportingsoftware(DRS).FoodChem2011;127(3):1300–8.
ShahidiF,ArachchiJKV,JeonYJ.Foodapplicationsofchitinandchitosans.Trends FoodSciTechnol1999;10(2):37–51.
ShiCM,ZhuY,RanX,WangM,SuY,ChengT.Therapeuticpotentialofchitosanand itsderivativesinregenerativemedicine.JSurgRes2006;133(2):185–92. TieraMJ,QiuXP,BechaouchS,ShiQ,FernandesJC,WinnikFM.Synthesisand
charac-terizationofphosphorylcholine-substitutedchitosanssolubleinphysiological pHconditions.Biomacromolecules2006;7(11):3151–6.
Tømmeraas K, Köping-Höggård M, Vårum KM, Christensen BE, Artursson P, SmidsrødO.Preparationandcharacterisationofchitosanswitholigosaccharide branches.CarbohydrRes2002;337(24):2455–62.
XuT, XinM, LiM, HuangH, Zhou S,Liu J. Synthesis, characterization,and antibacterialactivityofN,O-quaternaryammoniumchitosan.CarbohydrRes 2011;346(15):2445–50.
YangTC,ChouCC,LiCF.AntibacterialactivityofN-alkylateddisaccharidechitosan derivatives.IntJFoodMicrobiol2005;97(3):237–45.
Zhang B, Wang DF, Li HY, Xu Y, Zhang L. Preparation and properties of chitosan–soybeantrypsininhibitorblendfilmwithanti-Aspergillusflavus activ-ity.IndCropProd2009;29(2–3):541–8.