UNIVERSIDADE FEDERAL DE MINAS GERAIS
LUCAS ARAÚJO LIMA GÉO
FREQUÊNCIA, CORRELATOS E PERFIL NEUROPSICOLÓGICO DO TRANSTORNO DA COGNIÇÃO ASSOCIADO COM A INFECÇÃO PELO HIV
BELO HORIZONTE
LUCAS ARAÚJO LIMA GÉO
FREQUÊNCIA, CORRELATOS E PERFIL NEUROPSICOLÓGICO DO TRANSTORNO DA COGNIÇÃO ASSOCIADO COM A INFECÇÃO PELO HIV
Dissertação apresentada ao Curso de Mestrado do Programa de Pós-Graduação em Neurociências da Universidade Federal de Minas Gerais como requisito parcial à obtenção do título de Mestre em Neurociências.
Orientador: Prof. Dr. Leandro Fernandes Malloy-Diniz
Coorientação: Prof. Dr. Paulo Pereira Christo
Banca Examinadora:
Orientador: Prof. Dr. Leandro Fernandes Malloy-Diniz, Universidade Federal de Minas Gerais
Coorientador: Prof. Dr. Paulo Pereira Christo, Santa Casa de Belo Horizonte
Prof. Dr. Fernando Silva Neves, Universidade Federal de Minas Gerais
Prof. Dr. Paulo Marcos Brasil Rocha, Instituto Mineiro de Educação Superior/ Instituto Metropolitano de Ensino Superior
Prof. Dr. Maicon Rodrigues Albuquerque (Membro Suplente), Universidade Federal de Viçosa
Profa. Dra. Débora Marques Miranda (Membro Suplente), Universidade Federal de Minas Gerais
AGRADECIMENTOS
Gostaria de agradecer aos meus pais, minha irmã, tios, avós, primos, todos que me ajudaram nesta trajetória até o momento. Agradecer à Renalice, companheira, tradutora e amiga. Nina, minha cachorrinha, pela companhia absoluta.
Academicamente agradecer aos Prof. Dr. Leandro Malloy pela oportunidade e apoio, bem com o Prof. Dr. Paulo Christo que fomentaram o meu desenvolvimento na área. Especial carinho aos companheiros de laboratório que há anos me agüentam.
Agradecer aos meus mestres de longa data que me ensinaram o valor do conhecimento e o seu papel iluminador no meio das trevas da vida.
Para mim, ele estava sendo feito o canoeiro mestre, com o remo na mão, no atravessar o
rebelo dum rio cheio. – “Carece de ter coragem... Carece de ter muita coragem...” – eu relembrei. Eu tinha.
Resumo
GÉO, Lucas Araújo Lima. Frequência, correlatos e perfil neuropsicológico do transtorno da cognição associado com a infecção pelo HIV. Belo Horizonte, 2014. Dissertação (Mestrado em Neurociências)- Pós-graduação em Neurociências, Universidade Federal de Minas Gerais, 2014.
O Transtorno da Cognição Associado com a Infecção pelo HIV (HIV-associated
Neurocognitive Disorders - HAND) é causado pela ação direta do vírus HIV no Sistema
Nervoso Central (SNC) ou pela ação indireta dos processos metabólicos relacionados à
infecção. O quadro pode , em sua evolução, pode levar até mesmo à demência. O HAND
apresenta um padrão descrito como subcortical, ou seja, atinge estruturas cerebrais como a
substância branca e os núcleos da base. Entre as dificuldades cognitivas relatadas na literatura
em pacientes acometidos por HAND estão os déficits na memória episódica, havendo
interesse atual em avaliar se tal comprometimento é similar ao que ocorre em outras
demências, como na Doença de Alzheimer. Os objetivos do presente trabalho foram
caracterizar a frequência de HAND em uma população hospitalar infectada pelo HIV,
detectar correlatos clínicos e o padrão subcortical associados, além de avaliar o uso do teste
de aprendizagem e memória episódica auditivo-verbal. A frequênciatotal de HAND foi de
71,8% dos pacientes HIV/SIDA. Os fatores de risco encontrados foram o diagnóstico
de dislipidemia, baixo nível plasmático de hemoglobina e baixo nível educacional. Com
relação à memória episódica, o padrão subcortical foi observado nos pacientes acometidos
pela HAND em comparação aos pacientes com quadro neurodegenerativo, ou seja,
dificuldade na estratégia de busca da informação e não de seu armazenamento.
ABSTRACT
The HIV-associated Neurocognitive Disorders (HAND) is caused by direct action of the HIV
virus in the Central Nervous System (CNS) and by indirect action of related metabolic
processes associated with the infection - condition that can even drive to dementia. HAND
show a pattern described as subcortical which damage white matter brain structures and basal
ganglia. Impairment in episodic memory is usually describe in literature and is aim of study
by many researchers since is a typical difficulty in Alzheimer's disease. The objectives of this
study were characterizing the prevalence of HAND in a hospital population infected with
HIV, detect the risk factors and subcortical pattern as well as evaluate the use of
auditory-verbal learning test and episodic memory. The overall prevalence of HAND was 71.8% of
patients infected with HIV/AIDS. The clinical correlates found were the presence of
dyspilidemia, lower hemoglobin level and lower formal educational. The subcortical pattern
was observed in HAND; the performance demonstrated typical pattern of impairment in
information retrieval strategy but not in storage. The data showed a pattern of high
prevalence, risk factors peculiar to the population studied, with subcortical damage
characteristics typically described in the literature.
LISTA DE TABELAS
Tabela 3.1 Neuropsychological battery………... 30
Tabela 3.2 Descriptive statistics of demographic variables……… 31
Tabela 3.3 Prevalence of HIV-associated neurocognitive disorders……….. 31
Tabela 3.4 Clinical and laboratory characteristics of the sample……… 32
Tabela 3.5 Multiple regression analysis……….. 34
Tabela 4.1 Descritive statistcs of demographiv variables in AD and HAND groups…… 48
LISTA DE ABREVIATURAS E SIGLAS AIDS Síndrome da Imunodeficiência adquirida
AD Alzheimer's Disease
ANI Asymptomatic Neurocognitive Impairment ART Antiretroviral Therapy
BMI Body Mass Index
cART Combination Antiretroviral Therapy CD4 Grupamento de diferenciação 4 CNS Central Nervous System
CPE ART Penetration-Effectiveness ranking DA Doença de Alzheimer
MCD HIV-Associated Minor Cognitive Disorder MCI Amnestic Mild Cognitive Impairment MND Mild Neurocognitive Disorder
HAART Highly Active Antiretroviral Therapy HAD HIV-Associated Dementia
HAND Transtorno da Cognição Associado com a Infecção pelo HIV ou HIV-associated Neurocognitive Disorders
HCV Hepatitis C Virus
HIV Vírus da Imunodeficiência humana
IADLs Instrumental Activities of Daily Living Scale IHDS International HIV Dementia Scale
SPSS Statistical Package for Social Sciences TBI Traumatic Brain Injury
LISTA DE ANEXOS
ANEXO 1 - Artigo publicado com os dados básicos da dissertação
Neurocognitive Performance in Patients with AIDS in Brazil: a case-control study.
Paulo Pereira Christo, Post-doctorate; Lucas Araújo Lima Géo; Carlos Mauricio de Figueiredo Antunes, PHD.
SUMÁRIO
1 - INTRODUÇÃO ... 13
2 - OBJETIVOS ... 16
2.1 Objetivo geral ... 16
2.2 Objetivos específicos ... 16
3- Frequency and risk factors of HIV-associated neurocognitive disorders in a Brazilian population... 17
Abstract ... 17
3.1 INTRODUCTION... 18
3.2 METHODS... 19
3.2.1 Subects... 19
3.2.2 Neuropsychological assessment... 20
3.2.3 Data analysis... 21
3.3 RESULTS... 22
3.4 DISCUSSION ... 23
REFERENCES... 33
4 – COMPARISON OF HIV-ASSOCIATED NEUROCOGNITIVE DISORDERS AND ALZHEIMER DISEASE IN A VERBAL LEARNING EPISODIC TEST: SUBCORTICAL X CORTICAL PROFILE……….. 41
Abstract... 41
4.1 INTRODUCTION... 42
4.2 METHODS... 43
4.2.1 Participants... 43
4.2.2 Episodic Memory Assessment... 44
4.2.2.5 Data Analysis... 45
4.3 RESULTS... 45
1- INTRODUÇÃO
A Síndrome da Imunodeficiência Adquirida (sida-aids) foi descrita no inicio dos anos 80 e se disseminou pelo mundo tornando-se um dos maiores desafios de saúde pública das três últimas décadas. Aids é a manifestação clínica e/ou de resultados laboratoriais que indiquem deficiência imunológica da infecção pelo vírus HIV e que leva, em média, oito a dez anos para se manifestar. A Organização Mundial de Saúde (OMS) estima que, no mundo, aproximadamente 34 milhões de pessoas estão infectadas pelo vírus HIV ou apresentam a doença e que em 2011 ocorreram 1.7 milhões de mortes e cerca de 2.5 milhões de novos casos (UNAIDS, 2012). No Brasil, desde a identificação do primeiro paciente com Aids, em 1982, até junho de 2012, já foram identificados 656.701 casos da doença. Somente em 2011, foram notificados 38.776 casos.
O transtorno da cognição associado à infecção pelo HIV é conhecido na literatura como distúrbios neurocognitivo associado ao HIV (HIV-associated neurocognitive disorders - HAND), que apreende o estágio mais severo, demencial, mas também os estágios mais sutis, como o de comprometimento cognitivo leve e comprometimento cognitivo assintomático associado ao HIV. Demência por HIV contribui para morbidade da infecção e é um fator de risco para mortalidade (Sackor et al. 1996).
É de grande importância identificar o número de pacientes com danos cognitivos, uma vez que afetam a qualidade de vida, função laborativa e aderência à medicação (McArthur, 2004, Price et al. 1999).
A avaliação neuropsicológica tem um papel chave na identificação e diagnóstico de distúrbios cognitivos associados ao HIV e é usada para quantificar alterações em processos cognitivos associado com o tratamento. Testes neuropsicológicos são sensíveis para detectar distúrbios cognitivos na infecção pelo HIV-1 e devem incluir os seguintes domínios: 1-atenção/concentração; 2-rapidez do processamento da informação; 3- função executiva; 4-raciocínio/abstração; memória/aprendizado; 5-habilidade visuoespacial; e 6-funcionamento motor.
O perfil de comprometimento cognitivo pelo HIV é tipicamente subcortical (Cohen et al. 2010), porém com uma variabilidade grande na ocorrência dos domínios afetados, o que pode gerar grande dificuldade no processo avaliativo e até mesmo dúvidas diagnósticas com outros processos demenciais, até mesmo os considerados corticais, como Doença de Alzheimer (DA) (Sadek et al. 2007). Quando se define como demência subcortical, o esperado é um comprometimento típico em velocidade do processamento de informações e ou psicomotora, presença de sintomas psiquiátricos como depressão, ansiedade e apatia, bem como alterações em funções executivas (Woods et al. 2009, Christo, Géo & Antunes, 2013). Esta alteração nas funções executivas, que tem funcionamento preponderantemente cortical, se deve a estruturas mais internas no cérebro, como os núcleos da base. Para uma avaliação neuropsicológica eficaz, é necessária uma avaliação global do desempenho cognitivo, já que todos os domínios cognitivos podem estar alterados, mesmo que em menor intensidade (Christo, Géo & Antunes, 2013).
população brasileira ainda é pouco estudada, com resultados apontando a prevalência entre 52-65% (Rodrigues, Oliveira, Grinsztejn & Silva, 2013, de Almeida et al. 2013).
2 - OBJETIVOS
2.1 Objetivo geral
-Investigar a frequência dos transtornos HAND, bem como seus fatores de risco associados e seu perfil de comprometimento neuropsicológico.
2.2 Objetivos específicos
-Verificar se o padrão epidemiológico brasileiro é semelhante aos padrões internacionais, onde geralmente são descritos em países de primeiro mundo ou de extrema pobreza, como países africanos.
-Investigar características de vulnerabilidade da população brasileira para o desfecho cognitivo comprometido, com características sociodemográficas peculiares em comparação internacional.
-Investigar se pacientes com HAND apresentam um padrão de comprometimento de memória episódica característico de demências subcorticais
3- FREQUENCY AND RISK FACTORS OF HIV-ASSOCIATED NEUROCOGNITIVE DISORDERS IN A BRAZILIAN POPULATION
Abstract:
Background: It is estimated that in Brazil approximately 630,000 people currently live with HIV or AIDS. 1 can cause several neurological disorders, collectively known as HIV-associated neurocognitive disorders. Although the incidence of dementia-HIV has dropped since the advent of HAART, it continues to be a significant cause of morbidity in infected patients. In view of the importance of cognitive functions for productivity and performance of daily activities, the objective of the present study was to evaluate the frequency of neurocognitive deficits and their risk factors in a sample of patients with AIDS seen at a referral outpatient facility that provides care to HIV patients in the state of Minas Gerais, Brazil.
Methods: The sample was composed of 110 patients with AIDS and 64 control individuals. The patients were randomly recruited during their routine visit to the infectious disease specialist and were submitted to neurological and neuropsychological evaluation. This included medical history, structured neurologic and medical examination, as well as both functional and neuropsychological assessment and the prevalence of impairment by classifying the individuals in the groups considering test performance and IADL score due Frascati criteria.
provenance from cities other than the capital of the state, presence of dyslipidemias and hemoglobin levels. After a multivariate analysis there were differences between them concernig years of education, dyslipidemia e hemoglobin level.
Conclusion: HAND is quite frequent in patients with HIV in Brazil with asymptomatic form, the most prevalent manifestation. The presence of dysplidemia, low hemoglobin level and low formal education were the risk factors related to HAND.
Introduction
Approximately 34 million people worldwide are infected by HIV-1 (Global Report/Unaids, 2013). In Brazil since the beginning of the epidemic in 1980 until June 2011, 656.701 cases were registered. The incidence rate in Brazil was 20,2 cases/100,000 inhabitants in 2010 (Boletim epidemiológico AIDS-DST, 2011). It is estimated that in Brazil approximately 630.000 people currently live with HIV or AIDS (Christo, 2010).
HIV-1 can cause several neurological disorders, collectively known as HIV-associated neurocognitive disorders (HAND). HAND encompass a hierarchy of progressively more severe patterns of central nervous system (CNS) involvement, ranging from asymptomatic neurocognitive impairment (ANI), HIV-associated mild neurocognitive disorder (MND), to the more severe HIV-associated dementia (HAD; also known AIDS dementia complex, [ADC], or HIV encephalopathy) (Antinori et al. 2007).
Before the widespread use of HAART in the developed world, 20%–30% of individuals infected with HIV-1 developed a range of cognitive and motor symptoms that are collectively known as HAD. It has recently been estimated that ~10% of HIV-1 infected adults have HAD but that MND might be several times more common, involving perhaps as many as 30% of the HIV-1 infected population (Brew et al. 2009). This includes individuals who are not immune suppressed as well as those with end-stage acquired immunodeficiency syndrome (AIDS) (Grant, Sacktor & McArthur, 2005).
The Brazilian public health system provides antiretroviral drugs free of charge to patients with AIDS which makes the studies of HAND in this population important since Brazil is an emerging nation and the people have characteristics which are different from those in industrialized or underdeveloped countries.
In view of the importance of cognitive functions for productivity and performance of daily activities, the objective of the present study was to evaluate the frequency of neurocognitive deficits and their risk factors in a sample of patients with AIDS seen at a referral outpatient facility that provides care to HIV patients in the state of Minas Gerais, Brazil.
Methods Subjects:
The sample was composed of 110 patients with AIDS and 64 control individuals. The group of patients was composed of individuals followed up at the Infectious Diseases Outpatient Clinic, which is referral centre for care and treatment of HIV/AIDS in Belo Horizonte, Minas Gerais, Brazil. All patients were more than 18 years old and had no current history of diffuse or focal CNS disease. The control group was evaluated to determine normative values for the neurocognitive tests employed all participants were age older than 18. Subjects with a history of neurological disorders such as stroke and epilepsy, cognitive impairment identified by a lower score than expected for age/educational level in the Mini-Mental State Examination (Bertolucci, Brucki, Campacci & Juliano, 1994), presence of depressive symptoms identified by interview using the Beck Depression Scale (a score>12 indicates signs and symptoms of depression), and treatment with antidepressants, neuroleptics, anticonvulsant and mood-stabilizing agents were not included in this individual control sample.
Neuropsychological assessment.
Clinical and Neurological examination
This included medical history, structured neurologic and medical examination, as well as information about demographic, clinical and laboratory characteristics and comorbid conditions such as traumatic brain injury and past CNS opportunistic infections. This evaluation sought to ensure that these conditions, if present, were a static event since its beginning (static and acquired encephalopathy). Determination was performed to assess whether the neurocognitive impairment and functional disability are believed to be due to the effects of HIV on the brain, and are not readily attributable to comorbid conditions. This determination requires not only detailed information about the comorbid conditions themselves, but also clinical judgment about their severity, their likely impact on neurocognition and everyday functioning, and their timing in relation to the course of HIV disease and any functional limitations in everyday life.
Data Analysis
score 1 SD below on at least 2 cognitive domains; 2) no impairment on IADL. HIV normal cognitive functioning occurred when no criteria above were met.
We examined the prevalence of impairment by classifying the individuals in the groups considering test performance and IADL score. Analysis of variance (Anova) was used to investigate statistical differences in demographic and clinical variables among them. T-test, Mann-Whitney and chi-square tests were used to assess whether the two groups (normal versus presence of impairment) were statistically different from each other in demographic and clinical variables. Mann-Whitney U test was an alternative non-parametric test used to investigate differences between groups. A regression logistic model was adjusted for statistical significant variables observed in univariate analysis considering p<0.25. The final logistic model was adjusted for significant variables that could differentiates between the normal and impaired HIV cognitive groups considering p<0.05.
Results
A total of 110 patients with AIDS (cases) and 64 controls were assessed. Mean age, gender and years of education of participants groups are shown in Table 2. Age distribution was similar comparing cases and controls (t=0.47; p=0.64); controls have more years of education compared to cases (t=4.43; p<0.01). Regarding gender, a higher proportion of males were observed among cases (chi-square=20.29; p<0.01).
According to standardized neuropsychological scores, the patients were classified into neurocognitive diagnoses (Table 3). ANI was observed in 62/110 cases, HIV dementia in 5/110, MND was observed in 12 and 31 were normal.
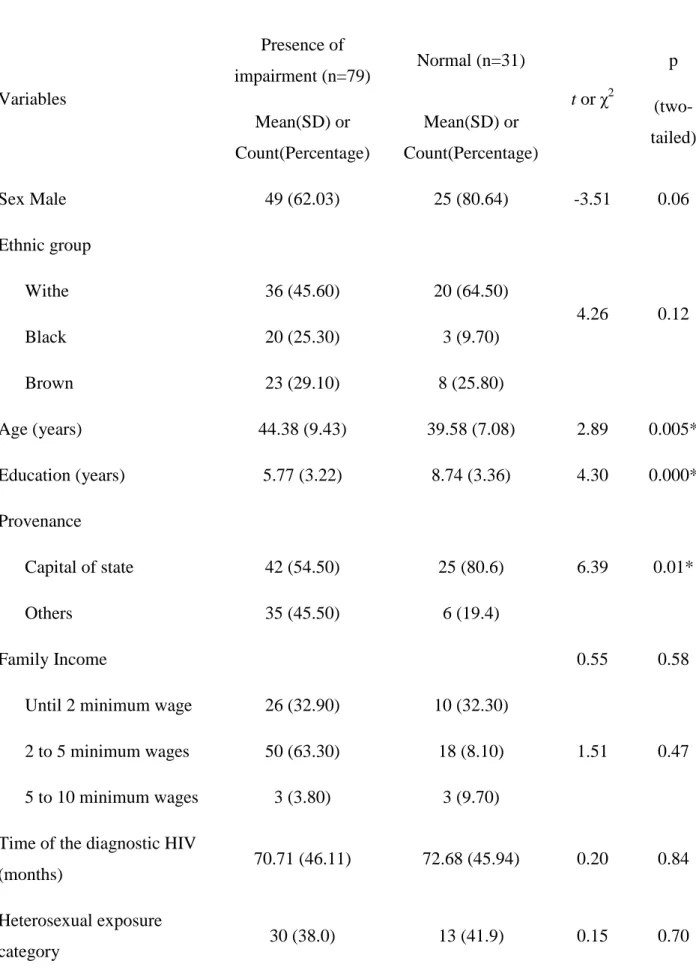
When comparing normal and impaired groups, there are differences between them concerning age (t=2.89; p=0.005) and years of education (t=4.30; p<0,001), provenance from cities other than the capital of the state (chi-square=6.39; p=0.01), presence of dyslipidemias (chi-square=4.46; p=0.04), hemoglobin levels (t=2.60; p<0.05). Moreover, there is no differences in gender (chi-square=3.51; p=0.06) time since diagnosis (t=0.20; p=0.84), mean CD4 count (t=0.18; p=0.86), mean viral load (t=1.57; p=0.12).
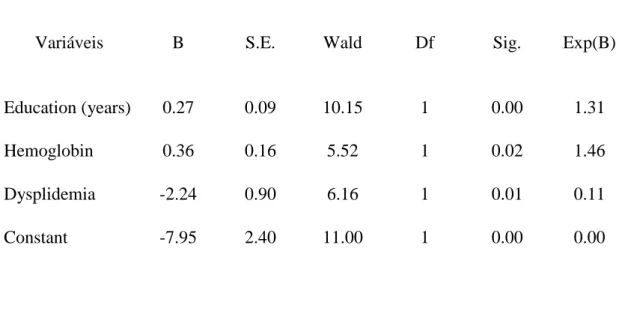
A regression model was adjusted for statistical significant variables observed on univariate analysis (p<0.25). Initially, the following variables were entered in the model: sex, age, years of study, provenance from cities other than the capital of the state, previous hospitalization, hepatitis B, hemoglobin level and presence of dyslipidemia. The final model indicated that years of study, hemoglobin and dyslipidemia could discriminate between normal and impairment HIV cognitive groups (see Table 5).
Discussion
et al. 2010)showed that the prevalence of HAND remains high despite HAART (52% of the patients in the study had neuropsychological impairment). The prevalence of asymptomatic neurocognitive impairment was highest at 33%, whereas that for HIV-associated dementia was only 2%, and 12% had mild neurocognitive disorder, supporting earlier evidence that whereas severe forms of neurocognitive impairment are occurring less frequently, HAND in its milder forms remains common (Heaton et al, 2010).
In research studies from sub-Saharan Africa, the prevalence of HAD ranges from 2.5%–54% (Kwasa , Cettomai , Lwanya & Lessons, 2012); these estimates vary widely likely due to differences in the sampled populations and methods for assessment of cognitive impairment. Recent study reported HAND in 69% of 200 patients with HIV in a Swiss patient samples who had maintained a good virological response (undetectable HIV RNA in plasma while on cART) over a median of 48 months (Simioni et al. 2012). In Brazilian population other researchs found similar results than shown in this work, with a prevalence of hand in 52% (only screening evaluation) (Rodrigues, Oliveira, Grinsztejn & Silva, 2013) and 58-65% in a full neuropsychological assessment (de Almeida et al. 2013). Similar epidemiological data of HAND where shown in other countries like Uganda 55-80%, United States 57-80% (Saktor et al. 2005), Cameron 85% (Atashili et al. 2013) and China 53% (Wang et al. 2013).
McArthur & Brew, 2010; McArthur, Steiner, Sacktor & Nath, 2010; Wojna & Nath, 2006). One of the important aspects defined in HAND is the identification of co-morbid etiologies to grade major confounds of HAND into secondary condition (findings are compatible with HAND or incidental), contributing (HAND), and confounding (unable to attribute abnormalities to direct effects of HIV). The main confounding etiologies identified by Antinori et al. (Antinori et al. 2007) were depression, traumatic brain injury (TBI), alcohol and/or substance abuse and co-infection with HCV. This the present study had no association of depression, alcoholism and presence of seizures with HAND and the group of patients also had no cases with TBI or other psychiatric disorders.
In Brazil there are insufficient data on the neurocognitive effects of HIV. These studies are important, since although the social-cultural level is lower than in developed countries, the HIV patient in Brazil has access to antiretroviral treatment free of charge and there are few Brazilian studies of cognitive assessment in patients with AIDS.
Given the rising prevalence of HIV/AIDS in the industrialized and developing world, it is likely that new HIV-associated neurologic syndromes will emerge as time progresses. Neurologic manifestations of AIDS is expected to be a major upcoming health issue among long term HIV seropositive survivors and AIDS patients. Today in developed countries, HAART has changed AIDS from a fatal disease to a more manageable chronic infection (del Palacio, Alvarez & Muñoz-Fernández, 2012). But even with aggressive therapy, the virus cannot be completely eradicated, and body regions that remain relatively isolated from the systemic circulation, such as the CNS, might provide a “sanctuary” for latent or slowly replicating virus.
secondary to neurocognitive disorders in HIV patients is currently a major challenge. Not infrequently, psychiatric symptoms are seen as "natural" response to virus infection. The detection of disorders generally requires rigorous investigation, because the symptoms can be subtle or remain unnoticed or unreported (Gallego, Barreiro & López-Ibor 2011). The diagnosis of HAND remains a challenge in HIV outpatient primary care settings in resource-limited regions. Potential reasons for this include: a lack of specialized personnel and diagnostic tests, and the inherent difficulties in making a clinical diagnosis of a complex disorder. We found a significant association of patient low educational level, dyslipidemia, low hemoglobin levels and origin of the interior city with the presence of cognitive impairment that shows the importance of a high specialized and complex treatment of the disease.
(1993) reported that the strongest predictor of HIV-dementia was low hemoglobin in the pre-cART era and more recent studies suggest that pre-cART reduces the prevalence of anaemia (Belperio & Rhew 2004). There is a paucity of studies in the post-cART era that have examined the association between anaemia and HAND.
With regard to the low educational level similar to other studies in HIV dementia as well as other dementias as Alzheimer's may be due to the brain reserve hypothesis (de Ronchi et al. 1998; Katzman, 1993). Highest level of education may delay the onset of cognitive impairment due to possible promotion of an extra brain reserve. It has also been reported that there is an increased risk of cognitive among older adults who are HIV positive (Becker, Lopez, Dew & Aizenstein, 2004).
Several additional studies have examined the association between HCV and risk of HAND (Cherner et al. 2005; Hilsabeck , Castellon & Hinkin, 2005; Letendre et al. 2005; Tozzi et al. 2005; Aronow, Weston, Pezeshki & Lazarus, 2008; Clifford et al. 2009). In all of these studies, HIV-infected participants who were co-infected with HCV had higher rates of global cognitive impairment. In this study the number of co-infected with hepatitis C virus was small making it impossible to analyze statistic, but the serology for hepatitis B was higher in patients with HAND.
baseline and current. In a pre-cART study, McArthur et al. (1997) showed that CSF, but not plasma HIV RNA levels, were significantly higher in those with HAND. However, more recent studies of patients on cART have not found this relationship (Sevigny et al. 2004).
No association was found between alcohol use and HAND. Alcohol has also been suggested as a risk factor for HAND, albeit reports have been conflicting (Jayadev & Garden, 2009).
Some studies have demonstrated beneficial effects on neurocognitive function by ART regimens ranked according to their predicted effectiveness in suppressing HIV replication within the CNS (Letendre et al. 2008; Heaton et al. 2010; Smurzynski et al. 2011). Better neurocognitive performance has been observed over a 15-week period in adult individuals beginning ART with regimens of higher CPE (Letendre et al. 2008). A cross-sectional study of 2636 adults (AIDS Clinical Trials Group Longitudinal Linked Randomized Trials [ALLRT cohort]) on effective ART (<50 HIV RNA copies/ml) demonstrated better neurocognitive performance in those receiving higher CPE ART (Smurzynski et al. 2011).
Risk factors for HAND found in this sample of patients, especially those not found, may be by specific characteristics of the study population or a limited sample. Future studies are needed to better characterize these risk factors, aiming to detect more clearly if there is a different pattern of these factors in the Brazilian population in comparison to international studies.
control group appeared, as well as lower education level of the control group compared to the clinical group.
Table 1: Neuropsychological battery
Cognitive domain
Neuropsychological Test Selected Neuropsychological Test
Processing speed
Coding WAIS-III Trail Making test part A Stroop test 1 and 2
Coding WAIS-III
Working
Memory/Attention
Paced Auditory Serial Addition Test- part A
Dígit Span WAIS-III
Paced Auditory Serial Addition Test- part A
Executive function
Five Points Test
Trail Making test part B Stroop test part 3
Five Points Test-
Episodic Memory and Learning
Rey Complex Figure record after 3 minutes
Rey Auditory Verbal Learning Test
Rey Complex Figure record after 3 minutes
Language
Semantic Verbal Fluency Foods Semantic Verbal Fluency Animals Fonemic Verbal Fluency F.A.S.
Semantic Verbal Fluency Foods
Fine motor coordination Nine Hole Peg Test Nine Hole Peg Test
Table 2: Descriptive statistics of demographic variables
Groups N Mean age (sd) Years education (sd) % men Cases 110 43.03 (9.07) 6.61 (3.51) 67.3 Controls 64 42.05 (15.20) 8.98 (3.23) 32.8
Table 3: Prevalence of HIV-associated neurocognitive disorders
Neurocognitive diagnoses Frequency Percent
Dementia-HIV 5 (4,5) 4,5
MND* 12 (10,9) 10,9
Asymptomatic (ANI) 62 (56,4) 56,4
Normal 31 (28,2) 28,2
Table 4: Clinical and laboratory characteristics of the sample
Variables
Presence of
impairment (n=79) Normal (n=31)
t or χ2
p (two-tailed) Mean(SD) or
Count(Percentage)
Mean(SD) or Count(Percentage)
Sex Male 49 (62.03) 25 (80.64) -3.51 0.06
Ethnic group
4.26 0.12
Withe 36 (45.60) 20 (64.50)
Black 20 (25.30) 3 (9.70)
Brown 23 (29.10) 8 (25.80)
Age (years) 44.38 (9.43) 39.58 (7.08) 2.89 0.005* Education (years) 5.77 (3.22) 8.74 (3.36) 4.30 0.000* Provenance
6.39 0.01* Capital of state 42 (54.50) 25 (80.6)
Others 35 (45.50) 6 (19.4)
Family Income 0.55 0.58
Until 2 minimum wage 26 (32.90) 10 (32.30)
1.51 0.47 2 to 5 minimum wages 50 (63.30) 18 (8.10)
5 to 10 minimum wages 3 (3.80) 3 (9.70) Time of the diagnostic HIV
(months) 70.71 (46.11) 72.68 (45.94) 0.20 0.84
Heterosexual exposure
Previous hospitalization 52 (65.8) 31 (51.6) 1.95 0.18
Depression 8 (10.10) 5 (16.10) 0.77 0.38
Smoking 22 (27.80) 8 (25.80) 0.047 0.83
Alcoholism 16 (20.30) 3 (9.70) 1.74 0.19
Dyslipidemia 19 (24.10) 2 (6.50) 4.46 0.04*
Past ONI d 16 (20.3) 4 (12.9) 0.81 0.84
Peripheral Neuropathy 29 (36.71) 7 (22.58) 2.02 0.16
Use ofARV 59 (93.70) 26 (100) 1.78 0.19
ARVa Score 4.97 (3.22) 6 (2.70) 1.13 0.19
Serology of hepatiise B 14 (20.60) 8 (29.60) 2.86 0.09
Hepatitis C 0 1 (4.20) 0.88 0.34
Initial CD4 count (cells/m3) 291.67 (294.99) 359.71 (263.36) 1.11 0.27 Current CD4 count (cells/m3) 491.96 (235.88) 501.11 (228.74) 0.18 0.86 Initial Viral Load (log) 3.98 (1.24) 3.96 (1.35) 0.08 0.94 Actual Viral Load (log) 2.62 (1.17) 2.62 (1.30) 0.01 0.99 Hemoglobin 13.61 (2.08) 14.77 (1.65) 2.60 0.01* Albumina 419.62 (493.87) 292.86 (459.12) 1.18 0.24
Seizures 14 (17.7) 3 (9.7) 1.10 0.25
Arterial hypertension 8 (10.1) 2 (6.5) 0.36 0.55
Diabetes Mellitus 1 (1.30) 0 0.40 0.53
Table 5: Multiple regression analysis
Variáveis B S.E. Wald Df Sig. Exp(B)
Education (years) 0.27 0.09 10.15 1 0.00 1.31
Hemoglobin 0.36 0.16 5.52 1 0.02 1.46
Dysplidemia -2.24 0.90 6.16 1 0.01 0.11
Constant -7.95 2.40 11.00 1 0.00 0.00
References:
1. Antinori A, Arendt G, Becker JT, et al. (2007). Updated research nosology for HIV-associated neurocognitive disorders. Neurology. 69:1789-1799.
2. Aronow HA, Weston AJ, Pezeshki, BB, Lazarus TS. (2008). Effects of coinfection with HIV and hepatitis C virus on the nervous system. AIDS Read.18:43-48.
3. Atashili J1, Gaynes BN, Pence BW, Tayong G, Kats D, O'donnell JK, Ndumbe PM, Njamnshi AK. (2013). Prevalence, characteristics and correlates of a positive-dementia screen in patients on antiretroviral therapy in Bamenda, Cameroon: a cross-sectional study. BMC Neurol. 15;13:86.
4. Becker JT, Kingsley L, Mullen J, et al. (2009). Vascular risk factors, and cognitive dysfunction in gay and bisexual men. Neurology. 73:1292-1299.
6. Belperio PS, Rhew DC. (2004). Prevalence and outcomes of anemia in individuals with human immunodeficiency virus: a systematic review of the literature. Am J Med. 116 Suppl 7A:27S-43S.
7. Bertolucci PHF, Brucki SMD, Campacci SR, Juliano Y. (1994). O Mini-exame do Estado Mental em uma população geral. Impacto da escolaridade. Arq. Neuropsiquiatr. 52:1-7.
8. Boletim epidemiológico AIDS-DST Ano VIII No1 publicado 28/11/2011. http://www.aids.gov.br/publicacao/2011/boletim_epidemiologico_2011.
9. Brew BJ, Crowe SM, Landay A, et al. (2009) Neurodegeneration and ageing in the HAART era. Journal of Neuroimmune Pharmacology. 4: 163–174
10.Cherner M, Letendre S, Heaton RK, et al. (2005). Hepatitis C augments cognitive deficits associated with HIV infection and methamphetamine. Neurology.64:1343-1347
11.Childs EA, Lyles RH, Selnes OA, et al. (1999). Plasma viral load and CD4 lymphocytes predict HIV-associated dementia and sensory neuropathy. Neurology. 52(3):607-613.
12.Christo PP. (2010). Cognitive alterations associated with HIV-1 infection and AIDS. Rev Assoc Med Bras. 56(2):242-7.
13.Christo PP, Géo LAL, Antunes CMF. (2013). Neurocognitive performance in patients with AIDS in Brazil: a case-control study. Clinical Neuropsychiatry. 10, 2, 107-110. 14.Clifford DB, Smurzynski M, Park LS, et al. (2009). Effects of active HCV replication
on neurologic status in HIV RNA virally suppressed patients. Neurology. 73:309-314 15.Cohen RA, Gongvatana A. (2010). The persistence of HIV-associated neurocognitive
16.de Almeida SM, Ribeiro CE, de Pereira AP, Badiee J, Cherner M, Smith D, Maich I, Raboni SM, Rotta I, Barbosa FJ, Heaton RK, Umlauf A, Ellis RJ. (2013). Neurocognitive impairment in HIV-1 clade C- versus B-infected individuals in Southern Brazil. J Neurovirol.19(6):550-6.
17.De Ronchi D, Fratiglioni L, Rucci P, Paternicò A, Graziani S, Dalmonte E. (1998). The effect of education on dementia occurrence in an Italian population with middle to high socioeconomic status. Neurology. 1998;50(5):1231-8.
18.del Palacio M, Alvarez S, Muñoz-Fernández MÁ. (2012). HIV-1 infection and neurocognitive impairment in the current era. Rev Med Virol. 22(1):33-45.
19.Gallego L, Barreiro P, López-Ibor JJ. (2011). Diagnosis and clinical features of major neuropsychiatric disorders in HIV infection. AIDS Rev. 13(3):171-9.
20.Global report: UNAIDS report on the global AIDS epidemic 2013.
21.Grant I, Sacktor N, McArthur JC. (2005). HIV neurocognitive disorders. In: Gendelman HE, Grant I, Everall I, Lipton SA, Swindells S, editors. The neurology of AIDS. 2nd ed., Oxford: Oxford University Press. 359-373.
22.Heaton RK, Clifford DB, Franklin DR Jr, et al. (2010). HIV-associated neurocognitive disorders persist in the era of potent antiretroviral therapy: CHARTER study. Neurology. 75:2087-2096.
24.Heaton RK, Franklin DR, Ellis RJ, et al. (2011). HIV-associated neurocognitive disorders before and during the era of combination antiretroviral therapy: differences in rates, nature, and predictors. J Neurovirol 17:3-16.
25.Hilsabeck RC, Castellon SA, Hinkin CH. (2005). Neuropsychological aspects of coinfection with HIV and hepatitis C virus. Clin Infect Dis. 41 Suppl 1:S38-S44. 26.Jayadev S, Garden GA. (2009). Host and viral factors influencing the pathogenesis of
HIV-associated neurocognitive disorders. J Neuroimmune Pharmacol. 4(2):175-189 27.Katzman R. (1993). Education and the prevalence of dementia and Alzheimer's
disease. Neurology. 43(1):13-20.
28.Kwasa J, Cettomai D, Lwanya E. (2012). Lessons learned developing a diagnostic tool for HIV-associated dementia feasible to implement in resource-limited settings: pilot testing in Kenya. PLoS One. 7(3):e32898.
29.Lawton, M.P., and Brody, E.M. (1969). Assessment of older people: Self-maintaining and instrumental activities of daily living. Gerontologist 9:179-186.
30.Letendre S, Marquie-Beck J, Capparelli E, et al. (2008). Validation of the CNS penetration-effectiveness rank for quantifying antiretroviral penetration into the central nervous system. Arch Neurol. 65:65-70.
31.Letendre SL, Cherner M, Ellis RJ, et al. (2005). The effects of hepatitis C, HIV, and methamphetamine dependence on neuropsychological performance: biological correlates of disease. AIDS.19 Suppl 3:S72-S78.
32.McArthur JC, Brew BJ. (2010). HIV-associated neurocognitive disorders: is there a hidden epidemic? AIDS. 24(9):1367-1370.
34.McArthur JC, McClernon DR, Cronin MF, et al. (1997). Relationship between human immunodeficiency virus-associated dementia and viral load in cerebrospinal fluid and brain. Ann Neurol. 42(5):689-698.
35.McArthur JC, Sacktor N, Selnes O. (1999). Human immunodeficiency virus-associated dementia. Semin Neurol. 19(2):129-50.
36.McArthur JC, Steiner J, Sacktor N, Nath A. (2010). HIV-associated neurocognitive disorders. Mind the gap. Ann Neurol. 67:699-714.
37.McArthur JC. (2004). HIV dementia: an evolving disease. J Neuroimmunol. 157:3-10. 38.McCutchan JA, Marquie-Beck JA, Fitzsimons CA, Letendre SL, Ellis RJ, Heaton RK,
Wolfson T, Rosario D, Alexander TJ, Marra C, Ances BM, Grant I; CHARTER Group. (2012). Role of obesity, metabolic variables, and diabetes in HIV-associated neurocognitive disorder. Neurology.78(7):485-92.
39.Mind Exchange Working Group. (2013). Assessment, diagnosis, and treatment of HIV-associated neurocognitive disorder: a consensus report of the mind exchange program. Clin Infect Dis. 56(7):1004-17.
40.Nachega JB, Trotta MP, Nelson M, et al. (2009). Impact of metabolic complications on antiretroviral treatment adherence: clinical and public health implications. Curr HIV/AIDS Rep. 6:121-129.
41.Rodrigues RA, Oliveira RL, Grinsztejn B, Silva MT. (2013) Validity of the International HIV dementia scale in Brazil. Arq Neuropsiquiatr. 2013;71(6):376-9. 42.Sacktor N, McDermott MP, Marder K, et al. (2002). HIV-associated cognitive
43.Sacktor NC, Wong M, Nakasujja N, Skolasky RL, Selnes OA, Musisi S, Robertson K, McArthur JC, Ronald A, Katabira E. (2005). HIV Dementia Scale: a new rapid screening test for HIV dementia. AIDS. 2;19(13):1367-74.
44.Selnes OA. (2005). Memory loss in persons with HIV/AIDS: assessment and strategies for coping. AIDS Read. 15(6):289-92, 294.
45.Sevigny JJ, Albert SM, McDermott MP, et al. (2004). Evaluation of HIV RNA and markers of immune activation as predictors of HIV-associated dementia. Neurology. 63:2084-2090.
46.Simioni S, Cavassini M, Annoni JM, et al. (2010) Cognitive dysfunction in HIV patients despite long-standing suppression of viremia. AIDS;24:1243-1250.
47.Smurzynski M, Wu K, Letendre S, et al. (2011). Effects of central nervous system antiretroviral penetration on cognitive functioning in the ALLRT cohort. AIDS. 25(3):357-365.
48.Tebas P. (2008). Insulin resistance and diabetes mellitus associated with antiretroviral use in HIV-infected patients: pathogenesis, prevention, and treatment options. J Acquir Immune Defic Syndr. 49 Suppl 2:S86-S92 .
49.Tozzi V, Balestra P, Lorenzini P, et al. (2005). Prevalence and risk factors for human immunodeficiency virus-associated neurocognitive impairment, 1996 to 2002: results from an urban observational cohort. J Neurovirol. 11(3):265-273 .
51.Valcour VG, Sacktor NC, Paul RH, et al. (2006). Insulin resistance is associated with cognition among HIV-1-infected patients: the Hawaii Aging With HIV cohort. J Acquir Immune Defic Syndr. 43(4):405-410.
52.Wang Z, Zheng Y, Liu L, Shen Y, Zhang R, Wang J, Lu H. (2013). High prevalence of HIV-associated neurocognitive disorder in HIV-infected patients with a baseline CD4 count ≤ 350 cells/µL in Shanghai, China. Biosci Trends. 7(6):284-9.
4- COMPARISON OF HIV-ASSOCIATED NEUROCOGNITIVE DISORDERS AND ALZHEIMER DISEASE IN A VERBAL LEARNING EPISODIC TEST: SUBCORTICAL X CORTICAL PROFILE
Abstract:
Background: HIV-associated Neurocognitive Disorders (HAND) is a typical neurological complication in HIV patients that damage subcortical structures of the brain. However Alzheimer Disease (AD) led to a cortical dementia with a central deficit in episodic memories.
Methods: We compared HAND (n=79) and AD (n=78) patients in Rey Auditory Verbal Learning Test (RAVLT) to find a differentiation of subcortical x cortical profiles, based in the differentiate in storage and retrieval of the information.
Results: The groups differ in A1 (U=2217,50, p=0,002), TOT (U=2483,00, p=0,036), LOT (U=2286,00, p=0,005) and REC (U=567,50, p<0,001), with better results for the HAND group. The other variables did not differ in our study (p>0,05). Excluding the dementia cases, we found the same result, better performance in HAND group in the same variables, A1 (U=1963,50, p=0,003), TOT (U= 2159,00, p=0,026), LOT (U=1947,50, p=0,002) and REC (U=510,50, p<0,001). In a comparison only using dementia subjects, even with a very small
numbers of (n=9) we found a significant difference in REC result (U=2,00, p=0,05) with a
better performance in HAND group. In effect size analyses of full sample we found small
effects for A1 (r= 0,24), TOT (r=0,17) and LOT (r=0,22) and large effects for REC (r=0,70).
Introduction
The HIV-Associated Dementia (HAD) is a well known neurological complication
caused by the directed and undirected action of the virus in CNS and its inflammatory
responses (Xia, 2011). Other earlier stages of the disease exist in a large number of
individuals with several impacts in their daily living activities with a presence of mild
impairment including since the asymptomatic neurocognitive impairment (ANI) to
HIV-associated mild neurocognitive disorder (MND), forming a cluster named HIV-HIV-associated
Neurocognitive Disorders (HAND) (Maj et al. 1994). In Brazilian population, HAND was
detected in 98 of 187 patients (52%) using a validated screening instrument (Rodrigues et al.
2013), the International HIV Dementia Scale (IHDS) while in a sample submitted to a full
neuropsychological evaluation the percentage of detection was 58-65% (de Almeida et al.
2013). In a previous study from our group (Christo et al., 2013) we detected the presence of
HAND in 71,8% of HIV infected population (n=110) using a broad neuropsychological and
clinical evaluation including tasks from Antinori criteria (Antinori et al. 2007).
The incidence of HAD has decreased after the use of HAART with a larger
expectancy of life but the prevalence of HAND has actually increased due to the enhanced
survival of HIV-1 infected patients receiving HAART (Saktor et al. 2002), probably
associated to more time of life exposed by the neuroinfection (Brew et al 2008). From aging
of HIV population emerges a new risk factor for other types of dementia, especially
Alzheimer's Disease (AD), tightly associated to aging processes and starts new challenges for
professionals of health (Xu & Ikezu, 2009). Therefore, is important to assess differences
between HAD and another types of dementia. This knowledge will be useful both considering
the elucidation of the brain mechanisms of HAD and for clinical purposes.
The dementia in HIV presents a “subcortical” pattern of symptomatology with a
demonstrated reduced volume both in subcortical and cortical areas in brain (Cohen et al.
2010). Deficits in episodic memory is a common symptom of HAND affecting treatment
adherence, especially in prospective memory, or, “remember to remember” (Woods et al.
2008). Sadek et al (Sadek et al. 2004) have found in HAND, Huntington Disease, other
subcortical dementia process and Alzheimer Disease deficits on remote memory but with a
benefit of a “clue” to retrieval the information on HAND and Huntington Disease, pointing to
a dissociation between cortical and subcortical dementias, involving the retrieval process in
fronto-subcortial structures and medial temporal structures involved in learning of the
information. A subcortical profile in verbal learning memory process are described in HAND
as well as a typical difficulty in the retrieval of the information (Delis, 1995).
This study intend to assess differences between a sample of subjects with pathological
aging presenting a cortical pattern of disease (including both amnestic mild cognitive
impairment and Alzheimer disease) and a group of patients presenting HAND or HAD. Our
hypothesis in this work is to seek a sub-cortical pattern in verbal learning episodic memory in
HAND, characterized by difficult to evoke episodic memories but with a benefit of a “clue”
for a retrieval process, a typically deficit on subcortical fronto-striatal structures. A true
deficit in storage of the information is associated to cortical-temporal structures, not benefited
by clues, a typical deficit in AD.
Method
Participants
Two groups were selected to this study at two different Hospitals in Belo Horizonte,
Minas Gerais, Brazil. The first group are composed by seventy-nine (n=79) regular patients
of Eduardo de Menezes (Infectious Diseases Outpatient Clinic), a referral centre of treatment
rigorous control for a epidemiological estimation of HAND. All participants are older than 18
years and have no current history of diffuse or focal CNS disease and/or systemic disease. All
these selected patients have diagnostic of HAND based on Antinori criteria (Antinori et al
2007) after a comprehensive neuropsychological assessment. The stages of HAND in this
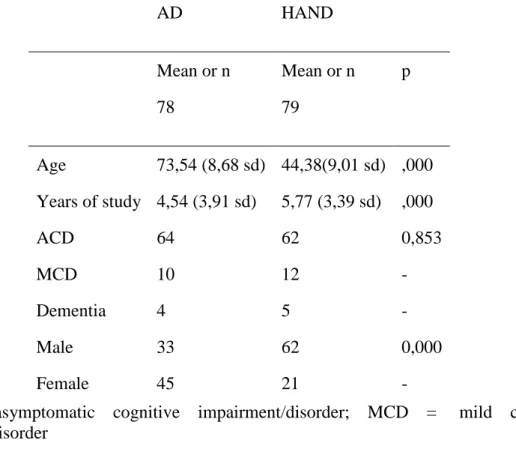
population were 1 - HIV associated dementia (n=5), 2- HIV-associated mild cognitive
disorder (n=12), 3- asymptomatic neurocognitive impairment (n=62).
A second clinical group involved older adults diagnosed with amnestic mild cognitive
impairment (MCI) or dementia probable due to AD. These participants underwent detailed
clinical and neuropsychological assessment as described elsewhere (de Paula et al., 2013).
Briefly it involve the assessment of clinical history according to the Mckhann and colleagues
(2011) and Albert and colleagues (2011) guidelines and cognitive assessment by the use of
the the Mini-Mental State Examination (Folstein, Folstein & McHugh, 1976), the Mattis
Dementia Rating Scale (Mattis, 1986), subtests from the CERAD Neuropsychological
Battery (Morris, 1989) and the Clinical Dementia Rating (Morris, 1993). Only patients with
Clinical Dementia Rating of 1 (AD) or 0,5 (MCI) were invited for participation.
Episodic Memory Assessment
We used the Brazilian version of the Rey’s Auditory Verbal Learning Test (RAVLT)
in both groups to access different aspects of verbal episodic memory among the two groups.
The test was developed for Brazilian-Portuguese speakers taking account the words length
and frequency (Malloy-Diniz et al., 2007) and it was validated for young and older adults of
this population (Salgado et al., 2011; de Paula et al., 2012). It consists of a 15 substantives
lists read aloud five times for the patient (A1, A2, A3, A4, A5); a distractor trial of 15 new
words (B1), a short (A6) and long term recall (A7) and recognition memory (REC). We
memory: short-term memory (trials A1 and B1), learning processes (Sum of words between
A1 and A5; the LOT index [Total-5*A1]), free recall (A6, A7), and recognition memory
(REC).
Data analysis
In previous analyses using Mann-Whitney U test and Chi Square analyses we found a
significant difference of age, sex and formal education in both groups (Table 1). That
difference was expected by the profile of the distinct populations, diseases and Health
Centers. To compare these two different populations, we calculated Z-Scores based on the
Brazilian normative studies for both young and older adults stratified by age and education
(Magalhães & Hamdan, 2010). The comparison of the groups was made with Student t test
considering p<0.05 and the effect size was calculate with r coefficient. We used the
Statistical Package for Social Sciences (SPSS) software version 20.0.
Results
The descriptive data are shown in Table 1. A total of 157 subjects were selected, 79 in
HAND group and 78 in AD group. No significant difference in the frequency of dementia
stage between groups (X² = 37,56, p= 0,853) were observed. Age (U=39,50, p<0,001), years
of study (U=1932,00, p<0,001) and sex (X²=15,59; p<0,001) was significantly different
between groups with an expected profile of younger (m= 42,38 years; sd= 9), more schooled
(m= 6,52 years of school; sd= 3,39) and typically male (73,4%) in HAND group. The AD
group are majority female (57,7%), elderly (m= 73,54 years; sd= 8,68) and less schooled (m=
Then we transform the scores in Z scores using a normative data of a Brazilian
population in RAVLT (Magalhães & Hamdan, 2010) corrected by age and scholarity in order
to correct the differences between the groups. The results are shown in Table 2. The groups
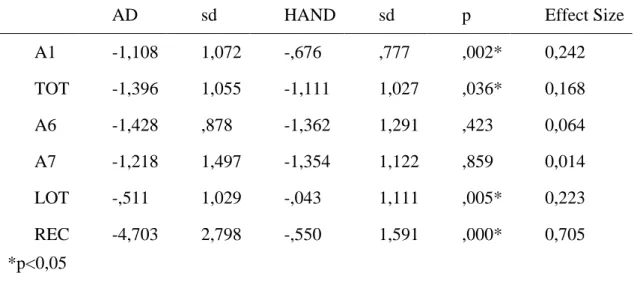
differ in A1 (U=2217,50, p=0,002), TOT (U=2483,00, p=0,036), LOT (U=2286,00, p=0,005)
and REC (U=567,50, p<0,001), with better results for the HAND group. The other variables
did not differ in our study (p>0,05). Excluding the dementia cases, we found the same result,
better performance in HAND group in the same variables, A1 (U=1963,50, p=0,003), TOT
(U= 2159,00, p=0,026), LOT (U=1947,50, p=0,002) and REC (U=510,50, p<0,001). In a
comparison only using dementia subjects, even with a very small numbers of (n=9) we found
a significant difference in REC result (U=2,00, p=0,05) with a better performance in HAND
group.
In effect size analyses of full sample we found small effects for A1 (r= 0,24), TOT
(r=0,17) and LOT (r=0,22) and large effects for REC (r=0,70).
Discussion
We have hypothesized that HAND group shown a subcortical pattern of performance
in episodic verbal memory and learning, in other words, difficult to evoque long term learned
facts but with a considerable help with clues to remember (Sadek et al. 2004). This pattern is
expected because of the damage in whiter matter and fronto-striatal structures in the HIV
neuroinfection (Xia et al. 2011). However in cortical dementia as classically described in
learned; the deficit is in the storage of the information not on the retrieval and the presence of
clues is irrelevant. The neuronal structures involved in storage of information was related to
hippocampus cortex and temporal lobe (Mulder et al. 2014), the former typical target in
Alzheimer Disease.
Our data analyses corroborate this hypothesis. In short term components and learning
stages, the performance in HAND group was slightly better than other group. The evocation
of the information which is a measure of long term memory had a similar result in both
groups. However in the presence of a clue to recognize the learned words, HAND group
demonstrated a superior performance suggesting that the information was stocked and the
difficulty was in the retrieval suggesting a specific damage on fronto-striatal structures. A
retrieval deficit was descripted even in visuospatial memory in HIV patients, pointing to a
fronto-striato-thalamo-cortical pathway damaged (Woods et al. 2013).
The effect size analyses shown that the impact of group differences are small for short
term memory and learning components, probably related to a more specific damage on
temporal structures in AD group. However the effect size in recognition component was
large, pointing to a very different pattern between groups what corroborate with the initial
hypothesis. This results was very similar to others works (Sadek et al. 2004; Delis et al.
1995), corroborating a subcortical profile.
Limitations of this study are clear and future works must improve the methodology.
We compare two very different populations, especially in a developing country like Brazil.
Age, sex and schooling can impact in the neuropsychological performance and the statistical
correction made is limited. Additional work must focus on longitudinal evaluation of HAND
and the presence of possible AD or use same or close age of groups.
Clinical and treatment implications of these results needs to be better discussed. The
and the possibility of HAND and AD was alarmant as the neurocognitive deficits involved
are different just like their causes. HAND population was likely to depend more of social
services compared to other HIV infected individuals (Umaki et al. 2013). Particularities in
HAND profile of episodic memory can change dramatically the treatment of patients
especially in adherence of medication (Woods et al. 2008) directly associated to memory
lapse. Others impacts in activities of daily living are probably associated too.
Our study focused in episodic verbal memory. Future studies should address other
differences in cognition assessing subcortical x cortical profile comparisons between HAND
and another types of pathological aging processes. .
Table 1 - Descriptive statistics of demographic variables in AD and HAND groups
AD HAND
Mean or n Mean or n p
78 79
Age 73,54 (8,68 sd) 44,38(9,01 sd) ,000 Years of study 4,54 (3,91 sd) 5,77 (3,39 sd) ,000
ACD 64 62 0,853
MCD 10 12 -
Dementia 4 5 -
Male 33 62 0,000
Female 45 21 -
Table 2 - Comparison of Z scores between groups and effect size
AD sd HAND sd p Effect Size
A1 -1,108 1,072 -,676 ,777 ,002* 0,242 TOT -1,396 1,055 -1,111 1,027 ,036* 0,168 A6 -1,428 ,878 -1,362 1,291 ,423 0,064 A7 -1,218 1,497 -1,354 1,122 ,859 0,014 LOT -,511 1,029 -,043 1,111 ,005* 0,223 REC -4,703 2,798 -,550 1,591 ,000* 0,705 *p<0,05
References:
1. Albert, M.S., DeKosky, S.T., Dickson, D., Dubois, B., Feldman, H.H., Fox, N.C., Gamst, A., Holtzman, D.M., Jagust, W.J., Petersen, R.C., Snyder, P.J., Carrillo, M.C., Thies, B., Phelps, C.H. (2011). The diagnosis of mild cognitive impairment due to Alzheimer's disease: recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Aging-Alzheimer's disease. Alzheimers Dement. 7(3):270-9.
2. Antinori A, Arendt G, Becker JT, et al. (2007). Updated research nosology for HIV-associated neurocognitive disorders. Neurology. 69:1789-1799
4. Christo PP, Géo LAL, Antunes CMF. (2013). Neurocognitive performance in patients with AIDS in Brazil: a case-control study. Clinical Neuropsychiatry. 10, 2, 107-110. 5. Cohen RA, Harezlak J, Gongvatana A, Buchthal S, Schifitto G, Clark U, Paul R,
Taylor M, Thompson P, Tate D, Alger J, Brown M, Zhong J, Campbell T, Singer E, Daar E, McMahon D, Tso Y, Yiannoutsos CT, Navia B; HIV Neuroimaging Consortium. (2010). Cerebral metabolite abnormalities in human immunodeficiency virus are associated with cortical and subcortical volumes. J Neurovirol.16(6):435-44. 6. de Almeida SM, Ribeiro CE, de Pereira AP, Badiee J, Cherner M, Smith D, Maich I, Raboni SM, Rotta I, Barbosa FJ, Heaton RK, Umlauf A, Ellis RJ. Neurocognitive impairment in HIV-1 clade C- versus B-infected individuals in Southern Brazil. (2013). J Neurovirol. Dec;19(6):550-6.
7. de Paula, J.J., Miranda, D.M., Nicolato, R., Moraes, E.N., Bicalho, M.A., Malloy-Diniz, L.F. (2013a). Verbal learning on depressive pseudodementia: accentuate impairment of free recall, moderate on learning processes, and spared short-term and recognition memory. Arq Neuropsiquiatr. 71(9A):596-9.
8. Delis, D. C., Peavy, G., Heaton, R., Butters, N., Salmon, D. P., Taylor, M., et al. (1995). Do patients with HIV-associated minor cognitive/motor disorder exhibit a “subcortical” memory profile? Evidence using the California Verbal Learning Test. Assessment. 2, 151–165.
9. Magalhães SS, Hamdan AC. (2010). The Rey Auditory Verbal Learning Test: normative data for the Brazilian population and analysis of the influence of demographic variables. Psychology & Neuroscience. 3, 1, 85 - 91
11.McKhann, G.M., Knopman, D.S., Chertkow, H., Hyman, B.T., Jack, C.R. Jr., Kawas, C.H., Klunk, W.E., Koroshetz, W.J., Manly, J.J., Mayeux, R., Mohs, R.C., Morris, J.C., Rossor, M.N., Scheltens, P., Carrillo, M.C., Thies, B., Weintraub, S., Phelps, C.H. (2011). The diagnosis of dementia due to Alzheimer's disease: recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement. 7(3):263-9.
12.Mulder ER, de Jong RA, Knol DL, van Schijndel RA, Cover KS, Visser PJ, Barkhof F, Vrenken H; for the Alzheimer's Disease Neuroimaging Initiative. (2014). Hippocampal volume change measurement: Quantitative assessment of the reproducibility of expert manual outlining and the automated methods FreeSurfer and FIRST. Neuroimage. 9. pii: S1053-8119.
13.Rodrigues RA, Oliveira RL, Grinsztejn B, Silva MT. (2013). Validity of the International HIV dementia scale in Brazil. Arq Neuropsiquiatr.71(6):376-9.
14.Sacktor N, McDermott MP, Marder K, et al. (2002). HIV-associated cognitive impairment before and after the advent of combination therapy. J Neurovirol. 8:136-142.
15.Sadek JR, Johnson SA, White DA, Salmon DP, Taylor KI, Delapena JH, Paulsen JS, Heaton RK, Grant I. (2004). Retrograde amnesia in dementia: comparison of HIV-associated dementia, Alzheimer's disease, and Huntington's disease. Neuropsychology.18(4):692-9.
17.Woods SP, Moran LM, Carey CL, Dawson MS, Iudicello JE, Gibson S, Grant I, Atkinson JH, HIV Neurobehavioral Research Center Group. (2008). Prospective memory in HIV infection: Is “remembering to remember” a unique predictor of self-reported medication management? Arch Clin Neuropsychol.23(3):257-70.
18.Woods SP, Hoebel C, Pirogovsky E, Rooney A, Cameron MV, Grant I, Gilbert PE; HIV Neurobehavioral Research Program Group. (2013). Visuospatial temporal order memory deficits in older adults with HIV infection. Cogn Behav Neurol. 26(4):171-80.
19.Xia C, Luo D, Yu X, Jiang S, Liu S. (2011). HIV-associated dementia in the era of highly active antiretroviral therapy (HAART). Microbes Infect.13(5):419-25.
5 - CONCLUSÕES E REPERCURSSÕES
Verificamos que em população brasileira, no estado de Minas Gerais, a frequência total de HAND foi de 71,8%, sendo 4,5% casos de demência, 10,9% comprometimento cognitivo leve e 56,4% casos de comprometimento cognitivo assintomático. Esta freqüência diagnóstica encontrada foi superior ao já reportado na literatura brasileira, de 52 a 65% (Rodrigues, Oliveira, Grinsztejn & Silva, 2013, de Almeida et al. 2013), o que pode ser explicado por uma metodologia mais completa de avaliação utilizada no presente estudo, bem como a amostra ser hospitalar, provavelmente com mais comprometidos. A necessidade de estudos específicos na população brasileira é requer atenção já que o Brasil possui uma realidade muito diferente dos países de primeiro mundo, ou países de extrema pobreza africanos, onde geralmente os estudos epidemiológicos internacionais ocorrem (Alkali, Bwala, Nyandaiti & Danesi, 2013; Joseph et al. 2013). Este resultado deve ser levado em consideração para a fundamentação de políticas públicas futuras já que a maioria da população infectada apresenta danos cognitivos que são diretamente ligados à danos em atividades laborativas, atividades de vida diária, inserção social, qualidade de vida e principalmente, aderência ao tratamento medicamentoso (Woods et al. 2008). Desconhecemos qualquer medida de política pública até esta presente data, com enfoque de divulgação e tratamento de danos cognitivos associados ao HIV no Brasil. Dos estimados 630.000 habitantes infectados por HIV no Brasil (UNAIDS/WHO 2011), aproximadamente 450 mil teriam HAND de acordo com a prevalência estimada neste estudo, um enorme contingente populacional que dificilmente receberá tratamento apropriado.
protetor cognitivo (de Ronchi et al. 1993) e que o nível educacional brasileiro é baixo, bem como serviços de saúde básica deficitários, o padrão populacional brasileiro encontra um risco considerável à HAND, especialmente em pacientes que não moram nas capitais estaduais. Outros dados chamaram atenção, em especial o fato de não encontrarmos associação do uso da medicação, bem como seu poder de penetrância, como fator de risco/proteção, o que já é discutido na literatura onde o uso do antiretroviral não tem uma associação claramente positiva com a prevenção e tratamento da HAND (Smurzynski et al. 2011).
Esta diferenciação em uma tarefa de aprendizagem verbal pode ser instrumento complementar em diagnóstico diferencial entre HAND e DA, uma crescente complicação diagnóstica devida ao envelhecimento da população infectada pelo HIV. Entretanto, diversos outros domínios cognitivos precisam de maior esclarecimento e outras possibilidades de técnicas neuropsicológicas diferenciais são necessárias e possíveis, pra isto futuras pesquisas deverão focar em populações mais semelhantes (idade, sexo e escolaridade) bem como um uso de testes padronizados e amplos ao máximo de domínios cognitivos possíveis. O uso clínico do Teste Auditivo-Verbal de Rey (RAVLT) é recomendado para se investigar o padrão subcortical x cortical das demências e o possível diferencial entre HAND e DA. O RAVLT é um teste validado e normatizado para a população brasileira (Malloy-Diniz et al. 2009), de fácil aplicação para profissionais da neuropsicologia treinados, com fácil adaptação em populações de baixa escolaridade.
Referências:
1. Alkali NH, Bwala SA, Nyandaiti YW, Danesi MA. (2013). NeuroAIDS in sub-Saharan Africa: a clinical review. Ann Afr Med. 12(1):1-10.
2. Christo PP, Géo LAL, Antunes CMF. (2013). Neurocognitive performance in patients with AIDS in Brazil: a case-control study. Clinical Neuropsychiatry.10, 2, 107-110. 3. Cohen RA, Harezlak J, Gongvatana A, Buchthal S, Schifitto G, Clark U, Paul R,
4. de Almeida SM, Ribeiro CE, de Pereira AP, Badiee J, Cherner M, Smith D, Maich I, Raboni SM, Rotta I, Barbosa FJ, Heaton RK, Umlauf A, Ellis RJ. (2013). Neurocognitive impairment in HIV-1 clade C- versus B-infected individuals in Southern Brazil. J Neurovirol. 19(6):550-6.
5. De Ronchi D, Fratiglioni L, Rucci P, Paternicò A, Graziani S, Dalmonte E. (1998). The effect of education on dementia occurrence in an Italian population with middle to high socioeconomic status. Neurology. 50(5):1231-8.
6. Delis DC, Peavy G, Heaton R, Butters N, Salmon DP, Taylor M, et al. (1995). Do patients with HIV-associated minor cognitive/motor disorder exhibit a “subcortical” memory profile? Evidence using the California Verbal Learning Test. Assessment. 2, 151–165.
7. Joseph J, Achim CL, Boivin MJ, Brew BJ, Clifford DB, Colosi DA, Ellis RJ, Heaton RK, Gallo-Diop A, Grant I, Kanmogne GD, Kumar M, Letendre S, Marcotte TD, Nath A, Pardo CA, Paul RH, Pulliam L, Robertson K, Royal W 3rd, Sacktor N, Sithinamsuwan P, Smith DM, Valcour V, Wigdahl B, Wood C. (2013). Global NeuroAIDS roundtable. J Neurovirol. 19(1):1-9.
8. Malloy-Diniz LF, Lasmar VAP, Gazinelli R, Fuentes D, Salgado JV. (2009) The Rey auditory-verbal learning test: applicability for the Brazilian elderly population. Revista Brasileira de Psiquiatria. v. 29, n. 4, p. 324-329.
9. McArthur JC. (2004). HIV dementia: an evolving disease. J Neuroimmunol. 157(1-2):3-10.
11.Rodrigues RA, Oliveira RL, Grinsztejn B, Silva MT. (2013). Validity of the International HIV dementia scale in Brazil. Arq Neuropsiquiatr. 71(6):376-9.
12.Sackor NC, BacellarH, Hoover DR et al. (1996). Psychomotor slowing in HIV infection: a predictor of dementia, AIDS and death. J Neurovirol. 2:404-410.
13.Sadek JR, Johnson SA, White DA, Salmon DP, Taylor KI, Delapena JH, Paulsen JS, Heaton RK, Grant I. (2004). Retrograde amnesia in dementia: comparison of HIV-associated dementia, Alzheimer's disease, and Huntington's disease. Neuropsychology.18(4):692-9.
14.Smurzynski M, Wu K, Letendre S, et al. (2011). Effects of central nervous system antiretroviral penetration on cognitive functioning in the ALLRT cohort. AIDS 25(3):357-365.
15.UNAIDS/WHO - Boletim epidemiológico AIDS-DST Ano VIII No1 publicado 28/11/2011. http://www.aids.gov.br/publicacao/2011/boletim_epidemiologico_2011. 16.Woods SP, Moore DJ, Weber E, Grant I. (2009). Cognitive neuropsychology of
HIV-associated neurocognitive disorders. Neuropsychol Rev.;19(2):152-68.
17.Woods SP, Moran LM, Carey CL, Dawson MS, Iudicello JE, Gibson S, Grant I, Atkinson JH; HIV Neurobehavioral Research Center Group. (2008). Prospective memory in HIV infection: Is “remembering to remember” a unique predictor of self-reported medication management? Arch Clin Neuropsychol. ;23(3):257-70.