MINISTÉRIO DA EDUCAÇÃO
UNIVERSIDADE FEDERAL DO RIO GRANDE DO NORTE CENTRO DE CIÊNCIAS DA SAÚDE
PROGRAMA DE PÓS-GRADUAÇÃO EM CIÊNCIAS DA SAÚDE
EFETIVIDADE DA SUPLEMENTAÇÃO DE ZINCO NA FORÇA,
RESISTÊNCIA E EQUILÍBRIO MUSCULAR EM IDOSAS: ensaio
clínico randomizado duplo cego
MARIA APARECIDA BEZERRA
MARIA APARECIDA BEZERRA
EFETIVIDADE DA SUPLEMENTAÇÃO DE ZINCO NA FORÇA,
RESISTÊNCIA E EQUILÍBRIO MUSCULAR EM IDOSAS: ensaio
clínico randomizado duplo cego
Dissertação apresentada ao Programa de Pós-Graduação em Ciências da Saúde da Universidade Federal do Rio Grande do Norte, como requisito para obtenção do Título de Doutor em Ciências da Saúde.
Orientador: Prof. Dr. José Brandão Neto
Coordenadora: Profa. Dra. Ivonete Batista
Araújo
B574i
Bezerra, Maria Aparecida.
Influência do zinco no desempenho muscular em mulheres jovens e idosas / Maria Aparecida Bezerra. – Natal/RN, 2013. 57f.: il.
Orientador: Prof. Dr. José Brandão Neto
Tese (Doutorado em Ciências da Saúde) – Programa de Pós-Graduação em Ciências da Saúde. Universidade Federal do Rio Grande do Norte. Centro de Ciências da Saúde.
1. Envelhecimento – Tese. 2. Nutrição – Zinco – Tese. 3. Força muscular – Tese. I. Brandão Neto, José. II. Título.
ii
MARIA APARECIDA BEZERRA
EFETIVIDADE DA SUPLEMENTAÇÃO DE ZINCO NA FORÇA,
RESISTÊNCIA E EQUILÍBRIO MUSCULAR EM IDOSAS: ensaio
clínico randomizado duplo cego
Aprovada em: ______/______/______
BANCA EXAMINADORA
______________________________________________________________ Prof. Dr. José Brandão Neto (Presidente – UFRN)
______________________________________________________________ Profa. Dra. Selma Sousa Bruno (Membro Interno – UFRN)
______________________________________________________________ Prof. Dr. Eryvaldo Sócrates Tabosa do Egito (Membro Interno – UFRN)
______________________________________________________________ Profa. Dra. Margareth de Fátima Formiga Melo Diniz (Membro Externo – UFPB)
iii
A Deus, fonte de sabedoria, de amor e de inspiração criadora. A Ele dedico este trabalho.
Aos meus pais, João (in memoriam) e Irene, pelo esforço que fizeram para que os filhos pudessem estudar.
Aos meus amados filhos, Amanda e Tiago, pelas palavras e ações de incentivo quando eu retornava para casa cansada da jornada de trabalho.
Às mulheres que fizeram parte dessa pesquisa, pelo compromisso assumido em todas as fases do trabalho e por tudo o que aprendi com elas.
iv
AGRADECIMENTOS
A Deus, fonte de vida e amor, que me amparou durante todas as etapas deste trabalho.
Ao Prof. Dr. José Brandão Neto, minha profunda gratidão, pela precisa e preciosa orientação ao longo desta importante fase de minha vida.
A Profa. Áurea Nogueira de Melo por sua dedicação voluntária à avaliação neurológica das pacientes, em todas as fases da pesquisa.
Aos colegas: Naira Josele Neves de Brito, Érika Dantas de Medeiros Rocha, Alfredo de Araújo Silva e Denise Dal'Ava Augusto pela ajuda nos procedimentos de avaliação em suas respectivas áreas de atuação.
À FAPERN e CNPq pelo financiamento da pesquisa.
Ao PPGCSA e aos docentes a ele vinculados.
A UFPB, especialmente aos colegas do Departamento de Fisioterapia, pelo incentivo nesta etapa acadêmica.
Às equipes do Programa de Saúde da Família dos Bancários, Timbó I e Timbó II pelo acolhimento, compromisso e dedicação com que nos receberam e acompanharam durante toda esta pesquisa.
Às voluntárias, cuja colaboração permitiu a realização deste trabalho.
v
RESUMO
Introdução: O decréscimo na função muscular durante o envelhecimento limita a capacidade funcional e, consequentemente, a independência física. Objetivo: Avaliar a efetividade do zinco na força, na resistência e no equilíbrio muscular em mulheres idosas atendidas em área de abrangência da Unidade de Saúde da Família do Sistema Único de Saúde. Metodologia: Ensaio clínico randomizado duplo cego placebo controlado com 38 participantes aleatoriamente distribuídos em 4 grupos: grupo-controle composto por 18 mulheres jovens, com idade entre os 20 e os 30 anos, assim subdivido: Jovem Placebo (n=9) ingeriu placebo (sorbitol 10%); Jovem Zinco (n=9) ingeriu 25mg do elemento zinco. Grupo experimental composto por 20 mulheres idosas, com idade entre os 60 e os 80 anos e subdividido: Idosa Placebo: (n=10) ingeriu placebo; Idosa Zinco (n=10) ingeriu 25mg do elemento zinco. O seguimento teve duração de 90 dias. A força, a resistência e o equilíbrio muscular foram estimados pelo pico de torque isocinético normalizado pelo peso corporal, do quadríceps (PT/kg QUA) e dos isquiotibiais (PT/kg IQS) nas velocidades angulares de 60°/s e de 180°/s por dinamometria Isocinética. Resultados: Na medida inicial PT/kg IQS 60°/s, não houve diferença significativa entre os grupos. Após 90 dias ocorreu redução significativa da força apenas no grupo Idosa Placebo: PT/kg IQS 60°/s =58,53±16,37 Nm em relação ao grupo Jovem Placebo: PT/kg IQS 60°/s = 84,15±27,60 Nm p=0,01. Quanto à resistência dos isquiotibiais (PT/kg IQS 180°/s), os dois grupos de idosas (Placebo e Zinco) eram significativamente menores do que o grupo Jovem Placebo na medida inicial. Após 90 dias, apenas o grupo Idosa Placebo tinha resistência significativa menor que o grupo Jovem Placebo. Efeito dentro de cada grupo: ocorreu aumento significativo de força e resistência dos isquiotibiais no grupo Idosa Zinco e diminuição significativa no grupo Idosa Placebo. A diferença de médias (Δ) entre Idosa Zinco e Idosa Placebo (teste t independente) dos isquiotibiais, após 90 dias, foi significativa tanto para força (PT/kg IQS60°/s Δ=8,97 Nm, p= 0,02) como para resistência (PT/kg IQS 180°/s Δ=11,88
Nm p=0,01). Conclusões: A diferença significativa entre as médias do inicio e as de seguimento, tanto de força como de resistência dos isquiotibiais entre Idosa Zinco e Idosa Placebo, mostra a vulnerabilidade desse músculo durante o envelhecimento. Essas perdas poderiam ser minimizadas com a suplementação de zinco. Isso indica que a nutrição adequada de zinco pode prevenir perda de força e resistência muscular em mulheres com mais de 60 anos.
Descritores: Envelhecimento. Zinco.Nutrição. Força muscular. Dinamômetro de
vi
LISTA DE ABREVIATURAS E SIGLAS
ERO Espécie reativa de oxigênio
DMO Densidade mineral óssea
Zn Zinco
vii
SUMÁRIO
1 INTRODUÇÃO ... 08
2JUSTIFICATIVA ... 12
3 OBJETIVOS ... 13
3.1 Objetivo geral ... 13
3.2 Objetivos específicos ... 13
4 MÉTODO ... 14
5 ANEXAÇÃO DO ARTIGO1 ... 15
6 ANEXAÇÃO DO ARTIGO 2 ... 40
7 COMENTÁRIOS, CRÍTICAS E CONCLUSÕES. ... 45
REFERÊNCIAS ... 48
ANEXO ... 51
1 INTRODUÇÃO
O envelhecimento populacional é uma realidade em países desenvolvidos e em desenvolvimento(1). A prevalência de múltiplas condições crônicas e incapacidade funcional são mais elevadas na senescência. Variações nas condições de saúde, no bem-estar, na capacidade funcional e nas necessidades de cuidado distinguem diferentes grupos de idosos. Tais variações poderão culminar com a Síndrome da Fragilidade caracterizada pela diminuição da massa corporal, fraqueza, fadiga, inatividade, redução da ingestão alimentar, sarcopenia, osteopenia, anormalidades no equilíbrio e na marcha(2). Essas características estão direta ou indiretamente interligadas com fatores, como: características demográficas, socioeconômicas e outros aspectos relacionados com a saúde(3).
Estilo de vida sedentário, característico dessa população, está associado com osteoartrite, consequente da inatividade física, redução da função mitocondrial, desajuste do estado redox celular, aumento de inflamação crônica sistêmica que torna o ambiente intracelular do músculo propenso à toxicidade de espécies reativas de oxigênio (ERO)(4). Esses fatores podem contribuir para a redução da massa muscular esquelética. Tal redução tem inicio discreto na terceira década e diminuição significativaa partir do final da quinta década em homens e mulheres(5).
A diminuição do músculo esquelético, tecido metabolicamente ativo, afeta a sua capacidade metabólica, particularmente as capacidades glicolíticas e respirátórias(6), potencializa aumento da gordura corporal, diminuição da aptidão aeróbica, da massa magra e da densidade mineral óssea(7).
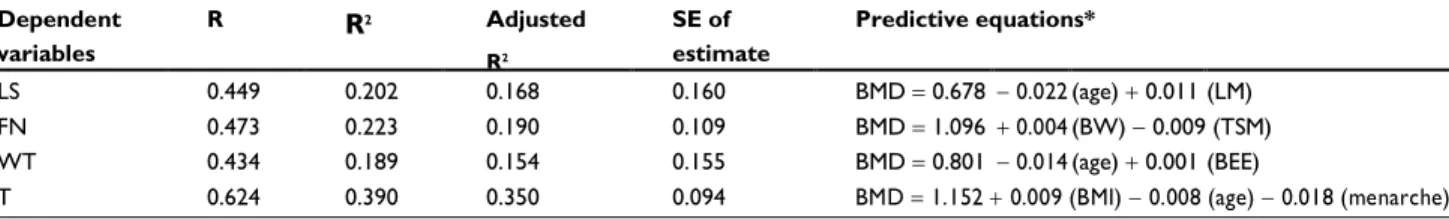
Estudo de nosso grupo de pesquisa mostrou que 20,2% da variabilidade da densidade mineral óssea na coluna lombar estava relacionada com a massa magra e tempo de menopausa; 22,3% de variabilidade da densidade mineral óssea do colo do fémur estava relacionada com o peso corporal e idade; 18,9% da variabilidade da densidade mineral óssea no triângulo de Ward estavam relacionadas com a idade e gasto energético basal; e 39% da variabilidade da densidade mineral no trocanter estavam relacionadas com o índice de massa corporal, idade e menarca(8).
no bidirecional processo epigenético: de genótipo para fenótipo e de fenótipo para genótipo(9).
O músculo esquelético sintetiza esse contexto e responde, de acordo com a tipologia de suas fibras, ao metabolismo aeróbico e anaeróbico. Fibras musculares de contração lenta (tipo I) têm metabolismo oxidativo e são ricas em capilares e mitocondrias. São requisitadas durante exercícios que aumentam a resistência muscualar. As fibras musclares de contração rápida (tipo IIa) têm perfil metabólico semelhante às fibras do tipo I. As fibras musculares glicolíticas de metabolismo anaeróbico (tipo IId e IIb) têm pouca mitocondria e pouca vascularização capilar. Estão envolvidas nos exercícios que visam aumentam a força muscular(10).
O grupos musclares isquitibias e quadricepes, que são objetos de nosso estudo, apresentam um percentual elevado de fibras musculares de contração rápida tipo II. Em estudo histoquimico, Garrett et al.(11) mostram que há uma proporção maior dessas fibras nos isquitibiais em comparação com o quadriceps. No bícepes femoral (cabeça longa), na área proximal, há um pencentual de 55,2% e na área distal, 53,8%; na cabeça curta do bíceps, na área central, o percentual é de 59,2%; no semitedinoso, na área proximal, 54,6% e na área distal, 60,4%; no semimembranoso, na área proximal, 51% e na área distal, de 50,5%. Para o quadríceps, no mesmo estudo, os músculos foram analisados apenas na área central. Mostra portanto, um percentual de fibras musculares tipo II no vasto lateral de 54,5%, no vasto intermédio de 45,7%, no vasto medial de 49,4% e no reto femoral de 57,7%(11).
Pessoas idosas com mais de 40% de fibras musculares tipo II apresentaram níveis mais baixos de peroxidação lipídica e desencadeiaram com mais eficiência o sistema contra ânio superóxido do que das pessoas que têm menos de 40% dessas fibras musculares(13). Também é reportado que há redução significativa da força muscular, da área de secção transversa muscular, diminuição de fibras tipo I e decréscimo de capilares por área de fibra muscular (14).
Essas transformações biológicas causadas por estilo de vida sedentário e fatores nutricionais podem mudar a capacidade mitocondrial e interferir no metabolismo oxidativo energético, o que pode tornar o organismo vulnerável a agentes agressores celulares, como os radicais livres, principalmente no envelhecimento, os quais estão relacionados com a sarcopenia e doenças crônicas(4, 12).
O estilo de vida fisicamente ativo das pessoas idosas, está associado com a compensação parcial da preservação da biogênese mitocondrial e com a capacidade antioxidante no músculo esquelético que pode retardar o início da sarcopenia(4).
A sarcopenia (redução da massa muscular) e a dinapenia (redução da força muscular) estão associadas com o aumento do estresse oxidativo e podem ser potencializadas devido à menor atividade das enzimas anaeróbicas e aeróbicas, do conteúdo de proteínas, e não apenas devido à diminuição da atividade física(6).
Existem evidências de que a deficiência de zinco no organismo pode afetar a função do músculo estriado (15) e pode induzir a apoptose de células musculares lisas vasculares. O estresse oxidativo, na deficiência de zinco, contribuiria para a apopitose dessas células(16).
O zinco é um abundante elemento de transição no cérebro, tem importante papel na estabilização da proteína básica de mielina e na formação da bainha de mielina(18), está envolvido no desenvolvimento e preservação das funções dos nervos periféricos(19), na preservação da quantidade de fibras musculares e no metabolismo energético dessas fibras musculares(20). Já mostrou exercer influência no trabalho total isocinético dos músculos extensores do ombro e do joelho em homens jovens(21).
Durante prolongados períodos de restrição de atividade motora, parece que são modificados mecanismos endógenos da homeostase do zinco. Zorbas et al.(22) mostram que ratos mantidos em hipocinesia, mesmo estes recebendo suplementação de zinco na dieta, esta não garantiu que o zinco penetrasse nos tecidos onde são normalmente depositados, como ocorre nos ossos e nos músculos. Foi obervado também aumento do zinco no plasma, na excreção fecal e urinária, o que resultou em significante perda de zinco corporal(22).
2 JUSTIFICATIVA
A redução do desempenho muscular em homens e mulheres está associada com a sarcopenia, que é uma das principais causas da redução da força e do desempenho muscular no envelhecimento(23) e que é caracterizada pela redução do número, do tamanho e do nível da vascularização das fibras musculares(13). A estimativa de sarcopenia aumenta à medida que as pessoas envelhecem. Em pessoas com idade entre os 60 e os 70 anos, o percentual de sarcopenia fica entre 5% e 13%. Já em pessoas com mais de 80 anos, essa estimativa aumenta para os percentuais de 11% a 50%(24).
A diminuição da força muscular relacionada com a idade foi denominada
dinapenia em 2008(25). Ela tem consequência significativa, durante o
envelhecimento, por aumentar o risco de limitações funcionais, de incapacidade e de mortalidade(26). É reportado atualmente que a dinapenia do quadríceps e do punho e não a sarcopenia, é preditor independentes de mortalidade(27).
O estudo da dinapenia em mulheres é um tema que tem relevância clínica, por ser fator de risco para a maioria das doenças crônicas relacionadas com o envelhecimento. Por outro lado, um aporte suplementar de zinco pode favorecer aumento da força muscular devido sua ação como antioxidante, estimulante imunológico e como agente inflamatório(16).
Considerando a realidade do decréscimo do desempenho muscular no envelhecimento e sua associação com a nutrição adequada de zinco, o presente estudo investigou a efetividade da suplementação de zinco (25 mg do elemento Zn++) no aumento da força, da resistência e do equilíbrio entre os músculos isquiotibiais e quadríceps em mulheres jovens e idosas. Portanto, este projeto atendeu à finalidade primordial da Política Nacional de Saúde da Pessoa Idosa (Portaria nº 2.528 de 19 de outubro de 2006)(28). Tal política visa a “recuperar,
3 OBJETIVOS
3.1 Objetivo geral
Avaliar a efetividade do zinco (25 mg do elemento Zn++) no desempenho dos músculos isquiotibiais e dos quadríceps nas velocidades angulares de 60°/s e de 180°/s entre mulheres jovens e idosas.
3.2 Objetivos específicos
Verificar se a suplementação de zinco em mulheres idosas diminuiu a diferença de força e resistência, em comparação com as mulheres jovens.
Observar se a suplementação de zinco aumentou a força, resistência e equilíbrio muscular em mulheres idosas.
4 MÉTODO
5 ANEXAÇÃO DO ARTIGO 1
ARTIGO 1
Título: Efetividade da suplementação de zinco na força, na resistência e no equilíbrio muscular em mulheres idosas: ensaio clínico randomizado duplo cego.
Periódico: Clinical Interventions in aging
ISSN: 1176-9092 (Print)
Qualis: A2 (Medicina II – CAPES) Fator de impacto: 2.083
ORIGINAL RESEARCH
Clinical Interventions in aging
Effectiveness of zinc supplementation on strength, endurance, and muscle balance in elderly women: a randomized double blind clinical trial.
Maria Aparecida Bezerra1
Simone Bezerra Alves1
Áurea Nogueira de Melo2
Érika Dantas de Medeiros Rocha3
Naira Neves de Brito3
José Brandão-Neto4
1 Departamento de Fisioterapia da Universidade Federal da Paraíba (João Pessoa,
Brasil);
2 Departamento de Pediatria da Universidade Federal do Rio Grande do Norte (Natal,
Brasil);
3 Pós-graduanda do Programa de Pós-graduação em Ciências da Saúde da
Universidade Federal do Rio Grande do Norte (Natal, Brasil);
4 Departamento de Medicina Clínica da Universidade Federal do Rio Grande do
Norte (Natal, Brasil).
Correspondente: José Brandão-Neto.
Av. Gal. Gustavo Cordeiro de Farias, s/n, Natal-RN, CEP 59012-570, Brazil. Tel +55 84 3342 9748
ABSTRACT
Introduction: The elderly generally show decreased muscle performance,
accompanied by low consumption of dietary zinc. It is reported that zinc has positive effects on muscle performance. The aim of this study was to evaluate the influence of zinc on strength, muscular endurance, and balance in older women. Methodology: A randomized double-blind placebo-controlled clinical trial with 38 participants randomly subdivided into 4 groups: a control group comprised of 18 young women aged between 20 and 30 years, thus subdivided: Young Placebo (n = 9) ingested a placebo (sorbitol 10%), Young zinc (n = 9) ingested 25 mg of zinc. An experimental group of 20 elderly women, aged between 60 and 80 years and subdivided as: Elderly Placebo: (n = 10) ingested a placebo; Elderly Zinc (n = 10) ingested 25 mg of zinc. The follow-up lasted 90 days. The strength, endurance, and muscular balance (hamstring/quadriceps) were estimated by isokinetic peak torque, normalized by body weight, of the quadriceps, and hamstrings in the angular velocities of 60°/s (force), and 180°/s (resistance). Results: Compared with the strength of the Young placebo hamstring group, the Elderly Zinc showed a proportional increase in strength, and the Elderly Placebo group was significantly reduced. The same was seen for hamstrings resistance, only that the Elderly Placebo group showed a significant reduction compared to the Young Placebo group. Effect within each group: a significant increase in strength and endurance of the hamstrings in the Elderly Zinc group, and a significant decrease in the Elderly Placebo group. Mean difference(Δ) between Elderly Zinc and Elderly Placebo for hamstring strength was significant for (PT/kg IQS60°/s Δ = 8.97 Nm, p = 0.02) and for resistance (PT/kg IQS 180°/s Δ = 11.88 Nm p = 0.01). Conclusions: The study showed that zinc may be effective in increasing the strength and endurance of the hamstring, (a vulnerable muscle), and prevents disproportionate reduction of its strength relative to the quadriceps, atypical muscle imbalance that increases the risk of falls in older people.
Introduction
Population aging is a reality on all continents1, and results in the need for the elderly to seek ways to address the related challenges; high prevalence of chronic disease, and functional disability2. All this may predispose this population to develop the Fragility Syndrome, compromising both their quality of life and interfering in their autonomy. Additional commitments and financial input from their families and the state are required to aid these people with their new needs in regard to personal care3. These factors are also directly or indirectly linked to demographic and socioeconomic factors, as well as to other aspects health2.
During aging, muscle performance is directly associated with decreased functional capacity4. Sarcopenia, characterized by reduced number, size5, and vascularization of the muscle fibers4, is directly related to accelerating muscle mass loss,after 60 years of age6, and has a direct effect on muscle strength reductions in the elderly 5.
Reduced muscle mass and strength are associated with increased oxidative stress, which may be potentiated due to lower anaerobic and aerobic enzyme activity, and protein losses, and not just due to decreased physical activity7.
There is evidence that zinc deficiency in the body can affect striate8 muscle function, and can induce apoptosis of vascular smooth muscle cells. Oxidative stress combined with zinc deficiencies, would contribute to this cellular apoptosis9.
Zinc is an abundant transition element in the brain, it plays an important role in the stabilization of basic myelin proteins, and the formation of the myelin sheath10, it is involved in the development and preservation of peripheral nervefunctions11, and in preserving the amount of muscle fibers, and their energy metabolism12. Zinc was also demonstrated to influence the total isokinetic work of the extensor muscles in the shoulders and knees in young men13.
In healthy aging, skeletal muscle shows a significant reduction in isokinetic muscle strength (especially in women), increased stiffness of the muscle fiber, reduced phosphorylation of the myosin light chains, and diminished actin and myosin cross-bridge kinetics, which results in low rates of force production15. There are also reductions in muscle cross-sectional area, type I fiber percentage, capillaritizedarea5 and a decline in mitochondrial oxidative capacity4.
Older people with an active lifestyle show partial compensation, preserving mitochondrial oxidative capacity. An active lifestyle helps retain antioxidant capacity. In contrast, in the sedentary elderly, mitochondrial function is compromised with deregulation of the redox function. This anomaly may increase chronic inflammatory processes, which make the intracellular spaces of skeletal muscle an environment prone to toxicity, as mediated by reactive oxygen species (ROS)16.
Proper zinc nutrition is essential to preserve muscle function8. Its deficiency, a characteristic of human aging, results in decreased immune response, and development of chronic degenerative diseases 17, which may predispose the elderly to long periods of restricted movement, and make them vulnerable to precipitate fecal and urinary excretion of zinc, thus decreasing corporalzinc14.
Decreased muscle strength related to age, called dynapenia by Clark and Maniniin 200818, has significant consequences for the aged, and increases the risks of functional limitations, disabilities and mortality 19. Study shows that dynapenia of the quadriceps and wrists are independent predictors of mortality20. Thus, we see the clinical relevance of dynapenia studies.
During human aging there is usually a gradual change from an active to sedentary lifestyle. The sedentary lifestyle is associated with chronic inflammatory disease, sarcopenia16, and dynapenia20. Zinc, being recognized as an anti–
Materials and methods
Patient and study design
The volunteers, 60 to 80 years old were selected from a population of healthy elders3, as routed by the doctors of three family health clinics in the 3rd Sanitary District of the city of João Pessoa (PB), and by active search of the medical records of families enrolled at these health clinics. After selection, a home visit (with the agent responsible for the covered area of the local Family Health Clinic) was made in order to inform the volunteers about the research, and to invite their participation.
The study included women; who practiced physical activities twice a week (at most), were residents in the areas covered by the study, which agreed to participate freely, and were aged from 20 to 30, and from 60 to 80. Women with a history of diseases such as diabetes mellitus, liver disease, thyroid disease, neurological disease, and rheumatoid arthritis were excluded. Besides these were excluded; users of medications that interfere with nerve function, and/or of pharmacological vitamin and mineral supplements, or which had a history of recent surgery. Those who were under hormone replacement therapy, or with mental illness, or had been bedridden during the last two months for more than two weeks, or whom did not agree to participate in the study were also excluded.
Initially, we pre-selected 56 women between 60 and 80 years of age. After initial assessments, 20 women were excluded: two for not being able to leave home during the evaluations, four were living in another neighborhood with their children, eleven were self-medicating with anti-inflammatory, analgesic, and/or vitamin supplements, three walked every day and practiced gym exercise three times a week.
Thirty-six women were referred for specialist clinical assessment in rheumatology, endocrinology, gastroenterology, cardiology, and neurology. At this stage, four with knee osteoarthritis, one with gastritis, two with diabetes, three with carbohydrate intolerance, and two with hypothyroidism were excluded.
the family health clinics of the older women, following all the procedures for evaluating the elderly.
Ethics
After being informed about all stages of the research, the reading and signing of the informed consent (as approved by the Ethics and Research Committee, Center of Health Sciences, Federal University of Paraiba (Protocol. 0193) was completed. Who agreed to be a volunteer then signed.
Experimental Design
Clinical randomized double blind controlled placebo. The professional responsible for carrying and storing bottles containing the zinc supplement and placebos was also responsible for group randomization, drawn randomly 1:1, randomly distributed into 4 groups. The control group included 18 young women between 20 and 30 years of age, and was subdivided into: Young Placebo: (n = 9) ingesting (10% sorbitol); Young Zinc: (n = 9) ingesting 25mg of elemental zinc/day. The experimental group comprised 20 elderly women aged between 60 and 80 years old, and was subdivided into: Elderly Placebo: (n = 10)ingesting a placebo(10% sorbitol); Elderly Zinc:(n = 10) ingesting 25 mg of zinc/day.
either zinc or placebo that they would use during the ninety days. In the end all of the assessment procedures were performed for the initial stage.
Instruments and data collection procedures
Anthropometric assessment
The weights and heights of the participants were obtained on a manual balance (Elmer) with a capacity of 150 kg, 100 g precision, and a metal scale of 200 cm with a precision of 1 cm, leveled and calibrated (Balmak; BK50F, Sao Paulo, SP, Brazil). The body mass index (BMI) was obtained from the ratio weight/height 2.
Evaluation of alimentary consumption
The food intake assessment was performed using an estimated weekly food intake based on three days, two days mid-week, and one day on the weekend. The volunteers were instructed to properly perform the technique of food accounting, noting the time of each meal, and all food consumed in their respective home measures.
The calculation of energy, macronutrients, fiber, calcium, iron, and zinc consumed (from the menus) was done through the Nut Win software version 1.5 a Nutrition Support Program, provided by the Department of Health Informatics, Federal University of São Paulo/UNIFESP. We also used the Brazilian Food Composition Table (TACO), provided by the Center for Studies and Research in Food (NEPA), State University of Campinas (UNICAMP)23.
Assessment of physical activity
Evaluation of strength, endurance, and muscle balance
Peak isokinetic torque, normalized by body weight(PT/kg) to assess strength, endurance, and muscle balance, 25 was measured at angular velocities of 60°/s, and 180°/s,on a Computerized Isokinetic Dynamometer (Biodex Multi-Joint System 3,Biodex System Biomedical Inc, New York, USA),at the Laboratory for Analysis of Muscular Performance, Department of Physical Therapy, UFRN.
The dynamometer was calibrated before each session, as described in the equipment manual. All tests were performed by the same physician in all phases of the study. Women performing the tests initiated a brief warm-up for five minutes on a stationary bicycle, adjusted to a resistance of 25 W at a speed of 20 km/h. They then conducted quadriceps stretching for both limbs. The stretching was conducted with the volunteers standing erect, the knee in complete flexion, and the hip extended to the maximum tolerable amplitude. Each maneuver was maintained for 30 seconds and repeated three times, at an interval of 30 seconds.
After stretching, each participant was positioned on the dynamometer chair with the backrest reclined (with respect to the vertical position) by 5°. The
participant’s trunk (thorax) is stabilized by means of two cross straps, and a transverse strap fixes the waist (pelvis). The support of the dynamometer lever arm was positioned in the distal region of the leg, 5 cm above the lateral malleolus, so that a complete arc of ankle dorsiflexionis allowed. The mechanical axis of rotation of the dynamometer was aligned with the lateral epicondyle of the femur (the axis of rotation of the knee joint). During all testing procedures, all women were told to hold firmly to the lateral seat support so as to keep all body segments stabilized.
three-minute rest period was maintained between sets to minimize the effects of fatigue.
Collection of biological material
8 mL of blood were collected: 2 mL for zincexamination (BD Vacutainer, Trace Element, Serum, BD Franklin Lakes, NJ,USA), 2 mL for hematologic tests (VacuetteK3EK3EDTA, Greiner Bio -One, Monroe, North Carolina, USA), 2 mL for biochemical tests, and 2 mL for hormonal dosage (Z Vacuetteserumclotactivator, GreinerBio-One, Monroe, North Carolina, USA).The samples for zinc were immediately kept at a temperature of 37oC in a stainless steel oven, suitable for metals. Six hours later, the tubes were taken, and the sera collected with plastic ferrules, and stored in plastic tubes with metal-free caps. Hemolyzed samples were discarded because red blood cells are richer in zinc than is plasma. All procedures related to the handling of zinc samples were followed according to international standards. The serum samples were stored in a freezer at -20ºC until analysis.
Laboratory analyzes
Hormones (GH, IGF - 1, IGFBP3, E2, LH, FSH, TSH, T4) were measured by chemiluminescence (Immulite 1000 systems), the blood count was performed by an auto-analyzer(Horiba ABX Diagnostics, Micros 60, Montpellier, France), glucose, total protein, albumin, alanine transaminase, aspartate transaminase, total bilirubin, urea, creatinine and lipids were analyzed by auto analyzer (DadeBehring, Dimension AR, Illinois, USA). Zinc was determined by atomic absorption spectrophotometry (AA - 240FS, Varian, Victoria, Australia) according to the manufacturer's instructions.
Statistical Analysis
torque, normalized by body mass (PT/kg) for the quadriceps and hamstring muscles, at the angular velocities of 60°/s and 180°/s, and the muscle equilibrium ratio (between hamstrings/quadriceps) were analyzed. We compared between the control group Young Placebo, and the experimental Elderly Placebo and Elderly Zinc groups(one-way ANOVA) at the beginning and at the end of testing. The difference of effects within each group was made by paired the Student's t test. The treatment effect, comparing the Zinc Elderly with the Placebo Elderly group was made from the difference between the averages of the initial and the final evaluations, by independent t test.
RESULTS
The final sample consisted of 20 elderly women and 18 young women. In table 1 are summarized the characteristics of homogeneity between the groups. After analyzing the data, the randomization layout of the groups was revealed. To compare the effectiveness of zinc on force (PT/kg 60°/s), on resistance (PT/kg180°/s), and on muscle balance (IQS/QUA) in aging, the control group of reference was the Young Placebo group, which was not affected by zinc supplementation.
In Table 2, the differences in averages (Δ), and standard deviations between the control group Young Placebo with the experimental groups Elderly Zinc and Elderly Placebo are expressed. In the initial measures of muscle strength APT/kg IQS 60°/s, the percentage difference between the groups Young Placebo and Elderly Zinc was 9.24%, between Young Placebo and Elderly Placebo it was 18.33%, no significant differences. Following 90 days of supplementation with 25 mg, the Elderly Zinc group had peak torque increased PT/kg IQS 60°/s by 8:18 Nm, with no significant difference in muscle strength continuing in the Young Placebo group. However, the Elderly Placebo group in contrast observed a decrease in PT/kg IQS 60°/s of 1.50 Nm from their initial measurements(A) representing a significant 30,45% reduction in strength when compared to the Young Placebo group.
reductions in strength difference between the Young Placebo, and Elderly Zinc groups at 22.83%, between the Young Placebo and Elderly Placebo groups it was 24%, the difference was not significant. As to muscle balance between the hamstrings and quadriceps (AIQS/QUA 60°/s), it was observed that the Young Placebo group showed lower muscular balance than the two elderly groups, which could expose their knees to damage. The evaluation, after 90 days of supplementation with 25 mg zinc revealed that the Elderly Zinc group maintained muscle balance within limits, but were 7.74% above the Placebo Young control group. However, in the Elderly Placebo group, there was a decreased muscle balance (DIQS/QUA 60°/s Nm) of 18.69% compared to the Young Placebo group.
Concerning hamstring muscle strength, in the initial (APT/kg IQS 180°/s),measurements a significant difference between the Young Placebo group and the Elderly Zinc group of 31.76%, and between the Young Placebo and Elderly Placebo group of26.86%, was observed, which shows decreasing hamstring strength with age. In the follow-up measurements, after 90 days of supplementation with zinc, increased hamstring resistance in the Elderly Zinc group was observed. The increase was enough to verify no significant differences between the Elderly Zinc and the Young placebo groups. However, in the Elderly Placebo group we observed decreased hamstring muscle strength, and a greater significant difference between the Young Placebo and the Elderly Placebo groups.
When the effect of zinc supplementation was compared in the same group between the initial measurement sand after 90 days, as seen in Table 3,the Elderly Zinc group showed significant increases (10.93%) in the strength of the hamstrings (PT/kg ISQ 60°/s), and significant increases (10.22%) in the quadriceps muscle (PT/kg QUA 60°/s). This represented an increase of 1.28%in the strength balance between the hamstrings and the quadriceps (ISQ/QUA 60°/s), approximating 60%. However, in the Elderly Placebo group seen in Table 4, there was a decrease of 2.56% in the strength of the hamstrings (PT/kg ISQ 60°/s), and significant increase of 9.80% in quadriceps strength (PT/kg QUA 60°/s), which represented a significant decrease of 12.78% in the balance of muscle strength (ISQ/QUA 60°/s).
represented an increase of 10.39% in the muscle strength balance (ISQ /QUA 180°/s). In contrast, it was found that for the Elderly Placebo group (in comparing the initial assessments with the subsequent 90 days of experiment), there was a decrease of 6.79% in the hamstring strength(PT/kg ISQ 180°/s), and 5.65% increase in quadriceps strength, this represented a decrease of 15.28% in the muscle strength balance (ISQ/QUA 180°/s).
The effect of zinc supplementation between the Elderly Zinc group, and the Elderly Placebo, seen in Table 5, shows a significant increase in both strength and endurance for the hamstring muscles and the quadriceps. It can be assumed that zinc supplementation of 25 mg may be effective in the prevention of muscle strength and endurance losses, while increasing muscle strength and endurance in healthy women over 60 years of age.
DISCUSSION
The main results of this study show that supplementation of 25 mg daily for a period of 90 days increased strength, endurance, and muscle balance in women between 60 and 80 years of age. This may contribute to the prevention of strength, endurance, and muscular balance losses common during aging.
insignificant. This shows that in the Young Placebo group, who increased their hamstring strengths by 12.66 that zinc may have been used in intramuscular structures thus reducing its availability in the plasma by 22.50%, leaving the group at the upper limit of zinc deficiency27. In the Elderly Placebo group, a significant reduction in serum zinc represented a reduction of force of 2.56%. This shows the importance of adequate amounts of zinc in the diet to preserve the strength of the hamstrings, since the supplemented Elderly Zinc group at 25 mg of zinc for ninety days, seems to have used the additional zinc, and secured increases in strength. This muscle group is extremely important in muscular balance for older people25. Zinc could also reduce or prevent hamstring injuries characteristic of aging 28.It is reported that to preserve muscle function, zinc is essential for proper nutrition8. Its deficiency is characteristic of human aging, and leads to immune response decreases, and the development of chronic degenerativediseases17.
Of interest in this study was the quadriceps strength. In the initial evaluation, strength of the quadriceps for the Young Placebo group was 28.24% higher than the Elderly Zinc group and larger by 29%than the Elderly Placebo group, significant differences. In the follow-up measure, the difference in strength between the groups decreased as the Elderly Zinc group increased by 10.22%, the group Elderly Placebo by 9.8%, and the Young Placebo group by only 3.46%. The proportionate gains of force, both in the hamstring muscles as well as the quadriceps for the Elderly Zinc group resulted in a better muscular balance. In contrast, in the Elderly Placebo group, decreased strength of the hamstrings to 1.50 Nm, and increased strength in the quadriceps of 12.53 Nm, led to a significant decrease in muscular balance from 51.59 ± 7.74 to 45.74 ± 8.24. Accordingly, with muscle imbalance, an overload of the quadriceps can occur, because the most requested of movements, from sitting to standing position, spares the hip extensor 29.
knee flexor and extensor muscles are inversely correlated with pain, stiffness, and functionality25.
A Brazilian study of the elderly with mean ages of 69 ± 3.64 years, established benchmarks for balance between the knee flexors and extensors (the hamstrings, and quadriceps)at an angular velocity of 60°/s: 47.95 ± 10.99% and an angular velocity of 180°/s: 59.59 ± 13, 40%32.In our study the approximate age of the women was by groups Elderly Zinc: 66.40 ± 6.20, and Elderly Placebo: 65.30 ± 5.03.Our study showed that at the angular velocity of 60°/s the muscular balance between flexors and extensors (during the initial evaluation of Elderly Placebo group) was 51.59 ± 7.74%, close to the reference values above. In the follow-up measurements, the balance between flexors and knee extensors of 45.74 ± 8.24% was lower, both in terms of their initial measurements as with the elderly of reference 32. However, in the Elderly Zinc group,(initial average of 58.09 ± 11.29% and final average of 58.84 ± 9.90), the average hamstring/quadriceps balance, the equilibrium force directed to the knee joint, was maintained at 22.27%,above the average benchmark for this parameter 32. These results indicate what zinc supplementation can do in addition to other factors that improve the health of the elderly 26, 27, and it may also be effective in increasing muscular strength, especially the hamstring muscles.
In the muscle balance measurements (ISQ/QUA180°/s), the Elderly Placebo group showed an initial mean of 61.69 ± 11.95, and a final average of 53.51 ± 6.21, the Elderly Zinc group showed an initial mean of 57, 98 ± 11.29, and final average of 64.70 ± 9.79. Therefore, the present study showed that the average strength of the muscle balance of the knee joint 90 days after the first assessment of Elderly Placebo group was 11.25% lower than the reference population. In contrast, the Elderly Zinc group after the same time was 7.9% higher than the reference population average 32. The increase in muscle balance in strength and resistance seen in this study could make it easier to change from the sitting position to the standing position, or the starting and maintaining walking speed for a longer time.
maintained close to 60%, and at the angular velocity of 180°/s they were close to 65%. This might help to focus future studies elucidating the role of zinc in muscle strength and endurance.
During aging, the hamstrings are most vulnerable to injury because a one-year increase in age increases the likelihood of tendon injury in this muscle group 1.3 times 33. Actions that develop increased muscle strength can be effective in improving the daily life of elderly women, and contribute to the maintenance and extension of their autonomy, of the functional capabilities of this population 34, 35.
The hamstrings are a muscle group, with a predominance of fast twitch type II muscle fibers. In healthy aging, there is a reduction of skeletal muscle fiber type I, of capillaritzed area4, of strength, and of cross-sectional area. In the hamstrings, the femoral biceps are made up of mainly 2c fibers that can become fast fiber or slow, depending on the demand for action requested 36.
Reduction of muscle mass and muscle strength is associated with increased oxidative stress and may be increased due to lowered activity of anaerobic and aerobic enzymes, and protein content, not just due to decreased physicalactivity7.
There is evidence that zinc deficiency in the body can adversely affect the function of striated muscle 8,and can induce apoptosis of vascular smooth muscle cells; oxidative stress with zinc deficiency could contribute to the apoptosis of these cells 9. Acute depletion of zinc in the body; also changes the working capacity of skeletal muscle 13. However, the effectiveness of zinc in the metabolism depends on lifestyle, because in experimental conditions of restricted mobility in mice, zinc supplementation was not effective in maintaining its metabolic balance 14.
To preserve muscle function, proper zinc nutrition is essential 8 because zinc deficiency in human aging causes a decrease in the immune response, and the development of chronic degenerativediseases17.
Public Health Action could minimize the effects of dynapenia and neuromuscular aging syndrome.
FINANCING AND CONFLICT OF INTEREST
This study was funded by FAPERN (Case Notice PPSUS 3 - in. 011/2009). The authors have no conflicts of interest in publishing this article.
REFERENCES
1. Lutz W, Sanderson W, Scherbov S. The coming acceleration of global population ageing. Nature. 2008;451(7179):716-9.
2. Remor CB, Bós AJG, Werlang MC. Características relacionadas ao perfil de fragilidade no idoso. Sci Med. 2011;21(3):107-12.
3. Alves LC, da Costa Leite I, Machado CJ. Perfis de saúde dos idosos no Brasil: análise da Pesquisa Nacional por Amostra de Domicílios de 2003 utilizando o método Grade of Membership Health profile of the elderly in Brazil: analysis of the 2003 National Household Sample Survey using. Cad Saúde Pública. 2008;24(3):535-46.
4. Frontera, WR, Hughes V A, Fielding R A, Fiatarone MA, Evans W J, Roubenoff R. Aging of skeletal muscle: a 12-yr longitudinal study.J Appl Physiol.
2000;88(4):1321-1326.
5. Morley JE. Sarcopenia in the elderly. FamPract. 2012;29(suppl 1):i44-i48.
6. Wang C, Bai L. Sarcopenia in the elderly: basic and clinical issues. Geriatr Gerontol Int. 2012;12(3):388-396.
7. Pastoris O, Boschi F, Verri M, Baiardi P, Felzani G, Vecchiet J, et al. The effects of aging on enzyme activities and metabolite concentrations in skeletal
muscle from sedentary male and female subjects. ExpGerontol.
2000;35(1):95-104
8. Grider A, Mouat MF, Scrimgeour AG. Consumption of a moderately Zn-deficient and Zn-supplemented diet affects soluble protein expression in rat soleus muscle. J Nutr Biochem. 2007;18(11):753-759
10. Tsang D, TsangYS, Ho WKK, Wong RNS. Myelin basic protein is a zinc-binding protein in brain: possible role in myelin compaction. Neurochem Res. 1997;22(7):811-819.
11. Ünal B, Tan H, Orbak Z, Kiki İ, Bilici M, Bilici N, et al. Morphological
alterations produced by zinc deficiency in rat sciatic nerve: a histological, electron microscopic, and stereological study. Brain Res. 2005;1048(1):228-234.
12. Maltin CA, Duncan L, Wilson AB, Hesketh JE. Effect of zinc deficiency on muscle fibre type frequencies in the post-weanling rat. Br J Nutr. 1983;50(03):597-604.
13. Van Loan M, Sutherland B, Lowe N, Turnlund J, King J. The effects of zinc depletion on peak force and total work of knee and shoulder extensor and flexor muscles.Int J Sport Nutr. 1999;9(2):125
14. Zorbas YG, Yaroshenko YN, Kuznetsov NK, Ivamov AL. Daily zinc supplementation effect on zinc deficiency in rats during prolonged restriction of motor activity. Biol Trace Elem Res.1997;60(1-2):101-113.
15. Miller MS, Bedrin NG, Callahan D M, Previs M J, Jennings M E, Ades PA, et al. Age-related slowing of myosin actin cross-bridge kinetics is sex specific and predicts decrements in whole skeletal muscle performance in humans. J Appl Physiol. 2013;115(7):1004-1014.
16. Safdar A, Hamadeh MJ, Kaczor JJ, Raha S, Tarnopolsky MA. Aberrant
mitochondrial homeostasis in the skeletal muscle of sedentary older adults. PLoS One. 2010;5(5):e10778. doi:10.1371/journal.pone.0010778.
17. Mocchegiani E, Romeo J, Malavolta M, Costarelli L, Giacconi R, Diaz LE, et al. Zinc: dietary intake and impact of supplementation on immune function in elderly. Age. 2013;35(3):839-860
18. Clark BC, Manini TM. Sarcopenia≠ dynapenia.J Gerontol A BiolSci Med Sci. 2008;63(8):829-34.
19. Clark BC, Manini TM. What is dynapenia? Nutrition. 2012;28(5):495-503.
20. Newman AB, Kupelian V, Visser M, Simonsick EM, Goodpaster BH,
Kritchevsky SB, et al. Strength, but not muscle mass, is associated with mortality in the health, aging and body composition study cohort. J Gerontol A BiolSci Med Sci. 2006;61(1):72-7.
21. Kahmann L, Uciechowski P, Warmuth S, Plümäkers B, Gressner AM, Malavolta M, et al. Zinc supplementation in the elderly reduces spontaneous
inflammatory cytokine release and restores T cell
22. Muzembo BA, Nagano Y, Eitoku M, Ngatu NR, Matsui T, Bhatti SA, et al. A cross-sectional assessment of oxidative DNA damage and muscle strength among elderly people living in the community. 1-9.Environ Health Prev Med. 2013;1-9. DOI 10.1007/s12199-013-0350-x
23. Universidade Estadual de Campinas (UNICAMP). Núcleo de Estudos e
Pesquisas em Alimentação (NEPA). Tabela Brasileira de Composição de Alimentos (TACO). 2 ed. Campinas, São Paulo, 2006.
24. MazoGZ, Mota J, Benedetti TB, Barros MVGD. Validade concorrente e
reprodutibilidade: teste-reteste do questionário de Baecke modificado para idosos. Ver Bras Ativ Fis Saúde. 2001;6(1):5-11.
25. Santos MLADS, Gomes WF, de Queiroz BZ, de Brito Rosa NM, Pereira DS,
Dias JMD, et al. Desempenho muscular, dor, rigidez e funcionalidade de idosas com osteoartrite de joelho. Acta Ortop Bras. 2011;(4):193-7.
26. Suplementação com zinco no tratamento da anorexia nervosa. Revista da Associação Médica Brasileira. 2013;59(04):321-324.
27. Yanagisawa H. Zinc deficiency and clinical practice. Japan Med Assoc J.2004;47(8):359-364.
28. Gabbe BJ, Bennell KL, Finch CF. Why are older Australian football players at greater risk of hamstring injury?. J Sci Med Sport. 2006;9(4):327-333
29. Meijer K, WillemsPJ, SavelbergHH. Muscles limiting the sit-to-stand movement: an experimental simulation of muscle weakness.GaitPosture. 2009;30(1):110-114.
30. Conboy IM, Conboy MJ, Smythe GM, Rando TA. Notch-mediated restoration
of regenerative potential to aged muscle. Science. 2003;302(5650):1575.
31. Rantanen T, Volpato S, Ferrucci L, Heikkinen E, Fried LP, Guralnik JM. Handgrip Strength and Cause‐Specific and Total Mortality in Older Disabled Women: Exploring the Mechanism.J AmGeriatr Soc. 2003;51(5):636-41.
32. Dias JMD, Arantes P, Alencar M, Faria J, Machala C, Camargos F, et al. Relacao isquiotibiais/quadriceps em mulheres idosas utilizando o
dinamometro isocinetico; Isokinetic hamstring/quadriceps ratio in elderly women. Rev bras fisioter. 2004;8(2):111-5
33. Verrall G, Slavotinek J, Barnes P, Fon G, Spriggins A. Clinical risk factors for hamstring muscle strain injury: a prospective study with correlation of injury by magnetic resonance imaging.Br J Sports Med. 2001;35(6):435-9.
34 Holloszy JO, Tseng BS, Marsh DR, Hamilton MT, Booth FW. Strength and
35. Offord E A, Karagounis LG, Vidal K, Fielding R, Meydani S, Penninger JM. Nutrition and the biology of human ageing: Bone health & osteoporosis / sarcopenia / immune deficiency. J Nutr Health Aging. 2013;17(8):712-716.
36. Dahmane R, Djordjevič S, Smerdu V. Adaptive potential of human biceps
femoris muscle demonstrated by histochemical, immunohistochemical and
mechanomyographical methods. Med Biol Eng Comput.
Table 1. Bioanthropometrics and health characteristics between Young Placebo, Elderly Zinc, and Elderly Placebo Groups
Young Placebo (n=9) ElderlyZinc (n=10) ElderlyPlacebo(n=10)
Age 24.11±2.97 66.40±6.20* 65.30±5.03*
Monthly income 1732.40±1080 1544.60±1111 2210.30±1865
Body mass(kg) 62.45±15.44 62.45±15.44 64.26±10.67
Height(m) 1.61±0.04 1.50±0.08* 1.51±0.03*
BMI(kg/m²) 23.75±5.41 25.96±5.33 27.82±4.03
Physical Activity 4.94±3.19 6.98±2.57 6.56±2.83
RBC(million/mm3) 4.45±0.19 4.48±0.35 4.56±0.30
Diet zinc(mg/dia) 7.42±1.15 5.74±2.17 6.54±2.09
Zinc (μg/mL) 1.081±0.06 1.068±0.11 1.079±0.04
Table 2. Peak torque; normalized by body mass of the quadriceps and hamstrings in the angular velocities of 60°/s and 180°/s, for Young Placebo, Elderly Placebo and Elderly Zinc Groups (one-way ANOVA).
Young Placebo (n=9)
Elderly
Zinc (n=10) P Δ1
Elderly Placebo (n=10)
p Δ2
APT/kg IQS-60°/s,Nm 73.50±25.59 66.71±13.13 0.73 6.79 60.03±18.56 0.30 13.47
DPT/kg IQS-60°/s,Nm 84.15±27.60 74.89±14.46 0.57 9.26 58.53±16.37 0.02 25.62*
APT/kg QUA- 60°/s,Nm 162.44±43.69 116.58±24.64 0.01 45.86* 115.34±29.24 0.013 47.10*
DPT/kg QUA 60°/s,Nm 168.25±59.75 129.84±29.61 0.11 38.41 127.87±30.04 0.13 40.38
APTIQS/QUA 60°/s,Nm 45.00±8.97 58.09±11.23 0.01 -13.09* 51.59±7.74 0.29 -6.59
DIQS/QUA 60°/s,Nm 54.29±18.23 58.84±9.90 0.71 -4.54 45.74±8.09 0.31 8.55
APT/kg IQS 180°/s,Nm 65.75±18.10 44.87±15.49 0.01 20.88** 48.09±8.63 0.03 17.66**
DPT/kg IQS 180°/s,Nm 68.06±21.74 53.71±9.18 0.10 14.35 45.03±11.34 0.006 23.03**
APT/kg QUA 180°/s,Nm 117.73±32.83 77.26±24.07 0.01 40.47** 79.33±15.55 0.006 38.40**
DPt/kg QUA 180°/s,Nm 125.42±41.46 85.09±19.88 0.01 40.33 84.06±18.54 0.009 41.36*
A IQS/QUA 180°/sNm 56.73±8.90 57.98±11.87 0.96 -1.24 61.69±11.95 0.59 -4.95
DIQS/QUA 180°/s,Nm 57.56±18.29 64.70±9.79 0.42 -7.13 53.51±6.21 0.75 4.05
A Zinc (μg/mL) 1.0816±0.06039 1.0683±0.1167 0.93 0.0132 1.0796±0.0472 0.99 0.0020
D Zinc (μg/mL) 0.8383±0.0865 1.006±0.1387 0.005 -0.1679 0.9738±.0736 0.02 -0.1354
Note - * Statistical significance (p ≤ 0.05).
Table 3. Peak torque (normalized by body mass) of the quadriceps and hamstrings at angular velocities of 60°/s and 180°/s of the Elderly Zinc group (t test stopped).
Note - * Statistical significance (p ≤ 0.05).
PT = peak torque, Nm = Newton meter; kg = body mass; ISQ = hamstrings, QUA = quadriceps, and ISQ/QUA = hamstrings to quadriceps ratio: Δ = difference between averages before and after
Elderly Zinc 95% confidence interval
Before After P Δ Lower Limit Upper Limit
PT/kg IQS 60°/s Nm 66.71±13.13 74.89±14.46 0.02 -8.18*
-14.99 -1.36
PT/kg QUA 60°/s Nm 116.58±24.64 129.84±29.61 0.01 -13.26*
-23.12 -3.39
IQS/QUA 60°/s Nm 58.09±11.23 58.84±9.90 0.85 -0.74
-9.39 7.90
PT/kg IQS 180°/s Nm 44.87±15.49 53.71±9.18 0.01 -8.84*
-15.60 -2.07
PT/kg QUA 180°/s Nm 77.26±24.07 85.09±19.88 0.01 -7.83*
-13.57 -2.08
IQS/QUA 180°/s Nm 57.98±11.87 64.70±9.79 0.07 -6.72
-14.22 0.78
Table 4. Peak torque normalized by body mass of the quadriceps and hamstrings in the angular velocities of 60°/s and 180°/s Elderly Pacebo group (t test stopped).
Note - * Statistical significance (p ≤ 0.05).
PT = peak torque, Nm = Newton meter; kg = body mass; ISQ = hamstrings,QUA = quadriceps, and ISQ/QUA = hamstrings to quadriceps ratio; Δ = difference between the averages before and after
ElderlyPlacebo Confidenceinterval95% Before (n = 10) Mean ± SD
After (n = 10) Mean ± SD
p Δ
LowerLimit UpperLimit
PT/kg IQS 60°/s Nm 60.03±18.56 58.53±16.37 0.61 1.50
-4.91 7.91
PT/kg QUA 60°/s Nm 115.34±29.24 127.87±33.04 0.02 -12.53*
-22.60 -2.45
IQS/QUA 60°/s Nm 51.59±7.74 45.74±8.24 0.001 5.84*
3.09 8.59
PT/kg IQS 180°/s Nm 48.09±8.63 45.03±9.18 0.32 0.06
-3.54 9.66
PT/kg QUA180°/s Nm 79.33±15.55 84.06±18.54 0.15 -4.73
-11.54 2.08
IQS/QUA 180°/s Nm 61.69±11.95 53.51±6.02 0.07 8.18
-1.03 17.39
Table 5. Difference of averages before and after 90 days (independent t test) of peak torque/body mass of the quadriceps and hamstrings, angular velocities of 60°/s and 180°/s, between Elderly Zinc and ElderlyPlacebogroups
Note - * Statistical significance (p ≤ 0.05).
PT = peak torque, Nm = Newton.meter; kg = body mass ; QUA = quadriceps, ISQ = hamstrings, Δ = average difference between
Elderly Zinc(n=10)
Elderly Placebo (n=10)
Confidence Interval
Mean ± SD Mean ± SD Δ P Lowest Highest
PT/kg IQS 60°/s Nm 8.29±9.44 -1.50±8.97 9.97* 0.02 1.13 18.44
PT/kgQUA 60°/s Nm 15.95±19.29 11.82±15.65 4.13 0.60 -12.30 20.56
PT/kg IQS 180°/s Nm 8.82±9.45 -3.06±9.15 11.88* 0.01 3.13 20.62
6 ANEXAÇÃO DO ARTIGO 2
ARTIGO 2
Título: Influence of basal energy expenditure and body composition on bone mineral density in postmenopausal women
Periódico: International Journal of General Medicine
ISSN: 1178-7074 (Electronic)
Qualis: B2 (Medicina II – QUALIS CAPES)
Open Access Full Text Article O r i g i n a l R e s e a r c h
Influence of basal energy expenditure
and body composition on bone mineral density
in postmenopausal women
This article was published in the following Dove Press journal: International Journal of General
Medicine 2 November 2012
Number of times this article has been viewed
Maria Aparecida Bezerra Quirino1 João Modesto-Filho2 Sancha Helena de Lima Vale3
Camila Xavier Alves3 Lúcia Dantas Leite4 José Brandão-Neto5
1Department of Physiotherapy, 2Department of Clinical Medicine, Universidade Federal da Paraíba, João Pessoa, Brazil; 3Postgraduate Health Science Program, 4Department of Nutrition, 5Department of Clinical Medicine, Universidade Federal do Rio Grande do Norte, Natal, Brazil
Background: The aim of this study was to investigate the influence of body mass index, body weight, lean mass, fat mass, and basal energy expenditure on bone mineral density in postmenopausal women.
Methods: This was a cross-sectional, descriptive study of a sample of 50 women, with mini-mum time since menopause between 1 and 10 years. Bone mineral density was assessed at the lumbar spine (L2–L4), femoral neck, Ward’s triangle, and trochanter using dual-energy X-ray absorptiometry. Body mass index, lean mass, fat mass, and basal energy expenditure were measured by bioimpedance.
Results: The mean age of the women was 51.493.86 years and time since menopause was3.50 2.59 years. Significant negative correlations were found between chronological age and lumbar spine, femoral neck, Ward’s triangle, and trochanteric bone mineral density. In regard to time since menopause, we also observed significant negative correlations with bone mineral density at the lumbar spine and Ward’s triangle. The following significant positive correlations were recorded: body mass index with bone mineral density at the femoral neck and trochanter; fat mass with bone mineral density at the femoral neck and trochanter; lean mass with bone mineral density at the lumbar spine, femoral neck, and trochanter; and basal energy expenditure with bone mineral density at all sites assessed. On the other hand, the multiple linear regression model showed that: 20.2% of bone mineral density variability at the lumbar spine is related to lean mass and time since menopause; 22.3% of bone mineral density variability at the femoral neck is related to body weight and age; 18.9% of bone mineral density variability at Ward’s triangle is related to age and basal energy expenditure; and 39% of bone mineral density vari-ability at the trochanter is related to body mass index, age, and menarche.
Conclusion: Changes in bone mineral density, specific for each skeletal site, are influenced byage, time since menopause, body weight, body mass index, lean mass, and basal energy expen-diture. Lean mass and basal energy expenditure positively influenced bone mineral density at the lumbar spine and Ward’s triangle, with a predominance of trabecular bone.
Keywords: women, menopause, bone mineral density, body composition, energy
expenditure
Correspondence: José Brandão-Neto Av Gal Gustavo Cordeiro de Farias, s/n, Natal-RN, CEP 59012-570, Brazil Tel 55 84 3342 9748
Fax 55 84 3342 9776 Email jbn@pq.cnpq.br
Introduction
adolescence, and early adulthood, until reaching peak bone mineralization. It is a negative predictor of osteoporosis and risk of fracture over time, and is influenced by genetic,
mechanical, nutritional, and hormonal factors.3
Peak bone mineralization in the entire skeleton, occurring on average at 18 years of age, varies little up to the age of 50 years, with a slight and progressive increase in BMD of around 0.2% per year in cortical bone-rich regions. However, in areas with a larger amount of trabecular bone, such as the proximal femur and the inner area of the vertebral body, an immediate decline is initiated at the age of 18 years, with an annual loss in BMD of 0.3% (trochanter), 0.4% (femoral
neck), 0.6% (Ward’s triangle), and 0.5% (lumbar spine).4
This
reveals a 50% loss of BMD, mainly in Ward’s triangle. One
study indicates that a 10% increase in peak bone miner-alization could delay the development of osteoporosis by 13 years, while a 10% increase in time since menopause would
delay it by only 2 years.5
As with BMD, lean mass in the third decade of life varies little until the fifth decade, showing a sharp decline from the
sixth decade onwards.6 The difference in muscle strength
between young and elderly individuals is less when these
values were adjusted for lean mass and muscle mass.7
Menopause is associated with diminished serum estrogen levels, which may provoke a decrease in BMD and lean mass,
and a rise in body fat.8 These alterations can affect gait and
balance in the elderly,9 reducing physical activity at work,
home, during leisure time, and in sport. This causes a decline in total and basal energy expenditure, which is influenced by age, sex, body composition, and hormonal factors, including
estrogen.10–12
Studies show that body weight has a positive influence on
BMD.13–15 However, this influence is different between
skeletal sites.15 There is no consensus regarding the effect of
body composition on BMD. Some research has shown that fat mass and lean mass are correlated with lumbar spine and hip
BMD, respectively.15 However, other studies demonstrate
that obesity does not protect against fracture in postmenopausal women. On the contrary, it is associated with
an increased risk of ankle and femur fractures.16 Lean mass
plays a relevant role in BMD, possibly acting positively on
cortical bone mass.17
Aging is accompanied by a decrease in lean mass and
basal energy expenditure.11 Individuals with low basal energy
expenditure are predisposed to gaining weight at the expense
of a proportional increase in fat mass.10,11 Studies show a
posi-tive association between basal energy expenditure and BMD in North American women, which is much more
significant than body weight.10,11
BMD in the first 10 years after menopause, we studied the influence of age, time since menopause, body mass index, fat mass, and basal energy expenditure on lumbar spine, femoral
neck, Ward’s triangle, and trochanteric BMD.
Materials and methods
Patients and study design
This was a cross-sectional, quantitative, descriptive study performed at the Lauro Wanderley University Hospital gynecology outpatient clinic of the Universidade Federal da Paraíba, Brazil. Participants were selected from those responding to posters put up at the hospital, university cam-pus, and family health units in nearby neighborhoods. The sample consisted of 50 women with minimum and maximum time since postmenopause of one and 10 years, respectively,
and body mass index between 18.5 and 39.9 kg/m2. A
stan-dard deviation of 6 and maximum error of estimation of 20% were used to calculate sample size, with a 5% significance level. All the women in the study were of mixed ethnicity. Exclusion criteria were: use of hormone replacement therapy; immunosuppressants, glucocorticoids, diuretics, anticon-vulsants, or calcium supplementation; tobacco or alcoholic beverages; previous surgery (colostomy and oophorectomy); and history of disease (neoplasia, diabetes mellitus, liver, kidney, and thyroid disorders, and rheumatoid arthritis).
All participants gave written informed consent. The project was approved by the research ethics committee of Lauro Wanderley University Hospital, Universidade Federal da Paraíba (protocol number 335/03). After assessment, the women were referred for specialized clinical follow-up.
Instruments and data
collection procedures
A form was used to record sociodemographic, clinical, and anthropometric data. Weight and height were measured while fasting and after bladder emptying. Subjects were barefoot, wearing Bermuda shorts and a t-shirt, and standing in the bipedal position, with their chin parallel to the floor. The head, buttocks, and heels were aligned with the stadiometer of a 150 kg anthropometric scale in 100 g increments and a 2 m metal rod in 1 cm increments (Filizola, Personalline E, São Paulo, Brazil). Body mass index was calculated to obtain classification of nutritional status as follows: eutrophic at
18.5–24.9 kg/m2; overweight at 25.0–29.9 kg/m2; and