Croat. Chem. Acta88 (1) (2015) 35–42. http://dx.doi.org/10.5562/cca2342
Original Scientific Article
Electrochemical Oxidation of Fragrances 4-Allyl and
4-Propenylbenzenes on Platinum and Carbon Paste Electrodes
Lai-Hao Wang,* Chia-Ling Chang, and Yi-Chun Hu
Department of Medical Chemistry, Chia Nan University of Pharmacy and Science, 60 Erh-Jen Road, Section 1, Jen Te, Tainan 71743, Taiwan
RECEIVED MAY 19, 2013; REVISED MARCH 10, 2014; ACCEPTED JULY 21, 2014 Abstract. The electrochemical oxidation behaviors of 4-allylbenzenes (estragole, safrole and eugenol) and 4-propenylbenzenes (anethole, asarone and isoeugenol) on platinum and carbon paste electrodes were in-vestigated in a Britton-Robinson buffer (pH = 2.93 and 10.93), acetate buffer, phosphate buffer solutions (pH = 2.19 and 6.67), and acetonitrile containing various supporting electrolytes examined lithium per-chlorate. Their oxidation potential with Hammett (free-energy relationships) and possible reaction mecha-nisms were discussed.
Keywords: electrochemical oxidation, 4-allylbenzenes, 4-propenylbenzenes
INTRODUCTION
Research on fragrances / flavors is popular; however, almost all studies focus on their wide application in the food, and cosmetics industries and in traditional medi-cine; as well as extraction methods and analytical methodologies.1–5 Fragrances that are air-sensitive may form peroxides, respiratory irritants, andaerosol parti-cles that cause inflammatory responses in the lungs. The photochemistry of some allylic compounds such as cis-trans isoeugenol and citral, were oxidized in the presence of atmospheric oxygen or photosensitized species (O3, OH, NO3, etc.).6–8 However, most reports have focused on the photochemical reaction of eugenol derivatives to for the synthesis of new flavors chemi-cals.9–14 The structurally related substituted 4-allyl-benzenes derivatives (eugenol, estragole and safrole) and 4-propenylbenzenes derivatives (isoeugenol, ane-thole and asarone), occur naturally in various tradi-tional foods, particularly in spices such as cloves, cinnamon and basil1. Some of them have been demon-strated to be an effective, inexpensive anesthetic agents, antioxidants and blood circulation enhancers. The major analytical methods for analyzing alkenyl-benzenes fragrances are gas chromatography and gas chromatography-mass spectrometry.15 There are few electrochemical theories reported in pharmaceutical formulations.16 4-allylbenzenes (4-allylanisole and eu-genol) and 4-propenylbenzenes (trans-anethole) are
studied to undergo electrochemical polymerization giving rise to either conducting or insulating films.17–19 The anodic methoxylation of alkenylbenzenes includes anethole and isosafrole on graphite and platinum, which can be converted to free aldehyde, a valuable fragrance compound and an intermediate for organic synthesis.20–23 The present work is concerned with the measurement of aromatic substituent effects and struc-tural elucidation of 4-allyl and 4-propenylbenzenes.
EXPERIMENTAL
Apparatus and Materials
Pure standard substances were purchased from bio-medical supply houses: eugenol, isoeugenol, and trans-anethole from Acros Organics, Geel, Belgium; -asarone, safrole from Sigma-Aldrich Fine Chemicals, Fluka, Swit-zerland; estragole from Aldrich Chemical Company, Inc., Milwaukee, WI. Supporting electrolytes were obtained as follows: tetrabutylammonium tetrafluoroborate (Bu4NBF4, Acros Organics, Thermo Fisher Scientific, Geel, Bel-gium), tetraethylammonium tetrafluoroborate (Et4NBF4, E. Merck, Chemical Co. Germany), tetrabutylammonium perchlorate (Bu4NClO4, Tokyo Chemical Industry Co., Ltd, Tokyo, Japan), tetraethylammonium perchlorate (Et4NClO4,Tokyo Chemical Industry Co., Ltd, Tokyo, Japan), and lithium perchlorate (LiClO4, Acros Organics, Thermo Fisher Scientific,Geel, Belgium). Other chemical reagents used were of analytical grade.
Voltammetric Measurements
The three voltammetric techniques, Sampling DC, linear Sweep and cyclic voltammetry, were all performed on a platinum and carbon paste electrodes. Cyclic voltamo-grams (CV) of the fragrances were taken on a platinum electrode in acetonitrile containing various supporting electrolytes, and Britton-Robinson buffer solutions (pH = 2.93 – 10.93) to monitor potential vs. current.
RESULTS AND DISCUSSION
Electrochemical Behavior of Fragrances 4-Allyl and 4-Propenylbenzenes on the Platinum Electrode
Supporting Electrolytes and Solvent Effects
There are several ways in which the supporting electro-lytes solvent system can influence mass transfer, the electron reaction (electron transfer), and the chemical reactions which are coupled to the electron transfer.24 The effects of supporting electrolytes and solvent com-position on fragrances 4-allyl and 4-propenylbenzenes peak potential (Ep) and peak current (ip) are listed in Table 1. Table 1 shows the peak current of fragrances in non-aqueous solvent (100 % acetonitrile) were higher than in aqueous-organic solvent (30 % acetonitrile) due to the higher background in non-aqueous solvent than that in aqueous-organic solvent. However, the peak potential of fragrances were less positive in aqueous organic solvents that are more suitable for oxidation. The electrooxidation process occurs in the heterogene-ous phase. For non-aqueheterogene-ous solvent, its molecules com-pletely cover the electrode surface to prevent the ad-sorption of fragrances. Furthermore in organic work, strongly basic anions or radical anions are often pro-duced and these are rapidly protonated by solvents like
Table 1. Effect of supporting electrolytes on the cyclic voltammetric peak potential (Ep) and peak current (ip) of α-asarone, trans-anethole, isoeugenol, safrole, estragole and eugenol at platinum electrode. The concentration of fragrances was 1 mmol dm–3; scan rate, v = 50 mV / s.
α-Asarone trans-Anethole Isoeugenol Safrole Estragole Eugenol
Ep/ V ip/ A Ep/ V ip/ A Ep/ V ip/ A Ep/ V ip/ A Ep/ V ip/ A Ep/ V ip/ A Bu4NBF4/ CH3CN 0.91
1.19 1.44 20.6 36.2 34.6 1.33 1.78 2.14 35.7 72.9 136 0.84 1.18 1.42 24.4 54.5 94.5 1.57 1.78 –(a) 62.0 62.6 –(a) 1.67 2.12 –(a) 80.1 98.2 – (a)
1.17 – (a) – (a)
44.9 – (a)
–(a) Et4NBF4/ CH3CN 0.99
1.31 1.86 30.3 28.8 45.9 1.31 1.68 2.11 28.8 46.9 98.0 1.10 1.25 1.52 26.2 38.9 45.8 1.51 1.88 –(a) 41.6 84.6 –(a) 1.67 2.13 –(a) 61.3 75.1 – (a)
1.23 – (a) – (a)
35.1 – (a)
–(a) Bu4NClO4/ CH3CN 1.20
1.43 1.71 31.6 37.6 49.0 1.26 1.65 2.06 49.2 69.6 150 1.10 1.27 1.51 29.0 41.5 42.4 1.51 1.80 –(a) 57.2 58.9 –(a) 1.67 2.06 –(a) 54.9 82.5 – (a)
1.27 – (a) – (a)
41.0 – (a)
–(a) Et4NClO4/ CH3CN 1.15
1.40 1.72 35.5 41.4 49.8 1.32 1.67 2.11 34.2 51.0 115 1.08 1.27 1.48 28.5 44.7 49.0 1.49 1.81 –(a) 41.7 42.1 –(a) 1.64 2.13 –(a) 69.7 83.7 – (a)
1.18 – (a) – (a)
41.7 – (a)
–(a) LiClO4/ CH3CN 1.13
1.36 1.73 32.8 33.3 37.9 1.25 1.60 2.00 32.8 59.6 114 1.00 1.18 1.43 27.6 45.4 55.3 1.39 2.09 –(a) 42.2 62.7 –(a) 1.61 2.07 –(a) 51.1 7.81 – (a)
1.16 – (a) – (a)
42.2 – (a)
–(a) Et4NBF4/ CH3CN,
w(CH3CN) = 30 % 0.76 0.99 12.7 21.7 1.11 1.57 24.2 67.17 0.61 –(a) 9.81 –(a) 1.18 –(a) 47.3 –(a) 1.37 –(a) 42.0 – (a)
0.85 – (a)
23.7 –(a) Et4NClO4/ CH3CN,
w(CH3CN) = 30 % 0.76 0.97 11.0 17.1 1.00 1.58 20.9 69.04 0.64 –(a) 9.00 –(a) 1.17 –(a) 51.8 –(a) 1.37 –(a) 48.9 – (a)
0.83 – (a)
18.9 –(a) LiClO4/ CH3CN,
w(CH3CN) = 30 % 0.76 0.97
12.9 21.1
1.11 – (a)
20.8 –(a) 0.69 –(a) 9.56 –(a) 1.19 –(a) 48.8 –(a) 1.38 –(a) 56.1 – (a)
0.92 – (a)
17.8 –(a) (a)
water or alcohol. These reasons explain why aqueous organic solvents are more suitable for the oxidation of the allyl and propenylbenzenes. The solubilities and specific resistance of Bu4NBF4/ CH3CN (s = 70 g / 100 ml, ρ = 37 Ω m) and Bu4NClO4/ CH3CN (s = 71 g / 100 ml, ρ = 31 Ω m) are very near. Therefore, the Ep and ip of the fragrances are very the similar.
The cation of the supporting electrolytes signifi-cantly influencing the safrole, estragole and eugenol on Ep and ip is confirmed by the results listed in Table 1. From Table 1, it is 1.51 V for Bu4NClO4 and 1.49 V for Et4NClO4 but the ip in CH3CN containing Bu4NClO4 (57.2 A) is 1.4 times that of in Et4NClO4 (41.7 A). These results can be accounted for by the presence of larger ion tetrabutylammonium than the tetraethyl-ammonium film on the platinum surface. However, the Ep of quaternary ammonium ion film on the Pt surface is very similar. Compared with quaternary ammonium ion and (R4N+) lithium ion (Li+), the Ep of the safrole in Bu4NClO4, Et4NClO4 and LiClO4, small cation size of lithium ion show less positive values (1.39 V) than quaternary ammonium ion (1.57 V) (Figure 1).Indeed, as reported in the literature,25 the bulky hydrophobic alkyl group stronger Van der Waals forces of cohesion between the ammonium groups, leading to a more com-pact hydrophobic adsorbed layer. The Ep of the estro-gole first peaks are expected to correspond to the pro-cesses of two a one-electron (Ep at 1.67 V and 2.13 V) and a one two-electron (Ep at 1.37 V) oxidation, in non-aqueous solvent (acetonitrile) and non-aqueous-organic sol-vent (30 % acetonitrile) (Figure 2). These data show the peak potentials shift more positively with 100 % acetoni-trile, because its molecules completely cover the elec-trode surface to prevent the adsorption of estragole. How-ever, 30 % acetonitrile point to an appreciable content of estragole adsorbed on the electrode surface.26
The total number of electrons is determined using controlled-potential coulometry using a platinum elec-trode. The accumulated charge (Q) is taken from the digital coulometer at a curve (potential corresponding to peak current) of the oxidation wave. Applying the equa-tion: Q = n F w / M where w is the weight of the sample in grams and M its molecular weight, the value of n for estrogole is found to be two electrons.19,27 The active oxidation group (OH) on benzene ring of estrogole because of between C=C double bond and benzene ring has not conjugation results a marked higher potential (1.61 V) than that has conjugation of trans-anethole (1.25 V) in acetonitrile containing LiClO4. A possible mechanism is given as below:
Substituted Group Effects
Insofar as electrons are transferred in the potential-determining step, the transition state is more electron rich than the reactant is, and electron-donating substit-uents will facilitate to oxidation process.28 Voltammet-ric oxidative groups (i.e. hydroxyl and methoxyl) are electron-donating substituents these attached to the 4-allylbenzene nucleus and 4-propenylbenzenes (struc-ture shown in Scheme 1) will affect the electronic dis-tribution within that nucleus. The substituents constant values can be quantitatively divided into the sum of Figure 2. The effect of nonqueous aprotic solvent (acetonitri-le) and 30 % acetonitrile containing Et4NBF4 on the cyclic voltamograms of estragole at platinum electrode.
independent inductive and resonance contributions.29 The following peak potentials are reported for substitut-ed 4-allylbenzenes in LiClO4/ CH3CN (w(CH3CN) = 100 %): hydroxyl +1.16 V, methoxyl +1.64 V and methylenedioxy +1.49 V. (Figure 3) Eugenol is oxi-dized more easily than the estragole and safrole. The same substituents at 4-propenylbenzenes have resonance effect because involves interaction between double bond and benzene ring, and give three peaks. Figure 4 demonstrates the effect of the hydroxyl and methoxyl substituents on the oxidation of isoeugenol and trans-anethole and both have three peaks.
The vibrational spectroscopic features of fragranc-es demonstrate the relationship between substituents and
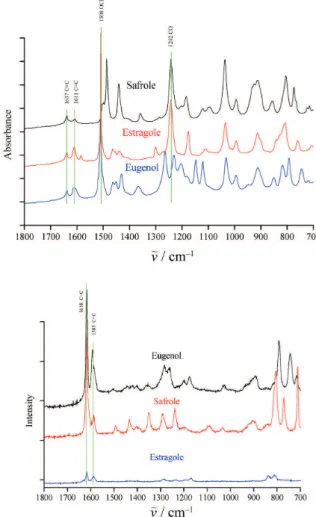
vinyl double bond. Both ATR-IR and Raman have C = C aromatic and conjugate bands about of 1600 cm–1 (Figure 5(a) and (b)).The ATR-IR spectra of 4-allylbenzenes (safrole, estragole and eugenol) have
Figure 5. (a) ATR-IR spectroscopy for structure of 4-allyl-benzenes (safrole, estragole ansd eugenol) (b) Raman spectro-scopy for structure of 4-allylbenzenes (safrole, estragole and eugenol)
Figure 3. The effect of substituted 4-allylbenzences at platinum electrode in nonqueous aprotic solvent (acetonitrile) containing lithium perchlorate (LiClO4/100%CH3CN).
Scheme 1. The fragrance structure of eugenol, estragole, safrole, isoeugenol, α-asarone , and trans-anethole.
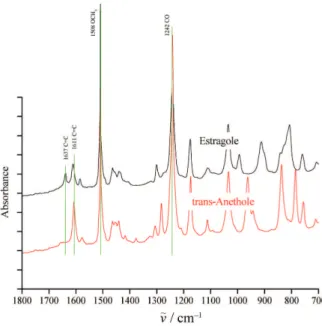
highly similar characteristics because of the presence of the same weak bands 1611 cm–1 for ν(C = C)ar and 1637 cm–1 for ν(C = C)vin, strong band 1508 cm–1 for ν(–OCH3) in estragole and eugenol, and strong band 1242 cm–1 for ν(C–O). On the other hand, the Raman spectra of these compounds show quite similar strong bands at 1584 cm–1 for ν(C = C)ar and 1618 cm–1 for ν(C = C)vin. However, from Figure 6 shows 1637 cm–1 for (C = C)vin in trans-anetholeis not apparent because of the resonance effect between the double bond and the benzene ring.
Fragrances on the Platinum and Carbon Paste Electrodes
pH Effects
The Ep and il in the Sampling DC voltammetric oxida-tion over a wide pH range are found to substantially vary from each other. The Ep and il obtained in the pres-ent work on the platinum and carbon electrodes are listed in Table 2. Notes, the Ep decreasing with an in-creasing pH does not obviouslly change in weak acidic media (pH = 2.93 – 5.39), but significantly decreased above pH = 6.14. Safrole and estragole differing from the other fragrances have two peaks above pH = 6.14 in alkaline media. However, there are two discrepancies between the voltammetric behaviors on the platinum (Pt) and carbon paste electrodes (CPE): (1) A higher value of limiting current (il) in strong acidic media (pH = 2.93 – 3.89) at CPE; but higher il in weak acidic media (pH = 6.18 – 6.83) at Pt; (2) Ep decreasing with an in-creasing pH at Pt is clearly more regular than that CPE. Our analyses of the effect of pH and supporting electro-lytes on the oxidation peak current and peak potential of fragrances in acidic solutions (pH = 2.19 – 6.83) and in the electrolyte of lithium perchlorate (pH = 6.04) showed the peaks shifted to a less to positive potential in acetate buffer and that the peak current in phosphate buffer (pH = 2.19) was higher than in the others (Figure 7). This indicates the oxidation of fragrances is strongly pH-dependent. The Ep–Ep/2 (Ep/2 = half- peak potential) values in pH = 2.93 – 8.81 at Pt and CPE electrodes are shown in Table 3. Ep–Ep/2 gave a range of 90–190 mV and 80–170 mV in acidic media at the Pt and CPE elec-trodes respectively. For a reversible charge transfer, the Ep–Ep/2 should be around 60 mV (Ep–Ep/2 = 47.7 mV /αna at 298 K). Hence, it may be concluded the mechanism for the oxidation of fragrance is an irreversi-ble charge transfer at both Pt and CPE electrodes in acidic media. At a pH below 6.14, only one-electron peak was observed since the second one-electron step is obscured by hydrogen evolution. The α-Asarone and eugenol un-dergoes oxidation in two steps (two one-electron), which are observed at pH above 6.83 and shown in Table 3.
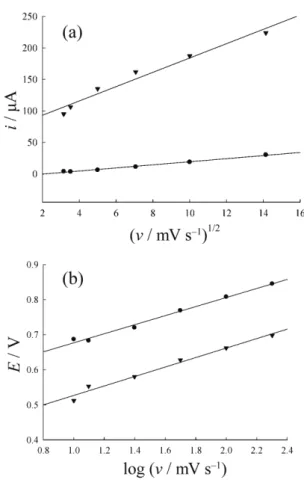
The effect scan rate on the electrooxidation of isoeugenol was examined in pH = 6.14 in the range of
10 mV/s to 800 mV/s. In this case the oxidative peak current was proportional to the square root of the scan rate on Pt and CPE electrodes, respectively. From Fig-ure 8 A, good linearity of the regression equation being y = 11.4 x + 70.3, the correlation coefficient r = 0.9900 for Pt electrode; y = 2.45 x – 5.27, the correlation coef-ficient r = 0.9961 for CPE. Under these conditions the currents were diffusion controlled. The relationship between peak potential and the logarithm of the scan rate (Figure 8(b), y = 0.14 x + 0.39, the correlation coef-ficient r = 0.9906 for Pt electrode; y = 0.13 x + 0.55, the correlation coefficient r = 0.9950 for CPE) can be used to roughly estimate the number of electrons involved in the catalytic oxidation.
Structure and Reactivity
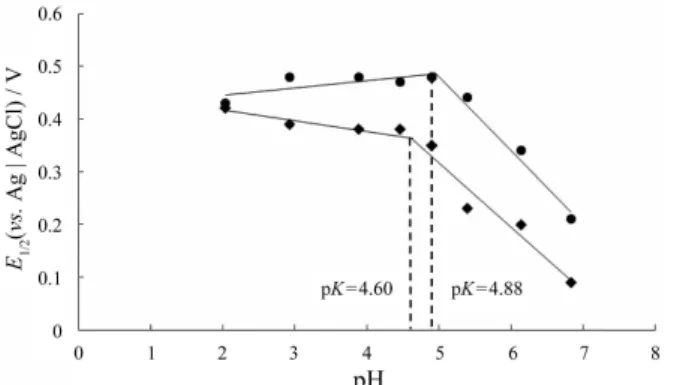
The two linear portions [plotted for (pK–1) > pH > (pK+1)] intersect at a pH value corresponding to pK. The E1/2 and pH values were input into the computer and using the simple regression method of the regres-sion analysis the best two equations were found. These were solved to find the pK values. The E1/2 vs. pH plot of α-asarone on Pt (pK = 4.60) and CPE (pK = 4.88) electrodes were given in Figure 9. By using the substit-uent constant (δH) value of trans-anethole as 0, the other substituent constants (δx) of fragrances calculated from pKH– pKx are –0.30, –0.40, –0.57, –2.0, –1.0, for eugenol,
α-asarone, safrole, estragole, and isoeugenol, respective-ly. Most of the data may be correlated by a modified Hammett equation, E1/2 = ρδx, ρ(–1.15) is a voltammetric reaction constant. The Hammett δ-ρ linear free-energy relationship is useful for evaluating substituent effects in a system. The rate of oxidation is greatly increased by the electron-donating substituent (–OH and –OMe).
L.-H. Wang
et
al.
,
Electroch
emical Oxida
tion o
f Fragr
ances Allyl and
4-Propen
ylbenzen
88
(2015)
35.
α–Asarone trans–Anethole Isoeugenol Safrole Estragole Eugenol
pH Pt CPE Pt CPE Pt CPE Pt CPE Pt CPE Pt CPE
Ep/ V ip/ A Ep/ V ip/ A Ep/ V ip/ A Ep/ V ip/ A Ep/ V ip/ A Ep/ V ip/ A Ep/ V ip/ A Ep/ V ip/ A Ep/ V ip/ A Ep/ V ip/ A Ep/ V ip/ A Ep/ V ip/ A
2.93 0,49 –43 0,59 –106 – (a) – (a) – (a) – (a) 0,38 –7,82 0,31 –64,5 0,87 –36,7 0,76 –24,31 1,09 –89,3 0,85 –36,7 0,47 –11,8 0,35 –75,3
3.89 0,45 –33,4 0,6 –90 0,4 –18,2 0,77 –1,92 0,32 –7,59 0,31 –62,3 0,86 –24,1 0,67 –24,2 1,12 –104 0,91 –13,4 0,44 –11,1 0,36 –54
4.46 0,48 –51,2 0,6 –84,8 0,38 –36,7 0,77 –0,13 0,32 –12,2 0,27 –14,7 0,89 –41,6 0,66 –9,34 1,1 –67,3 0,94 –30,6 0,45 –5,55 0,3 –45,4
5.39 0,29 –24,6 0,57 –46,6 0,32 –53,6 0,94 0,3 0,3 –15 0,38 –13,5 0,84 –47,5 0,63 –17,2 0,9 – (a)
–44,3 – (a)
0,58 0,89
–6,94 –27,4
0,38 –4,12 0,35 –46,1
6.14 0,26 –20,7 0,48 –48,9 0,33 – (a)
–65,7 – (a)
0,56 0,97
0,28 4,08
0,3 –91 0,35 –19,5 0,9 –31,6 0,62 –9,98 0,91 –57,7 – (a) – (a) 0,34
0,8 –65,6 –72,5
0,3 – (a)
–53,9 – (a)
6.83 0,22 0,76
–47,1 –44,2
0,28 0,55
–15,9 –39,9
0,32 0,95
–91 –138
0,53 0,91
–0,73 –0,23
0,22 0,8
–69,5 –56,1
0,32 – (a)
–13,9 – (a)
0,9 –32,1 0,58 –15,5 – (a) – (a) – (a) – (a) 0,26 0,74
–31 –40,7
0,3 –55,1
7.46 0,53 0,84
–17,4 –13,9
0,44 0,78
–70,3 –67
0,37 0,91
–88,7 –118
0,72 1,02
2,15 4,94
– (a) – (a) 0,29 –15,4 0,91 –13,4 0,56 –18,5 – (a) – (a) – (a) – (a) 0,25 0,65
–27,3 –33,2
0,22 0,55
–51,8 –34,6
8.27 0,6 0,8 1,11
–5,19 –9,12 –15,7
0,65 0,77
–45,4 –49
– (a) – (a) – (a) – (a) – (a) – (a) – (a) – (a) 0,93 –11,7 0,54 –6,91 – (a) – (a) – (a) – (a) – (a) – (a) 0,2 0,52
–46,4 –28,1
The positive potential order is estragole > safrole > eugenol due to the –OH strongly activating group than –OMe in the para-position. However, the overall rate enhancement arises from a sum of the groups’ inductive and resonance effects. Therefore, 4-propenylbenzenes (isoeugenol, α-asarone, and trans-anethole) have both inductive and resonance effects. The same substituents in the 4-allyl (eugenol) and 4-propenylbenzenes (isoeu-genol), when there is isoeugenol through-resonance between a reaction site that becomes electron-rich. Thus, the potential of isoeugenol shifts is less positive Figure 7. The effect of pH on the voltamograms for 9.09 × 10 –4 M of trans-anethole on platinum electrode and scan rate 50 mV/s. Britton-Robinson buffer solution (pH = 6.14, black line); LiCLO4 (pH = 6.04, red line); acetate buffer (pH = 4.29, green line); phosphate (pH = 2.09, yellow line); phosphate (pH = 6.67, blue line).
Table 3. Comparative linear sweep voltammetric behavior of fragrances in Britton-Robinson buffer on platinum (Pt) and carbon paste electrodes (CPE) where Ep is peak potential, and Ep/2 is half-peak potential
α-Asarone Eugenol
pH Pt CPE Pt CPE (Ep–Ep/2) / mV (Ep–Ep/2) / mV (Ep–Ep/2) / mV (Ep–Ep/2) / mV
2.93 180 180 100 90
3.89 190 190 100 90
4.46 170 190 100 80
5.39 140 190 100 80
6.14 90 160 170 100
6.83 120 170
140 100
130 170
80 – (a) 7.46 50
40
100 100
140 160
80 – (a) 8.27 70
60
50 160
– (a) – (a)
80 – (a) 8.81 70
60
70 120
– (a) – (a)
– (a) – (a) (a)
Not determined
than that of eugenol. The same as substituents of trans -anethole and eatragole in the benzene, likewise the potential of trans-anethole shifts less positively than that estragole.
CONCLUSION
The fragrances of the ally and propenyl derivatives of phenol and phenol ethers have similar of the irreversible oxidation potentials, and their potential is closely de-pendent on the structural factors. Thus, compared with various electron-donating groups and conjugation re-sults on platinum and carbon paste electrodes.
Acknowledgements. This work was financially supported by a grant from the National Science Council of the Republic of China (NSC 96-2113-M-041-003-MY3).
REFERENCES
1. D. Belsito, D. Bickers, M. Bruze, P. Calow, H. Greim, J. M. Hanifin, A. E. Rogers, J. H. Saurat, I. G. Sipesi, and H. Tagamij, (2010) S1–S46.
2. Jan C. R. Demyttenaere, Ch. 22, in: K. Hüsnü C. Başer, and G. Buchbauer (Eds.), Handbook of Essential Oils, Boca Raton, London, New York, CRC Press/Taylor & Francis, 2010, pp. 917–948.
3. I. A. Southwell, M. F. Russell, and N. W. Davies Flavour Fragr. J.
26 (2011) 336–340.
4. T. Karunasekara and C. F. Poole (2012) 159–165.
5. S. Furlanetto, S. Orlandini, I. Giannini, B. Pasquini, and S. Pinzauti 83 (2010) 72–77.
6. T. Shibamoto and S. Mihara Journal of Toxicology, Cut.& Ocular Toxico.2 (1984) 275–276.
7. H. H. Schunk, T. Shibamoto, H. K.Tan, and C. I. Wei Dev. in Food Sci.18 (1988) 1045–1068.
8. S. Mihara and T. Shibamoto30 (1982) 1215–1218.
9. Y. G. Davcheva, S. K. Ivanov, Z. D. Kalitchin, and S. A. Ivanov
Oxid. Commun.17 (1994) 17–23.
10. H. C. Chiang and S. Y. Li J. Chin. Chem. Soc.25 (1978) 141– 147.
11. G. Lear30 (1977) 1133–1136.
12. Y. H. Kuo, L. H. Chen, and L. M.Wa 39 (1991) 2196–2000.
13. K. Eskin (1979) 609–610.
14. E. M. Elgendy and S. A. Khayy44 (2008) 823–829.
15. L. H. Wang, C. C. Wang, and S. K. Chuang Asian J. Chem.22 (2010) 3835–3842.
16. L. H. Wang and J. C. Che (2011) 88–94. 17. A. Cihaner, H. N. Testereci, and A. M. Onal37
(2001) 1747–1752.
18. A. Ciszewski and G. Milczarek Electroanalysis13 (2001) 860– 867.
19. C. Demaille and A. J. Bard53 (1999) 842– 848.
20. X. J. Tang, J. J.Liang, X. Yan, and P. H. Li Huaxue Gongchengshi
22 (2008) 59–62.
21. I. M. Osadchenko and A. P. Tomilov79 (2006) 2035–2036.
22. Q. L. Zhong, X. H. Zhang, X. Q. Su, L. Zhang, Y. L. Liu, B. Ren, and Z. Q. Tian Wuli Huaxue Xuebao20 (2004) 94–97. 23. I. Barba, R. Chinchilla, and C. Gom55 (1990)
3270–3272.
24. D. T. Sawyer and J. L. Roberts, Experimental electrochemistry for chemists, John Wiley & Son, Inc., 1979, p. 184.
25. C. Mousty and G. Mousset New J. Chem.16 (1992) 1063–1070. 26. M. Blazquez, J. M.; Rodriguez-Mellado, and J. J. Rui
(1985) 1527–1532.
27. M. Iguchi, A. Nishiyama, Y. Terada, and S. Yamamur (1979) 1079–87.
28. P. Zuman and C. L.; Perrin, Organic polography, John Wiely & Son., 1969, p. 292.
29. C. D. Johnson, The Hammett equation, Cambridge University Press, 1973, p.11.