Гени за имуноглобулин и Т-ћелијски рецептор као
молекуларни маркери код деце с акутном
лимфобластном леукемијом
Jelena Lazić1, Lidija Dokmanović1, Nada Krstovski1, Jelica Predojević2, Nataša Tošić3, Sowa Pavlović3, Dragana Janić1
1Služba za hematologiju i onkologiju, Univerzitetska dečja klinika, Beograd, Srbija; 2Dečja klinika, Kliničkobolnički centar, Bawa Luka, Bosna i Hercegovina; 3Institut za molekularnu genetiku i genetičko inžewerstvo, Beograd, Srbija
UVOD
Akutnalimfoblastnaleukemija(ALL)jejedno odnajčešćihmalignitetadečjeguzrasta.Nastaje klonskomproliferacijomlimfoblasta,nezre lihprekursorskihćelijalimfoidnelozekojisu prethodnopodleglimalignojtransformaciji[1]. Zahvaqujućisavremenimprotokolima,intrate kalnojprimenilekovaizračewucentralnogner vnogsistema(CNS),danassekodoko80%deces ALLpostižedugotrajnakliničkaremisija,ko jajedefinisanaprisustvommaweod5%blastau kostnojsrži.Međutim,sobziromnatodasekod 1015%decerazvijerecidivbolesti,smatrase dakonvencionalnamorfološkaremisijamože bitiudruženasrezidualnimblastima,kojisu najčešćiuzrokponovneaktivacijebolesti[2].
Otkrivaweipraćewerezidualnihmalignih ćelijasenazivaminimalnarezidualnabolest (MRB)ipredstavqanajnižimerqivinivobo lesti[2].Kaonezavisniprognostičkifaktor, MRBomogućavastratifikacijunovihgrupari zikaipružaosnovuzapotpunoindividualni terapijskipristup[3].Odabirbolesnikapre magenetskomprofilubolestiomogućavapodelu nagrupukojazahtevaintenzifikacijuterapijei nagrupugdeseprimewujustandardniprotokoli lečewasasmawenimdozama,čimeseneugrožava ishodlečewa,aizbegavaseizlagawemnogimtok sičnimdejstvimacitostatika[4].
Tokomposledwedvedecenijezabeleženisupo kušajidaserazličitimmetodamaotkrijeipra tiMRB.MetodaizborazaMRBtrebadazadovoqi nekolikovažnihkriterijuma:senzitivnostod najmawe103,sposobnostrazlikovawamalignih
odnormalnihćelija,postojawestabilnihleuke mija–specifičnihmarkera,lakustandardizaci ju,brzodobijawerezultataimogućnostkvanti fikacije[5].Jednaodmetodajelančanareakcija umnožavawaDNK(engl.polyme ra se chain re ac tion – PCR).PCResejjesposobandaotkrijejednuleu
kemijskiizmewenućelijumeđumilionnormal nihćelija[6].ZapraćeweMRBkoristesemeto dekvalitativnaRT-PCR(engl.re ver se tran scrip ta se PCR)ikvantitativnaRQ-PCR(engl.real-ti me qu-an ti ta ti ve PCR),zasnovanenaotkrivawufuzionih
transkripataiantigenreceptorgena.
MRBuALLpodrazumevaotkrivaweipraćewe strukturnihfuzionihaberacijairearanžmana genskihlokusaimunoglobulina(IgH)iTćelij
skogreceptora(TCR).NasvojojpovršiniBlim
focitieksprimirajuimunoglobulin,aTlimfo
ci ti TCR,kojiprepoznajuantigene.Svakiklon
BiliTlimfocitanosireceptorjedinstvene specifičnostiodređenekombinacijomV(engl. va ri a ble–promenqiv),D(engl.di ver sity–razno
vrstan)iJ(engl.jo i ning–spajajući)genskihseg
menata,delovimalokusa,kakozaimunoglobulin, takoizaTCRzaantigen.Velikirepertoarraz
ličitihspecifičnihmestazaantigenpostiže
KRATAK SADRŽAJ
Uvod Akut na lim fo blast na le u ke mi ja (ALL) je ma lig na klo nal na bo lest i jed na od naj če šćih neo pla zmi deč jeg uz ra sta. Pri me nom sa vre me nih pro to ko la po sti žu se vi sok ste pen re mi si je i du go go di šwa sto pa pre ži vqa va wa. Uvo đe wem me to da mo le ku lar ne ge ne ti ke omo gu će ni su sub mi kro skop ska kla si fi ka ci ja i pra će we mi ni mal ne re zi du al ne bo le sti (MRB), od go vor ne za na sta nak re ci di va.
Ciq ra da Ciq ra da je bi lo is pi ti va we uče sta lo sti re a ran žma na gen skih lo ku sa za te ške lan ce imu no glo bu li na (IgH) i Tće lij ski re cep tor (TCR) i wi ho va ko re la ci ja s kli nič kim pa ra me tri ma.
Me to de ra da Is pi ta no je 41 de te obo le lo od ALL ko ji ma je na di jag no zi ra đe na mo le ku lar na de tek ci ja re a ran žma na gen skih lo ku sa za IgH i TCR me to dom lan ča nog umno ža va wa DNK (PCR). Po sma tra we raz vo ja MRB je ra đe no u in duk ci o noj fa zi le če wa, ka da se oče ku je mor fo lo ški od go vor na te ra pi ju.
Re zul ta ti Re a ran žman gen skog lo ku sa za IgH za be le žen je kod 82,9%, a za TCR kod 56,1% is pi ta ni ka. Po sle 33 da na in duk ci o ne te ra pi je re a ran žma ni za IgH gen ski lo kus su zabeleženi kod 39%, a za TCR kod 36,5% de ce.
Za kqu čak Ot kri va we ge net skih abe ra ci ja na mo le ku lar nom ni vou i wi ho va ko re la ci ja sa stan dard nim prog no stič kim pa ra me tri ma uka za la je na zna čaj no ve stra ti fi ka ci je ri zič nih gru pa, ko je zah te va ju pro me nu in ten zi te ta he mi o te ra pi je. Pra će we MRB se po ka za lo pre ci znim pre dik tiv nim fak to rom u na stan ku re ci di va bo le sti.
setokomsazrevawalimfocitakombinacijompojed noggenaizV, D i Jregiona.Varijabilnostsedodat
nouvećavadelecijomiinsercijomnukleotidaname stuspajawa,čimenastajesekvencajedinstvenazasva kiBiTlimfocitniklon[7].
TokomsazrevawaBiTlimfocitaodigravasepro cessomatskihrekombinacija,pričemusesekvenceod ređenogtipakombinujuistvarajugensakojegsepre pisujeinformacijazasintezuodgovarajućeglanca [8].Naovajnačingenerišeseogromandiverzitetan tigenskihreceptora,kojiseogledaupostojawuviše odbilion(1012)različitihklonovaBiTlimfoci
ta,svakisreceptoromrazličitespecifičnosti[9]. KlonspecifičniIgH i TCRrearanžmanisumar
kerispecifičnizatumoribolesnika,kojisemogu prepoznatinadijagnozivišeod95%pacijenatas limfoproliferativnimbolestima.Različitiklo nalniimunoglobulinskiiTCRrearanžmanimoraju
bititačnodefinisani,dabiserazlikovaliodslič nihpoliklonalnih,kojiseistovremenoswimaam plifikuju.Rearanžmaniimajuposebanznačajkaome tespecifičnezabolesnikazaotkrivaweipraćewe rezidualnebolestidecebezhromozomskihaberaci ja.Kodzdravihosobalimfocitnirepertoarjestabi lanipoliklonalan.Rekombinacijeseulimfoidnim progenitorimaodigravajupoodređenomhijerarhij skomredosledu,zbogčegaseuvišeod95%Bprekur sorskihALLmožeotkritiIgHrearanžman.
PotpunorearanžiraniIgHlokusinisuodlikais
kqučivoBleukemija,kaoštoseniTCRγrearanžma
nineotkrivajuiskqučivokodTmaligniteta.Znatan procenatBiTleukemijaseodlikujeistovremenom ekspresijomobarearanžiranalokusa[10].
AnalizomIgHrepertoaranadijagnoziseu3040%
BprekursorskihALLmožeotkritivišeodjedneklo nalnepopulacijeleukemijskihćelija.Oligoklonal nostsetakođemožeotkritisfrekvencijomodoko 10%zaimunoglobulinipribližno20%zaTCRgen
skelokuse.KadasuupitawuTALL,oligoklonalnost
TCRlokusasejavqakodmaweod5%,dokjeIgHuzorak
oligoklonalankod27%bolesnika[11].
Klonalnaevolucijajemodifikacijapostojećihre aranžmanakojomleukemijskiklondajevišepopula cijasubklonova.Poređeweshemeimunoglobulinai
TCRrearanžmananadijagnoziiurecidivubolesti
ukazujunapromenu,zavisnoodtipaleukemije,stepe nasazrevawaivrstesamogrearanžmana.Zbogmoguće pojavelažnonegativnihrezultata,stabilnostovih rearanžmanasemorauzetiuobzirkadaseonikori stekaomarkerizaposmatrawenapredovawabolesti. KakobarjedanrearanžiranIgH, TCRγ, od no sno TCRδ
alelostajestabilanu7590%BALL,odnosno90% TALL,preporučujeseposmatrawebardvamarkera, kolikojeidostupnokodvećinebolesnikasALL[12].
Rezultatimnogihstudijasupokazalidaseshema imunoglobulinskihiTCRrearanžmanamewasna
pretkombolesti.Sobziromnatodajekonfiguraci jagenskihlokusaspecifičanmarkerleukemijskeće lije,pomoćukojegseonamožeposmatrati,promenate konfiguracijeuvremenumožedaukažeinapromenu
samemalignećelijeiwenihbiološkihodlika.Od vijawerekombinacijanadostupnimimunoglobulin skimiTCRlokusimaukazujenatodajerekombina
tornisistemaktivan,amehanizmizatvarawahroma tinanefunkcionišukaokodnormalnihćelija.Sto gajemogućepretpostavitidainekidrugidelovige nomamogubitiotvorenizapristuprekombinaciji, usledčegamožedoćidonagomilavawamutacija.Ne keodwihmogubitiuzrokpromenefenotipaleuke mijskogklona,štonavodinazakqučakdajeklonalna evolucija,praćenakrozrearanžmaneimunoglobuli
na i TCR,modelnapretkabolesti[13].
CIQ RADA
Ciqradajebiodaseutvrdefrekvencijaiklonal nostrearanžmanaugenimazaIgH i TCRutrenut
kupostavqawadijagnozeiwihovakorelacijaskli ničkimistandardnimprognostičkimparametri mabolestikoddeceoboleleodALL.Posmatranisu irazvojMRB33.danaindukcionoglečewaikorela cijagenetičkihmarkeraspojavomrecidivaismrt nimishodom.
METODE RADA
Istraživawejeobuhvatilo41deteoboleloodALL (22dečakai19devojčica),uzrastaod1,9do16,5godina (medijanašestgodina),kodkojihjedijagnozaoboqe wapostavqenaizmeđu1.decembra2002.i1.jula2005. godine,anadgledanasudo1.avgusta2007.godine.Na Univerzitetskojdečjojklinici(UDK)uBeogradusu dijagnostikovanailečena32bolesnika,dokjekodde vetorodecedijagnozapostavqenanaUDK,alisuona lečenanaDečjojkliniciKliničkobolničkogcen trauBawaLuci.Ispitanicisuuprosekunadgleda nidvegodineitrimeseca(raspon0,753,7meseci).
Dijagnozajepostavqenanaosnovustandardnih citomorfoloških,citohemijskihiimunofenotip skihkriterijuma.
LečewejeizvedenopremaaktuelnomprotokoluALL IC-BFM 2002,kojisesastojioduobičajenihfazale
čewa:indukcije,konsolidacije,reindukcije(deter minisanarandomizacijom)ifazeodržavawa.Pro tokolnalažestratifikacijurizikauodnosunauz rast,inicijalnibrojleukocita,pozitivancitoge netskinalazt(9;22) i t(4;11),prisustvofuzionihge
na BCR/ABL i MLL/AF4,odgovornapronizonosmog
dana,odnosnoprocenatblastaukostnojsrži15.i 33.danalečewa.
MaterijalzaPCRanalizurearanžmanagenskih
lokusa(IgH i TCR)uzetjestandardnomaspiracijom
kostnesržiutrenutkupostavqawadijagnozei33.da nalečewa,kadaprotokolpredviđaulazakbolesnika umorfološkuremisiju.
nesržistandardnomfenolhloroformizoamilal koholnommetodom.
OtkrivaweIgHgenskihrearanžmanavršenojeme
todomPCR.Prajmerisukomplementarnisekvencama
kojeograničavajutrihipervarijabilnaregiona(CDR)
egzona,kojikodiravarijabilnidomenteškoglanca,i delevisokstepenhomologijeizmeđurazličitihV,od
no sno Jsegmenata.Naovajnačinomogućenojeumnoža
vawekompletnogIgHrepertoara.Normalanpoliklo
nalanuzorakzdravihindividuaotkrivasekao„smir” nadužiniizmeđu100i200bpipredstavqaveliki
brojtrakarazličitedužinekojepotičuodrazliči tihlimfocita.Pojavajednedominantnetrakeili višewihukazujenapostojaweklonalnihpopulaci jaćelijasidentičnimrearanžmanom,tj.VDJHkom
binacijomgenskihsegmenata(Slika1).Korišćenasu dvaparaprajmera,jersePCRamplifikacijaradiu
dvakoraka(engl.ne sted PCR):FR1 (5’-(G/C)AGGT(A/G) CAGCTG(G/C/T)(AT)G(GC)AGTC(G/A/T/C)G-3’) i J1H (5’-AC CTGAG GA GACGGTGAC CAGGGT-3’)–prvipar
prajmera;FR1 (5’-(G/C)AGGT(A/G)CAGCTG(G/C/T)(AT)
G(GC)AGTC(G/A/T/C)G-3’) i FR3A (5’-ACACGGC(C/T) (C/G)TGTAT TACTGT-3’)–drugiparprajmera[11].
Usloviamplifikacijesu:denaturacijana94°C(10
minuta),poslekojesledi35ciklusadenaturacijeta kođena94°C(15sekundi),anilingana55°C(20sekun
di)ielongacijena72°C(30sekundi).Reakcijaseza
vršavafinalnomelongacijomna72°C(petminuta).
OtkrivaweproizvodaPCRvršenojeelektroforezom
DNKnapoliakrilamidnomgelu.
OtkrivaweTCRγgenskihrearanžmanavršenoje
metodomPCR,prajmerimakojiomogućavajuumnoža
vawenajvećegdelaTCRγrepertoara.Normalanpoli
klonalanuzorakzdravihindividuaotkrivasekao „smir”nadužiniizmeđu400i500bp. Po ja va jed ne
dominantnetrakeilivišewihukazujenapostojawe klonalnihpopulacijaćelijasidentičnimrearanžma nom,tj.VDJγkombinacijomgenskihsegmenata(Sli
ka2).Ispitanicisadominantnomtrakomoznačeni sukaopozitivni,ito:sjednomkaomonoklonalni,sa dvebiklonalni,satritriklonalni,asavišeodtri kaooligoklonalni.Korišćenasudvaparaprajmera:
TCRγ1(5’-CTGTGA CA A CA AGTGTTGTTCCAC-3’) i
TCRγ2 (5’-GTGCTTCTAGCTTTCCTGTCTC-3’)–prvi
parprajmera;TCRγ3 (5’-GAG TACGCTGCCTA CA GA-GAGG-3’) i TCRγ4 (5’-CCACTGCCA A A GAGTTTCTT-3’)
–drugiparprajmera[14].
Usloviamplifikacijebilisu:denaturacijana 94°C(10minuta),poslekojesledi35ciklusadenatu
racijenatakođe94°C(45sekundi),anilingana65°C
(30sekundi)ielongacijena72°C(60sekundi).Reak
cijasezavršavafinalnomelongacijomna72°C(pet
minuta).OtkrivaweproizvodaPCRvršenojeelek
troforezomDNKnapoliakrilamidnomgelu.
REZULTATI
Kod70decejerađenoinicijalnootkrivawemoleku larnihrearanžmanazaIgH i TCRmetodomPCR,araz
vojMRBjeposmatranradibeležewaodgovoranapri mewenolečeweikorelacijeskliničkimprognostič kimfaktorima.Kodispitanihbolesnikabrojleuko cita(Le)ukrvnojslicinadijagnozijebiouprose
ku15400/mm3(opseg1300406400/mm3).CNSstatus1
(izostanakblastaulikvoruikliničkihznakovabo lestiCNS)ustanovqenjekod37dece(90,3%),CNS status2(netraumatskalumbalnapunkcijasamaweod petćelijapomikrolitruucerebrospinalnojtečno sti)utvrđenjekoddvadeteta(4,9%),dokjeCNSsta Сли ка 2. Ана ли за TCRγ-PCR про из во да на осмо по стот ном по ли-а кри лли-а мид ном ге лу
Figure 2. TCRγ-PCR analyzed products on 8% polyacrilamid gel 1 – здрав испитаник; 2 – пацијент са моноклоналним TCRγ; 3 – контрола PCR реакције; 4 – ДНК маркер (лествица од по 100 bp) 1 – healthy individual; 2 – patient with monoclonal TCRγ rearrangement; 3 – PCR reaction control; 4 – DNA marker (100 bp scale)
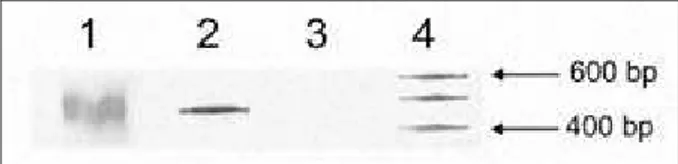
Сли ка 1. Ана ли за IgH-PCR про из во да на осмо про цент ном по ли-а кри лли-а мид ном ге лу
Figure 1. IgH-PCR analyzed products on 8% polyacrilamid gel 1 – здрав испитаник; 2 – ДНК маркер (лествица од по 100 bp); 3 – пацијент са моноклоналним IgH; 4 – пацијент са биклоналним IgH 1 – healthy individual; 2 – DNA marker (100 bp scale);
3 – patient with monoclonal IgH rearrangement; 4 – patient with biclonal IgH rearrangement
Rearanžmaniobagenskalokusaistovremenoutvr đenasukod19ispitanika(43,9%).Ugrupibolesnika
sa IgHrearanžmanom11dece(26,8%)jestratifikova
noustandardnirizik,18(43,9%)uintermedijarni,a petoro(12,2%)uvisokirizik.Međuispitanicimasa
TCRγrearanžmanomugrupiintermedijarnojrizi
kabiloje14dece(65,2%),doksuugrupistandardnog ivisokogrizikabilapočetiriispitanika(17,4%). PozitivnostnaBCR/ABLotkrivenajekodčetiriis
pitanika(11,8%)srearanžmanimaIgHgenskogloku
saipetispitanika(21,7%)saTCRγrearanžmanima.
NakonIafazeindukcionoglečewa,kod15dece(39%)
s IgHrearanžmanomotkrivenajeMRB:monoklonal
nostjezabeleženakodšestispitanika(14,6%),abi klonalnostioligoklonalnostkodpotri(7,3%).U grupibolesnikasaTCRγrearanžmanom,kod15dece
(65,2%)je33.danalečewauočenrezidualnimaligni klon.Monoklonalnostjezabeleženakod11ispita nika(47,8%),abiklonalnost,triklonalnostioligo klonalnostkodpojednogdeteta(4,3%)(Grafikon1). Kliničkaremisijajepostignutakod97,6%ispi tanika,ajednadevojčicajeumrlausledsepseprepro ceneremisije33.danalečewa.Ranimedularnireci divjedijagnostikovankodčetvorodece(9,4%).Smrt jenastupilakodtakođečetiriispitanika(9,4%):kod jednogdečakanakonrecidivabolesti,akodtride tetausledsepse.Transplantacijamatičnećelijehe matopoezejeurađenakodjednogdeteta,kodkojegjeot krivenBCR/ABLfuzionitranskript,kojijesadau
potpunojremisiji.
DISKUSIJA
UlečewuALLsepostiževisokstepenkliničkei morfološkeremisijekodvišeod95%dece,adugo godišwepreživqavawebezznakaaktivacijebolesti kodoko80%[15].Međutim,iporedprimenesavreme nihprotokola,kodoko15%bolesnikasejavqareci divbolesti,zaštasesmatraodgovornomMRB,kojase nemožeotkritikonvencionalnimmikroskopskim Табела 1. Клиничке одлике испитаника с откривеним генским
реаранжманима
Table 1. Clinical characteristics of patients with detected gene re-arrangements
Параметар Parameter
Генски реаранжмани
Gene rearrangements
IgH TCR
Укупан број испитаника
Total number of patients 34 23
Пол Sex
Мушки
Male 18 9
Женски
Female 16 14
Узраст (године) Age (years)
<6 19 9
≥6 15 14
Број леукоцита Number of leukocytes
<20000 23** 12
≥20000 11 11
Хепатомегалија Hepatomegaly
Да / Yes 21 12
Не / No 13 11
Спленомегалија Splenomegaly
Да / Yes 15 14
Не / No 19 9
Статус ЦНС CNS status
1 31 21
2 1 1
3 2 1
Имунофенотип Immunophenotype
B 26* 15
T 8 8
Морфологија бласта Morphology of blasts
L1 13 7
L2 21 16
Апсолутни број бласта 8. дана Absolute blast count on day 8
<1000 30 20
≥ 1000 4 3
Костна срж 15. дана Bone marrow on day 15
M1 25 17
M2 8 5
M3 1 1
Костна срж 33. дана Bone marrow on day 33
M1 32 22
M2 -
-M3 1 1
* p<0.05; ** p<0.01
36,5% 56,1%
39% 82,9% 40
35
30
25
20
15
10
5
0
IgH TCR
33. дан терапије Therapy day 33 Дијагноза On set
Графикон 1. Брзина елиминације малигног клона код деце са IgH и TCRγ реаранжманима
Graph 1. Speed of elimination of malignant clone in children with IgH and TCRγ rearrangements
tus3(kliničkiznaciinfiltracijeCNSimaweod petćelijapomikrolitruulikvorusapredominaci jomblastanacitospinu)takođeustanovqenkoddva deteta(4,9%).FABklasifikacijajepokazaladajepod
tipL1biozastupqenkod16dece(39%),doksumor
fološkeodlikepodtipaL2zabeleženekod25dece
(61%).ImunofenotipkojiodgovaraBćelijskojli nijijeutvrđenkod29dece(70,7%),dokjekod12de ce(29,3%)zabeleženaekspresijaantigenaspecifič nihzaTćelijskuliniju.Premapredviđenojstrati fikacijirizika,ustandardnirizik(SR)jesvrsta no12dece(29,3%),uintermedijarnirizik(IR)22 (53,7%),auvisokrizik(VR)sedamispitanika(17%).
Ugrupiod41ispitanikarearanžmanzaIgHgenski
lokusjezabeleženkod34deteta(82,9%):monoklonal nostkod17dece(41,5%),biklonalnostkod14(34,1%), triklonalnostkodjednogdeteta(2,4%)ioligoklo nalnostkodtriispitanika(7,3%).Uistojgrupiis pitanikarearanžmanzaTCRγgenskilokusjezabele
ženkod23deteta(56,1%):monoklonalnostkod20is pitanika(48,8%),biklonalnostkodtri(7,3%)itri klonalostkodjednogdeteta.Kliničkeodlikedecesa
metodama[16].Intenzifikacijaterapijepremagrupi rizikajevećprinciplečewaumnogimprotokoli manacionalnihimeđunarodnihkooperativnihgru pa[17].Mnogestudijeukazujunatodaseusmeravawem lečewapremavišestrukimprognostičkimfaktori mapostižepozitivanishodiznatnosmawujetoksič nostterapije[18].Otkrivawefuzionihgena,kaone zavisnihmolekularnihmarkera,pomoglajerazume vawunastankaleukemija[19]idirektnomusmerava wulečewa.Međutim,sobziromnatodasestruktur nehromozomskeaberacijejavqajukodtrećinedeces ALL[20],otkrivaweipraćewerearanžiranihgena zaIgH i TCRstvarajuuslovezapreciznustratifi
kacijirizikakodsvihbolesnika.
AnalizomIgHrepertoaranadijagnoziseu3040%
BprekursorskihALLmožeotkritivišeodjedne klonalnepopulacijeleukemijskihćelija.Kadasuu pitawuTALL,oligoklonalnostTCRlokusasejavqa
kodmaweod5%,aIgHlokusakod27%bolesnika[21].
Sdrugestrane,TCRrearanžmanisemoguotkritikod
3080%BprekursorskihALL.Ovafrekvencijajeu vezisastepenomsazrevawamaligneBćelije,jeršto jemawazrelostmalignoalterisanećelije,mawijei procenatotkrivawa,dokseIgHrearanžmanijavqa
juu1020%TALL.
Unašemistraživawu,rearanžmanzaIgHgenski
lokusjezabeleženkod82,9%ispitanika,ito:mono klonalnapopulacijaleukemijskiizmewenihćeli jakod41,5%,biklonalnakod34,1%,triklonalnakod 2,4%,aoligoklonalnakod7,3%.RearanžmanTCRγgen
skoglokusajezabeleženkod56,1%dece,odčegamono klonalnostkod48,8%,biklonalnostkod7,3%,atri klonalostkod2,4%bolesnika.
Kaoštosezastrukturnehromozomskeaberacije postavqapitawerazličitostiuodnosunaetničku osnovu,takoseraspodelaiklonalnostIgH i TCRre
aranžmanaispitujuuodnosunageografskoporeklo ilisocioekonomskeusloveživota.Skrideli( Scri-de li)iTone(To ne)[22]susumirawemrezultatabroj
nihmulticentričnihstudijazakqučilidasuinci dencijaiosobineanaliziranihrearanžmanaraz ličitiuodnosunaetničkupripadnostisocioeko nomskistatus.
MeđuispitanicimasaIgH i TCRrearanžmanima
genskoglokusakod7,3%decejeotkrivenBCR/ABLfu
zionitranskript.Ovajpodatakjeodsuštinskeva žnosti,jermewakliničkuslikuovihbolesnika,kao iočekivaniodgovornalečeweukoristustanovqe netranslokacije.Uobegrupedece–saIgH i TCRre
aranžmanom–višejebilodečaka,kojisuuproseku biliuzrastaod5,6do6,8godina,stimštojeugru piispitanikasaTCRrearanžmanombilovišede
cestarijeodšestgodina.
KodispitanikasaTCRrearanžmanombilajeoče
kivanovećamedijanabrojaleukocita,jerjeinicijal nopovišenavrednostleukocitapovezanasaTimu nofenotipom.
Uobegrupeispitanikabiojedominantanmor fološkipodtipL2,kaoiBimunofenotipblasta (76,5%prema65,2%),štodokazujedapotpunorearan
žiraniIgHlokusinisuodlikaiskqučivoBleuke
mija,odnosnoTCRrearanžmaniTleukemija.Vander
Velden(Van der Vel den)isaradnici[23,24]supotvr
dilidaseznatanprocenatBiTleukemijaodlikuje istovremenomekspresijomobarearanžiranalokusa.
Uobegrupenašihispitanikazabeleženjeloš odgovornapronizonosmogdanalečewa(11,8%pre ma13%),alisu,izuzevjednogdeteta,tobilibolesni cisatranslokacijomBCR/ABL.Takođe,kodjednogde
tetajeizostalakliničkaimorfološkaremisija33. danalečewa,štoseobjašwavawegovomPhpozitiv
nošćuiistovremenimotkrivawemrearanžmanaza
IgH i TCRγgenskilokus.
PoređeweshemeIgH i TCRrearanžmananadijag
nozitokompraćewaMRBiurecidivubolestiuka zujunapromene,itozavisnoodtipaleukemije,ste penasazrevawaivrstesamogrearanžmana.Kampana (Cam pa na)[25]jezakqučiodajeovakavrezultatpo
sledicapromeneIgH i TCRrearanžmanasnapredo
vawembolesti,štojeuočenoiunašemistraživawu. Konfiguracijagenskihlokusajespecifičanmarker leukemijskećelije,tesvakapromenauvremenuukazu jeinapromenusamemalignećelijeiwenihbiolo škihosobina[26].
Klonalnaevolucijajevažnaodlikaagresivnih Bprekursorskihleukemija,jerjeposmatrawemraz vojaMRBtokomrecidivabolestiuočenodajezna čajnovišedecekodkojejenapočetkubolestiusta novqenaoligoklonalnost.VanDongen(Van Don gen)
isaradnici[27]usvomraduobjavqenomjoš1998.go dinenavodedasudecakojazadržavajuoligoklonalnu populacijućelijatokomlečewaznatnokraćeuremi sijibolestiidajekodwihrizikodnastankasmr tidesetputaveći,štose,bezobziranamawiuzorak ispitanika,možeodnositiinabolesnikenašegis traživawa.Bjondi(Bi on di)isaradnici[28]suzakqu
čilidapraćeweMRBuranimfazamalečewaukazu jenapočetniodgovornaterapiju,hemiosenzitiv nostiklonalnostpreostalihblasta,štoje,usluča jupozitivnognalaza,prediktivnifaktorzanasta nakrecidiva;tojepotvrđenoiunašojstudiji.Među našimispitanicimakodkojihjeotkrivenaoligo klonalnostIgHrearanžiranihlokusa,kod7,3%wih
jedošlodorecidivabolesti,a11,8%jepreminulo, dokjemeđudecomsaTCRγrearanžmanomgenskoglo
kusarecidivdijagnostikovankod13%,asmrtniis hodkod17,4%bolesnika.
ProizvodidobijeniprimenomPCRmetodestva
rajudominantansignaluklonalnojproliferaciji uodnosunapostojeći,kojipotičeodnormalnepoli klonalnepopulacijelimfocita[29].Osetqivostot krivawazavisiodapsolutnogbrojazastupqenihleu kemijskihćelijaiapsolutnogbrojaBiTlimfocita ukostnojsrži,štopredstavqarazlogzakvantifi kacijuPCRproizvoda,jerpodatakoklonalnostini
jedovoqnosnažanprediktivnifaktor[30].Mono klonalniIgH i TCRgenskirearanžmanimoguseot
kritisasenzitivnošćuod0,51,5×101izperiferne
krvi,odnosno6×104izaspiratakosnesrži,štodi
Rezultatimnogihstudijapokazujudasekodoko50% decemožeotkritiamplifikovanPCRproizvodrea
ranžiranihIgH i TCRgenskihlokusanakrajuinduk
cije.Tadecasusigurnikandidatizanastanakreci divabolesti,arizikjeproporcionalanizmerenom nivouMRB[32].Stratifikacijarizikapremaovako ustanovqenimgenetskimmarkerimaomogućavaodre đivaweintenzitetaterapije,štoihčininezavisnim prognostičkimfaktorima.Individualnousmerava weterapijeomogućavasmawewetoksičnosticitostat skihagensakoddecesranomibrzomcitoredukcijom. Nastanakleukemijajedinamičanproceskojiuslo vqavatokbolestistalnimpromenamaupopulaci jimalignihćelija,dovodećidogenerisawaklonal nefenotipskepromenqivosti,čimedirektnoutiče naishodbolesti[33].Zahvaqujućiuvođewumoleku larnegenetikeukliničkudijagnostiku,otkrivasei pratipromenqivostmalignihćelija,kojajeodgovor nazavisokoproliferativne,agresivneirezistent nesubklonove[34,35].
ZAKQUČAK
RearanžmanIgHgenskoglokusaseotkrivakodčeti
ripetinebolesnika,aTCRγgenakodvišeodpolo
vine.Uobegrupebolesnikadominantnajemonoklo nalnapopulacijaleukemijskiizmewenihćelija(kod skoro50%dece),dokjeoligoklonalnapopulacijanaj mawezastupqena.Očekivanovećamedijanabrojale ukocitasebeležikoddecesaTCRγrearanžmanom
zbogkorelacijesaTimunofenotipom.Istovreme naekspresijaobarearanžiranagenskalokusajebi lačešćakoddecesaBimunofenotipom.Uobegru peispitanikanepovoqanishodbolestijebiopre dominantankoddecekodkojejeistovremenootkri veniFiladelfijahromozom.Izrazitovisokproce natulaskaukliničkuremisijujezabeleženkodsvih ispitanika,dokjeprocenatrecidivaismrtnogis hodausledrecidivanajmawikoddecebezgenetskih mutacija.Ovakavrezultatnesumwivoukazujenazna čajpočetnogotkrivawaiposmatrawamolekularnih markeratokomlečewa.
NAPOMENA
Ovajčlanakjerezultatradanaprojektubroj143051 MinistarstvazanaukuitehnološkirazvojRepu blikeSrbije.
LITERATURA
1. Greaves M. Childhood leukaemia. BMJ. 2002; 342(7332):283-7. 2. Cazzaniga G, Biondi A. Molecular monitoring of minimal residual
disease. In: Pui CH. Treatment of Acute Leukemias. New Jersey: Humana Press; 2003. p.537-547.
3. Cave H, van der Werff ten Bosch J, Suciu S, Guidal C, Waterkeyn C, Otten J, et al., for the European Organization for Research and Treatment of Cancer–Childhood Leukemia Cooperative Group. Clinical significance of minimal residual disease in childhood acute lymphoblastic leukemia. N Engl J Med. 1998; 339(9):591-8. 4. Stock W, Estrov Z. Studies of minimal residual disease in acute
lymphocytic leukemia. Hematol Oncol Clin North Am. 2000; 14(6):1289-305.
5. Netto GJ, Saad RD, Dysert PA. Diagnostic molecular pathology: current techniques and clinical applications. BUMC proceedings 2003; 16:379-83.
6. Gribben JG. Minimal residual leukemia. In: Nathan DG, Orkin SH, Ginsburg D, Look AT. Nathan and Oski’s Hematology of Infancy and Childhood. Vol 2. Philadelphia: W.B. Saunders; 2003. p.1259-1273. 7. Virella G. Immunoglobulin structure. In: Virella G. Medical
Immunology. 6th ed. New York: Informa Healthcare; 2007. p.55-64. 8. Burmester GR, Pezzutto A. Color Atlas of Immunology. Stuttgart:
Thieme; 2003.
9. Kuby J. Antibodies – structure and function. In: Goldsby RA, et al. Immunology. 5th ed. New York: WH Freeman; 2002. p.76-88. 10. Szczepanski T, Beishuizen A, Pongers-Willemse MJ, Hählen K, van
Wering ER, Wijkhuijs AJ, et al. Cross-lineage T cell receptor gene rearrangements occur in more than ninety percent of childhood precursor-B acute lymphoblastic leukemias: alternative PCR targets for detection of minimal residual disease. Leukemia. 1999; 13:196-205.
11. Green E, McConville CM, Powell JE, Mann JR, Darbyshire PJ, Taylor AM, et al. Clonal diversity of Ig and T-cell receptor gene
rearrangements identifies a subset of childhood B-precursor acute lymphoblastic leukemia with increased risk of relapse. Blood. 1998; 92(3):952-8.
12. Szczepanski T, Willemse MJ, Brinkhof B, van Wering ER, van der Burg M, van Dongen JJ. Comparative analysis of Ig and TCR gene rearrangements at diagnosis and at relapse of childhood precursor-B-ALL provides improved strategies for selection of stable PCR
targets for monitoring of minimal residual disease. Blood. 2002; 99(7):2315-23.
13. Germano G, del Giudice L, Palatron S, Giarin E, Cazzaniga G, Biondi A, et al. Clonality profile in relapsed precursor-B-ALL children by GeneScan and sequencing analyses. Consequences on minimal residual disease monitoring. Leukemia. 2003; 17:1573-82. 14. Čolović M, Pavlović S, Kragujac N, Čolović N, Janković G, Sefer D, et
al. Acquired amegakaryocytic thrombocytopenia associated with proliferation of γ/δ TCR+ t-lymphocytes and a bcr-abl (p210) fusion transcript. Eur J Haematol. 2004; 73:372-5.
15. Pui CH, Sandlund JT, Pei D, Campana D, Rivera GK, Ribeiro RC, et al. Improved outcome for children with acute lymphoblastic leukemia: Results of total therapy study XIIIB at St Jude Children’s Research Hospital. Blood. 2004; 104(9):2690-6.
16. Lightfoot TJ, Roman E. Causes of childhood leukemia and lymphoma. Tox App Pharm. 2004; 199:104-17.
17. Schultz KR, Pullen DJ, Sather HN, Shuster JJ, Devidas M, Borowitz MJ, et al. Risk and response based classification of childhood B-precursor acute lymphoblastic leukemia: A combined analysis of prognostic markers from the Pediatric Oncology Group (POG) and Children’s Cancer Group (CCG). Blood. 2007; 109(3):926-35. 18. Arico M, Conter V, Valsecchi MG, Rizzari C, Boccalatte MF, Barisone E,
et al. Treatment reduction of highly selected standard-risk childhood acute lymphoblastic leukemia. The AIEOP ALL-9501 study. Haematologica. 2005; 90(9):1186-91.
19. Mitelman F, Johansson B, Mertens F. The impact of translocations and gene fusions on cancer causation. Nat Rev Cancer. 2007; 7(4):233-5.
20. Cazzaniga G, Rossi V, Biondi A. Monitoring minimal residual disease using chromosomal translocations in childhood ALL. Best Prac Res Clin Haematol. 2002; 15(1):21-35.
21. Meleshko AN, Belevtsev MV, Savitskaja TV, Potapnev MP. The incidence of T-cell receptor gene rearrangements in childhood B-lineage acute lymphoblastic leukemia is related to
immunophenotype and fusion oncogene expression. Leuk Res. 2006; 30:795-800.
22. Scrideli CA, Tone LG. Ig and TCR gene rearrangements in childhood ALL: Is there ethnic and socio-economic diversity of
Dragana JANIĆ
Univerzitetska dečja klinika, Tiršova 10, 11000 Beograd, Srbija
Email: dr.janic@eunet.rs
23. Van der Velden VHJ, de Bie M, van Wering ER, van Dongen JJ. Immunoglobulin light chain gene rearrangements in precursor-B-acute lymphoblastic leukemia: Characteristics and applicability for the detection of minimal residual disease. Haematologica. 2006; 91:679-82.
24. Van der Velden VHJ, Szczepanski T, Wijkhuijs JM, Hart PG, Hoogeveen PG, Hop WC, et al. Age-related patterns of immunoglobulin and T-cell receptor gene rearrangements in precursor-B-ALL: implications for detection of minimal residual disease. Leukemia. 2003; 17:1834-44.
25. Campana D. Minimal residual disease studies in acute leukemia. Am J Clin Pathol. 2004; 122(Suppl):S47-57.
26. Willemse MJ, Seriu T, Hettinger K, D’Aniello E, Hop WC, Panzer-Grumayer ER, et al. Detection of minimal residual disease identifies differences in treatment response between T-ALL and precursor B-ALL. Blood. 2002; 99(12):4386-93.
27. van Dongen JJ, Seriu T, Panzer-Grumayer ER, Biondi A, Pongers-Willemse MJ, Corral L, et al. Prognostic value of minimal residual disease in acute lymphoblastic leukaemia in childhood. Lancet. 1998, 352:1731-8.
28. Biondi A, Valsecchi MG, Seriu T, D’Aniello E, Willemse MJ, Fasching K, et al. Molecular detection of minimal residual disease is a strong predictive factor of relapse in childhood B-lineage acute lymphoblastic leukemia with medium risk features. A case control study of the International BFM study group. Leukemia. 2000: 14:1939-43.
29. Szczepanski T. Why and how to quantify minimal residual disease in acute lymphoblastic leukemia? Leukemia. 2007; 21:622-6. 30. Neale GAM, Campana D, Pui CH. Minimal residual disease detection
in acute lymphoblastic leukemia: Real improvement with the real-time quantitative PCT method? J Pediatr Hematol Oncol. 2003; 25(2):100-2.
31. Van der Velden VHJ, Panzer-Grumayer ER, Cazzaniga G, Flohr T, Sutton R, Schrauder A, et al. Optimization of PCR-based minimal residual disease diagnostics for childhood acute lymphoblastic leukemia in a multi-center setting. Leukemia. 2007; 21:706-13. 32. Zhou J, Goldwasser MA, Li A, Dahlberg SE, Neuberg D, Wang H, et
al. Quantitative analysis of minimal residual disease predicts relapse in children with B-lineage acute lymphoblastic leukemia in DFCIALL Consortium Protocol 95-01. Blood. 2007; 110:1607-11. 33. Stanulla M, Cario G, Meissner B, Schrauder A, Moricke A, Riehm H,
et al. Integrating molecular information into treatment of childhood acute lymphoblastic leukemia – A perspective from the BFM Study Group. Blood Cells Mol Disease. 2007; 39:160-3. 34. Apostolidou E, Swords R, Alvarado Y, Giles J. Treatment of acute
lymphoblastic leukaemia: a new era. Drugs. 2007; 67(15):2153-71. 35. Lazić J. Korelacija prisustva minimalne rezidualne bolesti i
prognostičkih parametara u toku lečenja dece sa akutnom limfoblastnom leukemijom [magistarska teza]. Beograd: Medicinski fakultet Univerziteta u Beogradu; 2007.
SUMMARY
Introduction Acute lymphoblastic leukaemia (ALL) is a malig-nant clonal disease, one of the most common malignancies in childhood. Contemporary protocols ensure high remission rate and long term free survival. The ability of molecular genetic methods help to establish submicroscopic classification and minimal residual disease (MRD) follow up, in major percent responsible for relapse.
Objective The aim of the study was to detect the frequency of IgH and TCR gene rearrangements and their correlation with clinical parameters.
Methods Forty-one children with ALL were enrolled in the study group, with initial diagnosis of IgH and TCR gene rear-rangements by polimerase chain reaction ( PCR). MRD follow-up
was performed in induction phase when morphological remis-sion was expected, and after intensive chemiotherapy. Results In the study group IgH rearrangement was detected in 82.9% of children at the diagnosis, while TCR rearrangement was seen in 56.1%. On induction day 33, clonal IgH rearrange-ments persisted in 39% and TCR rearrangerearrange-ments in 36.5% of children.
Conclusion Molecular analysis of genetic alterations and their correlation with standard prognostic parameters show the importance of risk stratification revision which leads to new therapy intensification approach. MRD stands out as a precise predictive factor for the relapse of disease.
Keywords: acute lymphoblast leukaemia; minimal residual disease; IgH and TCR gene rearrangements
Immunoglobulin Genes and T-Cell Receptors as Molecular Markers in
Children with Acute Lymphoblastic Leukaemia
Jelena Lazić1, Lidija Dokmanović1, Nada Krstovski1, Jelica Predojević2, Nataša Tošić3, Sonja Pavlović3, Dragana Janić1
1Department of Haematology and Oncology, University Children’s Hospital, Belgrade, Serbia; 2Childrens Hospital, Clinical Centre, Banja Luka, Bosnia and Herzegovina;