w w w . r b h h . o r g
Revista
Brasileira
de
Hematologia
e
Hemoterapia
Brazilian
Journal
of
Hematology
and
Hemotherapy
Original
article
Do
immunoglobulin
G
and
immunoglobulin
E
anti-
l
-asparaginase
antibodies
have
distinct
implications
in
children
with
acute
lymphoblastic
leukemia?
A
cross-sectional
study
Gabriela
Galindo-Rodríguez,
José
C.
Jaime-Pérez
∗,
Mario
C.
Salinas-Carmona,
Sandra
N.
González-Díaz,
Ángeles
Castro-Corona,
Raúl
Cavazos-González,
Humberto
Trevi ˜no-Villarreal,
Alberto
C.
Heredia-Salazar,
David
Gómez-Almaguer
UniversidadAutónomadeNuevoLeón,Monterrey,Mexico
a
r
t
i
c
l
e
i
n
f
o
Articlehistory:
Received20June2016 Accepted28November2016 Availableonline24January2017
Keywords:
Acutelymphoblasticleukemia Allergy
Hypersensitivity
l-Asparaginase
Neutralizingantibodies Silentantibodies
a
b
s
t
r
a
c
t
Background:l-Asparaginaseisessentialinthetreatmentofchildhoodacute
lymphoblas-ticleukemia.IfimmunoglobulinGanti-l-asparaginaseantibodiesdevelop,theycanlead
tofasterplasmaclearanceandreducedefficiencyaswellastohypersensitivityreactions, inwhichimmunoglobulinEcanalsoparticipate.Thisstudyinvestigatedthepresenceof immunoglobulinGandimmunoglobulinEanti-l-asparaginaseantibodiesandtheirclinical
associations.
Methods:Under16-year-oldpatientsatdiagnosisofB-cellacutelymphoblasticleukemia confirmedbyflowcytometryandtreatedwithauniforml-asparaginaseandchemotherapy
protocolwerestudied.ImmunoglobulinGanti-l-asparaginaseantibodiesweremeasured
usinganenzyme-linkedimmunosorbentassay. Intradermalandprickskintestingwas performedtoestablishthepresenceofspecificimmunoglobulinEanti-l-asparaginase
anti-bodiesinvivo.Statisticalanalysiswasusedtoinvestigateassociationsoftheseantibodies withrelevantclinicaleventsandoutcomes.
Results:Fifty-onechildrenwerestudiedwith42(82.35%)havinganti-l-asparaginase
anti-bodies.InthisgroupimmunoglobulinGantibodiesaloneweredocumentedin10(23.8%) comparedtoimmunoglobulinEalonein18(42.8%)patients.ImmunoglobulinGtogether withimmunoglobulinEweresimultaneouslypresentin14patients. Childrenwho pro-duced exclusivelyimmunoglobulin Gor noantibodies hada lower event-freesurvival (p-value=0.024).Eighteenchildren(35.3%)relapsedwithfiveofnineofthisgroupwhohad negativeskintestssufferingadditionalrelapses(range:2–4),comparedtononeofthenine childrenwhorelapsedwhohadpositiveskintests(p-value<0.001).
∗ Correspondingauthorat:Edificio“Dr.RodrigoBarragán”2◦piso,HospitalUniversitario“Dr.JoséE.González”AvenidaMaderoyGonzalitos
S/N,ColoniaMitrasCentro,Monterrey,NuevoLeón,C.P.64460,Mexico. E-mailaddress:carjaime@hotmail.com(J.C.Jaime-Pérez).
http://dx.doi.org/10.1016/j.bjhh.2016.11.006
Conclusion: ChildrenwithacutelymphoblasticleukemiaandisolatedimmunoglobulinG anti-l-asparaginaseantibodieshadahigherrelapserate,whereasnoadditionalrelapses
developedinchildrenwithimmunoglobulinEanti-l-asparaginaseantibodiesafterthefirst
relapse.
©2017Associac¸ ˜aoBrasileiradeHematologia,HemoterapiaeTerapiaCelular.Published byElsevierEditoraLtda.ThisisanopenaccessarticleundertheCCBY-NC-NDlicense (http://creativecommons.org/licenses/by-nc-nd/4.0/).
Escherichia coli l-asparaginase is key in the treatment
of childhood acute lymphoblastic leukemia (ALL).1 High-intensityl-asparaginaseregimensresultinbetteroutcomes
than lower-dose schemes.2 The intravenous or intramus-cular route can be used to administer l-asparaginase; the
latter is well tolerated and does not appear to result in increased hypersensitivity reactions3 whereas the former is more immunogenic.4 More recently it was shown that theintravenousadministrationofpegylatedl-asparaginase
isalso associatedwith a higher riskof allergicreactions.5 Thel-asparaginasemoleculeishighlyreactive,hasa
com-plex quaternary structure and elevated molecular weight anditcanelicitproductionofimmunoglobulin(Ig)Ganti-l
-asparaginaseantibodies.Theseantibodiescancausesevere allergic and hypersensitivity reactions, albeit rarely fatal, in children suffering a severe reaction, mostly mediated byIgG and complement.3,6 In thesecases, substitution for l-asparaginase conjugated covalently with 5000 molecular
weightpolyethyleneglycol isindicated,although onethird ofthoseswitchedtothepegylatedenzymestillhaveallergic reactionsduetothefactthatthesourceofbothpreparations isthe same bacterium.7,8 Interestingly,treatment withthe enzymederivedfromErwiniachrysanthemi,whichcan substi-tutethetypicalvarietyofEscherichiacoli,maynotbenecessary forsomechildrenwithsevereallergiestoE.colil-asparaginase
whohavereceivedatleasthalfofintendeddoses.9Important aspectsforbettertherapeuticresultsandlessfrequentside effectsincludenewsourcesofl-asparaginasetoincreaseits
availability, improvedpharmacodynamics and pharmacoki-neticsandsafertoxicologicalprofile.10
Decreased efficacy of l-asparaginase due to high titers
of IgG antibodies may be due to neutralizing antibodies, increasedenzymeclearance,delayedabsorptionafter intra-muscular administration, and direct interference with its enzymaticactivity.11
Currently,thereareno commerciallyavailable,clinically validatedassaysforIgGorIgEanti-l-asparaginaseantibodies.
Moreover,thespecificityofanti-l-asparaginaseantibodiesto
predictinactivationhasbeenlowincomparisontomeasuring
l-asparaginaseactivityitself;manypatientsdevelopanti-l
-asparaginaseantibodieswithoutclinicalallergicreactionsor inactivationoftheenzyme,andantibodylevelsinchildren withandwithouthypersensitivityoverlap.12
Importantly,no correlationhasbeenfoundbetweenIgG antibodytitersandtheseverityoftheallergicreaction.13This isprobablybecauseIgGanti-l-asparaginaseantibodyassays
areusedasa surrogateforthe diagnosisofl-asparaginase
allergy,and non-allergic ALL children can develop specific IgG anti-l-asparaginase antibodies, rendering its
diagnos-tic utility controversial.14 Specific IgE anti-l-asparaginase
antibodies, on the other hand, contribute to clinical symptoms through mediator release from mast cells.15 Thus, controversy on the meaning of anti-l-asparaginase
antibodiesremainsalthoughitsprognosticsignificanceand clinical utilityhasbeenstudiedforover30years.16 Several importantquestionsremain,includingwhatistheassociation betweenIgEanti-l-asparaginaseantibodiesandALLclinical
events other than allergicreactions. Furthermore,the time duringwhichIgGandIgEantibodiescanbedetectedhasnot beenestablished.
ThisstudyinvestigatedtheproductionofIgGandIgE
anti-l-asparaginaseantibodiesinchildrendiagnosedwithBcell
ALLtreatedwithastandardizeddoseofE.colil-asparaginase
anddeterminedtheassociationofthesetwoantibodieswith theclinicalcourseandriskofrelapse.
Methods
Atransversaldescriptivecross-sectionalstudywasconducted intheHematology,Allergy,andImmunologyDepartments,of the“JoséEleuterioGonzález”UniversityHospitalofthe Uni-versidadAutónomadeNuevoLeón,Monterrey,Mexico.Under 16-year-oldpatients withdiagnosis ofB-cellALLconfirmed byflowcytometryatanystageoftreatmentafterinduction to remission therapy were included. Children taking anti-H1oranti-H2antihistamineswereexcluded.Thestudywas approvedbytheInstitutionalReviewBoardandEthics Com-mitteeoftheinstitutionandparentssignedinformedconsent forms.
Induction to remission therapy consistedof prednisone 60mg/m2, vincristine 1.5mg/m2, and six doses of l
-asparaginaseof6000IU/M2/intramuscularonDays8,12,16,
20,24,and36.Childrenwithhigh-riskALLreceivedtwo addi-tionaldosesofl-asparaginaseonDays2and8ofre-induction
andthreedosesofdoxorubicin(40mg/m2);tripleintrathecal
chemotherapyforcentralnervoussystem(CNS)prophylaxis wasadministeredfourtimes. Consolidationincludedsingle doses of cytosine arabinoside (1.5g/m2) and methotrexate
(1.5g/m2) administered in a one-day intravenous infusion.
Thiswasfollowedbyonemonthof6-mercaptopurinetaken dailyandweeklymethotrexate.Re-inductionincluded15days ofprednisone,threedosesofvincristine,twoofdoxorubicin forhigh-riskandoneforstandard-riskpatients,twodosesof
l-asparaginaseandtwooftripleintrathecalprophylaxis.Ten
daysafterre-induction,maintenancewasstartedfor90weeks with oral 6-mercapthopurine at 50mg/m2/day and weekly
methotrexatestartingat30mg/m2/dayandadjustedto
main-taintheabsoluteleukocytecountbetween3.0and5.0×103/L.
every three months during the second year, maintenance was suspended for a week in order to administrate one single dose of vincristine, triple intrathecal chemotherapy, and seven days of prednisone; this regimen was inspired onapreviouslypublishedprotocol.17ProphylaxisoftheCNS consistedintripleintrathecaltherapyincludingcytosine ara-binoside,methotrexate,andhydrocortisone.CNSirradiation wasreserved forpatients withinfiltrationatdiagnosis and thosesufferingCNSrelapse.Relapsedchildrenreceived addi-tionall-asparaginaseiftheydidnotdevelopclinicallyevident
hypersensitivitymanifestations.
ImmunoglobulinGanti-l-asparaginaseantibody
determination
IgG anti-l-asparaginaseserum antibodies were determined
employinganenzyme-linkedimmunosorbentassay(ELISA) following a previously reported method.18 Briefly, periph-eralblood collectedfrom patientsand tenhealthy controls wascentrifugedforfiveminutesat3000rpmand the sepa-ratedserumwasstoredat−70◦C.Leunase,10,000IU(Kioto,
Japan)wasdilutedin0.05Mcarbonate-bicarbonatebuffer(pH 9.4–5g/mL); 100L of this dilution were added, in
dupli-cate, to 96-well polystyrene ELISA plates (WWR Scientific Product,GA,USA)followedbyovernightincubationat4◦C. Thesupernatantwasdiscardedandtheplateswerewashed withphosphate buffered saline (PBS) containingTween-20, 0.1%.Phosphatebufferedsaline(300L),containing0.5%bovine serum albumin (BSA)(Sigma, St. Louis,MO,USA), 5%fetal bovineserum and 0.1%Tween-20,wasadded toeach well, followed by an incubation of 90min at room temperature (RT);excess supernatantwasdiscardedand thewells were washedthreetimeswiththePBS/tween-20solution.Forthe assay, 100L of plasma of patients and controls, diluted
1:3200insaline-tweenwereaddedinduplicatetoa96-well polystyrene plate to which l-asparaginase was previously
attached,asdescribedabove,18 includingnegativeand pos-itive controls. As no severe or anaphylactic reactions to
l-asparaginasedevelopedinthechildrenofthis study,
pos-itive IgG anti-l-asparaginase control serum was obtained
fromsensitizedmice.Briefly,15mgofLeunase(KyowaHakko Kogyo Co., Japan) in incomplete Freund adjuvant solution was injected into the peritoneum of Balb/c mice between sixand eightweeksofage.Additionalimmunizationswere givenonDays15and30injecting10mgoftheenzymeinthe sameFreundsolution.OnDay35post-immunization,blood was taken from the retro-orbital vascular plexusand cen-trifugedat3000rpm;finally,miceserawerepooledandtested; the mixed serum withthe highest IgG anti-l-asparaginase
titer, as assayed above, was the positive control. Negative controlsconsisted ofsera from non-immunizedmice, nor-malhumansera,and diluent alone. Plateswere incubated withcontinuousagitation at37◦Cforonehour; the super-natantwasdiscardedandthewellswashedthreetimeswith saline-Tween-20;150Lofasecondaryperoxidase-conjugated
monoclonalgoatanti-humanIgGantibody(Sigma,St.Louis, MO,USA)wasaddedtoeachwellandincubatedat37◦Cfor onehour.Fourwasheswerefollowedbytheadditiontoeach wellof100Lofasubstrate-chromogensolutioncontaining
o-phenylenediaminedihydrochloride (OPD):2HCl(Sigma, St.
Louis,MO,USA),hydrogenperoxide,andcitratebuffer,then incubated for30minatroomtemperatureinthedark.The reactionwasstoppedbyadding100Lofa1.0Mphosphoric
acid solution; l-asparaginase antibodies were expressedas
opticaldensity(OD)readings.Samplesweredefinedas posi-tiveifthenaturallogofthe1:3200ODreadingwasgreaterthan twostandarddeviationsabovethenegativecontrolprocessed mean.19
ImmunoglobulinEanti-l-asparaginaseantibodydetection
Due to the lack of a commercially available standardized assay forIgEanti-l-asparaginaseantibodies,wedecidedto
assess IgE-mediated type I hypersensitivity reaction to l
-asparaginaseinvivo.Thus,twotypesofvalidatedskintesting wereperformed,aprickskintest(PST)20andintradermalskin test (IST).21 ThePSTwas carriedout byapplying onedrop of a30M l-asparaginasesolution (Leunase, Kyowa Hakko
Kogyo Co.,Japan) followedbya skinpuncture using a dis-posable plasticlancet(Duotip® by Lincoln Diagnostic,Inc., Decatur,IL).Thesolutionwasleftincontactwiththeskinfor 15min.Afterwardsthepapuleanderythemawereobserved andthediameterwasmeasuredinmillimetersbythesame experiencedallergistinallcasesusingamillimeterscale.The ISTwas performedbyinjecting 10Lofthesame sterilel
-asparaginasesolution;after15min,thepapuleanderythema weremeasuredinmillimetersinthesamewayasforthePST. Anintradermalinjectionof10Lof1%histaminephosphate
solution was the positive controlfor the skintest; normal salinesolutionwasthenegativecontrol.Awhealof3mmor largerthanthenegativecontrolwasinterpretedasapositive test.20
Statisticalanalysis
The Chi-square test was used to analyze frequencies, the Mann–Whitney test for independent variables with non-normal distribution, andSpearman’s method were usedto study IgG–IgE correlations. The Kaplan–Meier method was usedtocompareevent-freesurvival(EFS)betweenchildren whodidanddidnothaveIgGantibodies,andbetweenthose withandwithoutanIgEresponsetol-asparaginaseas
eval-uatedusingthePSTandIST.TheStatisticalPackageforthe SocialSciences(SPSS–v20)wasemployed.
Results
Fifty-onepatientswerestudied;pertinentdescriptivedataare showninTable1.Childrenreceivedamedianofeightdoses (range:5–14)ofl-asparaginase.Accordingtotheclinicalfiles
andelectronicrecords,onlythree(5.8%)patientssufferedany allergic/hypersensitivityreactionconsistingofurticaria/skin rashafterreceiving8,8,and13l-asparaginasedoses,
respec-tively;therewerenoanaphylacticreactions.
Intotal42/51(82.35%)patientshadIgG,IgE,orboth
anti-l-asparaginase antibodies; ten (23.8%) had exclusively IgG
Table1–Importantcharacteristicsof51childrenwith acutelymphoblasticleukemia.
Characteristic
Age,years–median(range) 8(4–17)
Gender–n(%)
Male 27(53)
Female 24(47)
Riskgroup–n(%)
Standard-risk 23(45.1)
High-risk 28(54.9)
Clinicalstatusatthetimeofthestudy–n(%)
Maintenance(≥1year) 27(52.9)
Surveillance 16(31.3)
Relapse 8(15.6)
Anti-l-asparaginaseantibodies–n(%)
IgG(ELISA) 24(47.0)
IgE(invivo) 18(35.3)
None 9(17.7)
bothIgGplusIgEantibodies.NoIgGorIgEantibodieswere foundin9/51(17.64%)children.
With regard to associations between risk group and
antibodies, 19/23 (82.6%) standard-risk patients developed antibodies comparedto23/28(81.2%) high-riskchildren(p -value>0.05).Whenantibodydistributionwasstudieditwas foundthatsix(21.4%)high-riskpatientsdevelopedIgG anti-bodies,comparedtofour(17.4%)inthestandard-riskgroup, whileIgEantibodiesweredetectedin13(46.4%)vs.five(21.7%), andIgGtogether withIgEantibodies infour(14.3%)vs.ten (43.46%)high-riskandstandard-riskchildren,respectively(p -value=0.38).
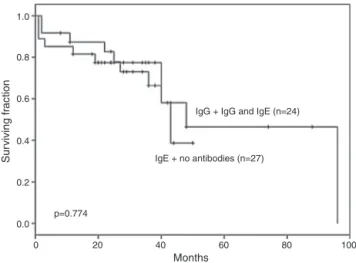
NodifferencewasdocumentedinEFSforALLchildrenwith IgG and IgG plusIgEanti-l-asparaginase antibodies (n=24) vs. those with no antibodies or with IgEalone (n=27 – p -value=0.774;Figure1).
Neither the presence nor the titer of IgG anti-l
-asparaginaseantibodiesinfluencedtheresponsetoinduction or re-induction to remission therapy (p-value=0.19). Fur-thermore, no difference in response to ALL induction or
IgG + IgG and IgE (n=24)
IgE + no antibodies (n=27)
0 20
p=0.774
40 Months
60 80 100
1.0
0.8
0.6
0.4
0.2
Sur
viving fr
action
0.0
Figure1–Nodifferencewasfoundintheevent-free survivalofacutelymphoblasticleukemiachildrenwith immunoglobulin(Ig)GandIgGplusIgEanti-l-asparaginase
antibodiesvs.IgEantibodiesandnoantibodies.
1.0
0.8
0.6
0.4
0.2
0.0
0 20 40 60
Months
80 100
IgE + IgE and IgG (n=32)
IgG + no antibodies (n=19)
p=0.024
Sur
viving fr
action
Figure2–Event-freesurvivalwassignificantlylower amongchildrenwhoproducedonlyimmunoglobulin(Ig)G anti-l-asparaginaseantibodiesandthosewithno
antibodiescomparedtochildrenwithIgEantibodiesin isolationorcombinedwithIgGantibodies.
re-inductiontherapywasdocumentedbetweenchildrenwith apositiveoranegativeskintest(p-value=0.82).Table2 com-pares thetypeand prevalenceofantibodies inthe current studysamplewiththeresultsofreportsintheliterature.
Prickskintestingwasperformedinall51childrenwith28 (54.9%)havingpositiveresults;23(45.1%)patientswith nega-tivePSTweresubmittedtoISTwithonlyfourhavingpositive results.Thus,32(62.7%)of51childrenhadspecificIgEanti-l
-asparaginaseantibodiesdemonstratedbyapositiveskintest
invivo.
Eighteen(35.3%)patientsofthewholegrouprelapsed,two (8.7%)ofthe23standard-riskchildrenand16(57.1%)inthe28 high-riskgroup(p-value<0.001).
The EFS of the 19 patients who developed IgG anti-l
-asparaginaseantibodies onlyand thosewithno antibodies was significantly lower than in the remaining32 patients, [36 months (range: 27–40) vs. 96 months (range: 62–99);p -value=0.024–Figure2].
No statistically significant difference in EFS was doc-umented for children with no antibodies (n=9) against
l-asparaginase compared to those havingIgG, IgE or both
(n=42)antibodies(p-value=0.583–Figure3).
Eighteen (35.3%) patients relapsed at a median of 20.5 months(range:1–96),tenrelapsedduringfirstmaintenance, fiveaftercessationoftherapy,andthreeseveralyearsafter completing treatment.Nine (50%)hadexclusivelyIgG
anti-l-asparaginaseantibodies andnine (50%)had positiveskin
testsindicatingthepresenceofIgEanti-l-asparaginase
Table2–Anti-l-asparaginaseantibodiesidentifiedinthisstudyandinrepresentativepublications.
Reference n IgG IgE IgG+IgE IgM None
Currentstudy 51 10(19.6%) 18(35.3%) 14(27.5%) 9(17.6%)
Panosyanetal.1 1001 611(61.0%) 390(39.0%)
Zalewska-Szewczyketal.8 13 5(38.5%) 8(61.53%)
Wooetal.13 152 54(35.5%) 98(64.5%)
Zalewska-Szewczyketal.14 47 20(42.5%) 19(40.4%) 8(17.0%)
Cheungetal.16 13 7(53.8%) 6(46.2%)
Kawediaetal.19 35 28(80.0%) 7(20.0%)
Ig:Immunoglobulin.
1.0
0.8
0.6
0.4
Sur
viving fr
action
0.2
0.0
0
p=0.583
No antibodies n=9
IgG or IgE or both n=42
20 40
Months
60 80 100
Figure3–Nosignificantdifferenceinevent-freesurvival wasfoundforacutelymphoblasticleukemiachildrenwith noantibodiescomparedtothosewithimmunoglobulin (Ig)G,IgEorbothIgGandIgEanti-l-asparaginase
antibodies.
Discussion
Therelevanceofantibodiesdirectedagainstl-asparaginase
in children with ALL has been consistently highlighted in the Berlin–Frankfurt–Münster (BFM) studies and recently reviewed.8,12Adversereactionstotheenzymecanbe medi-ated by IgG, complement or IgE antibodies, or more than oneatthe same time.22 Aneutralizing natureofIgG anti-l-asparaginaseantibodies leading to accelerated plasmatic
clearance, with an important decrease in l-asparaginase
activity, has been reported23; the titer of these antibodies can increase considerably after switching to pegylated l
-asparaginasefollowingthedevelopmentofhypersensitivity.24 Interestingly,thedifferentrolesoftheseanti-l-asparaginase
antibodyclasses and their correlation with clinical allergy manifestationsandthedevelopmentofsilentantibodieshas notbeendiscussed.
Allergic reactions are associated with the appearance of antibodies, which have been reported to increase l
-asparaginaseclearanceandtoreduceorevenneutralizethe catalyticactivityoftheenzyme.14,25ALLpatientswhodevelop anti-l-asparaginaseantibodiesmaythushavepooroutcomes
becauseoflowl-asparaginaseactivity.14,19
Areportonthediagnosticutilityofserumantibody test-ingin410childrenwithALLreceivingl-asparaginasefound
that169(41.2%)hadsomedegreeofclinicalallergyand148 (87%)hadIgGanti-l-asparaginaseantibodies;oftheremaining
241patientswithnoallergy,89(36.9%)hadIgGantibodies.IgE antibodieswerenotdetermined.26
Thegoalofthecurrentstudywastoinvestigatethe pres-ence of bothIgG and IgEantibodies to l-asparaginase and
to explore their clinical correlation withthe courseof the disease.Over80%ofchildrendevelopedantibodies,almost 60% of these were IgG and only these were associated to lowerEFSmostprobablyduetotheirl-asparaginase
neutral-izingnature;theincidenceofanti-l-asparaginaseantibodies
inthisstudysample(82.35%)wassimilartothe87%reported inalargeprospectivestudy.27Remarkably,IgGandIgE anti-bodies weredetectedinsomecasesseveralyears afterthe patient’s lastexposuretotheenzymethus confirmingthat
l-asparaginase isstrongly immunogenic and that immune
memory mechanismsare activeandoperationalduringthe evolution and therapy ofALL. It isimportant to point out that currentrecommendations statethatanti-asparaginase antibody and asparagine measurements are not indicated for clinical decisionmaking outside the contextof clinical trials.12
Furthermore,itisimportanttounderscorethatthereare patientswithdecreasedseruml-asparaginaseactivityandno
demonstrableIgGantibodiestotheenzyme,suggestingthat additional factors are involvedinthis phenomenon. These factors includethenumberofdosesandintensityofthel
-asparaginaseregimen,concomitantadministrationofstrong immunosuppressivedrugsaspart ofthe chemotherapeutic regimenandenhancedenzymeclearanceassociatedto pro-teasedegradation.
Itisnoticeable that giventhefrequencyand severityof reactions tol-asparaginaseinchildrenwithALL, few
stud-ieshaveanalyzedthecorrelationofIgEwithclinicalevents. Thecurrentstudyfoundaprevalenceforpositiveskintests of 63% (32/51) employing the PST and IST, although only threeofthesechildren(9.4%)developedhypersensitivity reac-tions,suggestingthatproductionofIgEanti-l-asparaginase
reactions in the childrenof this study could be related to theroutinepracticeinourinstitutionofpre-medicationwith anti-histaminestopreventallergicreactionstotheenzyme, thelow-dosel-asparaginaseregimenused(6000IU/M2
com-paredto 10,000IU/M2 in other reports)8,13,21 and the lower mediannumberofl-asparaginasedoses[8(range:5–15)
com-paredto13.5 (range:4–20)inotherreports].Both doseand numberofdoses aredirectlyrelatedtothedevelopmentof hypersensitivity events.3 Interestingly,despitethe factthat high-risk ALL childrenreceived morel-asparaginasedoses
thanstandard-riskpatients(9vs.6),therewasnodifference inthe rateof anti-l-asparaginase antibodies produced.We
hypothesizethatthiscouldbeduetotheexistenceofa criti-calthresholdofimmunizingeventstol-asparaginase,beyond
whichl-asparaginase-non-respondersremainantibody-free.
Genetic factors may influence the likelihood of develop-ingclinicalhypersensitivityreactions;usingagenome-wide approach,itwasreportedthatgeneticvariationsinGRIAIwere associatedwithasparaginaseallergies.29
Thebasophilactivation test (BAT),inwhich thesurface expressionofthe degranulation/activationmarker(CD203c) onbasophilsisdetected,isconsideredareliabletoolfor diag-nosing IgE-mediatedallergies. Recently, it hasbeen shown that BAT isa useful markerfor identifying l-asparaginase
allergybecauseofitshighsensitivityandspecificity,and com-biningtheBATwithanl-asparaginase-specificIgGassayisthe
mostaccuratemethodofidentifyingl-asparaginaseallergy.30
Thisstudyfoundapositivecorrelationbetweenthe pres-enceofIgGanti-l-asparaginaseantibodiesandALLrelapse,
confirmingpreviousstudiesthatfoundanegativeinfluence ofneutralizingIgGantibodies,manifestedaslowerEFSand overallsurvival.1,3,15Remarkably,thepresenceofIgE antibod-iesdocumentedbyskintestingwasnotassociatedtoahigher relapserate.Thisisprobablybecausethisclassof antibod-ieslacksneutralizingactivity;onthecontrary,therewasan associationbetweennegativeskintestsandelevatedriskof additionalrelapses.Thus,childrenwhosufferedafirstrelapse and had negative skin tests had a statistically significant increasedriskofsubsequentrelapses:55% comparedto0% inthosewithpositiveskintests(p-value<0.0001).We hypoth-esizethatchildrenwithmorethanonerelapseandnegative skintestsmighthaveadditionaldefectiveimmune surveil-lanceandbluntingofthecriticalprocessofhost-dependent secondaryeliminationoftheresidualleukemicclone,thereby favoringALLrelapses.Inthisrespect,both,humoraland cel-lular immune functions are decreased during ALL and its therapy31;regainingimmunefunctioncantakeseveralyears toaccomplish,andeventhen,therecoveryofIgGsubclasses canbe impaired.32 Additionally, relapsedchildrenwith IgE antibodiesmayhavealimitedcapacitytoproduceIgG neu-tralizingantibodiesandthenhavealowerriskofrelapse.
ThehypothesisthatchildrenwithIgGanti-l-asparaginase
antibodies, in isolation or associated with IgE antibodies, would clear the drug from the blood and children would relapse morefrequentlythan those withoutIgG antibodies wasnotconfirmedbyKaplan–Meieranalysis(p-value=0.774). Inaddition,thehypothesisthatIgEanti-l-asparaginase
anti-bodies,aloneorincombination withIgGantibodies,would bedetrimentalbecausechildrenwhodevelopedIgE antibod-ieswouldnotreceivethesameamountofl-asparaginasedue
tosuperveningallergicreactionswasnotconfirmed.Onthe contrary,IgEseemed toprotectchildrenwiththisantibody, aloneorincombinationwithIgGantibodies(p-value=0.024). Accordingly,IgEmightplayadualroleinALL,asanegative factor duetoits participationinhypersensitivityreactions, forcingthecessationoftherapyandtheswitchingofenzyme preparations,orasapositivesurrogateindicatorforresidual immunecompetenceandlessadditionalrelapses.Moreover, thereisthepossibilityofsomeinteraction,eitherpositiveor negative,betweentheeffectsofIgGandIgEantibodies.These resultsappearequivocal,whichmaybeduetothesmall num-ber ofchildrenineach group; the literaturehowever, does notofferadefinitiveanswerinthisrespectyetand interest-ingly,therearenostudies documentingalinkbetweenthe presenceofIgEanti-l-asparaginaseantibodiesanddecreased
serumactivityofl-asparaginase.Thelastconsensus
recom-mendationsconsiderthatthedevelopmentofeitherantibody isdetrimentaltochildrenwithALL.12However,whenthe cur-rentsmallgroupwasanalyzed,nosignificantdifferencewas found,althoughthecomparisonshowedahigherEFS,albeit non-significant (p-value=0.583), inthe no-antibodies group (n=9;median68months)vs.thosewithanytypeofantibody (n=42;median45months).Theseresultssuggestthatthere aredifferentimplicationsaccordingtotheclassofantibody present.IgGantibodiesareassociatedtoabadprognosisand IgEhas eithera negativeassociation dueto hypersensitiv-ityreactions, orapositiveassociationconferringresistance tosubsequentrelapses,probablyasasurrogateindicatorof residual immune competence in children, leading to final clearanceoftheleukemicclone.
Limitationsinourproof-of-conceptstudyincludethesmall sample size and its retrospective design. Additionally, the relapseratewashigherthanexpected,reflectingthefactthat mostpatientsreferredtoourcenterhaveunfavorableclinical andhematologiccharacteristicsatdiagnosisandweretreated withalow-moderatedoseintensityprotocol,aswellasthe knowngreaterincidenceofhigh-riskchildrenintheHispanic population.33Anothermajorlimitationistheheterogeneity oftheclinicalstagesatthetimeofthesingledetermination ofIgG and IgEantibodies forthis cross-sectional, proof-of-concept study,and thus inorder toconfirmthese findings aprospective,sufficientlypoweredstudy,includingbalanced groupsatallmajortimepointsoftreatment,isrequired.
In conclusion,childrenwithonlyIgG antibodiesagainst
l-asparaginase suffered more relapses than those without
theseantibodiesorwhenIgEwassimultaneouslypresentand patients withIgEpositive skintests forthe enzymehad a decreasedriskofsufferingmorethanonerelapse.Forty-five yearsaftertheinitialreport,34criticalaspectsoftheimmune responsetol-asparaginaseinALLarestillundefined;
prospec-tivestudiesaimedatdecipheringtheintricatenatureofthis responsemediatedbyIgGandIgEantibodiesarenecessaryto definitivelyestablishtheirinteractionsandinfluenceonthe outcomesofALLofchildhood.
Conflicts
of
interest
r
e
f
e
r
e
n
c
e
s
1. PanosyanEH,SeibelNL,Martin-AragonS,GaynonPS, AvramisIA,SatherH,etal.Asparaginaseantibodyand asparaginaseactivityinchildrenwithhigher-riskacute lymphoblasticleukemia:Children’sCancerGroupStudy CCG-1961.JPediatrHematolOncol.2004;26(4):217–26. 2. AvramisVI,PanosyanEH.
Pharmacokinetic/pharmacodynamicrelationshipsof asparaginaseformulations:thepast,thepresentand recommendationsforthefuture.ClinPharmacokinet. 2005;44(4):367–93.
3. ShinnickSE,BrowningML,KoontzSE.Managing
hypersensitivitytoasparaginaseinpediatrics,adolescents, andyoungadults.JPediatrOncolNurs.2013;30(2):63–77. 4. NesbitM,ChardR,EvansA,KaronM,HammondGD.
Evaluationofintramuscularversusintravenous
administrationofl-asparaginaseinchildhoodleukemia.AmJ PediatrHematolOncol.1979;1(1):9–13.
5. MacDonaldT,KulkarniK,BernsteinM,FernandezCV.Allergic reactionswithintravenouscomparedwithintramuscular pegaspargaseinchildrenwithhigh-riskacutelymphoblastic leukemia:apopulation-basedstudyfromthemaritimes, Canada.JPediatrHematolOncol.2016;38(5):341–4.
6. HijiyaN,vanderSluisIM.Asparaginase-associatedtoxicityin childrenwithacutelymphoblasticleukemia.Leuk
Lymphoma.2016;57(4):748–57.
7. FigueiredoL,ColePD,DrachtmanRA.AsparaginaseErwinia chrysanthemiasacomponentofamulti-agent
chemotherapeuticregimenforthetreatmentofpatientswith acutelymphoblasticleukemiawhohavedeveloped
hypersensitivitytoE.coli-derivedasparaginase.ExpertRev Hematol.2016;9(3):227–34.
8. Zalewska-SzewczykB,AndrzejewskiW,BodalskiJ. Developmentofanti-asparaginaseantibodiesinchildhood acutelymphoblasticleukemia.PediatrBloodCancer. 2004;43(5):600–2.
9. YenHJ,ChangWH,LiuHC,YehTC,HungGY,WuKH,etal. OutcomesfollowingdiscontinuationofE.colil-asparaginase uponsevereallergicreactionsinchildrenwithacute lymphoblasticleukemia.PediatrBloodCancer. 2016;63(4):665–70.
10.LopesAM,Oliveira-NascimentoL,RibeiroA,TairumCAJr, BreyerCA,OliveiraMA.Therapeuticl-asparaginase: upstream,downstreamandbeyond.CritRevBiotechnol. 2017;37(1):82–99.
11.EbeidEN,KamelMM,AliBA.Detectionofanti-asparaginase antibodiesduringtherapywithE.coliasparaginasein childrenwithnewlydiagnosedacutelymphoblasticleukemia andlymphoma.JEgyptNatlCancInst.2008;20(2):
127–33.
12.vanderSluisIM,VroomanLM,PietersR,BaruchelA, EscherichG,GouldenN,etal.Consensusexpert recommendationsforidentificationandmanagementof asparaginasehypersensitivityandsilentinactivation. Haematologica.2016;101(3):279–85.
13.WooMH,HakLJ,StormMC,SandlundJT,RibeiroRC,Rivera GK,etal.Hypersensitivityordevelopmentofantibodiesto asparaginasedoesnotimpacttreatmentoutcomeof childhoodacutelymphoblasticleukemia.JClinOncol. 2000;18(7):1525–32.
14.Zalewska-SzewczykB,AndrzejewskiW,MłynarskiW, Jedrychowska-Da ´nskaK,WitasH,BodalskiJ.The anti-asparaginesantibodiescorrelatewithl-asparagines activityandmayaffectclinicaloutcomeofchildhoodacute lymphoblasticleukemia.LeukLymphoma.2007;48(5): 931–6.
15.KörholzD,WahnU,JürgensH,WahnV.Allergicreactionsin treatmentwithl-asparaginase.SignificanceofspecificIgE antibodies.MonatsschrKinderheilkd.1990;138(1): 23–5.
16.CheungN-KV,ChauIY,CocciaPF.Antibodyresponseto
Escherichiacolil-asparaginase:prognosticsignificanceand clinicalutilityofantibodymeasurement.JPediatrHematol Oncol.1986;8(2):99–104.
17.HillFG,RichardsS,GibsonB,HannI,LilleymanJ,KinseyS, etal.Successfultreatmentwithoutcranialradiotherapyof childrenreceivingintensifiedchemotherapyforacute lymphoblasticleukaemia:resultsoftherisk-stratified randomizedcentralnervoussystemtreatmenttrialMRC UKALLXI(ISRCTN16757172).BrJHaematol.
2004;124(1):33–46.
18.LanversC,VieiraPinheiroJP,HempelG,WuerthweinG,BoosJ. Analyticalvalidationofamicroplatereader-basedmethodfor thetherapeuticdrugmonitoringofl-asparaginaseinhuman serum.AnalBiochem.2002;309(1):117–26.
19.KawediaJD,LiuC,PeiD,ChengC,FernandezCA,HowardSC, etal.Dexamethasoneexposureandasparaginaseantibodies affectrelapseriskinacutelymphoblasticleukemia.Blood. 2012;119(7):1658–64.
20.AntunesJ,BorregoL,RomeiraA,PintoP.Skinpricktestsand allergydiagnosis.AllergolImmunopathol(Madr).
2009;37(3):155–64.
21.NetoHC,RosarioN.StudyingspecificIgE:invivoorinvitro. AllergolImmunopathol.2009;37(1):31–5.
22.PietersR,HungerSP,BoosJ,RizzariC,SilvermanL,Baruchel A,etal.l-Asparaginasetreatmentinacutelymphoblastic leukemia:afocusonErwiniaasparaginase.Cancer. 2011;117(2):238–49.
23.AsselinB,RizzariC.Asparaginasepharmacokineticsand implicationsoftherapeuticdrugmonitoring.Leuk Lymphoma.2015;56(8):2273–80.
24.WillerA,GerssJ,KönigT,FrankeD,KühnelHJ,HenzeG,etal. Anti–Escherichiacoliasparaginaseantibodylevelsdetermine theactivityofsecond-linetreatmentwithpegylatedE.coli
asparaginase:aretrospectiveanalysiswithintheALL-BFM trials.Blood.2011;118(22):5774–82.
25.AmylonMD,ShusterJ,PullenJ,BerardC,LinkMP,WharamM, etal.Intensivehigh-doseasparaginaseconsolidation improvessurvivalforpediatricpatientswithTcellacute lymphoblasticleukemiaandadvancedstagelymphoblastic lymphoma:aPediatricOncologyGroupstudy.Leukemia. 1999;13(3):335–42.
26.LiuC,KawediaJD,ChengC,PeiD,FernandezCA,CaiX,etal. Clinicalutilityandimplicationsofasparaginaseantibodiesin acutelymphoblasticleukemia.Leukemia.2012;26(11):2303–9. 27.YangL,PanettaJC,CaiX,YangW,PeiD,ChengC,etal.
Asparaginasemayinfluencedexamethasone
pharmacokineticsinacutelymphoblasticleukemia.JClin Oncol.2008;26(12):1932–9.
28.LeeC,GianosM,KlaustermeyerWB.Diagnosisand managementofhypersensitivityreactionsrelatedto commoncancerchemotherapyagents.AnnAllergyAsthma Immunol.2009;102(3):179–87.
29.ChenSH,PeiD,YangW,ChengC,JehaS,CoxNJ,etal.Genetic variationsinGRIA1onchromosome5q33relatedto
asparaginasehypersensitivity.ClinPharmacolTher. 2010;88(2):191–6.
30.HinoM,ShimojoN,OchiaiH,InoueY,AndoK,ChikaraishiK, etal.ExpressionofCD203conbasophilsasamarkerof immunoglobulinE-mediatedl-asparaginaseallergy.Leuk Lymphoma.2014;55(1):92–6.
leukaemia–aprospectivestudyof20paediatricpatients.BrJ Haematol.2009;147(3):360–70.
32.KristinssonVH,KristinssonJR,JonmundssonGK,JonssonOG, ThorssonAV,HaraldssonA.Immunoglobulinclassand subclassconcentrationsaftertreatmentofchildhood leukemia.PediatrHematolOncol.2001;18(3):167–72.
33.PuiCH,MullighanCG,EvansWE,RellingMV.Pediatricacute lymphoblasticleukemia:wherearewegoingandhowdowe getthere?Blood.2012;120(6):1165–74.
34.PetersonRG,HandschumacherRE,MitchellMS.