w ww . e l s e v i e r . c o m / l o c a t e / b j p
Original
Article
Gamma
radiation
treatment
activates
glucomoringin
synthesis
in
Moringa
oleifera
Tsifhiwa
Ramabulana
a,
Risimati
D.
Mavunda
b,c,
Paul
A.
Steenkamp
a,d,
Lizelle
A.
Piater
a,
Ian
A.
Dubery
a,
Ashwell
R.
Ndhlala
e,
Ntakadzeni
E.
Madala
a,∗aDepartmentofBiochemistry,UniversityofJohannesburg,AucklandPark,SouthAfrica bDepartmentofPhysics,UniversityofJohannesburg,AucklandPark,SouthAfrica cSouthAfricanNuclearEnergyCorporation,Pretoria,SouthAfrica
dCouncilofScientificandIndustrialResearch,Biosciences,NaturalProductsandAgro-processingGroup,Pretoria,SouthAfrica eAgriculturalResearchCouncil,VegetableandOrnamentalPlants,Pretoria,SouthAfrica
a
r
t
i
c
l
e
i
n
f
o
Articlehistory:
Received13February2017 Accepted14May2017 Availableonline24August2017
Keywords: Gammaradiation Glucosinolates Metabolitefingerprinting Oxidativestress UHPLC-qTOF-MS
a
b
s
t
r
a
c
t
Plantsareaveryrichsourceofpharmacologicallyrelevantmetabolites.However,therelative concentra-tionsofthesecompoundsaresubjecttothegeneticmake-up,thephysiologicalstateoftheplantaswellas environmentaleffects.Recently,metabolicperturbationsthroughtheuseofabioticstressorshaveproven tobeavaluablestrategyforincreasingthelevelsofthesecompounds.Oxidativestress-associated stress-ors,includingionizingradiation,havealsobeenreportedtoinducemetaboliteswithvariousbiological activitiesinplants.Hence,theaimofthecurrentstudywastoinvestigatetheeffectofgammaradiation ontheinductionofpurportedanti-cancerousmetabolites,glucomoringinanditsderivatives,inMoringa
oleiferaLam.,Moringaceae.Here,anUHPLC-qTOF-MS-basedtargetedmetabolicfingerprintingapproach
wasusedtoevaluatetheeffectofgammaradiationtreatmentontheafore-mentionedhealth-beneficial secondarymetabolitesofM.oleifera.Followingradiation,anincreaseinglucomoringinandthree acyl-atedderivativeswasnoted.Assuch,thesemoleculescanberegardedascomponentsoftheinducible defensemechanismofM.oleiferaasopposedtobeingconstitutivecomponentsasithaspreviouslybeen assumed.Thismightbeanindicationofapossible,yetunexploredroleofmoringinagainsttheeffects ofoxidativestressinM.oleiferaplants.Theresultsalsosuggestthatplantsundergoingphoto-oxidative stresscouldaccumulatehigheramountsofglucomoringinandrelatedmolecules.
©2017SociedadeBrasileiradeFarmacognosia.PublishedbyElsevierEditoraLtda.Thisisanopen accessarticleundertheCCBY-NC-NDlicense(http://creativecommons.org/licenses/by-nc-nd/4.0/).
Introduction
Glucosinolates(GS)aresecondarymetabolitesfoundinalmost
all plants of the order Brassicales (Fahey et al., 2001; Mithen,
2001).These compoundsarediversein origin,side chain
mod-ification,degradation and final biological functions (Grubb and
Abel,2006), and comprise short- and long-chain aliphatic
glu-cosinolates (Ile, Leu, Val, Ala and Met), indolic glucosinolates
(Trp) and aromatic glucosinolates (Tyr and Phe)(Brown et al.,
2003;Clarke,2010;AgerbirkandOlsen,2012;Leoneetal.,2015).
Undernormalconditions,GSarechemicallystable,however,
dur-ingplantwoundresponsesthesecompoundsarehydrolyzedby
theenzymemyrosinasetoproduceisothiocyanates,nitriles,
thio-cyanates,epithionitrilesand oxazolidineswhichareresponsible
∗ Correspondingauthor.
E-mail:emadala@uj.ac.za(N.E.Madala).
forthereportedbiologicalactivitiesthereof(BonesandRossiter, 2006; Zandalinasetal., 2012).GS-derivedmolecules arehighly
water-solubleduetothehydroxyl-aminosulfategroupanda
-thioglucosylresidueattachedtothevariableR-groupontheGS
skeletal structure (Clarke, 2010; Vo et al., 2013; Förster et al., 2015a; Leone et al., 2015), thereby contributing to a high
bio-availabilityfollowinghumanconsumption.Inplants,GSareknown
toberesponsivetobothbioticandabioticstresses,andhavebeen
shown tobeinduced byvarious environmentalfactorssuchas
solarradiation,temperaturevariationandclimatechanges(Bones
andRossiter,2006;Zandalinasetal.,2012).Almostallthe
afore-mentionedstressorsofplantsareassociatedwithoxidativestress
(Bajguzand Hayat,2009; Demidchik,2015), therebysuggesting
a possible role of these compounds in mitigating the damages
imposedasaresultofsuchstress,aphenomenonwhichhasalso
been extended tohuman-related diseases (Tumer et al., 2015;
Williamsonetal.,1998).
http://dx.doi.org/10.1016/j.bjp.2017.05.012
T.Ramabulanaetal./RevistaBrasileiradeFarmacognosia27(2017)569–575
Recently, the GS components (4-(␣-l
-rhamnopyranosyloxy)-benzyl glucosinolate followed by three isomeric acetyl-4-(␣-l
-rhamnopyranosyloxy)-benzylglucosinolate(Ac-isomer-GSI,II,III)
ofMoringa oleiferaLam.have beenreportedtopossessindirect anti-oxidantactivityduetotheabilitytoregulateananti-oxidant
enzymaticprocessesinmammaliansystems(Tumeretal.,2015).
GShavealsobeenreportedtocontrolthedamagesofother
physio-logicalconditionsassociatedwithoxidativestresssuchasreducing
therisks of several cancers (colon, bladder and breast cancer)
(Björkmanetal.,2011).IthasbeenshownthatGShavetheability
toinactivatephaseIenzymes(cytochromeP-450)ortostimulate
phaseIIenzymes(glutathione-S-transferase),therebyeliminating
carcinogenicmetabolites(ZhangandTalalay,1994).Morerecently,
consumptionof GS derivedcompounds suchas isothiocyanates
(ITC)hasbeenshowntobebeneficialformammals,sincetheylead
totheup-regulationofxenobioticmetabolism(phaseIImetabolic
enzymes),associatedwithanincreasesintheantioxidant
capac-ity,thusleadingtoimprovedprotectionagainstvariouschronic
physiologicalconditions(TrakaandMithen,2009).
Aspreviouslymentioned,thelevelsofGSinplantsaresubject
toseveralenvironmentalfactorsand,assuch, createconditions
favoringtheproductionofthesecompoundsbyplants,ashasbeen
investigated(Försteretal.,2015a).AnincreaseinaGS,
glucotropae-olin,duetoUV-Bradiationtreatmentofnasturtium(Tropaeolum
majusL.)plantshasbeenreported(Schreineretal.,2009). Differ-entformsofradiationareknowntoinduceoxidativestressinplants (Esnaultetal.,2010;Hollósy,2002;KovácsandKeresztes,2002),
andthustheinvolvementofGScouldbetocontrolthedamagesof
radiation-inducedoxidativestress.
Moringaoleiferaisaversatileandwidelycultivatedspeciesin
themonogenericfamilyofMoringaceae,andisknowntocontain
GSmolecules(Fahey,2005;Försteretal.,2015a;Moyoetal.,2011; PopoolaandObembe,2013).AlmostallpartsoftheM.oleiferaplant
containvaryingamountofaromaticGSs,withtheleavescontaining
thehighestlevels(Clarke,2010;Moyoetal.,2011).Someofthe
reportedpharmacologicalpotencyoftheplanthavebeendirectly
correlatedtothepresenceoftheseGSs(Clarke,2010).
Recently,wehaveshownthatM.oleiferadoesnotproduceother
highlysoughtafterpharmacologicallyrelevantmetabolites(rutin
foranexample)incomparisontootherrelatedspecies,M.
oval-ifolia(Makitaetal.,2016).Moreover,wefurtherspeculatedthat
productionofsuchmetabolitescouldbeinfluencedbyvarious
fac-torssuchasenvironmentalconditionsand thegeneticmake-up
oftheplants(Makitaetal.,2016).Elsewhere,thelevelsof
health-promotingmetaboliteshavebeenshowntobeaffectedbyionizing
radiation(Ramabulana etal.,2015,2016).Radiationis apotent
inducerofoxidativestress,andithasbeenusedtoidentify metabo-liteswithanti-oxidativepropertiesinvariousplants(Mittler,2002).
Withincreasingevidenceontheanti-oxidativepropertiesofGS
molecules(Guerrero-Beltránetal.,2012)andaspotentialagents
for ameliorating oxidative stress-associated diseases (
Dinkova-KostovaandKostov,2012),itisimportanttostudybioticandabiotic
factorswithpotentialofenhancingthelevelsofthesecompounds.
Assuch,inthecurrentstudy,apotentformofradiation,namely
gammaradiationwasusedtotriggeroxidativestressinM.oleifera
leaves.SubsequentperturbationsinthelevelsofGSswere
moni-toredusingUHPLC-ESI-qTOF-MS-basedfingerprinting.
Materialsandmethods
Plantmaterial
TwomontholdMoringaoleiferaLam.,Moringaceae,plantswere
obtainedfrom thePatience Wellness Centre farm in
Lebowak-gomo,South Africa.The plantspecieswasauthenticated, and a
voucherspecimen(withvouchernumberNEM001)wasprepared
anddepositedattheDepartmentofBotany,Universityof
Johan-nesburg,SouthAfrica.
Gammaradiationprocedure
Plants were irradiatedas previously described (Ramabulana
etal.,2015,2016).IrradiationwasperformedatNuclearEnergy
CooperationofSouthAfrica(NECSA)(Phelindaba,Pretoria,South
Africa). Briefly, fifteen plants were irradiated with a Cobalt-60
source(atadoserateof22kGy/h)insideawell-protectedchamber, alongwithfifteennon-irradiatedcontrolplants.Variousradiation
doses(0.1–8kGy)weretestedand2kGydosewasfoundtobemore
potentasshownpreviously(Ramabulanaetal.,2015).Total
radi-ationdoseabsorbedbyplantswasfurtherconfirmedbyHarwell
PerspexPolyMethylMethacrylateAmber(PMMA)3042
dosime-ters(HarwellCo,UnitedKingdom).
Metaboliteextraction
From the optimization results achieved with our preceding
studies, plant leaf material was harvested a day (24h)
post-radiation and dried at 50◦C for 72h(Ramabulana et al., 2015,
2016).Thedried plantmaterial wasground andextracted with
80%aqueousmethanolasdescribedbyRamabulanaetal.(2015,
2016).Theextractswereconcentrated,reconstitutedin50% aque-ousmethanolandstoredat−20◦Cuntilanalyzed.
Chromatographyandmassspectrometryanalyses
Threetechnicalrepeatsofthehydromethanolicextracts(5l)
were analyzed using an Acquity UHPLC equipped with an
AcquityBEHC18reversephasecolumn(150mm×2.1mm,1.7m)
(WatersCorporation,MA,USA).ThemobilephaseAconsistedof
0.1%formic acidin deionizedwater, while themobile phase B
consistedof0.1%formicacidinacetonitrile(RomilPureChemistry,
Cambridge,UK).Theelutiongradientstartedat98%Auntil5%at
26minfor2min,andthenreturnedtoinitialconditionsof98%Aat
28minfor2minwitharuntimeof30minataconstantflowrate
of0.4ml/min.Chromatographicseparation/elutionwasmonitored
usinga photodiode-arraydetector(PDA)collecting20 spectra/s
betweenthe200and500nmrange.Inaseconddetection,aSynapt
G1high-definitionmassspectrometer(MS) wasusedoperating
inbothpositiveandnegativeelectrosprayionization(ESI)modes.
Briefly,thefollowingMSconditionswereusedasoptimal
exper-imentalconditions:thecapillaryvoltageof2.5kV,multichannel
platedetectorpotentialof1600V,sampleconepotentialof30V,
desolvationtemperatureof450◦C,sourcetemperatureof120◦C,
conegasflow of 50l/hand desolvation gasflow of550l/h.For
MSfragmentationexperiments,theMSacquisitionmethodwith
lowcollisionenergyrampof10–30eVandahighcollisionenergy
rampof15–60eVwasusedtogeneratetypicalMSE
fragmenta-tionpatterns.MassLynxTMand MarkerLynxTM software(Waters
Corporation,MA, USA) were used tovisualize and analyzethe
UHPLC-qTOF-MSrawdatasoastogeneratedatamatrixforfurther
statisticalmodeling.
Metaboliteidentificationandstatisticalanalyses
TheUHPLC-ESI-MSdatacollectedinnegativeionizationmode
wasanalyzedusingMarkerLynxTMXSsoftwareforpeakalignment,
peak finding, peak integration and retention time (Rt)
correc-tionwiththefollowingparameters:Rt rangeof1–27min,mass
rangeof 100–1000Da, masstoleranceof0.05Da,Rt windowof
0.2min.Datawasnormalizedtototalintensity(area).Theacquired
T.Ramabulanaetal./RevistaBrasileiradeFarmacognosia27(2017)569–575
Sweden)forPrincipalcomponentanalysis(PCA)andOrthogonal
projection to latent structures-discriminant analysis (OPLS-DA)
computation(Ramabulanaetal.,2015)and,usingthesemodels,
possiblebio-markersshowingdifferentialaccumulationacross
dif-ferenttreatmentswereidentified(Madalaetal.,2012;Ramabulana etal.,2015).ThedatamatrixwasalsoexportedtoMicrosoftExcel
and,usingtheareaunderthepeakcorrespondingtothe
respec-tivemasses(m/z)ofknownGSmoleculesfromM.oleifera(Förster
etal.,2015a,b),weresearchedforandfurtherusedtocreate
box-and-whiskersplotsusingSPSSversion22 software(IBM,United
StatesofAmerica,www.ibm.com/SPSSStatistics).Furthermore,GS
moleculeswithstatisticalsignificancewerecomputedusingthe
studentt-testinMicrosoftExcel.Here,ap-valueof<0.01indicates thatthefoldincreasesoftheidentifiedmetabolitesarestatistically significant.
Tofurtherconfirmtheidentificationofmetabolites,the
frag-mentationpatternsgeneratedwiththeuseofdifferentcollision
energies were compared with the already existing knowledge.
Briefly,themolecularformulaeofallthepeakscorrespondingto
GSmoleculeswerecomputedandselectedbasedonthecriterion
thatthesearewithin5mDamassaccuracywhencomparedtothe
calculatedmassofthecorrespondingmolecules.Metaboliteswere
thusannotatedaccordingtotheMetabolomicStandardsInitiatives,
level2identification(Sumneretal.,2007).
Resultsanddiscussion
Gammaradiation isan inducerof oxidative stressthat
sub-sequently activates complicated defense mechanisms in plants
(Ahujaetal.,2014;Esnaultetal.,2010).M.oleiferaisableto
syn-thesizeGSaspartofitssecondarymetabolites.Thepredominant
GSmoleculeinthisplantspeciesis4-(␣-l
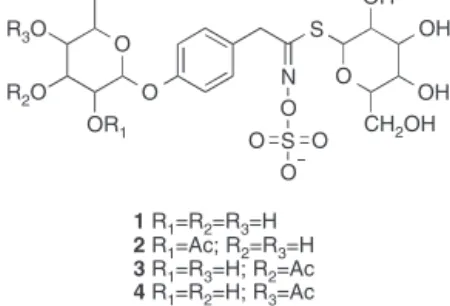
-rhamnopyranosyloxy)-benzylglucosinolate(1),knownasglucomoringin(Clarke,2010;
deGraafetal.,2015;Tumeretal.,2015).Thestructuraluniqueness
oftheseGSderivesfromthepresenceofasecondglycosylresidue
inadditiontothealreadyglycosylatedsidechain(Amagloetal.,
2010).Otherstructuralderivativesofglucomoringinsuchasthe
acylatedformsthereofhavealsobeenreportedinthisplant(Fig.1) (Försteretal.,2015a),makingthesemoleculesinterestingtostudy.
Moreremarkably,glucomoringinhasalwaysbeenthoughttoexist
onlyinM.oleifera.However,ithasalsobeenrecentlyreportedin Noccaeacaerulescensbuttheauthorscouldnotidentifythe acety-latedforms(deGraafetal.,2015).Thissuggeststheacylationof
glucomoringintobeanexclusivephenomenonofM.oleifera.
Inthecurrentstudy,gammaradiation-inducedoxidativestress
resultedinchangestothemetabolomeinM.oleiferaplants(Fig.2, Table1).UsinganUHPLC-ESI-qTOF-MS-basedtargetedmetabolite
fingerprinting approach, increasedlevels of GS molecules were
foundinplantsirradiatedwitha2kGydoseofgammaradiation
as compared to the control plants (Fig. 2; Table 1). Here, the
box-and-whiskers plots display an increase in the
concentra-tionsof glucomoringin and related GS molecules in M. oleifera
followinggammaradiationtreatment(Fig.2).Theaboveresults
provideasemi-quantitativeoverviewoftheamountofGSandits
derivativessince there arenocommerciallyavailablestandards
ofthesemoleculestoachieveabsolutequantification.Moreover,
the results indicate that the fold increase in the identified GS
werestatisticallysignificant,withalmostallhavingp-valuesof
less than 0.01 as shown in Table 1. Interestingly, it should be
re-emphasizedthatadoseof2kGywasfoundtobemorepotent
and non-lethal, thus inducing the highest levels of GS and its
derivatives.Preliminaryoptimizationshowedlowerdoses(0.1,0.5
and1.0kGy)tominimallyaffectthelevelsofGSanditsderivatives
butthelevelsabove2kGysuchas4kGand8kGywerefoundto
belethal,thuskillingtheplantsimmediatelyafterradiation.The
above phenomenon was also highlighted in studies conducted
withanotherplant,Phaseolusvulgaris(Ramabulanaetal.,2015).
Furthermore, the characterization of these metabolites was
achievedbymeansofaccuratemassMSresults(asshowninFig.3)
withtheuseoffragmentationpatternsandcomparisontoalready
publisheddata.Brieflymolecule1withprecursorion([M−H]−)at m/z570.0927(C20H29NO14S2)andRtof3.17minwasidentifiedas
4-(␣-l-rhamnosyloxy)-benzylglucosinolate(glucomoringin).The
acylatedformsofglucomoringin(2–4)producedisobaricprecursor ionsatm/z612.102(C22H31NO15S2).Interestingly,thesemolecules
elutedatdifferentRtand,inaccordancewithalreadypublished
results(Försteretal.,2015a;Tumeretal.,2015),thesethreeisomers
wereidentifiedasacetyl4-(␣-l-rhamnopyranosyloxy)-benzylGS
isomerI(2),II(3)andIII(4)elutingatRtof5.60min,6.46minand 9.63minrespectivey(Bennettetal.,2003;Försteretal.,2015a,b) (Table1).
R3O
R2O
CH2OH OH OH OH
S
S N
OR1
1 R1=R2=R3=H
2 R1=Ac; R2=R3=H
3 R1=R3=H; R2=Ac
4 R1=R2=H; R3=Ac O
O
O O
O
O O
Thepresenceoftheseacetylatedisomersposeanother
interest-ingbutchallengingdimensiontoourresultssincethefunctionof
100
0
3.00 4.00 5.00 6.00 7.00 8.00 9.00 10.00 11.00 12.00Time
9.60 612.1011
Ac-Isomer-GS III
Ac-Isomer-GS II Ac-Isomer-GS I
α-Isomer-GS
9.46 612.1042 5.60
612.1013 3.17
570.0936
%
Fig.1. UHPLC-ESI-qTOF-MSanalysesinnegativeionizationmodeofhydromethanolicextractsfrom2kGygammairradiatedMoringaoleiferashowingbasepeakintensity(BPI) chromatogramsof4-(␣-l-rhamnopyranosyloxy)-benzylglucosinolate(␣-rhamnoGS),acetyl-4-(␣-l-rhamnopyranosyloxy)-benzylglucosinolateisomerI(Ac-isomer-GSI),
T.Ramabulanaetal./RevistaBrasileiradeFarmacognosia27(2017)569–575
800
A
B
C
D
600
Peak intensity
P
eak intensity
400
200
0
200
100
100
50
0
Control Treated Control Treated
Control Treated Control Treated
120
100
80
60
40
20
0
500
400
300
200
100
0
Fig.2.Box-and-whiskersplotsshowingrelativecompositionoffouridentifiedglucosinolates(moringinderivatives),increaseddueto2kGygammaradiationtreatment ofM.oleiferawithstatisticalsignificanceofp<0.01.(A)4-(␣-l-Rhamnopyranosyloxy)-benzylglucosinolate;(B)acetyl-4-(␣-l-rhamnopyranosyloxy)-benzylglucosinolate
isomerI;(C)acetyl-4-(␣-l-rhamnopyranosyloxy)-benzylglucosinolateisomerII;and(D)acetyl-4-(␣-l-rhamnopyranosyloxy)-benzylglucosinolate,isomerIII.
Table1
Gammaradiation-inducedglucomoringinmoleculesinMoringaoleifera.
Peak Rt(min) Compoundname Mass(m/z) Elementalcomposition p-values Foldchange
1 3.09 4-(␣-l-Rhamnopyranosyloxy)-benzylglucosinolate(1) 570.0922 C20H29NO14S2 1.7×10−5 22
2 5.60 Acetyl-4-(␣-l-rhamnopyranosyloxy)-benzylglucosinolateisomerI(2) 612.1029 C22H31NO15S2 1.4×10−5 30
3 6.46 Acetyl-4-(␣-l-rhamnopyranosyloxy)-benzylglucosinolateisomerII(3) 612.1004 C22H31NO15S2 3.7×10−5 51
4 9.54 Acetyl-4-(␣-l-rhamnopyranosyloxy)-benzylglucosinolateisomerIII(4) 612.1044 C22H31NO15S2 1.5×10−8 10
thismodificationandtheeffectonthebiologicalactivityof glu-comoringinarenotknown.Thepresence ofstructurallyrelated (isomeric) metabolitesin plants is a knownphenomenon with aclassicalexamplebeingpositionalisomersofchlorogenicacids (Ncubeetal.,2014,2016).However,thepresenceofpositional iso-mersofchlorogenicacidsinplantsisalsonotfullyunderstood;but recentlyithasbeenspeculatedtobeastrategydeployedbyplants
toincreasetheconcentrationofthesemoleculesthrough
diversi-fication,soastocreatearichreservetobeutilizedwhenneeded (Karaköseetal.,2015).Assuch,thesamephenomenoncouldbetrue forthecaseofM.oleiferabutmoreresearchisneededtovalidatethis
hypothesis.Althoughalltheseisomersincreasedconcomitantly,
therelativeabundancelevelsinirradiatedplantsdiffered(Fig.1),
suggestingvaryingstabilityamongstthesecompounds.However,
elsewheretheseacetylisomerswerefoundtobeaffectedbythe
typeof extractionmethodand significantrearrangementswere
noted,withastandardofacetyl-isomer-GSIIIbeingconvertedto
acetyl-isomers-GSIandIIinabufferedsystemduetoanapparent
acetylmigration(Försteretal.,2015a).Thus,itcanbepostulated thatthediversityofGSmoleculesinM.oleiferacouldbetheresultof
bothenzymaticandnon-enzymaticreactionsinabiologicalsystem
respondingtoanoxidativestressenvironment.
EventhoughtheMSdatawasacquiredusingbothpositiveand
negativeESImodes,onlytheESInegativedatawasfoundtobe
suitableforidentificationoftheGSmoleculesandthiscouldbedue tothefactthatthesemoleculesareinherentlynegativelycharged).
TheaccurateMSspectraofthesemoleculescollectedatelevated
collisonenergy(30eV)areshowninFig.3.
Usingacombinationofmultivariateandunivariatestatistical
models(datanotshown),underlyingdifferencesinpeak
intensi-tiesoftheextractsobtainedfrombothcontrolandirradiatedplants
werenoted.ThesedifferencesinthelevelsofGSmoleculesisan
indicationofinduction oftheglucomoringinbiosynthesis
path-wayinreponsetotheoxidativestresstriggeredbytheradiation
treatment.Aspreviouslystated,GSmoleculeshavebeenshown
toaccumulatein plantsirradiatedwithUV-radiation(Schreiner
etal.,2009),whileotherresearchhasreportedthesemolecules
to be constitutively present in M. oleifera leaf extracts (Fahey,
2005;Försteretal.,2015b; Jansenetal.,2008;Vo etal.,2013).
T.Ramabulanaetal./RevistaBrasileiradeFarmacognosia27(2017)569–575
100
100 200 300 400 500 600 700 800 900 1000
0
%
100
100 200 300 400 500 600 700 800 900 1000
0
%
100
100 200 300 400 500 600 700 800 900 1000
0
%
100
100 200 300 400 500 600 700 800 900 1000
0
%
259.0064 328.0791 424.0306
570.0905
A
B
C
D
571.0988
650.0465 716.1448 952.1398
958.2131 708.1854
613.1076 612.0959
533.1224 323.1260
191.0526
193.0438
367.0981
612.0970
533.1294
613.1055
708.1782 966.2234
858.1284 693.0696
613.1198 612.1079
549.2479 259.0061 370.0934
Fig.3. SpectraofidentifiedGSsinM.oleiferaleafextractsofplantsirradiatedwith2kGydoseofgammaradiation.(A)4-(␣-l-Rhamnopyranosyloxy)-benzylglucosinolate;
(B)acetyl-4-(␣-l-rhamnopyranosyloxy)-benzylglucosinolateisomerI;(C)acetyl-4-(␣-l-rhamnopyranosyloxy)-benzylglucosinolateisomerII;and(D)acetyl-4-(␣-l
-rhamnopyranosyloxy)-benzylglucosinolateisomerIII.
induciblecomponentsofthisplantspeciesasthesewerefound
toincreaseuponradiationtreatment.Generally,GSmoleculesare
knownto respondagainst plantwounding (Bodnaryk, 1992), a
phenomenonwhichisinevitableduringleafharvestingandcould
furtherexplainwhythesecompoundsarereportedinnon-induced
leaveselsewhere(Rodríguez-Pérez et al.,2015).Previously,the
distributionand presence of these molecules inM. oleifera has
alsobeenreportedwithmixedoutcomes.Forinstance,onlyone
glucomoringinmoleculewasidentifiedinthecurrentstudybut
three distinct glucomoringin molecules were identified in M.
oleiferafromMadagascar(Rodríguez-Pérez etal.,2015).As
pre-viouslystated, glucomoringinwas alsorecentlyidentified in N.
caerulescens plants, but the distribution was only limited to a
fewsamplesanalyzedandabsentinotheraccessions/cultivars(de
Graafetal.,2015).Thesameauthorsconcludedthatthese differ-encesareduetoregionalgeneticvariationratherthantheinitially
thoughtenvironmentalfactorssuchas metaltoxicity (deGraaf
et al.,2015).Geneticvariation was furtherusedtojustify why
glucomoringinwasneverdetectedinsomespeciesrelatedtoN.
caerulescens(Tolràetal.,2000;Asadetal.,2013).Recently,
acetyl-(4-␣-l-rhamnopyranosyloxy)-benzyl GS isomers were foundto
onlyaccumulateinsome,butnotallM.oleiferaplantsofthesame ecotype(Försteretal.,2015b).Takentogether,alltheaboveresults
areanindicationthatthepresenceandrelativeconcentrationof
these compounds are subject tounderlying cellular conditions
or geneticmakeup of plants. Assuch, not allGS-containing M.
oleiferaplantswillhavesimilarGS-mediatedbio-activities. There-fore,studiesofconditionswiththeabilitytoincreasethelevelsof GSmoleculesinplantscapableofGSsynthesisareimportant.Inthis
regard,thedistributionofGSmoleculesinM.oleiferahasbeen stud-iedbyvaryingthecultivationconditionssuchassulfurfertilization
andwateravailability,andithasbeenshownthattheGScontent
increasedunderawater-deficientregiment,withtheeffectmore
pronouncedinselectedecotypes(Försteretal.,2015b).Thisagain
highlightstheimportanceofgeneticvariationandabioticstress
conditions.
Inthecurrentstudy,anincreaseinGScontentduetogamma
radiationwasnotedand,moreimportantly,alltheirradiatedplants exhibitedaconsistentresponse.Ingeneral,theinvolvementofGSs
againstoxidativestresscausedbybioticandabioticstresseshas
beenreportedelsewhere(Björkmanet al.,2011; Sardansetal.,
2011;Zhangetal.,2011;Zandalinasetal.,2012).Accumulation
oftheGScontentinplantstreatedwithradiation(i.e.UVlight)
hasbeenreportedinT.majus(Schreineretal.,2009), Arabidop-sisthaliana(Wangetal.,2011)andbroccoli(Pérez-Balibreaetal., 2008;Mewisetal.,2012).Therefore,takentogether,theincreasein
GSmoleculesinresponsetoamorepotentstimulatorofoxidative
stressintheformof gammaradiationisanindicationof
possi-bleanti-oxidativepropertiesofthesemoleculesinplants.Hitherto, thereareverylimitedreportsonthedirectanti-oxidantactivityof
GSmoleculesandwhetherthesecompoundsfunctionas
indepen-dententitiesorsynergistically(Försteretal.,2015a,b).Thoughthe
currentresultshasindicatedgammaradiationasapotentinducer
ofmedicinallyimportantmetabolites,careneedstobetakensince
thistypeofradiationisknowntocauseirreversibledamagesto
foodvitaminssuchasvitaminC(Dionísioetal.,2009).Assuch,
pro-longedexposuretomilderformsofradiationcanbeusedinstead
T.Ramabulanaetal./RevistaBrasileiradeFarmacognosia27(2017)569–575
Conclusion
Thestudyrepresentsaproofonconceptmanipulationof
health-beneficialneutraceuticalsinamedicinalplantwheretheinducer
leavesnochemicalresidue.Here,thetargetedmetabolite
profil-ingconfirmsthepresenceof structurallydiverseglucomoringin
moleculesinM.oleiferaand demonstrates therelative
accumu-lationpost-gammaradiationtreatment.Thecurrent resultsalso
showtheGSmoleculesofM.oleiferatobepartoftheinducible
defensemechanismofplantsratherthanconstitutivecomponents
aspreviously perceived.Our resultssupportsan inplanta
anti-oxidativeroleforglucomoringinandacylatedderivativesfromM.
oleifera, and by extension in thehuman body when consumed
asherbalsupplement. Assuch, consumptionof non-inducedM.
oleiferaleafmaterialdoesnotnecessarilyguaranteethereported
activitiesassociated withthesemolecules.However, theuseof
radiationmayprovideanattractivewaytoenhanceGScontent
and,as such, Moringa plants grown underlight intensive
envi-ronmentscontributingtophoto-oxidativestress,areexpectedto
containahighercontentthereof.Moreover,irradiatedplantsare
alsoexpectedtoexhibitenhancedpharmacologicalpropertiesand,
assuch,futurestudiesshouldfocusonevaluationandbiological
testingofextractspreparedfromirradiatedplants.
Authors’contributions
NEM,RDMandIADconceivedofthestudy,TRconductedthe
experiments.TR,ARN,NEMandPASanalyzedtheMSdata.NEM,
RDM,LAPandIADsupervisedtheprojectandLAPparticipatedin
criticalreadingofthemanuscript.Allauthorsreadandapproved
thefinalmanuscript.
Conflictsofinterest
Theauthorsdeclarenoconflictsofinterest.
Acknowledgments
SouthAfricanNationalResearchFoundation(NRF),University
ofJohannesburgandNuclearEnergyCorporationofSouthAfrica
(NECSA)arethankedforfinancialsupport.MrManfredRellingis
thankedforhisassistancewithradiationexperiments.
References
Agerbirk,N.,Olsen,C.E.,2012.Glucosinolatestructuresinevolution.Phytochemistry 77,16–45.
Ahuja,S.,Kumar,M.,Kumar,P.,Gupta,V.K.,Singhal,R.K.,Yadav,A.,Singh,B.,2014. Metabolicandbiochemicalchangescausedbygammairradiationinplants.J. Radioanal.Nucl.Chem.300,199–212.
Amaglo,N.K.,Bennett,R.N.,LoCurto,R.B.,Rosa,E.S.,LoTurco,V.,Giuffrida,A.,Curto, A.,Lo,Crea,F.,Timpo,G.M.,2010.Profilingselectedphytochemicalsand nutri-entsindifferenttissuesofthemultipurposetreeMoringaoleiferaL.,grownin Ghana.FoodChem.122,1047–1054.
Asad,S.A.,Young,S.,West,H.,2013.Effectofnickelandcadmiumonglucosinolate productioninThlaspicaerulescens.PakistanJ.Bot.45,495–500.
Bajguz,A.,Hayat,S.,2009.Effectsofbrassinosteroidsontheplantresponsesto environmentalstresses.PlantPhysiol.Biochem.47,1–8.
Bennett,R.N.,Mellon,F.,Foidl,N.,Pratt,J.H.,Dupont,M.S.,Perkins,L.,Kroon,P.,2003. Profilingglucosinolatesandphenolicsinvegetativeandreproductivetissues ofthemulti-purposetreesMoringaoleiferaL.(Horseradishtree)andMoringa stenopetalaL.J.Agric.FoodChem.51,3546–3553.
Björkman,M.,Klingen,I.,Birch,A.N.E.,Bones,A.M.,Bruce, T.J.A.,Johansen,T.J., Meadow,R.,Mølmann,J.,Seljåsen,R.,Smart,L.E.,Stewart,D.,2011. Phytochemi-calsofBrassicaceaeinplantprotectionandhumanhealth–influencesofclimate, environmentandagronomicpractice.Phytochemistry72,538–556.
Bodnaryk,R.P.,1992.Effectsofwoundingonglucosinolatesinthecotyledonsof oilseedrapeandmustard.Phytochemistry31,2671–2677.
Bones,A.M.,Rossiter,J.T.,2006.Theenzymicandchemicallyinduceddecomposition ofglucosinolates.Phytochemistry67,1053–1067.
Brown,P.D.,Tokuhisa,J.G.,Reichelt,M.,Gershenzon,J.,2003.Variationof glu-cosinolateaccumulationamongdifferentorgansanddevelopmentalstagesof Arabidopsisthaliana.Phytochemistry62,471–481.
Clarke,D.B.,2010.Glucosinolates,structuresandanalysisinfood.Anal.Chem.9660, 310–325.
deGraaf,R.M.,Krosse,S.,Swolfs,A.E.M.,teBrinke,E.,Prill,N.,Leimu,R.,vanGalen, P.M.,Wang,Y.,Aarts,M.G.M.,vanDam,N.M.,2015.Isolationand identifica-tionof4-␣-rhamnosyloxybenzylglucosinolateinNoccaeacaerulescensshowing intraspecificvariation.Phytochemistry110,166–171.
Demidchik,V.,2015.Mechanismsofoxidativestressinplants:fromclassical chem-istrytocellbiology.Environ.Exp.Bot.109,212–228.
Dinkova-Kostova,A.T.,Kostov,R.V.,2012.Glucosinolatesandisothiocyanatesin healthanddisease.TrendsMol.Med.18,337–347.
Dionísio,A.P.,Gomes,R.T.,Oetterer,M.,2009.Ionizingradiationeffectsonfood vitamins:areview.Braz.Arch.Biol.Technol.52,1267–2127.
Esnault,M.,Legue,F.,Chenal,C.,2010.Ionizingradiation:advancesinplantresponse. Environ.Exp.Bot.68,231–237.
Fahey,J.,2005.Moringaoleifera:areviewofthemedicalevidenceforits nutri-tional,therapeuticand prophylacticproperties,Part1. TreesLife J. 1,15, http://www.tfljournal.org/article.php/20051201124931586.
Fahey,J.W.,Zalcmann,A.T.,Talalay,P.,2001.Thechemicaldiversityanddistribution ofglucosinolatesandisothiocyanatesamongplants.Phytochemistry56,5–51. Förster,N.,Ulrichs,C.,Schreiner,M.,Müller,C.T.,Mewis,I.,2015a.Developmentof
areliableextractionandquantificationmethodforglucosinolatesinMoringa oleifera.FoodChem.166,456–464.
Förster,N.,Ulrichs,C.,Schreiner,M.,Arndt,N.,Schmidt,R.,Mewis,I.,2015b. Eco-typevariabilityingrowthandsecondarymetaboliteprofileinMoringaoleifera: impactofsulfurandwatervailability.J.Agric.FoodChem.63,2852–2861. Guerrero-Beltrán,C.E.,Calderón-Oliver,M.,Pedraza-Chaverri,J.,Chirino,Y.I.,2012.
Protectiveeffectofsulforaphaneagainstoxidativestress:recentadvances.Exp. Toxicol.Pathol.64,503–508.
Grubb,C.D.,Abel,S.,2006.Glucosinolatemetabolismanditscontrol.TrendsPlant Sci.11,89–100.
Hollósy,F.,2002.Effectsofultravioletradiationonplantcells.Micron33,179–197. Jansen,J.J.,Allwood,J.W.,Marsden-Edwards,E.,vanderPutten,W.H.,Goodacre,R., vanDam,N.M.,2008.Metabolomicanalysisoftheinteractionbetweenplants andherbivores.Metabolomics5,150–161.
Karaköse,H.,Jaiswal,R.,Deshpande,S.,Kuhnert,N.,2015.Investigationofthe pho-tochemicalchangesofchlorogenicacidsinducedbyultravioletlightinmodel systemsandinagriculturalpracticewithSteviarebaudianacultivationasan example.J.Agric.FoodChem.63,3338–3347.
Kovács,E.,Keresztes,Á.,2002.EffectofgammaandUV-B/Cradiationonplantcells. Micron33,199–210.
Leone,A.,Spada,A.,Battezzati,A.,Schiraldi,A.,Aristil,J.,Bertoli,S.,2015.Cultivation, genetic,ethnopharmacology,phytochemistryandpharmacologyofMoringa oleiferaleaves:anoverview.Int.J.Mol.Sci.16,12791–12835.
Madala,N.E.,Steenkamp,P.A.,Piater,L.A.,Dubery,I.A.,2012.Collisionenergy alter-ationduringmassspectrometricacquisitionisessentialtoensureunbiased metabolomicanalysis.Anal.Bioanal.Chem.404,367–372.
Makita,C.,Chimuka,L.,Steenkamp,P.,Cukrowska,E.,Madala,E.,2016.Comparative analysesofflavonoidcontentinMoringaoleiferaandMoringaovalifoliawiththe aidofUHPLC-qTOF-MSfingerprinting.SouthAfricanJ.Bot.105,116–122. Mewis,I.,Schreiner,M.,Nguyen,C.N.,Krumbein,A.,Ulrichs,C.,Lohse,M.,Zrenner,
R.,2012.UV-Birradiationchangesspecificallythesecondarymetaboliteprofile inbroccolisprouts:inducedsignalingoverlapswithdefenseresponsetobiotic stressors.PlantCellPhysiol.53,1546–1560.
Mithen,R.,2001.Glucosinolates–biochemistry, geneticsandbiological activity. PlantGrowthRegul.34,91–103.
Mittler,R.,2002.Oxidativestress,antioxidantsandstresstolerance.TrendsPlant Sci.7,405–410.
Moyo,B.,Masika,P.J.,Hugo,A.,Muchenje,V.,2011.Nutritionalcharacterizationof Moringa(MoringaoleiferaLam.)leaves.AfricanJ.Biotechnol.10,12925–12933. Ncube,E.N.,Mhlongo,M.I.,Piater,L.,Steenkamp,P.,Dubery,I.,Madala,N.E.,2014. Analysesofchlorogenicacidsandrelatedcinnamicacidderivativesfrom Nico-tianatabacumtissueswiththeaidofUPLC-QTOF-MS/MSbasedonthein-source collision-induceddissociationmethod.Chem.Cent.J.8,1–10.
Ncube,E.N.,Steenkamp,P.A.,Madala,N.E.,Dubery,I.A.,2016.Chlorogenicacids biosynthesisinCentellaasiaticacellsisnotstimulatedbysalicylicacid manipu-lation.Appl.Biochem.Biotechnol.179,685–696.
Pérez-Balibrea,S.,Moreno,D.A.,Garcia-Viguera,C.,2008.Influence oflighton health-promotingphytochemicalsofbroccolisprouts.J.Food.Agric.Environ. 88,904–910.
Popoola,J.O.,Obembe,O.O.,2013.Localknowledge,usepatternandgeographical distributionofMoringaoleiferaLam.(Moringaceae)inNigeria.J. Ethnopharma-col.150,682–691.
Ramabulana,T.,Mavunda,R.D.,Steenkamp,P.A.,Piater,L.A.,Dubery,I.A.,Madala, N.E.,2015.SecondarymetaboliteperturbationsinPhaseolusvulgarisleavesdue togammaradiation.PlantPhysiol.Biochem.97,287–295.
Ramabulana,T.,Mavunda,R.D.,Steenkamp,P.A.,Piater,L.A.,Dubery,I.A.,Madala, N.E.,2016.Perturbationofpharmacologicallyrelevantpolyphenoliccompounds inMoringaoleiferaagainstphoto-oxidativedamagesimposedbygamma radia-tion.J.Photochem.Photobiol.B:Biol.156,79–86.
T.Ramabulanaetal./RevistaBrasileiradeFarmacognosia27(2017)569–575
Sardans,J.,Pe˜nuelas,J.,Rivas-Ubach,A.,2011.Ecologicalmetabolomics:overview ofcurrentdevelopmentsandfuturechallenges.Chemoecology21,191–225. Schreiner,M.,Krumbein,A.,Mewis,I.,Ulrichs,C.,Huyskens-Keil,S.,2009.Short-term
andmoderateUV-Bradiationeffectsonsecondaryplantmetabolismindifferent organsofnasturtium(TropaeolummajusL.).Innov.FoodSci.Emerg.Technol.10, 93–96.
Sumner,L.W.,Amberg,A.,Barrett,D.,Beale,M.H.,Beger,R.,Daykin,C.A.,Fan, T.W.-M.,Fiehn,O.,Goodacre,R.,Griffin,J.L.,Hankemeier,T.,Hardy,N.,Harnly,J., Higashi,R.,Kopka,J.,Lane,A.N.,Lindon,J.C.,Marriott,P.,Nicholls,A.W.,Reily, M.D.,Thaden,J.J.,Viant,M.R.,2007.Proposedminimumreportingstandards forchemicalanalysisChemicalAnalysisWorkingGroup(CAWG)Metabolomics StandardsInitiative(MSI).Metabolomics3,211–221.
Tolrà,R.P.,Alonso,R.,Poschenrieder,C.,Barceló,D.,Barceló,J.,2000. Determi-nationofglucosinolatesinrapeseedandThlaspicaerulescensplantsbyliquid chromatography-atmosphericpressurechemicalionizationmassspectrometry. J.Chromatogr.889,75–81.
Traka,M.,Mithen,R.,2009.Glucosinolates,isothiocyanatesandhumanhealth. Phy-tochem.Rev.8,269–282.
Tumer,T.B.,Rojas-Silva,P.,Poulev,A.,Raskin,I.,Waterman,C.,2015.Directand indi-rectantioxidantactivityofpolyphenol-andisothiocyanate-enrichedfractions fromMoringaoleifera.J.Agric.FoodChem.63,1505–1513.
Vo,Q.V.,Trenerry,C.,Rochfort,S.,Wadeson,J.,Leyton,C.,Hughes,A.B.,2013. Synthe-sisandanti-inflammatoryactivityofaromaticglucosinolates.BioorganicMed. Chem.21,5945–5954.
Wang,Y.,Xu,W.-J.,Yan,X.-F.,Wang,Y.,2011.Glucosinolatecontentandrelated geneexpressioninresponsetoenhancedUV-BradiationinArabidopsis.African J.Biotechnol.10,6481–6491.
Williamson,G.,Faulkner,K.,Plumb,G.W.,1998.Glucosinolatesandphenolicsas antioxidantsfromplantfoods.Eur.J.CancerPrev.7,17–21.
Zandalinas,S.I.,Vives-Peris,V.,Gómez-Cadenas,A.,Arbona,V.,2012.Afastand precisemethodtoidentifyindolicglucosinolatesandcamalexininplantsby combiningmassspectrometricandbiologicalinformation.J.Agric.FoodChem. 60,8648–8658.
Zhang,W.J.,Björn,L.O.,2009.Theeffectofultravioletradiationontheaccumulation ofmedicinalcompoundsinplants.Fitoterapia80,207–218.
Zhang,J.,Sun,X.,Zhang,Z.,Ni,Y.,Zhang,Q.,Liang,X.,Xiao,H.,Chen,J.,Tokuhisa, J.G.,2011. Phytochemistrymetaboliteprofilingof Arabidopsisseedlingsin response to exogenoussinalbin and sulfurdeficiency. Phytochemistry 72, 1767–1778.