w w w . r b h h . o r g
Revista
Brasileira
de
Hematologia
e
Hemoterapia
Brazilian
Journal
of
Hematology
and
Hemotherapy
Original
article
Everolimus
as
a
single
agent
in
refractory
or
relapsed
Hodgkin’s
lymphoma:
the
Brazilian
Named
Patient
Program
Experience
Talita
Máira
Bueno
da
Silveira
da
Rocha
∗,
Sergio
Costa
Fortier,
Thais
Rodrigues
da
Cunha
Fischer,
Guilherme
Fleury
Perini,
Rafael
Dezen
Gaiolla,
Laura
Fogliatto,
Marcia
Torresan
Delamain,
Andressa
Fragoso
da
Costa,
Nelson
Siqueira
de
Castro,
Wolney
Gois
Barretos,
Cármino
Antonio
de
Souza,
Valéria
Buccheri,
Carlos
Sérgio
Chiattone
FaculdadedeCiênciasMédicasdaSantaCasadeSãoPaulo(FCMSCSP),SãoPaulo,SP,Brazil
a
r
t
i
c
l
e
i
n
f
o
Articlehistory:
Received2September2016 Accepted23March2017 Availableonline11May2017
Keywords: Everolimus
Hodgkin’slymphoma Refractory
a
b
s
t
r
a
c
t
Background:Despiteallthescientificprogressthathasbeenmadeonunderstandingthe
disease,prognosisforpatientswithrelapsedandrefractoryHodgkin’slymphomaremains poorandthetreatmentispalliativeinthemajorityofthecases.Thus,theaimofthisstudy wastopresenttheresultsonthecompassionateuseofeverolimusinagroupofpatients whoweremonitoredatninedifferentcentersinBrazil.
Methods:A10-mgoraldoseofeverolimuswasgiventoeachpatientdaily.Responsetimewas
evaluatedfromthebeginningofmedicationuseuntillossofresponse,toxicityormedical decisiontoceasetreatment.
Results:Thirty-threepatientswereevaluated.Themedianageatthebeginningof
medica-tionadministrationwas29years.Patientshadreceivedamedianoffivepriortherapies. Overallresponseratewas45.4%,with13patientsachievingpartialresponse,twoachieved clinicalresponse,14remainedwithstabledisease,twohaddiseaseprogression,andtwo werenotevaluated.Patientsreceivedamedianof14cycles.Progression-freesurvivalwas ninemonths,andoverallsurvivalwasestimatedtobe36months.Threepatientsused themedicationformorethanfouryears.Themostfrequentlyreportedadverseeventswere thrombocytopeniaandhypercholesterolemia.Threepatientshadpulmonarytoxicity.Grade IIIandIVadverseeventsoccurredin39%ofthepatients.
Conclusion:Everolimuswasfoundtoprovidearesponseinagroupofpatientswithrefractory
orrelapsedHodgkin’slymphomawhohadadequatetolerabilitytothedrug.
©2017Associac¸ ˜aoBrasileiradeHematologia,HemoterapiaeTerapiaCelular.Published byElsevierEditoraLtda.ThisisanopenaccessarticleundertheCCBY-NC-NDlicense (http://creativecommons.org/licenses/by-nc-nd/4.0/).
∗ Correspondingauthorat:HematologyandHemotherapyDisciplineoftheMedicalClinicDepartment,FaculdadedeCiênciasMédicasda
SantaCasadeSãoPaulo(FCMSCSP),RuaDr.CesárioMottaJr.,112,SantaCecília,01221020SãoPaulo,SP,Brazil. E-mailaddress:tasilveira6@yahoo.com.br(T.M.Rocha).
http://dx.doi.org/10.1016/j.bjhh.2017.03.008
Introduction
Itisknownthatalmost80%ofthepatientswithHodgkin’s lymphoma (HL) can be cured at first treatment. How-ever, 10–15% will relapse and 5% of treated patients will not respond to the initial therapy and will become pri-mary refractory. For these patients, the gold standard therapyishigh-dose chemotherapyfollowed byautologous hematopoietic stem cell transplantation (HSCT). This sec-ondtreatmentcanstillrescue50%ofrelapsedandrefractory patients.1
Relapsedor refractorypatientsare amajorconcernand choosingthemostsuitabletreatmentforthemremainsa chal-lenge.Inthesecases,despitealltheprogressthathasbeen madeinthelastyears,prognosisremainspoor,andtreatment willbepalliativeinthemajorityofcases.2,3Inaddition,elderly patientswhoarenotsuitableforallogeneicHSCTbecauseof thetoxicityoftheregimen,areagroupforwhomchoosingthe correcttherapyisdifficult.4
InAugust2011,theanti-CD30conjugatedantibody, bren-tuximab vedotin, was the first drug approvedfor patients withrelapsedandrefractorydiseaseafterautologousHSCT. However,althoughthisantibodyshowedgoodresponsesin chemo-refractorypatients,witha75%overallresponserate (ORR – complete response/partial response), most patient responsesendwithinsixmonths,withonlyafew maintain-ing a response after20 months.5 This treatment modality isgenerallyveryinterestingasabridgeforallogeneicHSCT. Consideringthesamegroupofpatients,anotherdrug, anti-PD1(nivolumab),was approvedbythe AmericanFood and DrugAdministration(FDA)recentlyaftertheresultsofaphase IIstudyshowedaveryimpressiveORRof87%.
AnotherphaseIIstudyexaminedtheuseofeverolimusin refractoryandrelapsedpatientsandshowedanORRof47%, withverygoodandprolongedresponsesandfewtoxicities.6 Everolimusisamechanistictargetoftherapamycin(mTOR) inhibitorandisthefocusofthecurrentpaper.
mTORisaserine/threoninekinaseinvolvedincellgrowth, metabolismandcellsurvival,regulationofthedifferentstages of the cell cycle, and controlling the checkpoints that are responsibleforthecell’sresponsestoDNAdamage.7–9 Further-more,thereisevidencethatmTORisanimportantcomponent ofangiogenesis, especially in the phosphatidylinositol-4,5-bisphosphate3-kinase(PI3K)/Akt/mTORactivation pathway. Thisaxisisdirectlyassociatedwiththevascularendothelial growthfactor(VEGF),asitsactivationleadstothe transcrip-tion of the HIF-1a pathway, which consequently increases VEGFlevels.10
It is known that in HL there is an activation of the PI3K/Akt/TORC1 axis with consequent cell proliferation, angiogenesis,andtumorcellsurvival.10–12 Oneinvitrostudy demonstrated that everolimusacts onanother pathway of HL tumorcells, namely, the CCAAT/enhancer binding pro-tein beta (C/EBPb) pathway, decreasing activation offactor nuclear kappa B (NF-kB) and consequently inhibiting cell proliferation.13
Therefore,theaimofthisstudyistopresenttheresultson thecompassionateuseofeverolimusinagroupofpatients whoweremonitoredatninedifferentcentersinBrazil.
Methods
and
patients
Thisstudyisaretrospectiveanalysisofrefractoryandrelapsed HLpatientsenrolled inaNamedPatientProgram involving ninecentersinBrazil.Thefirstpatientstartedthetreatment inNovember2010andthelast,inMarch2015.
Inorder tobeconsidered forthe currentstudy,patients were required to be fully eligible, that is, to be consid-ered refractory/relapsed after autologousand/or allogeneic HSCT.TheEasternCooperativeOncologyGroup(ECOG)score requestedwas<2.Patientswerealsorequiredtobe≥18years old,withanabsoluteneutrophilcount(ANC)≥1000×106/L, platelets ≥75,000×106/L, hemoglobin ≥8g/dL,serum creat-inine ≤2× upper limit, serum total bilirubin ≤2× upper limitofnormal(ULN)andaspartateaminotransferase(AST) ≤3×ULN.Itwasrecommendedthatthemedicationshould not be given topatients who had recently received radio-therapy(within fourweeks)orimmunosuppressivetherapy (within three weeks), those who were using chronic sys-temic immunosuppressive agents, such as corticosteroids, hadseverehemorrhagicdiathesisorpresentedsevere uncon-trolled comorbidities(such as diabetesmellitus,infections, severe liver disease, lung disease with severe functional impairment).Moreover,femalepatientswhowerepregnant orwerebreastfeedingdidnotreceivethemedication.
Theinitialdoseof10mg/daywassuggestedbythe manu-facturerbasedonpreviousphaseIandIIstudies.Decreasing thedoseto5mg/dayor5mgeveryotherdaywasallowedwhen adverseeventsoccurred.
Data were requested from the 13 centers that had had patientswhoparticipatedintheNamedPatientProgram,but onlyninecenterssenttherequesteddata.
ThestudywasapprovedbytheEthicsCommitteesofall participatingcenters(HospitalSantaCasaSãoPaulo, Hospi-talSantaMarcelinadeSãoPaulo,HospitaldasClínicasdeSão Paulo–FMUSP,HospitalIsraelitaAlbertEinstein,Hospitaldas ClínicasdaUNICAMP,HospitaldasClínicasdeBotucatu, Hos-pitaldasClínicasdaUFRS,HospitaldoCâncerdeBarretosand HospitalSantaRitadeCássia).Informedconsentwasobtained fromallpatientsincludedinthestudy.
AnExcelspreadsheetwas senttoall participating insti-tutions, who were asked to provide the following data: patient’s initials; gender; histologic subtype; date of birth; dateofdiagnosis;stageofthediseaseatdiagnosis; interna-tionalprognosticscore(IPS)orearly-stageriskfactor(bulky mediastinal mass,2 ormore nodalsites, elevated erythro-cyte sedimentation rate); treatment response; number of relapses; number oflines oftreatment; dateofautologous HSCT(ifperformed);dateofallogeneicHSCT(ifperformed); date everolimus began being administered; best response observed; response assessmentmethod [positron emission tomography–computed tomography (PET–CT) or computed tomography(CT)];responseduration;dateeverolimus admin-istration was ceased; reason for treatment interruption; degreeoftoxicity;dateofdeathordateoflastcontact.
disappearanceofall clinicaland radiologicevidenceofthe disease;apartialresponsewasdefinedasshowingagreater than50%reductioninthenumberofsitesthatwereaffected bythedisease;andrefractorinesswasdefinedasnotmeeting theabovecriteria.
The time of response assessment was defined by the attendingphysician.Atthereferencecenter,SantaCasaSão Paulo,assessmentswereconductedeveryfourmonthswith CTand/orPET–CT,dependingonavailability.Patient’s medi-cationwasmaintainediftheyshowedaresponseoriftheir diseasewasstable.Becauseassessmentswere madeat dif-ferent centers,the criteria forinterruptingtreatment were individualandweremostlyrelatedtodiseaseprogressionand toxicity.Someofthepatientshadnodaldiseaseprogression, butastheyshowedclinicalresponsewithoutconstitutional symptoms,theyweremaintainedundertreatmentuntilloss ofclinicalresponse.
Statisticalanalysis
Standarddescriptiveanalyseswerecarriedout.The descrip-tive assessment of the stage of the disease comprised chemotherapy;first-lineresponse;numberoflinesof treat-ment; type of HSCT performed; age at the beginning of everolimus administration; time between diagnosis and beginning oftreatment witheverolimus;response to treat-ment; duration of medication use; cause of treatment interruption;adverseevents.
Overallsurvivalwasdefinedasthetimebetweendateof diagnosis and date ofdeath or last follow-up. Progression freesurvival(PFS)wasdefinedasthetimebetweenthedate ofinitial diagnosis and the date ofdisease progressionor deathfromanycause,whichevercamefirst.TheKaplan–Meier estimatorwasusedtoexaminesurvivalcurves,whichwere comparedusingthelogranktestintheStatisticalPackagefor theSocialSciences(SPSS)version20.
Theresponseratewasestimatedastheratiobetweenthe numberofresponsesandthenumberofpatientswhowere eligibleforassessment.Abimodal95%confidenceinterval(CI) wasusedtocalculateoverallresponse.
ToxicitywasclassifiedasgradesI,II,IIIandIVforanyevent relatedtotheuseofmedication.
Results
Data from the 33 patients with refractoryand/or relapsed HLwhosetreatmentwitheverolimuswasinitiatedbetween November2010andMarch2015wereevaluated(Table1).
Whenassessingthenumberoflinesoftreatmentthathad previouslybeenused bythepatient, the averagecoincided withthemedian,fivechemotherapyand/orradiotherapy regi-mens(range:3–7).
TwoofthepatientshadnotbeensubmittedtoHSCTprior toreceivingeverolimus;twenty-sevenhadundergone autolo-gousHSCTalone,twohadundergoneallogeneicHSCTalone (oneduetomobilizationfailure,andonewithnodata)and twopatientshadundergoneautologousandallogeneicHSCT.
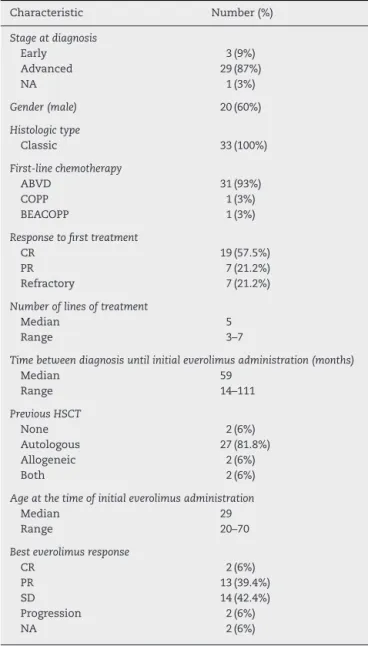
Table1–Patientcharacteristics(n=33).
Characteristic Number(%)
Stageatdiagnosis
Early 3(9%)
Advanced 29(87%)
NA 1(3%)
Gender(male) 20(60%)
Histologictype
Classic 33(100%)
First-linechemotherapy
ABVD 31(93%)
COPP 1(3%)
BEACOPP 1(3%)
Responsetofirsttreatment
CR 19(57.5%)
PR 7(21.2%)
Refractory 7(21.2%)
Numberoflinesoftreatment
Median 5
Range 3–7
Timebetweendiagnosisuntilinitialeverolimusadministration(months)
Median 59
Range 14–111
PreviousHSCT
None 2(6%)
Autologous 27(81.8%) Allogeneic 2(6%)
Both 2(6%)
Ageatthetimeofinitialeverolimusadministration
Median 29
Range 20–70
Besteverolimusresponse
CR 2(6%)
PR 13(39.4%)
SD 14(42.4%)
Progression 2(6%)
NA 2(6%)
NA:notassessable;HSCT:hematopoieticstemcelltransplantation; ABVD:adryamicin,bleomycin,vinblastineanddacarbazine;COPP: cyclophosphamide,oncovin,procarbazine,prednisone;BEACOPP: bleomycin, etoposide, adriamycin, cyclophosphamide, oncovin, procarbazine, prednisone; CR: complete response; PR: partial response;SD:stabledisease.
Themediantimebetweendiagnosisandthebeginningof treatmentwitheverolimuswas1652days(54months;range 14–111months).
TheassessmentswereconductedusingCTin16patients, PET–CTinninepatients,acombinationofCTandPET–CTin fivepatientsandphysicalexaminationwastheonly assess-mentemployedintheevaluationofonepatient.
100
90
80
70
60
50
40
30
20
% PFS
10
0
0 10 20 30
Months
40 50 60
Figure1–Progressionfreesurvival(PFS)in33eligible patientstreatedwitheverolimus.PFS:progressfree survival.
anoverallresponse,45%[15/33;95%CI:28.5–62.4%]responded tothetreatmentwitheverolimus.
Mediantreatment time inthis cohort was 317 days(10 months).Thirteenpatientsunderwenttreatmentforoverone year,andthreepatientshadbeenreceivingtreatmentformore thanfouryears.
With a median follow-up time of 24 months, median survivalrate was 62%, and the estimated median survival rate was 36 months. PFS was nine months (95% CI: 7–10) (Figures1and2).
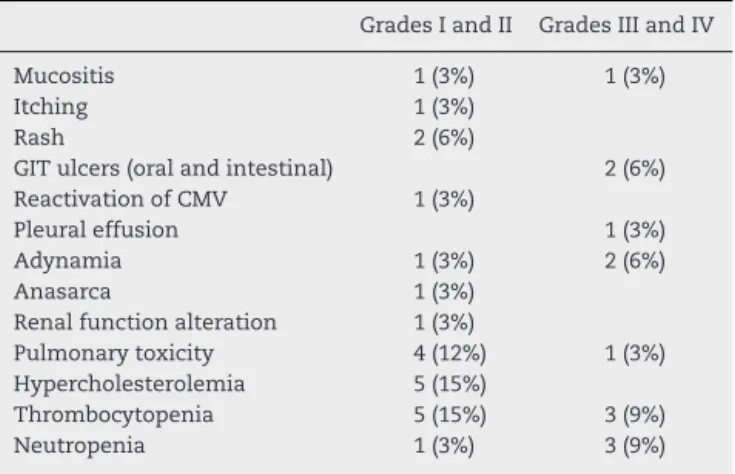
TheadverseeventsreportedweregradeIandII mucosi-tisinonepatient(3%);gradeIIIandIVmucositisinone(3%); itchingreportedonlyatthebeginningofmedicationuse in one(3%);gradeIskinrashintwo(6%);oralulcersinone(3%); intestinalulcersinone(3%);reactivationofcytomegalovirus (CMV)inone(3%);emergenceofpleuraleffusioninone(3%); adynamiaintwo(6%);anasarca inone(3%); alteredkidney functioninone(3%);andpulmonarytoxicitywiththeonsetof coughing,wheezingandpulmonaryinfiltratesinfive(15.1%) (Table2).Hypercholesterolemiawasdescribedinfive(15.1%) patients,someofwhomrequiredlipid-loweringagents.With regardtohematologictoxicity, five(15.1%)had gradeIand II thrombocytopenia, and three (9%) had grade III and IV
100
90
80
70
60
50
40
30
20
10
0
0 10 20 30
Months
% OS
40 50 60
Figure2–Overallresponsein33eligiblepatientstreated witheverolimus.OS:overallsurvival.
Table2–AdverseeventsintheBraziliancohort.
GradesIandII GradesIIIandIV
Mucositis 1(3%) 1(3%)
Itching 1(3%)
Rash 2(6%)
GITulcers(oralandintestinal) 2(6%) ReactivationofCMV 1(3%)
Pleuraleffusion 1(3%)
Adynamia 1(3%) 2(6%)
Anasarca 1(3%)
Renalfunctionalteration 1(3%)
Pulmonarytoxicity 4(12%) 1(3%) Hypercholesterolemia 5(15%)
Thrombocytopenia 5(15%) 3(9%) Neutropenia 1(3%) 3(9%)
GIT:Gastrointestinaltract;CMV:Cytomegalovirus.
thrombocytopenia.GradeIandIIneutropeniawasreported inonepatient(3%),and gradeIIIand IVinthree(9%).One patient(3%)hadadiagnosisofacutemyeloidleukemia(AML) whileundergoingtreatmentwitheverolimus;thispatienthad beenusingeverolimusfor13monthsandhadalready under-gonesixlinesoftreatment,includingHSCT.Onepatient(3%) wasdiagnosedwithpulmonarytuberculosiswhileusingthe medication.
Inthree(9%)patientsmedicationwasdiscontinueddueto death(notreportedwhetherbyprogressionortoxicity), toxic-itywasthereasonforinterruptioninfive(15.1%)patientsand progressionin17(51.5%).Treatmentforonepatientwas dis-continuedduetothediagnosisofAML,andanotherhadavery goodpartialresponseandwasreferredforallogeneic trans-plantation.Fivepatientsremainedintheprogramandwere stillreceivingthe medicationwhilethisanalysiswasbeing conducted.
Discussion
Patients withprimaryrefractoryHL who are notallergenic HSCTcandidates,orinwhomthediseaserelapsesfollowing thistreatmentmodality,presentaverypoorprognosis.1New drugsandtheirincorporationinthetreatmentareessentialto increaseresponseandsurvivalratesinthisspecificgroupof patients.OneexampleismTORinhibitorswiththeirrationale useinHLbeingbasedonstudiesthatdemonstrateactivation ofthePI3Kpathwayinthistypeoftumor.7
Thisstudyisnotaclinicaltrialbutaretrospectivestudy on the use of anoralexperimental medication ina group ofpreviouslytreatedpatientswhohadfewalternativesand thechanceofapotentialincreaseinsurvival.Itisimportant toemphasizethat,bythetimeeverolimuswasavailablefor compassionateuse,brentuximabvedotin(anti-CD30)wasnot approvedforuseinBrazil;thus,therewerefewtherapeutic optionsforrelapsedandrefractoryHLpatients.Atthattime, onlyonepatienthadimportedandusedbrentuximabvedotin andhadsuffereddiseaseprogressionwithinashorttime.
events.Therefore,weconsideradverseeventscouldbe under-reported,especiallygradeIandIIevents.Nevertheless,the mostseverecases,inwhichtreatmentwasreducedor inter-rupted,havebeenfullyreported.
Another limitation was the inability to standardize the rangeandcriteriaforevaluatingpatients.Becausethese anal-yses were carried out at the centers responsible for the patients’treatment,someofthedataamongthemore objec-tive responses may have been overestimated. Two of the patientshadnodalprogression,buttheirclinicalresponsewas maintained.Therefore, theyremainedinthe programuntil lossofclinicalresponsewasobserved.Oneofthosepatients was still using the medication four years after starting to receiveit.
Itisimportanttohighlightthatthecontinuoususeofthe medication,despite nodal progression, is considered to be appropriateaposteriori,giventheimpossibilityofnew treat-mentmodalitiesatthatmomentandthebenefittothepatient, whoremainedclinicallywellandwhosediseaseprogression wasveryslow.
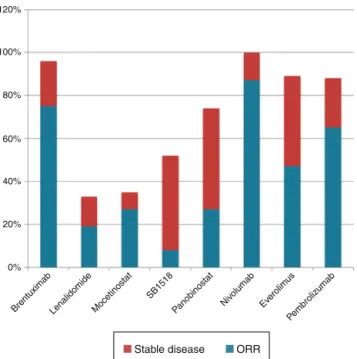
Therearefewpublishedstudiesontheuseofthis medi-cationinthispopulation.Moreprecisely,thereisonephase IIstudycarriedoutintheUSAthatassessed19patients,of whomonly16usedeverolimusformorethanonecycle(28 daysoftreatment).6InthisUScohorttheORRwas47%(9/19; 95% CI:24–71%), witheight (42%) patients showingpartial responseandone(5.2%)patienthavingcomplete response. IntheBraziliancohort,theORRwas45.4%,with13 (39.3%) patientsachieving partialresponseand two(6%), complete response.Inaddition,twoupdatesoftheUSphaseIIstudy publishedas conferenceabstracts demonstrated that, with greaternumbersofpatients(38and57),responserateswere only37%of38and42%of57,with34%of38and33.3%of57 patientsshowingpartialresponse,and2.6%of38and8.8%of 57showingcompleteresponse(Figure3).13,14
120%
100%
80%
60%
40%
20%
0%
Brentuximab Lenalidomide Mocetinostat SB1518
Panobinostat Niv olumab
Everolim us
Pembrolizumab
Stable disease ORR
Figure3–Drugresponsesinpatientswithrefractoryand relapsedHodgkin’slymphoma.ORR:overallresponserate.
OnlytheUSphaseIIstudy6canbeusedforfurtheranalysis andcomparisonofdatagiventhatresultsofitsupdated ver-sions,with38and57patients,respectively,havenotyetbeen fullypublished.16IntheUSstudy,themediantimebetween HL diagnosis and the beginning ofeverolimus administra-tion was 3.5years;whileintheBrazilian cohortit was4.5 years.Themediantimebetweenthebeginningofeverolimus use and responsewas 3.6 monthsin the US study. Unfor-tunately, wecouldnotperformthis analysisduetolackof objective dataintheBraziliancohort.AlsointheUSstudy, response duration time was 7.1 months (95% CI:3.9–14.8) withfourpatientsusingthemedicationforover12months with noprogression. Inthe Brazilian cohort, PFSwas nine months(CI95%:7–10)withamediantreatmentdurationof ten months,whileinthe USstudy PFSwas 6.2months(CI 95%:5.9–9.5).
Thelongtreatmentdurationmaybeexplainedbythefact, mentionedabove,thattherewasagroupofpatients main-tainedintheprogramevenafternodaldiseaseprogression. Itseemsthesepatientsmightstillhaveclinicalbenefitsfrom thetreatmentfornotshowingsignsofconstitutional symp-toms.Onretrospectivelyreviewingthisconduct,itcouldbe considered appropriate,asthesepatientshavebeen receiv-ingthemedicationforoverfouryears,withexcellentquality oflifeandveryslownodalprogression.Itisworth mention-ingthatsuchalongresponsetimecouldbeassociatedwith thefactthatthreepatientshavecontinuedusingthe medica-tionforoverfouryears,whichshowsitsgoodtolerabilityand safety.
Overall survival was estimated in the US study at 25.2 months(95%CI:13-notyetavailable;medianfollow-uptime was24months)versus36months(95%CI:15–56)inthe Brazil-iancohort(themedianfollow-upwasalso24months).
IntheUSstudy,treatmentdiscontinuationhadthe follow-ingreasons:progressionin16(84%)patients;pneumoniain one(5%); and infection leadingtodeathinone (5%).In its updatedversion,progressionisdescribed asthe reasonfor discontinuationin11(29%)patients.6
IntheBraziliancohort,progressionofthediseasewasthe reasonfordiscontinuationin17(51%)patients,whiletoxicity wasthereasoninsix(18%)andone(3%)patientwasdiagnosed withAML;onewasreferredtoallogeneicHSCTfollowinga good responsewiththemedication.Inthis present cohort, three(9%)patientsreportedly interruptedtreatmentdueto deathwithoutfurtherspecifications.
Another significant piece of data was that only a few patientsinthe Braziliancohort were referredtoallogeneic HSCTafterexhibitingresponseselicitedbyeverolimus.This decision was made by each center with the underlying reasonsbeingthehightolerabilitytoeverolimusassociation withthepatients’qualityoflifeversusthehighriskrelatedto allogeneicHSCTaswascorroboratedbytherecentapproval ofeverolimus inBrazil, the inappropriate infrastructure to perform this procedure at centers and patients’ refusal to undergoHSCT.Nodataonthisconcernwerepublishedeither intheUSphaseIIstudyorintheupdatedresults.
Everolimusisstillunderstudyandcombinationsofother antineoplastic agents have been tested. A phase I study with everolimus associated with panobinostathad a good ORR(43%), albeit with high toxicity.16 Other combinations arebeingtestedsuchasthecombinationofeverolimusand lenalidomide,andthecombinationofsorafenibandhistone deacetylaseinhibitors.17
In comparison with other drugs used for refractory patients,everolimushaslessresponsecomparedto brentux-imabvedotin(79%),5pembrulizumab(65%),18andnivolumab (87%),19 but its response ishigher than those ofSB1518,20 lenalidomide,21mocetinostat22andpanobinostat.23Itis evi-dentthatthereisagroupofpatientswhomaybenefitfrom using this medication, while there is another group that eitherdoesnotshowanyresponseorwhoseresponsetothe druglastsonlybriefly.Identifyingthesubgroupofresponding patientswouldhaveasignificantimpactonoptimizing med-icationusewithrespecttoresponseratesandsignificantand permanentadverseeventsandcosts.
Inconclusion,webelievethatthisstudyisrelevantevenif itsmethodologyisretrospective.Everolimusisstillan exper-imentalmedication and has been proven tobe activeand improvetheresultsinagroupofselectedpatientsinwhich there are few therapeutic possibilities, suchas those with refractoryandrelapsedHL.Furthermore,becauseofthegood responsesobservedinasubgroupofpatients,theseresults allowthestudyofthismedicationcomparedtoother medica-tionsthathavedifferentmechanismsofaction.
Compliance
with
ethical
standards
Theinstitutionalreviewboardofeachparticipatinginstitution approvedthisstudydesign,andthisstudywasconductedin accordancewiththeDeclarationofHelsinki.Writteninformed consentwasobtainedfromallpatientsbeforetheirinclusion inthestudy.
Conflicts
of
interest
Theauthorsdeclarenoconflictsofinterest.
r
e
f
e
r
e
n
c
e
s
1. vonWasielewskiR,MengelM,FischerR,HansmannML, HübnerK,FranklinJ,etal.ClassicalHodgkin’sdisease. Clinicalimpactoftheimmunophenotype.AmJPathol. 1997;151(4):1123–30.
2.MartinezC,CanalsC,SarinaB,AlessandrinoEP,KarakasisD, PulsoniA,etal.Identificationofprognosticfactorspredicting outcomeinHodgkin’slymphomapatientsrelapsingafter autologousstemcelltransplantation.AnnOncol. 2013;24(9):2430–4.
3.vonTresckowB,DiehlV.Anupdateonemergingdrugsfor Hodgkinlymphoma.ExpertOpinEmergDrugs.
2014;19(2):215–24.
4.BollB,BorchmannP,ToppMS,HänelM,ReinersKS,EngertA, etal.Lenalidomideinpatientswithrefractoryormultiple relapsedHodgkinlymphoma.BrJHaematol.
2010;148(3):480–2.
5.YounesA,GopalAK,SmithSE,AnsellSM,RosenblattJD, SavageKJ,etal.ResultsofapivotalphaseIIstudyof brentuximabvedotinforpatientswithrelapsedorrefractory Hodgkin’slymphoma.JClinOncol.2012;30(18):2183–9. 6.JohnstonPB,InwardsDJ,ColganJP,LaplantBR,KabatBF,
HabermannTM,etal.APhaseIItrialoftheoralmTOR inhibitoreverolimusinrelapsedHodgkinlymphoma.AmJ Hematol.2010;85(5):320–4.
7.GuariniA,MinoiaC,GiannoccaroM,RanaA,IacobazziA, LapietraA,etal.mTORasatargetofeverolimusin refractory/relapsedHodgkinlymphoma.CurrMedChem. 2012;19(7):945–54.
8.BraderS,EcclesSA.Phosphoinositide3-kinasesignalling pathwaysintumorprogression,invasionandangiogenesis. Tumori.2004;90(1):2–8.
9.JiangBH,LiuLZ.RoleofmTORinanticancerdrugresistance: perspectivesforimproveddrugtreatment.DrugResist Update.2008;11(3):63–76.
10.PassamFH,AlexandrakisMG,KafousiM,FotinouM, DarivianakiK,TsirakisG,etal.Histologicalexpressionof angiogenicfactors:VEGF,PDGFRalpha,andHIF-1alphain Hodgkinlymphoma.PatholResPract.2009;205(1):11–20. 11.VezinaC,KudelskiA,SehgalSN.Rapamycin(AY-22,989),a
newantifungalantibiotic.I.Taxonomyoftheproducing streptomyceteandisolationoftheactiveprinciple.JAntibiot. 1975;28(10):721–6.
12.FossHD,AraujoI,DemelG,KlotzbachH,HummelM,SteinH. Expressionofvascularendothelialgrowthfactorin
lymphomasandCastleman’sdisease.JPathol. 1997;183(1):44–50.
13.Doussis-AnagnostopoulouIA,TalksKL,TurleyH,DebnamP, TanDC,MariatosG,etal.Vascularendothelialgrowthfactor (VEGF)isexpressedbyneoplasticHodgkin–Reed–Sternberg cellsinHodgkin’sdisease.JPathol.2002;197(5):677–83. 14.ChesonBD,FisherRI,BarringtonSF,CavalliF,SchwartzLH,
ZuccaE,etal.Recommendationsforinitialevaluation, staging,andresponseassessmentofHodgkinand non-Hodgkinlymphoma:theLuganoclassification.JClin Oncol.2014;32(27):3059–68.
15.ChesonBD,PfistnerB,JuweidME,GascoyneRD,SpechtL, HorningSJ,etal.Revisedresponsecriteriaformalignant lymphoma.JClinOncol.2007;25(5):579–86.
16.JohsntonPB,Pinter-BrownLC,RogerioJ,WarsiG,ChauQ, RamchandrenR,etal.Everolimus(EVE)for
relapsed/refractoryclassicalHodgkinlymphoma(cHL): open-label,single-arm,phaseIIstudy.JClinOncol.2012;30, abstr8028.
17.OkiY,BuglioD,FanaleM,FayadL,CopelandA,RomagueraJ, etal.PhaseIstudyofpanobinostatpluseverolimusin patientswithrelapsedorrefractorylymphoma.ClinCancer Res.2013;19(24):6882–90.
19.AnsellSM,LesokhinAM,BorrelloI,HalwaniA,ScottEC, GutierrezM,etal.PD-1blockadewithnivolumabinrelapsed orrefractoryHodgkin’slymphoma.NEnglJMed.
2015;372(4):311–9.
20.YounesA,RomagueraJ,FanaleM,McLaughlinP,Hagemeister F,CopelandA,etal.PhaseIstudyofanoveloralJanuskinase 2inhibitor,SB1518,inpatientswithrelapsedlymphoma: evidenceofclinicalandbiologicactivityinmultiple lymphomasubtypes.JClinOncol.2012;30(33):4161–7. 21.FehnigerTA,LarsonS,TrinkausK,SiegelMJ,CashenAF,Blum
KA,etal.Aphase2multicenterstudyoflenalidomidein
relapsedorrefractoryclassicalHodgkinlymphoma.Blood. 2011;118(19):5119–25.
22.YounesA,OkiY,BociekRG,KuruvillaJ,FanaleM,NeelapuS, etal.MocetinostatforrelapsedclassicalHodgkin’s
lymphoma:anopen-label,single-arm,phase2trial.Lancet Oncol.2011;12(13):1222–8.
23.YounesA,SuredaA,Ben-YehudaD,ZinzaniPL,OngTC, PrinceHM,etal.Panobinostatinpatientswith