w w w . r b h h . o r g
Revista
Brasileira
de
Hematologia
e
Hemoterapia
Brazilian
Journal
of
Hematology
and
Hemotherapy
Original
article
Blood/Injection
Fear
Scale:
Portuguese
version,
cultural
adaptation
and
psychometric
properties
in
a
large
sample
of
primary
health
care
users
Miriane
Lucindo
Zucoloto
∗,
Edson
Zangiacomi
Martinez
UniversidadedeSãoPaulo(USP),RibeirãoPreto,SP,Brazil
a
r
t
i
c
l
e
i
n
f
o
Articlehistory:
Received13February2017
Accepted23May2017
Availableonline27June2017
Keywords:
Fear Blood Injections Translating Psychometrics
a
b
s
t
r
a
c
t
Background:Blood/injectionphobiamayhaveimportantconsequencesforhealth.These
phobicindividuals,inmostcases,avoidcontactwithhealthsystems,postponeoravoid
medicalprocedures,avoidinvasivetreatmentsanddonotparticipateinhealth
promo-tionandearlydetectionofdiseaseinitiativessuchasvaccination,consultations,preventive
examsorblooddonation.Thus,specificandvalidatedinstrumentsarenecessarytoassess
thisvariable.In addition,a lack ofstudieson this thememaybeassociatedwith the
lowavailabilityofinstruments.ThisstudyaimedtoproposeaPortugueseversionofthe
Blood/InjectionFearScale(BIFS-P)andassessitspsychometricproperties.
Methods:Translationandback-translationwereperformed.Contentvaliditywasassessedin
twostepsbyapanelof20experts.Thepsychometricpropertieswereassessedinastratified
andrepresentativesampleofprimaryhealthcareserviceusersofRibeirãoPreto,
southeast-ernBrazil.Exploratoryandconfirmatoryfactoranalyseswereconductedusingapolychoric
correlationmatrix.
Results:Atotalof1054primaryhealthcareusersparticipated;79.7%werefemaleandthe
meanagewas40.6(standarddeviation=15.16)years.Accordingtotheexploratoryfactor
analysis,theitemscanbegroupedintothreeorfivefactorswithbestfitsbeingdetectedfor
thethree-andfive-factormodelsinconfirmatoryfactoranalysis.
Conclusion:Blood/InjectionFearScale(Portugueseversion)iseasytounderstandandapply
inthegeneralpopulation,showedadequatepsychometricproperties,andrepresentsan
alternativeintheassessmentofblood/injectionphobiaforfuturestudies.
©2017Associac¸ ˜aoBrasileiradeHematologia,HemoterapiaeTerapiaCelular.Published
byElsevierEditoraLtda.ThisisanopenaccessarticleundertheCCBY-NC-NDlicense
(http://creativecommons.org/licenses/by-nc-nd/4.0/).
∗ Correspondingauthorat:DepartmentofSocialMedicine,RibeirãoPretoMedicalSchool,UniversidadedeSãoPaulo(USP),Avenida
Bandeirantes,3900,14049-900MonteAlegre,RibeirãoPreto,SP,Brazil.
E-mailaddress:mirianezucoloto@gmail.com(M.L.Zucoloto).
http://dx.doi.org/10.1016/j.bjhh.2017.05.006
1516-8484/©2017Associac¸ ˜aoBrasileiradeHematologia,HemoterapiaeTerapiaCelular.PublishedbyElsevierEditoraLtda.Thisisan
Introduction
Bloodandinjectionphobiasarecharacterizedbyanextreme
aversionofseebloodorreceiveinjectionsorbesubjectedto
invasivemedicalprocedures.1
AccordingtoKoseandMandiracioglu,2amongthevarious
typesofspecificphobias,therearetwotypesofblood/injection
phobiadescribedinthescientificliteraturethatareclassified
according tothe presenceor absence of a vasovagal
reac-tion.Thevasovagalreaction,characterizedbyasuddeninitial
increaseinheartrateandbloodpressurefollowedbya
sud-dendrop,isadysphasiccardiovascularresponseelicitedin
the face ofthe exposureofthe individual to situations of
fear.Forthisreason,thevasovagalreactionisaccompanied
bysymptomssuchasdiscomfort,nausea,pallor,expressions
ofdisgust/loathing,andevenfainting,all ofwhicharevery
commonamongpatientswithbloodandinjectionphobias.2–4
Unlikeothertypesofspecificphobias,bloodandinjection
phobiasmayhaveimportantconsequencesforhealth.Inmost
cases,theseindividualsavoidcontactwithhealthsystems,
postponeoravoidmedicalprocedures,avoidinvasive
treat-mentsanddonotparticipateinhealthpromotionandearly
detectionofdiseaseinitiativessuchasvaccination,
consulta-tions,preventiveexamsorblooddonation.5,6
Inthefaceofthesedirectimplicationstothehealth,the
developmentandvalidationofspecificinstrumentsto
evalu-ateblood/injectionphobiaarenecessary.Someinstruments
wereproposed forthis investigationinthegeneral
popula-tion,suchastheBlood-InjectionSymptomScale(BISS)7and
theBlood/InjectionFearScale(BIFS).2
TheBIFSwasproposedbyKoseandMandiraciogluin20072
andconsistsof20itemsdividedintotwofactors,fearofblood
andfearofinjectionsandthesymptomsinvolved.Theoriginal
versionofthisscalewaspublishedinEnglishanduntilnow,
ithasnotbeentranslatedtootherlanguages.
Few studies have been conducted to investigate
blood/injection phobia in the Brazilian population.8,9 To
thebestofourknowledge,thereisonlyoneBrazilianstudy,
publishedbyD’ElReyandPaciniin2005;itinvestigatedthe
prevalenceofbloodandinjectionphobiasinarepresentative
sampleofthe generalpopulationofthe city ofSão Paulo.8
Amongtheresults,thecombinedprevalenceofbloodor
injec-tion phobiaswas 4.1%,withthis fear beingmorecommon
amongwomenandamongindividualswithlittleschooling.
Itisbelievedthatthelowavailabilityofinstrumentsthat
areappropriateandadaptedforuseinthegeneralpopulation
isassociatedwiththelownumberofepidemiologicalstudies
inBrazilonthistypeofphobia.Themajorityofstudiesinthe
areaperformedinBrazilreferstodiagnosticandtreatment
techniquesandare fromthe psychologyand/orpsychiatric
fields.10
Objectives
Inlightoftheforegoing,thisstudywasconductedtopresenta
PortugueseversionoftheBIFSandevaluateitspsychometric
propertiesinarepresentativesampleofprimaryhealthcare
usersinRibeirãoPreto,southeasternBrazil.
Methods
Instrumentcharacteristics
BIFS is composed of 20 items divided into two factors
accordingtotheoriginalproposal;thefirstis‘fearof
injec-tions’ (Items 1–12) and the second “fear of blood” (Items
13–20).2 Responses use a 5-point Likert scale (1=Strongly
agree;2=Agree; 3=Neither agreenordisagree;4=Disagree;
5=Stronglydisagree).2
Translation,backtranslationandculturaladaptation
Threebilingualtranslators,whosenativelanguageis
Brazil-ian Portuguese, performed the translation of the BIFS,
independently. The two researchers responsible for the
study, who are also bilingual, compared the three
trans-lated versions. Thus, the Portuguese version of BIFS was
defined.
The Portuguese version of the instrument was sent to
a translator fluent in Brazilian Portuguese whose native
languageisEnglishtoperformthebacktranslation.This
trans-latorwasnotawareofthecontentoftheoriginalscaleandwas
notinformedthatitwasaback-translation.Theback
trans-lationwas importanttoconfirmthatthe translationofthe
instrumentdidnotchangethemeaningoftheitemsaccording
totheoriginalproposal.
Afterthesesteps,thePortugueseversionoftheBIFS
(BIFS-P)wasobtainedandhaditspsychometricpropertiesevaluated
inthisstudy.
Contentvalidity
ToevaluatetheobjectivityandrelevanceoftheBIFS-P,the
con-tentofitsitems wasassessedindependentlybyapanelof
eightjudges,healthprofessionals,withexperiencein
hema-tologytransfusionmedicineand/orinprofessionalservicesin
primaryhealthcare.Judgeswereinformed aboutthe
objec-tivesof thestudy and were asked toclassify each itemof
the instrumentasadequate ornotadequate foruse inthe
targetpopulation.Suggestionsofmodificationsand
simplifi-cationofitemswere requestedinthesecases.Thecontent
validityindex(CVI)wascalculatedforeachitemconsidering
that IVC≤0.70 is indicative ofthe need ofa new
transla-tion (i.e., 30% or moreofthe judges classifiedthe item as
notadequate).11Accordingtothesuggestionsofthejudges,
someitemsweremodifiedaimingtosimplifyandimprovethe
understandingpriortotheapplicationoftheinstrumentinthe
targetpopulation.
In a second phase of the content analysis, a panel of
20 judges with experience in hematology and transfusion
medicine was invited to classify, also independently, each
item ofthe instrumentaccording toitsessentiality
(essen-tial,usefulbutnotessentialornotnecessary)toevaluate‘fear
ofbloodorinjections’.Thecontentvalidityratiowas
calcu-latedaccordingtotheproposalofLawshe.12Thesignificance
ofthedecisionwasinaccordancewiththeproposalofWilson
Trainingofinterviewers
Beforestartingdatacollection,thethreeinterviewers
respon-sibleforthisstepparticipatedinatrainingsessiononhow
toapplytheinstrumentandallthestrategiesregardingthe
approach,explanationoftheresearchobjectivesand
instruc-tionstopatientswerediscussed.
Facevalidity
Thepre-testoftheBIFS-Pinthetargetpopulationwas
con-ductedin30primaryhealthcareusersofRibeirãoPretoina
singlehealthcarecliniclocatedinthecentralregion,which
catersfordifferentmedicalspecialties.Theincomprehension
index(proportion)ofeachitemwascalculatedand,if
neces-sary,theitemwasrewritten.
Datacollection
Thesamplewascomposedbyadultindividuals(≥18years),
residentsinRibeirãoPreto,southeasternBrazil,whoare
pri-maryhealth care users in the municipality. Ribeirão Preto
isamediumsizedcityofSãoPauloStatethathas
approxi-mately670,000inhabitants.Themunicipalityisdividedinto
five health districts,North, West, Central, Southand East.
Atthe timeof datacollection, there were 41 primary
gov-ernmenthealthcareclinicsinthemunicipality,whichwere
classifiedaccordingtothedistrictinwhichtheyarelocated
andtothePaulistaIndexofSocialVulnerability(PSVI)
preva-lentintheirarea.ThePSVIisdevelopedbytheSEADE(State
System ofData Analysis)from censusdata, with the
geo-graphicareasbeingclassifiedintosixdistinctgroupsofsocial
vulnerability.14Healthcareclinicsweregroupedinto12strata
accordingtothedistrictwheretheyarelocatedandthe
pre-dominantIPVS.TheclinicswherethePSVIwasequalto1or
2weregroupedinthesamestratumandtheclinicswithPSVI
equaltoorgreaterthan4weregroupedinanotherstratum.
Itshouldbenotedthatthisstudyispartofawiderproject
thataimstoevaluatetheassociationofsomevariableswith
blooddonation.Thus,thesamplesizewasobtained
consider-ingastratifiedsamplingplan,15a95%confidencelevelandan
absoluteprecisionof3%fortheestimativeoftheproportionof
blooddonors.Thisproportion,formaximizationofvariance,
wasconsideredequalto50%ineachstratum.Thus,a
sam-plesizeof1054interviewswasestimated,withthenumberof
interviewstobeconductedineachstratumbeingproportional
to their estimated population size. The healthcare clinics
wheretheinterviewswereconductedwereselectedrandomly
withineachstratum.InadditiontotheBIFS-P,aquestionnaire
aboutsociodemographiccharacteristicsandgeneralhealthof
theparticipantswasusedtocharacterizethesample.
PsychometricpropertiesofBIFS-P
FactorialvalidityoftheBIFS-Pwasassessedusingexploratory
factoranalysis(EFA)andconfirmatoryfactoranalysis(CFA).
EFA was performed using polychoric correlation methods
implementedusingtheSASsoftware.TheCFAwasalso
per-formedusingthepolychoriccorrelationsmatriximplemented
inthesoftware Mplus,version 6.0.Theestimation method
usedinCFAwastheweightedleastsquaresmeanand
vari-anceadjusted(WLSMV).TheratioofChi-squaretoitsdegrees
offreedom(2/df),comparativefitindex(CFI),Tucker–Lewis
index (TLI) and root mean square error of approximation
(RMSEA) were considered as goodness of fit indices. The
fit ofthe modelswasconsideredadequate when2/df was
≤2.0,CFIandTLIwas≥0.90and RMSEAwas<0.10.16 Items
with factor weights () lower than 0.40 were considered
underweightitemsforthecorrespondingfactor.Modification
indices,obtainedfromtheLagrangemultipliers,wereusedto
verifytheexistenceofcorrelationsbetweenerrors.16
Compar-isonsbetweenthetestedmodelswereperformedconsidering
their respectivefit ingeneral, through the goodness of fit
indices,withemphasisontheRMSEAindex.17 RMSEA
esti-matesshowhowwelltheparametersofthemodelreproduce
the population covariance and can be considered a
parsi-moniouscorrectionindex.Thus,lowervaluesofRMSEAare
indicativeofbettermodels.17,18
Factorial invariance of the models was assessed using
multigroup analysis considering the Chi-square difference
(2).Thisanalysis isused toevaluatethe stability ofthe
modelsindifferentsamples.Forthis,thetotalsamplewas
randomlydividedintotwoequalpartsthe‘testsample’and
the‘validationsample’.Inthisanalysistheequivalenceof
fac-torialweights[metricinvariance()],thefactorialweightsand
intercepts[scalarinvariance(Int)],andthefactorialweights,
interceptsandvariances/covariances[strictinvariance(Cov)]
wereevaluated.16,19
Ethicalaspects
ThisstudywasapprovedbytheEthicsCommitteeonHuman
ResearchoftheHospitaldasClínicasinRibeirãoPreto(CAAE:
38148814.2.0000.5440),withdatacollectionbeingapprovedby
theDepartmentofHealthofRibeirãoPreto.Inaddition,this
studywasconductedinaccordancewiththeHelsinki
Declara-tionasrevisedin2008.Onlyadultindividuals(≥18years)who
agreedandsignedinformedconsentformsparticipated.The
authors oftheoriginalscaleauthorizedthetranslation and
validationoftheinstrument.
Results
Contentvalidity
ThefirststepofthecontentvalidityprocessoftheBIFS-Pwas
performed byapaneloffourmale andfour female judges
withameanageof48.8(SD13.3)yearswhohavebeen
health-careprofessionalsfor20.6yearsonaverage(SD15.1).Ofthese,
37.5%workinhematology/transfusionmedicineservicesand
62.5%areengagedinprimaryhealthcareactivities.According
totheCVIvalues(CVI≥0.75forallitems),therewasnoneed
fornewtranslationsofanyitemoftheBIFS-P.However,some
importantsuggestionsweremadeandconsideredtosimplify
andadapttheinstrumentforthetargetpopulation.
Thesecondstepofthecontentvalidityprocesswas
per-formed by 20 judges, who did not participate in the first
stepofthisevaluation;80%werefemale,themeanagewas
Table1–OriginalandPortugueseversionofBlood/InjectionFearScaleandtheresultsofcontentvalidityratio(CVR)
estimatedforeachitem.
Item Portugueseversion Originalversion CVR
1 Eutenhomedodesentirdoraotomar
injec¸ão
I’mafraidofpainduetoreceiving injection
0.90
2 Eujátiveexperiênciasruinsaotomar
injec¸ão
I’vepreviousbadexperienceabout receivinginjection
0.20a
3 Euevitoolharaenfermeirapreparara seringadeinjec¸ão
Iavoidtowatchthenursepreparethe syringe
−0.30a
4 Eutenhomedodetomarinjec¸ão Iamafraidtoreceiveinjections 0.70 5 Eutenhomedodeveroutraspessoas
tomareminjec¸ão
Iamafraidtoseeothersreceiveinjections −0.30a
6 Euevitotomarinjec¸ão Iavoidreceivinginjections −0.20a 7 Otamanhodaagulhamedeixacommedo Needlesizefrightensme 0.40a 8 Eusintomal-estarquandotomoinjec¸ão IfeeldisgustedwhenIreceiveinjections 0.80 9 Eumeincomodocomapossibilidadede
veroutraspessoastomandoinjec¸ão
Iworryaboutthepossibilityofseeing othersreceiveinjections
−0.50a
10 Eumeincomodocomapossibilidadede terquetomarinjec¸ão
Iworryaboutthepossibilityofhavingto receiveinjections
0.40a
11 Eudesmaioquandotomoinjec¸ão IfaintafterIreceiveinjections 0.80 12 Eudesmaioquandovejooutraspessoas
tomandoinjec¸ão
IfaintwhenIseeothersreceiveinjections 0.00a
13 Euevitoverosanguedeoutraspessoas Iavoidseeingothers’blood 0.00a 14 Eumeincomodocomapossibilidadede
verosanguedeoutraspessoas
Iworryaboutthepossibilityofseeing others’blood
−0.50a
15 Eusintomal-estarquandovejomeu própriosangue
IfeeldisgustedwhenIseemyownblood 0.60
16 Euevitoolharparaomeuprópriosangue Iavoidlookingatmyownblood 0.30a 17 Eutenhomedodeolharparaomeu
própriosangue
Iamafraidofthesightofmyownblood 0.30a
18 Eudesmaioquandovejosangue IfaintwhenIseetheblood 0.60 19 Ocheirodasaladeaplicac¸ãome
incomoda
I’minfluencedbysmellsintheroom −0.30a
20 Eusintomal-estarquandovejoosangue deoutraspessoas
IfeeldisgustedwhenIseeothers’blood −0.20a
CVR:contentvalidityratio;CVR20;0.05=0.438. a Valuesbelowtheminimumsignificantvalue.
professionalsfor10.6(SD8.2)yearsonaverage.Ofthese,10%
areengagedinactivitiesofattendingthepopulationin
pri-maryhealthcareservices,10%arenursingstaffintransfusion
medicineorblooddonationservices,and80%areengagedin
othertypesofactivitiesinservicesofhematology/transfusion
medicineorblooddonation.Thecontentvalidityratio(CVR)
wasestimatedandispresentedinTable1.
OnlyItems1,4,8,11,15,and18wereconsideredessentials
bythejudges.
Facevalidity
Inthepre-testofthescaleinthetargetpopulation,30patients
wereinterviewed(female:73.3%;meanage:36.97±15.1years)
inasinglehealthcarecliniclocatedinthecentralregionofthe
municipalitywhichattendsdifferentspecialties.The
incom-prehensionindexwasnullforallitems,indicatingthatthere
wasnoneedforrewrites.
Psychometricproperties
Inthedatacollectionstepwhichaimstoevaluatethe
psy-chometric properties of the BIFS-P, 1055 users of primary
healthcareservicesinthemunicipalitywereinterviewedin
12 healthcare clinics (female: 79.7%;mean age: 40.6±15.2
years). Regarding the perception ofhealth, 69.7% classified
theirhealthas‘good’, 26.5%as‘regular’,and 3.8%as‘bad’.
Arterial hypertensionand diabetes mellituswere the most
prevalentchronicdiseasesinthesampleat22.0%and8.0%,
respectively.
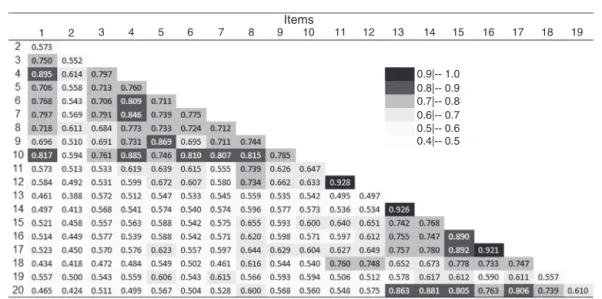
Figure1presentsthepolychoriccorrelationsmatrixforthe
20itemsoftheBIFS-P.Highercorrelationcoefficients(>0.90)
weredetectedbetweenItems11and12,13and14,and16and
17.Items2and19werenotstronglycorrelatedwithanyother
itemoftheBIFS-P,withcorrelationcoefficientsbetween0.39
and0.62.
Table2showstheresultsoftheEFA.AccordingtotheEFA,
theitemscanbegroupedinuptofivefactors,withFactors4
and5beingresponsibleforprovidingamorespecificfeatureof
theBIFS-P.Thesefactorsarerelatedtofearand/orreactionson
seeingtheirownblood(Factor4)andtofearand/orreactions
toseeingthebloodofothers(Factor5).
Thus,threefactorialstructuresoftheBIFS-Pweretested
intheCFA:(1)originalfactorialstructurewithtwofirst-order
factors(fearofblood/fearofinjections);(2)factorialstructure
Items
1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19
0.9|-- 1.0 0.8|-- 0.9 0.7|-- 0.8 0.6|-- 0.7 0.5|-- 0.6 0.4|-- 0.5
Figure1–PolychoriccorrelationsmatrixfortheitemsofthePortugueseversionoftheBlood/InjectionFearScale.Darker colorswereattributedtohighercorrelationcoefficients.
Table2–Exploratoryfactoranalysis(EFA)performedfor
thePortugueseversionofBlood/InjectionFearScale
appliedtoasampleofprimaryhealthcareusersof
RibeirãoPreto,southeasternBrazil.
Items Factor1 Factor2 Factor3 Factor4 Factor5
1 0.94824 −0.04576 0.01594 0.01025 −0.05050
2 0.53484 0.03142 0.14454 0.03172 −0.00915
3 0.76520 0.13205 −0.08722 0.03735 0.05435
4 1.00683 −0.02188 0.02150 0.00254 −0.07423
5 0.40865 0.04349 0.06960 −0.01315 0.53536
6 0.76514 0.06143 0.10821 −0.04096 0.01040
7 0.85282 0.08602 −0.08119 0.04364 0.03388
8 0.53039 0.08319 0.32310 0.01095 0.07290
9 0.33508 0.02865 0.03671 0.00579 0.65536
10 0.81127 0.04275 0.09071 −0.01493 0.05383
11 0.13787 0.01650 0.94704 −0.06475 −0.04700
12 0.09631 0.01645 0.83284 −0.00927 0.08024
13 0.07872 0.96400 −0.04550 −0.03915 −0.03175
14 0.07691 0.96147 −0.00046 −0.07278 −0.00220
15 0.06794 0.48897 0.12326 0.48440 −0.04881
16 0.07099 0.45754 −0.00549 0.59339 0.00287
17 0.05699 0.48089 0.03722 0.52252 0.04549
18 −0.10038 0.41821 0.54167 0.17653 −0.03061
19 0.30834 0.33451 0.02683 0.09117 0.11449
20 −0.02867 0.83920 0.08419 0.07572 0.02694
Theshadingillustratesthegroupingofitemsaccordingtofactor.
factorialstructurewith5first-orderfactorsresultingfromEFA.
Thesestructuresare calledModel 1,Model2and Model3,
respectively.
Table3presents the resultsof theCFA forthe factorial
structurestestedfortheBIFS-P.Itshouldbementionedthat
itwasnecessarytoexcludeItem19duetoitslowfactorial
weightinallanalysesandlowercorrelationcoefficientswith
alltheotheritemsofthescaleinordertohaveadequatefits
ofallmodels(Figure1).
Despitetheappropriatefactorweightsofallitemsineach
factorinthethreemodelstested(>0.60),theoriginal
struc-turewithtwofactors(Model1)doesnotpresentanadequatefit
Table3–Factorialweights(),goodnessoffitindicesof
theconfirmatoryfactoranalysis(2/df,CFI,TLI,and
RMSEA),modificationindices,andcorrelationbetween
errorsadoptedforthethreefactorialstructurestested
forthePortugueseversionofBlood/InjectionFearScale
inasampleofprimaryhealthcareusersofRibeirão
Preto,southeasternBrazil.
Estimative Model1 Model2 Model3
0.65–0.95 0.66–0.99 0.67–0.99
2 2726.79 1294.88 989.64
2/df 18.94 9.25 7.50
CFI 0.97 0.99 0.99
TLI 0.97 0.98 0.99
RMSEA 0.13 0.09 0.08
RMSEA–95%CI 0.13–0.14 0.08–0.09 0.07–0.08
MI ≤546.13 ≤92.52 ≤63.00
CBE 7.00 8.00 10.00
CFI:comparativefitindex;TLI:Tucker–Lewisindex;RMSEA:root meansquareerrorofapproximation;95%CI:95%confidence inter-val; MI: modification indices; CBE: correlations between errors considered.
Model1:originalstructureconsideringtwofirst-orderfactors. Model2:Structurewiththreefactorsobtainedinexploratoryfactor analysis.
Model3:Structurewithfivefactorsobtainedinexploratoryfactor analysis.
tothedataconsideringthegoodnessoffitindices.Evenafter theexclusionofItem19andthemodelre-specification(seven correlations betweenerrorsconsidered),Model 1presented resultsofRMSEAhigherthanadequateandhighmodification indices,whichcanbeconsideredapooradjustment(Table3).
Ontheotherhand,Models2and3presentedadequatefitto
thedata,withgoodnessoffitindiceswithinacceptablelimits
(afterre-specificationofthemodelsandtheexclusionofItem
19).Despitehighlevelsof2/df,thisindexisoverestimated
inlargersamples,andshouldbeevaluatedtogetherwiththe
Structure with three factors (Model 2)
Structure with five factors (Model 3)
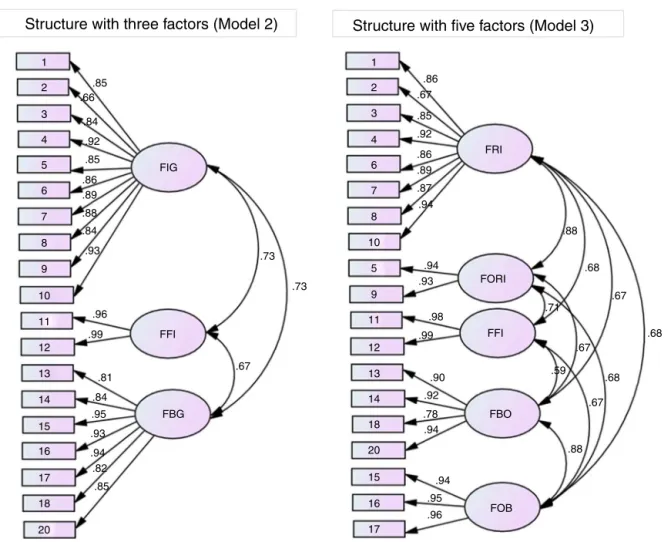
1 1 2 3 4 6 7 8 10 5 9 11 12 13 14 18 20 15 16 17 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 20 FIG FFI FBG FOB FBO FFI FORI FRI .86 .67 .85 .92 .86 .89 .87 .94 .94 .93 .98 .99 .90 .92 .78 .94 .94 .95 .96 .88 .67 .68 .68 .67 .67 .68 .88 .71 .59 .73 .73 .67 .96 .93 .84 .88 .89 .86 .85 .92 .84 .66 .85 .99 .81 .84 .95 .93 .94 .82 .85Figure2–DistributionofitemsofthePortugueseversionofBlood/InjectionFearScaleineachfactorandthelocalfit()of Models2and3ofthedataobtainedintheconfirmatoryfactoranalysis.FIG:fearofinjectioningeneral;FFI:faintingdueto fearofinjection;FBG:fearofbloodingeneral;FRI:fearofreceivinginjection;FORI:fearofseeingothersreceivinginjection; FFI:faintingduetofearofinjection;FBO:fearofseeingbloodofothers;FOB:fearofownblood.
Models2and3,observingtheRMSEAvaluesandtheir
con-fidenceintervals,itisclearthatnomodelhasabetterfitas
bothmodelsareproperlyadjustedtothedataandboth
struc-turescanbeconsideredintheassessmentoffearofbloodand
injectionsinthissample.
Distributionoftheitemsineachfactorconsideredinthe
confirmatoryfactoranalysisofModels2and3andthefactorial
weights()arepresentedinFigure2.Thenamesofthefactors
wereadoptedinaccordancewiththetheoreticalcontentof
theitemswhengrouped.
All items presented adequate factorial weights for the
correspondingfactors(≥0.66),whichconfirmsanadequate
distributionofitemsintheexploratoryfactoranalysis.
Model 2 (structure with three factors) presented
equiv-alenceoffactorial weights(metric invariance –: 2=18.7;
p-value=0.29), of factorial weights and intercepts (scalar
invariance – Int: 2=85.2; p-value=0.10), and of factorial
weights,interceptsand variances/covariances(strict
invari-ance–Cov:2=67.7;p-value=0.09)consideredverystablein
differentsamples.Model3(structure withfivefactors)
pre-sentedequivalenceoffactorialweightsindifferentsamples
(metricinvariance–:2=20.6;p-value=0.11)withpoor
sta-bility.
Discussion
There is a lack of studies and instruments to assess
blood/injection phobia in the general Brazilian population,
sincemostoftheinstrumentsavailablearespecificto
psychi-atryandfocusondiagnosisandtreatment.Inaddition,little
isknownabouttheprevalenceofthistypeofphobiaand
asso-ciatedfactorsintheBrazilianpopulation;thisisanimportant
gapinthescientificliteratureinthefaceoftheimportant
con-sequencesthatfearofbloodandinjectionmayhaveonthe
individual’shealth.
RegardingtheCVRestimatedfortheitems,onlysixitems
were considered essentialbythe judges (Table1).Ofthese
items,fourarerelatedtothefearofreceivinginjectionsand
adversereactionsandtwoarerelatedtothereactionssuchas
discomfort/feelingillonseeingtheirownbloodand/or
faint-ing atthesightofblood.As90.0% ofthejudges arehealth
professionals who performsomekind ofactivity in
hema-tology/transfusion medicine/blood donation services, these
results suggest that there may have been a trend by the
professionalsongivemoreimportancetotheseitems.
considered as a complementary analysis and may not be
used onlyas justification forthe exclusion ofitems of an
instrument.20,21
Inthepolychoriccorrelationsmatrixpresented(Figure1),
mostitemsoftheBIFS-Pwerestronglycorrelatedaccording
toparticipants’responses.Despitethehighcorrelation
coef-ficient(>0.90)betweenthreepairsofitems(11and12,13and
14,and16and17),theseitemsshowedhighfactorweightsin
allthemodelstestedintheCFA(Figure2)andforthisreason
theyhavebeenmaintainedintheinstrument.However,this
isnotthecaseofItem19,relatedtothesmellofthe
injec-tionapplicationroom.Thisitem,besidesthelowcorrelation
withallotheritemsofthescale(Figure1),presentedlow
fac-torweightinallmodelstested,andhinderedtheirfit.Forthis
reason,weoptedtoexcludeItem19intheanalysis,andthis
exclusionwasguidedbothbythelowfactorialweightdetected
andbytheclassificationasanon-essentialiteminthecontent
analysis.
Thechoice ofthe best factorial structureto beused to
assessthefearofblood/injectionsinthegeneralpopulation
isworthsomeconsideration.Firstly,bothmodels(withthree
andfivefactors)presentedadequatefittothedata,
suggest-ingthatbotharesuitabletomeasuretheconstruct.However,
Model2providesamorecomprehensivemeasureguidedby
alargergroupingofitems.Model3,inturn,providesamore
specificmeasure,whichcandistinguishmorepreciselythe
situationoffearandreactions.Thus,thechoiceofthe
struc-turetobeusedwilldependontheobjectivesofeachstudyto
beconducted.Furthermore,Model2wasstronglystableinthe
analysisoffactorialinvarianceinrandomsubsamples,which
allowsustosuggest itsuse instudies ofBrazilianusers of
healthcareservices.
WithrespecttotheoverallscoreoftheBIFS-P,theauthorsof
theoriginalproposalsuggestthesumofallanswers.However,
therearestrongargumentsthatcontradicttheuseofthesum
ofresponseswhenusingpsychometricscalesmainlydirected
atthedifferentfactorialweightsofitemsanddistributionof
factorsindistinctpopulations.20,21Thus,wesuggestusingthe
arithmeticmeanforthecomputationofanoverallscoreora
scoreforeachfactorseparately.
WeconcludethattheBIFS-Pisanimportantinstrument
inthefaceofadequatepsychometricpropertiesfoundinthe
sampleofprimaryhealthcareusers andits simplicityand
easeofunderstandingwithinthepublichealthcontext.We
suggestthatotherstudiesshouldbeconductedtoassessthe
psychometric properties ofthe BIFS-P in different
popula-tions such aspatients with chronic disease,blood donors,
elderly people, high school students, students in
health-carecourses,amongothers,aimingtoexpandtheutilityof
this instrument and contributeto other areas ofcollective
health.
Financial
support
This study was supported by the Fundac¸ão de Amparo
à Pesquisa do Estado de São Paulo (FAPESP) under grant
#2014/14020-6;andtheCoordenac¸ãodeAperfeic¸oamentode
PessoaldeNívelSuperior(CAPES).
Conflicts
of
interest
Theauthorsdeclarenoconflictsofinterest.
Acknowledgements
This study was funded by the Fundac¸ão de Amparo à
PesquisadoEstadodeSãoPaulo(FAPESP)2014/14020-6andthe
Coordenac¸ãodeAperfeic¸oamentodePessoaldeNívelSuperior
(CAPES).
r
e
f
e
r
e
n
c
e
s
1.HagopianLP,CrockettJL,KeeneyKM.Multicomponent treatmentforblood-injury-injectionphobiainayoungman withmentalretardation.ResDevDisabil.2001;21(2):141–9.
2.KoseS,MandiraciogluA.Fearofblood/injectioninhealthy andunhealthyadultsadmittedtoateachinghospital.IntJ ClinPract.2007;61(3):453–7.
3.TrijsburgRW,JelicicM,VandenBroekWW,PlekkerAEM, VerheijR,PasschierJ.Exposureandparticipantmodellingina caseofinjectionphobia.PsychotherPsychosom.
1996;65(1):57–61.
4.BuodoG,SarloM,PoliS,GiadaF,MadalossoM,RossiC,etal. Emotionalanticipationratherthanprocessingisalteredin patientswithvasovagalsyncope.ClinNeurophysiol. 2012;123(7):1319–27.
5.ArmstrongT,HemmingerA,OlatunjiBO.Attentionalbiasin injectionphobia:overtcomponents,timecourse,andrelation tobehavior.BehavResTher.2013;51(6):266–73.
6.MiloyanB,EatonWW.Blood-injection-injuryphobiainolder adults.IntPsychogeriatr.2016;28(6):897–902.
7.PageAC,BennettKS,CarterO,SmithJ,WoodmoreK.The Blood-InjectionsSymptomScale(BISS):assessingastructure ofphobicsymptomselicitedbybloodandinjections.Behav ResTher.1997;35(5):457–64.
8.D’ElReyGJ,PaciniCA.Prevalênciadafobiade
sangue-injec¸ão-ferimentosemamostradapopulac¸ãodeSão Paulo-SP.PsicolArgum.2005;23(43):53–9.
9.D’ElReyGJ,PeroniPC.Estudodeconfiabilidadedaversãoem portuguêsdaEscaladeSintomasdeSangue-Injec¸ão(BISS). Psicologia.2007;12(11):1–11.
10.GuimarãesAM,SilvaNetoAC,VilarAT,AlmeidaBG, AlbuquerqueCM,FermoseliAF.Transtornosdeansiedade: umestudodeprevalênciasobreasfobiasespecíficasea importânciadaajudadapsicologia.CadGrad.
2015;3(1):115–28.
11.PolitDF,BeckCT.TheContentValidityIndex:areyousureyou knowwhat’sbeingreported?Critiqueandrecommendations. ResNursHealth.2006;29(5):489–97.
12.LawsheCH.Aquantitativeapproachtocontentvalidity.Pers Psychol.1975;28(4):563–75.
13.WilsonFR,PanW,SchumskyDA.Recalculationofthecritical valuesforLawshe’scontentvalidityratio.MeasEvalCouns Dev.2012;45(3):197–210.
14.FerreiraMP,DiniNP,FerreiraSP.Espac¸osedimensõesda pobrezanosMunicípiosdoEstadodeSãoPaulo:Índice PaulistadeVulnerabilidadeSocial–IPVS.SãoPauloPerspect. 2006;20(1):5–17.
15.ScheafferRL,MendenhallW,OttRL,GerowK.Elementary SurveySampling.7thed.Boston:CengageLearning;2011.
17.LeónDA.AnáliseFatorialConfirmatóriaatravésdos SoftwaresReMplus.PortoAlegre,Brazil:Universidade FederaldoRioGrandedoSul;2011.
18.KlineRB.Principlesandpracticeofstructuralequation modeling.NewYork:TheGuilfordPress;1998, 354pp.
19.KaplanD.Structuralequationmodeling:foundationsand extensions.ThousandOaks,CA:SagePublications;2000.
20.AnastasiA.Psychologicaltesting.6thed.NewYork: MacmillanPublishingCompany;1988.