w w w . r b h h . o r g
Revista
Brasileira
de
Hematologia
e
Hemoterapia
Brazilian
Journal
of
Hematology
and
Hemotherapy
Original
article
Quantitative
flow
cytometric
evaluation
of
CD200,
CD123,
CD43
and
CD52
as
a
tool
for
the
differential
diagnosis
of
mature
B-cell
neoplasms
Elissandra
Machado
Arlindo
a,∗,
Natália
Aydos
Marcondes
a,b,
Flavo
Beno
Fernandes
b,
Gustavo
Adolpho
Moreira
Faulhaber
a,baUniversidadeFederaldoRioGrandedoSul(UFRGS),PortoAlegre,RS,Brazil bLaboratórioZanol,PortoAlegre,RS,Brazil
a
r
t
i
c
l
e
i
n
f
o
Articlehistory:
Received16February2017 Accepted2May2017 Availableonline29May2017
Keywords:
MatureB-cellneoplasms CD200
CD52 CD43 CD123
a
b
s
t
r
a
c
t
Background:DistinctionbetweenmatureB-cellneoplasmscanbedifficultdueto overlap-pingofimmunologicfeaturesandclinicalmanifestations.Thisstudyinvestigatedwhether quantifyingmeanfluorescenceintensityoffourmonoclonalantibodiesinaflowcytometry panelisusefulforthedifferentialdiagnosisandcharacterizationofthesedisorders.
Methods:TheexpressionsofCD52,CD200,CD123andCD43wereanalyzedinsamplesfrom 124patientswithmatureB-cellneoplasms.Thequantitativeestimationoftheseantigens wasassessedbymeanfluorescenceintensity.
Results:Thecasesincludedwere78chroniclymphocyticleukemias,threeatypicalchronic lymphocyticleukemias, sixmarginal zone lymphomas,11 splenic marginal zone lym-phomas,ninelymphoplasmacyticlymphomas,sixmantlecelllymphomas,twohairycell leukemias,twohairycellleukemiasvariant,fivefollicularlymphomas,oneBurkitt lym-phomaandonediffuselargeB-celllymphoma.ThemeanfluorescenceintensityofCD200 washigherinatypicalchroniclymphocyticleukemia,chroniclymphocyticleukemiaand hairycellleukemiacases.CD123showedhighermeanfluorescenceintensitiesinhairycell leukemiacells.Chroniclymphocyticleukemia,atypicalchroniclymphocyticleukemiaand mantlecelllymphomahadhigherexpressionofCD43andallfollicularlymphomacases hadverylowmeanfluorescenceintensityvalues.CD52expressionwasconsistentlypositive amongallcases.
Conclusion:Quantitativeevaluationofthesemarkerscanbeausefuladditionaltooltobetter identifysometypesofmatureB-cellneoplasms.
©2017Associac¸ ˜aoBrasileiradeHematologia,HemoterapiaeTerapiaCelular.Published byElsevierEditoraLtda.ThisisanopenaccessarticleundertheCCBY-NC-NDlicense (http://creativecommons.org/licenses/by-nc-nd/4.0/).
∗ Correspondingauthorat:RuaRamiroBarcelos,2350,90035-903PortoAlegre,RS,Brazil.
E-mailaddress:elissandra.machado@ufrgs.br(E.M.Arlindo).
http://dx.doi.org/10.1016/j.bjhh.2017.05.002
Introduction
Mature B-cell neoplasms (MBCN) account for around 80% ofall lymphoidneoplasmsandcompriseabroadspectrum of disorders with different morphologies, clinical aspects, geneticsandimmunophenotypes.However,theyhaveamore maturelymphoidprogenitorincommoncomparedto imma-tureneoplasms.AccordingtotheWorldHealthOrganization (WHO),immunophenotypicsimilaritiesofthesecellsata cer-tainstageofmaturationinconjunctionwithmorphological, geneticandclinicalfindingsallowthesediseasestobe classi-fiedanddiagnosed.1
Immunophenotypingbyflowcytometryisafastand cost-effectivetechniquewidelyusedforthediagnosisandfollow upofhematologicaldisorders.Multiparametricflow cytom-etry (MFC) has become more complex in the diagnosis of MBCNduetotheavailabilityofseveralmarkers,inaddition tothe highnumberofentities.Usually,theanalysisis per-formedseparatelyforeachsamplealiquotlabeledwithfourto eightmonoclonalantibodies(MoAbs);anexpertinterpretsthe immunophenotypicprofileofneoplasticB-cellsandspecifies adiagnosis.Preferred markersare those ableto differenti-ateB-cellsfromothercells,definethematurationstageand identifyphenotypic aberrations. Amongthese are the pan B-cellmarkers(CD19andCD20)andthoseusedforthe dif-ferentialdiagnosis (CD10,CD5, CD103,CD43, CD23,CD49d, CD81,CD200,CXCR5,immunoglobulin(Ig)MandKappaand Lambdachains).Adifferentialdiagnosiscanbedifficult,due tooverlappingimmunophenotypicfeaturesandsimilar clin-icalmanifestations,suchascasesofdifferentiationbetween lymphoplasmacyticlymphoma(LPL)andmarginalzone lym-phoma (MZL), or between diffuse large B-cell lymphoma (DLBCL)and follicularlymphoma.For thefinaldiagnosis,it isimportanttointegrateresultsofMFCwithmorphological, clinicalandcytogeneticanalysis.1,2
Somemarkersare knownfortheirexpressioninMBCN, suchas the positivity ofCD52,3 and negativity ofCD43 in
MZL,4the usefulnessofCD200inthedifferential diagnosis
betweenchroniclymphocyticleukemia(CLL)andmantlecell lymphoma(MCL),5andtheroleofCD123inthediagnosisof
hairycellleukemia(HCL).6However,thequantitativeanalysis
ofthesemarkersinMCBNisseldomdiscussed,andits expres-sionmaybeimportantnotonlyforthedifferentialdiagnosis, butalsoforprognosisandpotentialtherapeuticfactors,given theexistenceoftargetdrugsagainstsomeofthesemarkers.
Theaimofthisstudywastoevaluatequantitativelythe expressionofCD200,CD123,CD43andCD52inMBCN.
Methods
All MCBNcases diagnosedina referencelaboratory inthe southofBrazilfromOctober2014toJune2015were consecu-tivelyincludedinthestudy.Specimenssentforreassessment werenotincluded.ThestudywasapprovedbythelocalEthics Committee;writteninformedconsentwasdeemed unneces-saryafterthesignatureofacommitmenttermfortheuseof biologicalmaterialandassociatedinformationbyresearchers.
The panel with the four MoAbs under study was dis-tributedusingthefollowingfluorescences:CD123FITC(clone 7G3),CD52PE(clone4C8),CD43APC(clone1G10)andCD200 PerCP-Cy5.5(cloneMRCOX-104).AllMoAbswerepurchased fromBDBiosciences(SanDiego,USA).About1,000,000cells from whole blood or bonemarrow samplesanticoagulated with EDTAwere incubated for20min inthe dark atroom temperaturewitheachMoAb.Redbloodcellswerelysedby incubation withExcellyse I(EXBIO, Praha, CZ),followed by incubation withdistilled water. Samples were washed and resuspendedinphosphatebufferedsaline(PBS).Allreagents were usedaccordingtothemanufacturer’sinstructions. All sampleswereprocessedwithin48hofcollection.7
Immediatelyafterpreparation,sampleswereanalyzedon aFACSCaliburflowcytometerusingCellQuestTMProsoftware
(BDBiosciences,SanDiego,CA,USA).About100,000eventsper samplewereobtained.InfinicityTMFlowCytometryversion1.7
software (Cytognos,SL, ES)wasused fordataanalysis.For thegatingstrategy,debriswereremoved,basedon forward-scatter (FSC) and side-scatter(SSC) distribution. Neoplastic cellswereinitiallyidentifiedbypositivityoftheCD19andCD20 and theninaccordancewiththeexpressionofotherpanel markersforMBCN.Themeanfluorescenceintensities(MFIs) ofneoplasticcellswererecordedinarbitraryunitsfrom0to 104.
Reproducibility ofthe fluorescence intensities was pre-served bycalibration and daily quality controlprocedures. Calibritebeads(BD Biosciences,SaoDiego, USA)were used inordertoensurestandardization.Alldiagnosiswere estab-lishedinaccordancewiththeWHOcriteria.1
Medians andinterquartile ranges foreach MFIofMoAb werecalculated.TheMann–Whitneytestwasusedto calcu-latethestatisticalsignificanceofdifferencesbetweengroups. BurkittlymphomaandDLBCLwerenotincludedinthe sta-tistical analysis because there was only one case of each disease. Differences were considered significant when the
p-value<0.05.All statisticalanalyseswere performedusing theStatisticalPackagefortheSocialSciencesv.18.0software (SPSS,Chicago,USA).
Results
Immunophenotypicanalysiswasperformedin124samples –67ofperipheralblood(54%)and57ofbonemarrow(46%) –frompatientsdiagnosedwithMBCN.Patientmeanagewas 69.3±12.1yearsoldand51.6%weremen.Thedistributionof differential diagnosesofMBCNisdescribedinTable1.The most common diagnosis, as expected, was CLL due to its highprevalence,andtherarestwereBurkittlymphomaand DLBCL. Therateofneoplastic B-cellsamongtotalcells per samplerangedfrom3.7%to97.1%,withameanof52.3±25.4%. MedianMFIsforeachMoAbofthedifferentgroupsareshown inTable2.
The MFI of CD200 was higher in atypical chronic lym-phocytic leukemias (aCLL),CLL and HCL ascanbe seenin
400,0
300,0
200,0
100,0
70,0
50,0
30,0
10,0 4,0
,0
aCLL BL CLL DLBCL
CD200 MFI
FL HCL HCLv LPL MCL MZL SMZL
Figure1–BoxplotshowingMFIofCD200in124casesofmatureB-cellneoplasms.aCLL:atypicalchroniclymphocytic
leukemia;BL:Burkittlymphoma;CLL:chroniclymphocyticleukemia;DLBCL:diffuselargeB-celllymphoma;FL:follicular
lymphoma;HCL:hairycellleukemia;HCLv:hairycellleukemiavariant;LPL:lymphoplasmacyticlymphoma;MCL:mantle
celllymphoma;MZL:marginalzonelymphoma;SMZL:splenicmarginalzonelymphoma.
Table1–DistributionofmatureB-cellneoplasms.
Diagnosis Number
ofcases
(%)
Atypicalchroniclymphocyticleukemia 3 2.4
Burkittlymphoma 1 0.8
Chroniclymphocyticleukemia 78 62.9
DiffuselargeB-celllymphoma 1 0.8
Follicularlymphoma 5 4.0
Hairycellleukemia 2 1.6
Hairycellleukemiavariant 2 1.6
Lymphoplasmacyticlymphoma 9 7.3
Mantlecelllymphoma 6 4.8
Marginalzonelymphoma 6 4.8
Splenicmarginalzonelymphoma 11 8.9
comparedtoLPL(p-value<0.001),MCL(p-value<0.001), follicu-larlymphoma(p-value=0.001),MZL(p-value<0.001)andSMZL (p-value<0.001).TheMFIofCD200washigherinLPLcompared
toMCL(p-value=0.003).TheMFIofCD200washigherinHCL comparedtoMCL(p-value=0.046),MZL(p-value=0.046)and SMZL(p-value=0.030).TheMFIofCD200 ofHCL wasabout 6-foldhigherwhencomparedtohairycellleukemiavariant (HCLv),howeverduetothesmallnumberofcasesincluded it wasnot possibleto show statisticaldifference in CD200 expressionbetweenthesetwoneoplasms(p-value=0.121).
CD123hadhigherMFIsinHCLcellsasshowninFigure2. The MFIofCD123 washigher inHCL comparedto CLL (p -value=0.016),LPL(p-value=0.034),MCL(p-value=0.046),MZL (p-value=0.046)andSMZL(p-value=0.030).TheMFIofCD123 washigherinSMZLcomparedtoCLL(p-value=0.003),LPL (p-value=0.037)and follicular lymphoma(p-value=0.050).MFI ofCD123hadatrendtobehigherinHCLcomparedtoHCLv (p-value=0.121).
Figure 3illustratestheexpressionofCD43.All follicular lymphomacaseshadverylowMFIvalues.TheMFIofCD43 was higher in aCLL compared to MZL (p-value=0.034) and
Table2–MedianMFIvaluesofevaluatedmarkersperdiseasecategory.
Diagnosis CD200 CD123 CD43 CD52
Atypicalchroniclymphocyticleukemia 113.7(70.4/122.2) 7.5(5.7/7.6) 133.7(74.5/154.3) 765.2(546.5/814.7)
Burkittlymphoma 4.5(4.5/4.5) 5.9(5.9/5.9) 57.0(57.0/57.0) 143.0(143.0/143.0)
Chroniclymphocyticleukemia 61.1(41.4/89.2) 8.0(5.6/10.7) 179.8(112.8/278.7) 777.9(469.3/1101.0) DiffuselargeB-celllymphoma 3.8(3.8/3.8) 6.1(6.1/6.1) 160.9(160.9/160.9) 549.5(549.5/549.5)
Follicularlymphoma 2.6(1.8/11.4) 6.4(4.5/10.0) 6.8(3.6/13.1) 686.1(455.9/820.2)
Hairycellleukemia 220.3(163.1/277.5) 83.2(61.3/105.0) 79.0(44.4/113.7) 956.4(911.3/1001.6) Hairycellleukemiavariant 36.1(22.1/50.1) 22.0(14.4/29.5) 14.6(11.9/17.2) 990.3(705.9/1274.8) Lymphoplasmacyticlymphoma 14.0(12.30/40.2) 9.0(6.7/11.1) 20.7(6.5/25.3) 697.1(447.7/1150.8) Mantlecelllymphoma 3.5(2.1/4.1) 13.9(8.6/30.9) 90.1(48.6/226.7) 704.5(538.7/1375.5) Marginalzonelymphoma 8.3(4.5/13.2) 11.7(8.1/16.0) 7.8(5.0/23.6) 1326.1(737.5/1847.0) Splenicmarginalzonelymphoma 7.6(4.0/22.9) 12.3(9.4/16.1) 7.1(6.5/8.6) 1297.6(1096.9/1861.8)
150,0
100,0
50,0
40,0
30,0
20,0
10,0 6,0
3,0
,0
aCLL BL CLL DLBCL
CD123 MFI
FL HCL HCLv LPL MCL MZL SMZL
Figure2–BoxplotshowingMFIofCD123in124casesofmatureB-cellneoplasms.aCLL:atypicalchroniclymphocytic
leukemia;BL:Burkittlymphoma;CLL:chroniclymphocyticleukemia;DLBCL:diffuselargeB-celllymphoma;FL:follicular
lymphoma;HCL:hairycellleukemia;HCLv:hairycellleukemiavariant;LPL:lymphoplasmacyticlymphoma;MCL:mantle
celllymphoma;MZL:marginalzonelymphoma;SMZL:splenicmarginalzonelymphoma.
600.0
500.0
400.0
300.0
200.0
100.0
70.0
50.0
30.0
10.0
0
aCLL BL CLL DLBCL
CD43 MFI
FL HCL HCLv LPL MCL MZL SMZL
Figure3–BoxplotshowingtheMFIofCD43in124casesofmatureB-cellneoplasms.aCLL:atypicalchroniclymphocytic
leukemia;BL:Burkittlymphoma;CLL:chroniclymphocyticleukemia;DLBCL:diffuselargeB-celllymphoma;FL:follicular
lymphoma;HCL:hairycellleukemia;HCLv:hairycellleukemiavariant;LPL:lymphoplasmacyticlymphoma;MCL:mantle
celllymphoma;MZL:marginalzonelymphoma;SMZL:splenicmarginalzonelymphoma.*Oneoutliercase(MFI=1.1).One
outliercase(MFI=106.3).
SMZL(p-value=0.010). TheMFIofCD43was higher inCLL comparedtoHCLv(p-value=0.021),LPL(p-value<0.001), fol-licularlymphoma(p-value=0.001),MZL(p-value<0.001)and SMZL(p-value=0.003).
The MFI of CD43 was higher in MCL compared to LPL (p-value=0.013), follicular lymphoma (p-value=0.033), MZL (p-value=0.025)andSMZL(p-value=0.001).
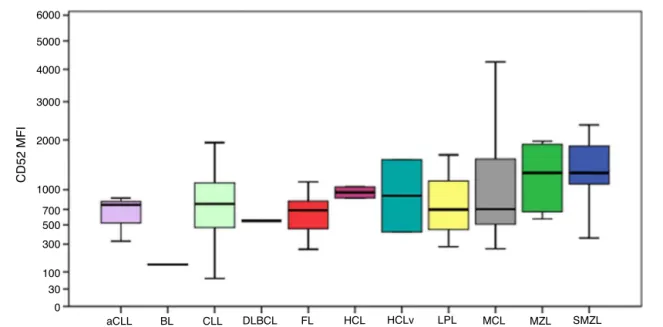
CD52expressionwasconsistentlypositiveamongallcases, as shown in Figure 4. The MFI of CD52 was higher in SMZLcomparedtoCLL(p-value=0.001),aCLL(p-value=0.036), LPL(p-value=0.030)andfollicularlymphoma(p-value=0.037).
Despite theBurkittlymphomagrouponlyhavingonecase, theMFIofCD52seemedconsiderablylowercomparedtothe remaininggroups.
Discussion
CD200 isa membraneglycoprotein ofthe immunoglobulin superfamily,expressedindendriticcells,neuronsandT-cell lymphocytes.8Differentresearchgroupshaveidentifiedthe
6000
5000
4000
3000
2000
1000
700 500
300
100
30
0
aCLL BL CLL DLBCL
CD52 MFI
FL HCL HCLv LPL MCL MZL SMZL
Figure4–BoxplotshowingtheMFIofCD52in124casesofmatureB-cellneoplasms.aCLL:atypicalchroniclymphocytic
leukemia;BL:Burkittlymphoma;CLL:chroniclymphocyticleukemia;DLBCL:diffuselargeB-celllymphoma;FL:follicular
lymphoma;HCL:hairycellleukemia;HCLv:hairycellleukemiavariant;LPL:lymphoplasmacyticlymphoma;MCL:mantle
celllymphoma;MZL:marginalzonelymphoma;SMZL:splenicmarginalzonelymphoma.
versusMCL,includingoneanalyzingaBrazilianpopulation.9
Thecurrentstudyhadsimilarresults,and,althoughoneCLL caseshowedlowexpressionofthismarker,100%ofMCLcases had significantly lower expressionscompared to CLL. In a recentstudy ofCLL patients, caseswith lowMFIofCD200 hadashortertime-to-treatment comparedtopatientswith higherexpressionofthe marker.10 Aspreviouslydescribed
intheimmunohistochemicalanalysisbyPillaietal.,CD200 canbeextremelyhelpfulinthedifferentialdiagnosisbetween HCLandHCLv11;inthepresentstudy,HCLcasesshoweda
trendtohavehigherexpressionsthanitsvariantform.There wasnodifferenceinCD200expressionbetweenCLLandaCLL (CD5-negative)cases.
CD200appearstobe usefulin thedifferential diagnosis between CD5-positiveLPL and CLL, as it was lower inthe formercomparedtothelatter.However,ifanalyzed qualita-tively,thisdifferencecouldnothavebeennoted,since30%of casesshowedintermediatepositivity,whichisinagreement withwhatwas describedinapreviousstudyanalyzingthe expressionofthismarkerbyimmunohistochemistry.12CD200
hasalsobeendescribedasusefultodifferentiatebetweenCLL andMZL;theresultsofthepresentstudyconfirmthefindings describedbyimmunohistochemistrywithalowerexpression ofthemarkerinMZLandSMZL.8
CD123isasubunitoftheinterleukin-3receptor.Ithasbeen described in several hematological neoplasms.13 Recently,
CD123 expressionwas studied quantitatively using MFI by Garcia-Dabrioetal.whocorrelateditsimplicationasa prog-nosticfactorindenovoacutemyeloidleukemia(AML),14and
SL-101,ananti-CD123antibody-conjugate,isunderstudyfor the treatment ofCD123-positive AML.15 Theresults ofthe
currentstudyareinagreementwithpreviousstudies investi-gatingthehigherexpressionofthismarkerinHCL,16,17since
higherMFIvalueswereidentifiedinHCLcasesandatrendof
HCLtohavehigherCD123expressioncomparedtoHCLvcases wasalsoobserved.Thisstudyidentifiedahigherexpressionof CD123inSMZLcomparedtoCLL,LPLandfollicularlymphoma cases.
According toaprevious study,CD43expressionis simi-laramongCD10-positiveMBCN(follicularlymphoma,Burkitt lymphomaandDLBCL),oneofthemostcomplicated differ-entialdiagnoses.18Allfivecasesoffollicularlymphomahad
extremelylowexpressionswhencomparedtoaCLL,CLLand MCL, andthe onlycaseofDLBCLexpressedthis markerat anintermediateintensityasdescribedbypreviousstudies,19
however,analysisofthisgroupwasimpairedbythelow fre-quencyofcases.
DuringtheontogenyofB-cells,CD43isexpressedinearly stagesandislostinintermediatestages,butitisexpressed again in plasma cells and activated mature B-cells.20 In
agreementwiththeliterature,theseresultsidentifiedhigher expressioninaCLL,CLLandMCL,andlowerexpressioninMZL andSMZL,probablyduetoclonalityintheintermediatestage ofthedisease.DespiteCD43notbeingarelevantmarkerfor thedifferentialdiagnosisofMCLandMZL,cautionshouldbe takenintheinterpretationofthesedatawithrespecttothe differential diagnosisofMCLversusCD5-positiveMZL.One MCLcase(16.0%)hadalowexpressionofthismarkersimilarto MZLandoneMZLcase(5.8%)showedintermediateexpression similartothatseeninMCL.
CD52isacellsurfaceglycoproteinwhosefunctionispoorly understood and is expressed in lymphocytes, monocytes, macrophagesandafewdendriticcells.21 Itsexpressionhas
beendescribedinseveralMBCNandpositivityforthismarker involves specific treatments for diseases such as CLL and
LPL.3,22,23Furthermore,solubleCD52hasbeenidentifiedasa
markerofdiseaseactivityinCLL.24Thisstudyidentifiedthe
resultsseemtobeincontrastwiththosedescribedbyother studies,suchasRodigetal.whoreportednegativityforsome diseasessuchasBurkittlymphomaand DLBCL,3thelowest
MFIsinthe samples ofthe present study were exactly for Burkittlymphomaand DLBCL.Chuang etal. analyzedMCL casesandidentifiedthatonly60%ofcaseswerepositivefor CD52,butthedifferenceintheimmunohistochemical tech-niqueusedmayexplainthedistinctresults.25
Sincefluorescenceintensitymeasuresareimportant deter-minants in the analysis of leukemias and lymphomas,26
reliable methods for measuringMFI are importantfor the correctdatainterpretation,and,consequently,correct classi-ficationofhematologicalmalignancies.Forthisreason,terms suchas“weak”and“strong”areuseful,but,asHendersonetal. suggested,perhapsquantitativevaluescouldbeusedinorder tofurtherexploretheinformationprovided.27
There are numerousvariables involvedin the quantita-tivedeterminationoffluorescenceintensity,somerelatedto thespecificityinthechosenMoAb,sampletype, anticoagu-lantemployed,autofluorescence,typeoffixation,cytometer compensationand unitofmeasurementusedtoreportthe data,amongothers.Even whenallthesevariables arewell controlled,somecautionininterpretingthedatashouldbe taken,butgiventheincreasinguniversalstandardizationin thefield ofimmunophenotyping, thistypeofanalysismay gainground,allowingforcomparativefigureswithgreater reli-abilityovertime.Untilthen,eachcentercandeveloptheuse ofdatainMFIaccordingtotheir caseseries, inaddition to usingthedataavailableintheliterature,aslongasitis crit-icallyinterpretedandthelimitationstothistypeofanalysis areunderstood.
Thisstudyhassomelimitations.Themainoneisthatonly immunophenotypingand biopsy resultsascomplementary testsforthediseaseentitydefinitionwereaccessible.Besides, fewcasesofnon-CLLcases,suchasBurkittlymphomaand DLBCL,wereavailable.
Conclusion
Inconclusion,theresultsofthisstudyshowtheusefulness ofalreadyknownmarkersinMBCNsuchasCD200inthe dif-ferentialdiagnosis ofCLLand MCL.Thesedatasuggestthe usefulnessofmorecomplexanalysesquantifyingMFIinthe differentialdiagnosis ofMBCN, suchasCD52expressionin SMZLversusLPL,andCD43expressioninMZL,LPLandSMZL comparedtoMCLandCLL.Nevertheless,theseresultsshould befurtherexploredinanalysesusingalargersamplesize.
Conflicts
of
interest
Theauthorsdeclarenoconflictsofinterest.
Acknowledgments
WeacknowledgetheFundodeIncentivoaPesquisa(FIPE)of HospitaldeClínicasdePortoAlegre(HCPA)forfinancial sup-port.
r
e
f
e
r
e
n
c
e
s
1.SwerdlowSH,InternationalAgencyforResearchonCancer WorldHealthOrganization.WHOclassificationoftumoursof haematopoieticandlymphoidtissues.4thed.Lyon,France: InternationalAgencyforResearchonCancer;2008.
2.vanDongenJJ,LhermitteL,BöttcherS,AlmeidaJ,
vanderVeldenVH,Flores-MonteroJ,etal.EuroFlowantibody panelsforstandardizedn-dimensionalflowcytometric immunophenotypingofnormal,reactiveandmalignant leukocytes.Leukemia.2012;26(9):1908–75.
3.RodigSJ,AbramsonJS,PinkusGS,TreonSP,DorfmanDM, DongHY,etal.HeterogeneousCD52expressionamong hematologicneoplasms:implicationsfortheuseof alemtuzumab(CAMPATH-1H).ClinCancerRes. 2006;12(23):7174–9.
4.BergerF,FelmanP,ThieblemontC,PradierT,BaseggioL, BryonPA,etal.Non-MALTmarginalzoneB-celllymphomas: adescriptionofclinicalpresentationandoutcomein124 patients.Blood.2000;95(6):1950–6.
5.PalumboGA,ParrinelloN,FargioneG,CardilloK,ChiarenzaA, BerrettaS,etal.CD200expressionmayhelpindifferential diagnosisbetweenmantlecelllymphomaandB-cellchronic lymphocyticleukemia.LeukRes.2009;33(9):1212–6.
6.DelGiudiceI,MatutesE,MorillaR,MorillaA,
Owusu-AnkomahK,RafiqF,etal.Thediagnosticvalueof CD123inB-celldisorderswithhairyorvillouslymphocytes. Haematologica.2004;89(3):303–8.
7.DavisBH,DasguptaA,KussickS,HanJY,EstrelladoA. Validationofcell-basedfluorescenceassays:practice guidelinesfromtheICSHandICCS–partII–preanalytical issues.CytometryBClinCytom.2013;84(5):286–90.
8.DorfmanDM,ShahsafaeiACD200.(OX-2membrane glycoprotein)expressioninbcell-derivedneoplasms.AmJ ClinPathol.2010;134(5):726–33.
9.SandesAF,deLourdesChauffailleM,OliveiraCR,MaekawaY, TamashiroN,TakaoTT,etal.CD200hasanimportantrolein thedifferentialdiagnosisofmatureB-cellneoplasmsby multiparameterflowcytometry.CytometryBClinCytom. 2014;86(2):98–105.
10.MiaoY,FanL,WuYJ,XiaY,QiaoC,WangY,etal.Low expressionofCD200predictsshortertime-to-treatmentin chroniclymphocyticleukemia.Oncotarget.
2016;7(12):13551–62.
11.PillaiV,PozdnyakovaO,CharestK,LiB,ShahsafaeiA, DorfmanDM.CD200flowcytometricassessmentand semiquantitativeimmunohistochemicalstaining distinguisheshairycellleukemiafromhairycell leukemia-variantandotherB-celllymphoproliferative disorders.AmJClinPathol.2013;140(4):536–43.
12.AlapatD,Coviello-MalleJ,OwensR,QuP,BarlogieB, ShaughnessyJD,etal.Diagnosticusefulnessandprognostic impactofCD200expressioninlymphoidmalignanciesand plasmacellmyeloma.AmJClinPathol.2012;137(1): 93–100.
13.MorettiS,LanzaF,DabustiM,TieghiA,CampioniD,
DominiciM,etal.CD123(interleukin3receptoralphachain). JBiolRegulHomeostAgents.2001;15(1):98–100.
14.García-DabrioMC,HoyosM,BrunetS,TormoM,RiberaJM, EsteveJ,etal.Complexmeasurementsmayberequiredto establishtheprognosticimpactofimmunophenotypic markersinAML.AmJClinPathol.2015;144(3):484–92.
16.Stetler-StevensonM,TembharePR.Diagnosisofhairycell leukemiabyflowcytometry.LeukLymphoma.2011;52Suppl. 2:11–3.
17.VenkataramanG,AguharC,KreitmanRJ,YuanCM,
Stetler-StevensonM.CharacteristicC.D103,CD123expression patterndefineshairycellleukemia:usefulnessofCD123and CD103inthediagnosisofmatureB-celllymphoproliferative disorders.AmJClinPathol.2011;136(4):625–30.
18.SchniederjanSD,LiS,SaxeDF,LechowiczMJ,LeeKL, TerryPD,etal.Anovelflowcytometricantibodypanelfor distinguishingBurkittlymphomafromCD10+diffuselarge B-celllymphoma.AmJClinPathol.2010;133(5):718–26.
19.MaXB,ZhengY,YuanHP,JiangJ,WangYP.CD43expression indiffuselargeB-celllymphoma,nototherwisespecified: CD43isamarkerofadverseprognosis.HumPathol. 2015;46(4):593–9.
20.WikénM,BjörckP,AxelssonB,PerlmannP.InductionofCD43 expressionduringactivationandterminaldifferentiationof humanBcells.ScandJImmunol.1988;28(4):457–64.
21.HaleG,XiaMQ,TigheHP,DyerMJ,TheWaldmannH. CAMPATH-1antigen(CDw52).TissueAntigens. 1990;35(3):118–27.
22.RossmannED,LundinJ,LenkeiR,MellstedtH,OsterborgA. VariabilityinB-cellantigenexpression:implicationsforthe
treatmentofB-celllymphomasandleukemiaswith monoclonalantibodies.HematolJ.2001;2(5):300–6.
23.OwenRG,HillmenP,RawstronAC.CD52expressionin Waldenstrom’smacroglobulinemia:implicationsfor alemtuzumabtherapyandresponseassessment.Clin Lymphoma.2005;5(4):278–81.
24.VojdemanFJ,HermanSE,KirkbyN,WiestnerA, vanT’VeerMB,TjønnfjordGE,etal.SolubleCD52isan indicatorofdiseaseactivityinchroniclymphocyticleukemia. LeukLymphoma.2017:1–7.
25.ChuangSS,HuangWT,HsiehPP,TsengHH,CampoE, ColomerD,etal.MantlecelllymphomainTaiwan:
clinicopathologicalandmolecularstudyof21casesincluding onecyclinD1-negativetumorexpressingcyclinD2.PatholInt. 2006;56(8):440–8.
26.BorowitzMJ,BrayR,GascoyneR,MelnickS,ParkerJW, PickerL,etal.U.S.-CanadianConsensusrecommendationson theimmunophenotypicanalysisofhematologicneoplasiaby flowcytometry:dataanalysisandinterpretation.Cytometry. 1997;30(5):236–44.