Article
0103 - 5053 $6.00+0.00
*e-mail: sarbani277@yahoo.com
Lewis Acid Free High Speed Synthesis of Nimesulide-Based Novel
N
-Substituted Cyclic Imides
Kavitha Kankanala,a,b Vangala Ranga Reddy,c Khagga Mukkantia and Sarbani Pal*,b
aJNT University Hyderabad, Kukatpally, Hyderabad-500085, India
bDepartment of Chemistry, MNR Degree and PG College, Kukatpally, Hyderabad-500072, India
cDr. Reddy’s Laboratories Ltd. Integrated Product Development, Bachupally,
Hyderabad-500090, India
A primeira síntese de novas imidas cíclicas derivadas de nimessulida foi realizada via reação de uma imina preparada a partir de nimessulida com anidridos apropriados na presença de acetato de sódio. Usando este processo, uma variedade de imidas cíclicas N-substituídas foi preparada em bons rendimentos em ácido acético glacial. Alguns dos compostos sintetizados mostraram atividades anti-inlamatórias quando testados in vivo.
The irst synthesis of nimesulide-based novel cyclic imides has been accomplished via the reaction of an amine prepared from nimesulide with appropriate anhydrides in the presence of sodium acetate. Using this process a variety of N-substituted cyclic imides was prepared in good yields in glacial acetic acid. Some of the compounds synthesized showed anti-inlammatory activities when tested in vivo.
Keywords: nimesulide, anhydride, cyclic imide, anti-inlammatory activities
Introduction
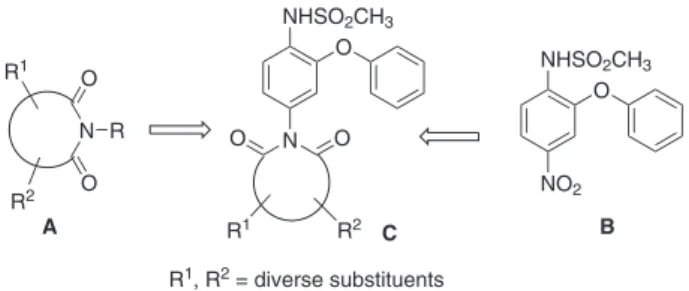
N-Substituted cyclic imides A (Figure 1) represent an important class of bioactive molecules that show a wide range of pharmacological activities such as androgen receptor antagonistic, anti-inflammatory, anxiolytic, antiviral, antibacterial, and antitumor properties.1-11 On the other hand N-(4-nitro-2-phenoxy phenyl) methanesulfonamide or nimesulide B (see Figure 1), a preferential cyclooxygenase-2 (COX-2) inhibitor is one of the well known non-steroidal anti-inlammatory drugs (NSAIDs) that has been utilized to treat pain and other inlammatory diseases. Because of their common anti-inlammatory properties and our interest in nimesulide derivatives12 as potential anti-inlammatory agents we decided to prepare compound C having structural features of both A and B.
In spite of their extensive pharmaceutical and industrial use only a limited number of procedures are available for the synthesis of A.13,14 These include (i) the dehydrative condensation of an anhydride and an amine at high
temperature,15 (ii) the cyclization of the amic acid in the presence of acidic reagents,16 (iii) N-alkylation of maleimide with alcohols under Mitsunobu reaction conditions,13 (iv) Lewis acids or hexamethyldisilazane catalyzed synthesis of N-alkyl and N-arylimide derivatives17 and dehydrative cyclization of acid anhydrides, imides and dicarboxylic acids with substituted amines in the presence of DPPOx and Et3N.18 However, many of these methodologies suffer from several drawbacks such as (i) the use of expensive catalysts or Lewis acids and carcinogenic solvent media and (ii) formation of numerous by-products leading to the poor yields of products. Moreover, only a narrow range of imide derivatives can be synthesized by using these methods.
N O
O R
A
N
O O
NHSO2CH3
O
NO2
NHSO2CH3
O
B C
R1
R2
R1 R2
R1, R2 = diverse substituents
While microwave assisted addition of amines to phthalic anhydride has been studied,19 no signiicant difference was observed when the reaction was carried out by microwave or conventional heating in DMF.20 Moreover, microwave-assisted solvent free synthesis of cyclic imides required the use of TaCl5-silica gel as catalyst.21 To overcome all these issues, we herein report the Lewis acid free rapid synthesis22 of novel N-substituted cyclic imides derived from nimesulide under conventional heating.
Results and Discussion
The starting compound 1 required for our study was prepared12 in quantitative yield from nimesulide B via reducing its nitro group as shown in Scheme 1.
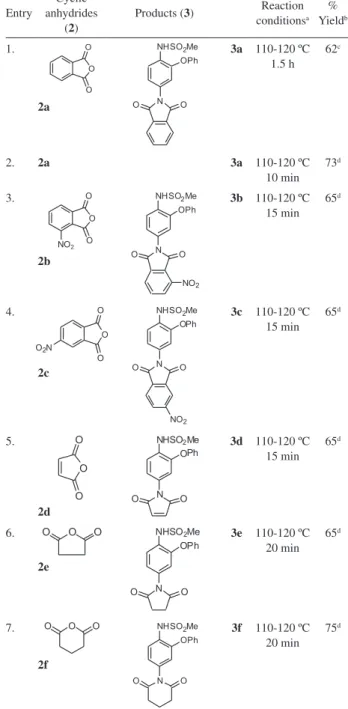
The aromatic amine was then treated with a variety of cyclic anhydrides 2 (Scheme 1) and the results are summarized in Table 1. In the beginning of our study, the reaction of aromatic amine 1 with phthalic anhydride 2a was examined under a conventional condition. After reluxing the reaction mixture in glacial acetic acid for 1.5 h the desired imide 3a was isolated in 62% yield (Entry 1, Table 1). In order to improve the product yield the above reaction was carried out in the presence of NaOAc in the same solvent.
The reaction was completed within 10 min affording 3a in 73% yield (Entry 2, Table 1). A further accelerating effect was observed when the reaction was carried out in the presence of NaOAc providing 3a in 62 and 73% yield respectively(Entry 1 and 2, Table 1). Encouraged by these observations, especially in the presence of NaOAc (Entry 2, Table 1) when the reaction was completed within 10.0 min, we decided to asses the generality of this process for the synthesis of other related derivatives. As indicated in Table 1 that nitro substituted phthalic anhydrides (2b and 2c) reacted with 1 even slower than 2a (Entries 3 and 4 vs 2, Table 1) in the presence of sodium acetate. Among the aliphatic anhydrides, the six-membered anhydride 2f (Entry 7, Table 1) produced the best result. All these reactions were also performed under conventional heating condition without sodium acetate (c.f. Entry 1, Table 1) and the results were compared with that of sodium acetate
NO2
NHSO2CH3
O
B
Sn/HCl
90 oC, 3h
NH2
NHSO2CH3
O
1
O O
O
N
O O
NHSO2CH3
O
3 2
Scheme 1. Preparation of N-substituted cyclic imides from nimesulide.
Table 1. Synthesis of novel N-substituted cyclic imides
Entry
Cyclic anhydrides
(2)
Products (3) Reaction conditionsa
% Yieldb
1.
2a
3a 110-120 ºC 1.5 h
62c
2. 2a 3a 110-120 ºC
10 min 73d
3.
2b
3b 110-120 ºC 15 min
65d
4.
2c
3c 110-120 ºC 15 min
65d
5.
2d
3d 110-120 ºC 15 min
65d
6. 2e
3e 110-120 ºC 20 min
65d
7. 2f
3f 110-120 ºC 20 min
75d
aAll the reactions were carried out using compound 1 (1.0 mmol), cyclic
anhydride 2 (1.0 mmol) and anhydrous NaOAc (100 mg, 1.2 mmol).
catalysed method. Nevertheless, all the compounds prepared are novel and well characterized by spectral and analytical data.
Having synthesized a range of nimesulide-based novel cyclic imides we decided to examine anti-inlammatory activities of selected compounds e.g. 3a, 3b, 3c, 3e and 3f. The anti-inlammatory activity of these compounds was evaluated in a carrageenan-induced rat model
of inflammation23 using indomethacin as a reference
compound. At a dose of 100 mg kg-1 (i.p.) compound 3f
showed 20, 25, 45 and 40% inhibition of edema after 1, 2, 3 and 4h when indomethacin showed 24, 43, 66 and 93%
inhibition at 10 mg kg-1 at the same time points. Though
the other compounds were also found to be active (15-25% inhibition of edema at various time points) when dosed at 100 mg kg-1 the compound 3f was found to be the best among them.
Conclusions
We have described a simple and rapid synthesis of novel cyclic substituted imides24 that were designed from a well known drug nimesulide. The aromatic amine prepared from nimesulide was reacted with a variety of cyclic anhydrides in the presence of sodium acetate to afford the desired products within few minutes.Overall, due to the shorter reaction time and simple operational procedure that does not require the use of expensive catalysts / solvents, would ind wide application in the synthesis of various nimesulide-based cyclic imides of potential pharmacological interest. Some of the compounds synthesized showed anti-inlammatory activity when tested in rats.
Experimental
Melting points were determined by open glass capillary method on a Cintex melting point apparatus and are uncorrected. IR spectra were recorded on a Perkin-Elmer spectrometer using KBr pellets. 1HNMR spectra were recorded on a Bruker ACF-300 machine and a Varian 300 and 400 MHz spectrometer using CDCl3 or DMSO-d6, with reference to tetramethylsilane as an internal reference. 13CNMR spectra were recorded on a 75 MHz spectrometer. Elemental analyses were performed by Varian 3LV analyzer series CHN analyzer. Mass spectra were recorded on a Jeol JMC D-300 instrument by using Electron ionization at 70 ev. All reactions were monitored by TLC on pre-coated silica gel plates. Column chromatography was performed on 100-200 mesh silica gel (SRL, India) using 10-20 times (by weight) of the crude product. All the anhydrides used are commercially available.
Preparation of cyclic amides (3)
Typical procedure for the preparation of 3a
To a mixture of compound 1 (278 mg, 1.0 mmol) and phthalic anhydride 2a (148 mg, 1.0 mmol) in glacial acetic acid (3 mL) was added anhydrous sodium acetate (100 mg, 1.2 mmol) and the mixture was allowed to relux for 10 min. After completion of the reaction (as indicated by TLC) the mixture was added to crushed ice (50 g) and stirred. The solid separated was iltered and dried. The crude product was puriied by column chromatography followed by re-crystallization from methanol.
N-[4-(1,3-dioxo-1,3-dihydroisoindol-2-yl)-2-phenoxy-phenyl]methanesulfonamide (3a)
White solid; mp 230-232 ºC; Rf 0.95 (CHCl3: Ethylacetate = 9:1), IR (KBr) ν
max/cm
-1: 1714, 1HNMR (400 MHz, CDCl3) d 3.0 (s, 3H), 6.8 (m, 2H), 7.1 (m, 2H), 7.2 (m, 2H),7.4 (m, 2H), 7.7 (m, 3H), 7.9 (m, 2H); 13CNMR (75 MHz, CDCl3) d 40.0 (CH3), 116.1 (CH), 118.9 (CH), 121.1 (CH), 122.1 (CH), 123.8 (CH), 124.8 (CH), 127.6 (C), 128.6 (C), 130.2 (CH), 131.5 (C), 134.5 (CH), 147.2 (C), 155.2 (C), 166.8 (C); MS 408 (M+, 100%); Elemental analysis found: C, 61.70, H 3.94, N 6.89; C21H16N2O5S requires C, 61.75; H, 3.95; N, 6.86%.
N-[4-(4-nitro-1,3-dioxo-1,3-dihydroisoindol-2-yl)-2-phenoxy-phenyl]methanesulfonamide (3b)
Light green solid; mp 214-216 ºC; Rf 0.68 (CHCl3: Ethylacetate = 9:1); IR (KBr) ν
max/cm
-1: 1732; 1HNMR (400 MHz, DMSO-d6) d 3.0 (s, 3H), 7.0 (s, 1H), 7.1 (d,
J 7.8 Hz, 2H), 7.2 (m, 2H), 7.4 (t, J 7.8 Hz, 2H), 7.6 (d,
J 8.3 Hz, 1H), 8.0 (t, J 7.8 Hz, 1H), 8.2 (d, J 6.7 Hz, 1H), 8.3 (d, J 7.4 Hz, 1H), 9.5 (bs, 1H, NH); 13CNMR (75 MHz, CDCl3) d 39.9 (CH3), 116.0 (CH), 119.0 (CH), 120.8 (CH), 122.2 (CH), 125.0 (CH), 127.4 (CH), 127.6 (C), 128.3 (CH), 128.9 (C), 130.3 (CH), 135.8 (CH), 147.2 (C), 155.1 (C); MS 453 (M+, 100%); Elemental analysis found: C, 55.69, H 3.35, N 9.19; C21H15N3O7S requires C, 55.63; H, 3.33; N, 9.27%.
N-[4-(5-nitro-1,3-dioxo-1,3-dihydroisoindol-2-yl)-2-phenoxyphenyl]methanesulfonamide (3c)
Light yellow solid; mp 192-194ºC; Rf 0.86 (CHCl3: Ethylacetate = 9:1); IR (KBr) ν
max/cm
-1: 1719; 1HNMR (400 MHz, DMSO-d6) d 3.0 (s, 3H), 7.0-7.3 (m, 5H), 7.4 (t, J 6.4 Hz, 2H,), 7.6 (d, J 8.3 Hz, 1H,), 8.2 (d, J 8.3 Hz, 1H,), 8.5 (d, J 1.5 Hz, 1H,), 8.6 (dd, J 7.7 and 1.9 Hz, 1H), 9.6 (bs, 1H, NH); 13CNMR (75 MHz, CDCl
MS 453 (M+, 100%); Elemental analysis found: C, 55.57, H 3.30, N 9.31; C21H15N3O7S requires C, 55.63; H, 3.33; N, 9.27%.
N-[4-(2,5-dioxo-2,5-dihydropyrrol-1-yl)-2-phenoxyphenyl] methanesulfonamide (3d)
Pale yellow solid; mp 145-148 ºC; Rf 0.80 (CHCl3: Ethylacetate = 9:1); IR (KBr) ν
max/cm
-1: 1725, 1708; 1HNMR (400 MHz, CDCl3) d 3.0 (s, 3H), 6.80 (s, 2H), 6.88 (d, J 6.0 Hz, 1H), 6.96 (bs, 1H, NH), 7.05 (d, J 9.0 Hz, 2H), 7.12 (dd, J 12.0 Hz, J 6.0 Hz, 1H), 7.18 (t, J 9.0 Hz 1H), 7.40 (t,
J 12.0 Hz, 2H), 7.75 (d, J 12.0 Hz, 1H); 13CNMR (75 MHz, CDCl3) d 39.7 (CH3), 115.6 (CH), 118.9 (CH), 121.2 (CH), 121.6 (CH), 124.7 (CH), 127.5 (C), 128.2 (C), 130.2 (CH), 134.1 (CH), 147.2 (C), 155.2 (C), 169.0 (CO); MS 359 (M+, 100%); Elemental analysis found: C, 56.90, H 3.96, N 7.97; C17H14N2O5S requires C, 56.98; H, 3.94; N, 7.82%.
N-[4-(2,5-dioxo-pyrrolidin-1-yl)-2-phenoxyphenyl]methane-sulfonamide (3e)
Off white solid; mp 225-228 ºC; Rf 0.33 (CHCl3: Ethylacetate = 9:1); IR (KBr) ν
max/cm
-1: 1707; 1HNMR (300 MHz, CDCl3) d 2.7 (s, 4H), 3.1 (s, 3H), 6.8 (s, 1H), 7.1( m, 3H,), 7.3 (m, 1H), 7.4 (m, 3H), 9.5 (s, NH,); 13CNMR (75 MHz, CDCl3) d 28.3 (CH2), 39.8 (CH3), 115.9 (CH), 119.1 (CH), 120.8 (CH), 122.1 (CH), 124.8 (CH), 128.1(C), 128.6 (C), 130.2 (CH), 147.2 (C), 155.1 (C), 175.7 (CO); MS 361 (M+, 100%); Elemental analysis found: C, 56.70, H 4.50, N 7.74; C17H16N2O5S requires C, 56.66; H, 4.47; N, 7.77%.
N-[4-(2,6-dioxo-piperidin-1-yl)-2-phenoxyphenyl]methane-sulfonamide (3f)
White solid; mp 126-128 ºC; Rf 0.38 (CHCl3: Ethylacetate = 1:1); IR (KBr) ν
max/cm
-1: 1671; 1HNMR (400 MHz, DMSO-d
6) d 1.7 (m, 2H), 2.4 (m, 4H), 3.0 (s,
3H), 7.0 (dd, J 8.8 and 1 Hz, 2H), 7.2-7.3 (m, 4H), 7.42 (m, 2H), 10.0 (1H, NH); 13CNMR (75 MHz, DMSO-d
6)
d 20.2 (CH2), 32.9 (CH2), 35.3 (CH2), 108.6 (CH), 113.8 (CH), 119.1 (CH), 122.3 (C), 123.8 (CH), 127.8 (CH), 129.9 (CH), 138.3 (C), 151.0 (C), 155.9 (C), 170.7 (C), 174.0 (CO); Mass 375 (M+, 100%); Elemental analysis found: C, 57.69, H 4.86, N 7.52; C18H18N2O5S requires C, 57.74; H, 4.85; N, 7.48%.
Anti-inlammatory activity
The anti-inlammatory activity of ive compounds (3a, 3b, 3c, 3e and 3f) was evaluated according to the method reported in the literature23 where a pedal inlammation in rat paws was induced by sub plantar injection of 0.1 mL
carrageenan (0.2%) suspension in gum acacia into the right hind of the rats. Male adult albino Wister rats (100-120 g) were divided into 12 groups of six animals each. The rat paw thickness was measured with a Veriner caliper before and 1 h after the carrageenan injection to detect the carrageenan induced inlammation. Each test compound at a dose of 100 mg kg-1 was injected i.p. to a separate group of
rats 1 h after carrageenan injection. Control group received the vehicle (5% gum acacia), while the reference group
received indomethacin, 10 mg kg-1.
The difference between the thicknesses of the two paws was taken as a measure of edema. The measurement was carried out at zero, 1, 2, 3 and 4 h after injection of the test compounds, reference drug, and the vehicle.
Acknowledgments
The authors (K. Kavitha and S. Pal) thank Mr. M. N. Raju, the chairman of MNR Educational Trust for his support and encouragement.
References
1. Salvati, M. E.; Balog, A.; Shan, W.; Wei, D. D.; Pickering, D.; Attar, R. M.; Geng, J.; Rizzo, C. A.; Gottardis, M. M.; Weinmann, R.; Krystek, S. R.; Sack, J.; An, Y.; Kish, K.; Bioorg. Med. Chem. Lett.2005,15, 271.
2. Kossakowski, J.; Jarocka, M.; Il Farmaco2001,56, 785; Sondhi, S. M.; Rani, R.; Roy, P.; Agarwal, S. K.; Saxena, A. K.; Bioorg. Med. Chem. Lett.2009, 19, 1534.
3. Ishizumi, K.; Kojma, A.; Antoku, F.; Chem. Pharm. Bull.1991,
39, 2288.
4. Shibata, Y.; Shiehita, M.; Sasaki, K.; Nishimura, I. L.; Hashimoto, Y.; Iwasaki, S.; Chem. Pharm. Bull. 1995,43, 177. 5. Jindal, D. P.; Bedi, V.; Jit, B.; Karkra, N.; Guleria, S.; Bansal,
R.; Palusczak, A.; Hartmann, R.W.; Il Farmaco2005,60, 283. 6. Wang, J. J.; Wang, S. S.; Lee, C. F.; Chung, M. A.; Chern, Y.
T.; Chemotherapy1997, 43, 182.
7. Machado, A. L.; Lima, L. M.; Arau´jo, Jr., J. X.; Fraga, C. A. M.; Koatz, V. L. G.; Barreiro, E. J.; Bioorg. Med. Chem. Lett.
2005,15, 1169.
8. Kenji, S.; Hideko, N.; Yoshihiro, U.; Yoshikazu, S.; Kazuharu, N.; Motoji, W.; Konstanty, W.; Tadafumi, T.; Tetsuji, A.; Yuji, Y.; Kenji, K.; Hitoshi, H.; Bioorg. Med. Chem.2005,13, 4014. 9. Mayer, A.; Neuenhofer, S.; Angew. Chem., Int. Ed. 1994,33,
1044.
10. Miguel, F. B.; Gema, D.; Beatriz, S.; Cynthia, R.; Simmon, R.; Teresa, B.; Eur. J. Med. Chem.2002,37, 541.
12. Pericherla, S.; Mareddy, J.; Rani, D. P. G.; Gollapudi, P. V.; Pal, S.; J. Braz. Chem. Soc. 2007, 18, 384.
13. Walker, M. A.; J. Org. Chem.1995,60, 5352; Walker, M. A.;
Tetrahedron Lett.1994,35, 665.
14. Dorta, R. L.; Francisco, C. G.; Sua´rez, E.; Tetrahedron Lett.
1994,35, 1083.
15. Da Settimo, A.; Primoiore, G.; Settimo, F. Da; Simorini, F.; LaMotta, C.; Martinelli, A.; Boldrini, E.; Eur. J. Med. Chem.
1996,31, 49.
16. Mehta, N. B.; Phillips, A. P.; Lui, F. F.; Brooks, R. E.; J. Org. Chem.1960,25, 1012.
17. Reddy, P. Y.; Kondo, S.; Toru, T.; Ueno, Y.; J. Org. Chem.1997,
62, 2652.
18. Abdel-Aziz, A. A.-M.; Eur. J. Med. Chem.2007, 42, 614. 19. Bose, A. K.; Manhas, M. S.; Ghush, M.; Raju, V. S.; Tabei, K.;
Urbanczyk-Lykewaska, Z.; Heterocycles1990,30, 741.
20. Westaway, K. C.; Gedye, R. N.; J. Microw. Power Electromagn. Energy1995, 30, 219.
21. Chandrasekhar, S.; Takhi, M.; Uma, G.; Tetrahedron Lett.1997,
38, 8089.
22. Akula, M. R.; Kabalka, G. W.; Synth. Commun. 1998,28, 2063. 23. Nargund, L. V. G.; Reedy, G. R. N.; Haripbasad, V.; J. Pharm.
Sci. 1994, 83,246.
24. For the synthesis of cyclic unsubstituted imides, see: Mederski, W. W. K. R.; Baumgarth, M.; Germann, M.; Kux, D.; Weitzel, T.; Tetrahedron Lett.2003,44, 2133; Hijji, Y.; Benjamin, E.;
Heterocycles2006, 68, 2259.
Received: December 22, 2009