Ar
ti
cle
J. Braz. Chem. Soc., Vol. 21, No. 10, 1819-1824, 2010. Printed in Brazil - ©2010 Sociedade Brasileira de Quím ica
0103 - 5 05 3 $ 6 .00+ 0.00
* e-m ail: m arcelosob ral@ ltf .uf p b .b r
3’,8’’-Biisokaempferide, a Cytotoxic Bilavonoid and Other Chemical Constituents
of Nanuza plicata (V elloz iaceae)
Meri Emili F. Pinto,a Marcelo Sobral da Silva,*,a Elisabete Schindler,b José Maria Barbosa Filho,a Ramon dos Santos El- Bachá,b Marianna V ieira S. C astello- Branco,a
Maria de Fatima A g raa and Josean Fechine T avaresa
aLaboratório de Tecnologia Farmacêutica, Univers idade Federal da P araíba, CP 5 0 0 9 ,
5 8 0 5 1 - 9 7 0 João P es s oa- P B, Brazil
bLaboratório de N euroq uímica e Biologia Celular ( LabN q ) , I ns tituto de Ciências da Saúde ( I CS) ,
Univers idade Federal da Bahia, 4 0 1 1 0 - 9 0 2 Salvador- BA , Brazil
F oi isolado das f olh as de N anuza p licata um nov o b if lav onóide, ch am ado 3 ’,8’’-b iisocam p f erideo (1) , j untam ente com os conh ecidos com p ostos am entolav ona (2) , ácido
p atag ônico (3) , ( 4aR ,5 S,6 R ,8aR ) -5 -[ 2-( 2,5 -dih idro-5 -m etóx i-2-ox of uran-3 -il) etil] -3 ,4,4a,5 ,6 ,7 ,8, 8a-octah idro-5 ,6 ,8a-trim etilnaf taleno-1-ácido carb ox ílico (4) , caf eoilq uinato de m etila (5) , ácido
3 ,5 -di-caf eoilq uínico (6) e luteolina (7) . O s com p ostos 3, 4, 5 e 6 são relatados p ela p rim eira
v ez em Velloziaceae. A s estruturas dos com p ostos f oram elucidadas com b ase em m étodos esp ectroscóp icos, esp ecialm ente R M N e E M . A citotox icidade de 3 ’,8’’-b iisocam p f erideo f oi
estudada em células de g liob lastom a h um ano ( G L -15 ) . A concentração ef etiv a, q ue p roduziu m orte em 5 0% das células ap ós 7 2 h f oi 3 6 ,5 μm ol L-1. A lterações na m orf olog ia celular, incluindo
retração e deg radação de citop lasm a, f oram ob serv adas q uando as células f oram tratadas com concentrações a p artir de 20 μm ol L-1 de 3 ’,8’’-b iisocam p f erideo p or 7 2 h .
A nov el b ilav onoid, nam ed 3 ’,8’’-b iisok aem p f eride (1) , along w ith th e k now n com p ounds
am entolav one (2) , p atag onic acid (3) , ( 4aR ,5 S,6 R ,8aR ) -5 -[ 2-( 2,5 -D ih y dro-5 -m eth ox y -2-oxof uran-3 -y l) eth y l] -uran-3 ,4,4a,5 ,6 ,7 ,8,8a-octah y dro-5 ,6 ,8a-trimeth y lnap h th alene-1-carb ox y lic acid (4) ,
5 -caf f eoy lq uinic acid m eth y l ester (5) , 3 ,5 -di-caf f eoy lq uinic acid (6) and luteolin (7) , w ere isolated
of th e leav es f rom N anuza p licata. T h e com p ounds 3, 4, 5 and 6 are rep orted f or th e irst tim e
in Velloziaceae. T h e structures of th e com p ounds w ere elucidated on th e b asis of sp ectroscop ic m eth ods, esp ecially NM R and M S analy ses. T h e cy totox icity of 3 ’,8’’-b iisok aem p f eride w as
studied in cultures of h um an g liob lastom a G L -15 cells. T h e ef f ectiv e concentration, w h ich k illed 5 0 % of cells af ter 7 2 h w as 3 6 .5 μm ol L-1. C h ang es in cellular m orp h olog y , including retraction
and deg radation of cy top lasm , w ere ob serv ed w h en cells w ere treated w ith concentrations f rom 20 μm ol L-1 of 3 ’,8’’-b iisok aem p f eride f or 7 2 h .
K eyw ords: 3 ’,8’’-b iisok aem p f eride, N anuza p licata, cy totox icity , Velloziaceae
I ntroduction
Velloziaceae is a f am ily of m onocoty ledonous p lants containing ab out 27 0 sp ecies of th e trop ical
Brazilian lora occurring in rock y ields.1 T h ese p lants
liv e under conditions of h ig h solar irradiation and low
w ater av ailab ility .2 Sp ecies of th is f am ily contain h ig h
am ounts of diterp enes w ith a sk eleton of th e clerodane,2
cleisth antane, isop im arane,3 k aurane,4 and lab dane5 ty p es,
b esides lav onoids m onoisop reny lated, C-alk y lated and
b ilav onoids.6 -9 T h e g enus N anuza com p rises th ree sp ecies,
N . p licata, N . almeidae and N . luetzelburgii. N anuza
p licata ( M art.) L . B. Sm . & A y ensu ( Sy nony m y : V ellozia p licata and X erop hy ta p licata) , is k now n com m only as “canela d’em a”. I n earlier studies th e com p ounds
3 -g erany l-4-h y drox y b enzoate10-11 and th e b if lav onoid
am entolav one,12 w ere isolated f rom sp ecies collected in
th e States of R io de J aneiro and M inas G erais, Brazil,
resp ectiv ely . I n th is w ork , w e rep ort th e isolation and
3 ’,8’’-Biisok aem p f eride, a C y totox ic Bilav onoid and O th er C h em ical C onstituentsJ. Braz. Chem. Soc.
1820
b ilav onoids, 3 ’,8’’-b iisok aem p f eride (1) , a new natural
p roduct, and am entolav one (2) ,13 tw o diterp enes of th e
clerodane ty p e, p atag onic acid (3)14 and ( 4aR ,5 S,6 R ,8aR )
5 [ 2( 2,5 dih y dro5 m eth ox y 2ox of uran3 y l) eth y l] -3 ,4,4a,5 ,6 ,7 ,8,8a-octah y dro-5 ,6 ,8a trim eth y lnap h th
alene-1-carb ox y lic acid (4) ,15 tw o caf f eoy lq uinic acid deriv ates,
5 -caf f eoy lq uinic acid m eth y l ester (5)16 and 3 ,5
-di-caf f eoy lq uinic acid (6)17 -18 and a lav one, luteolin (7)19
( F ig ure 1) . T h e com p ounds 3, 4 , 5 and 6 are rep orted f or
th e irst tim e in Velloziaceae.
I nterest in th e p ossib le h ealth b eneits of lav onoids h as
increased b ecause som e h um an f eeding studies sup p ort a
p rotectiv e ef f ect of th eir consum p tion in cancer20 and also
ag ainst cy totox ic insults induced b y ox idativ e stress and
am y loid b sug g esting th eir th erap eutic p otential against
neurodeg enerativ e diseases.21 T h e rep lacem ent of anim al
tech niq ues w ith non-anim al ones is im p erativ e in saf e
tox icolog ical ev aluations of new com p ounds. M odels using
cultured cells to ev aluate th e cy totox icity of ch emicals
h av e th e adv antag e of b eing sim p le and q uick w ith a low
ev aluation cost.22 T h e inv estig ation of new m olecules f or
g liob lastom a treatm ent is relev ant b ecause desp ite current adv ances in th erap y , including surg ical resection f ollow ed b y radiation and ch em oth erap y , th e p rog nosis f or p atients
rem ains p oor.23 I n th is p ap er, th e cy totox icity of 1 to h um an
g liob lastom a G L -15 cells w as ev aluated in vitro.
R esults and D iscussion
T h e eth anol ex tract f rom N . p licata af f orded lav onoids,
clerodane diterp enes and deriv ativ es of ch lorog enic acid.
T h e new b ilav onoid (1 ) w as isolated in th e f orm of a
y ellow solid w ith m p 3 40-3 42º C , and th e H R -E SI -M S m ass
sp ectrum sh ow ed a m olecular ion p eak at 5 99.5 3 3 1 [ M +
H ] , com p atib le w ith th e m olecular f orm ula C3 2H23O12. T h e
inf rared sp ectrum in K Br sh ow ed ab sorp tion at 3 3 5 0, 3 25 0
and 16 5 1 cm-1, ch aracteristic of O H and carb ony l g roup s,
resp ectiv ely . T h e 13C NM R in C D
3O D ( 5 00 M H z) sh ow ed
th e p resence of 3 2 sig nals w h ere 20 sig nals w ere attrib uted
to non-h y drog enated carb ons, 8 sig nals corresp onding to 10
m eth y nic carb ons and 2 to m eth ox y carb ons. T h e ch emical
sh if ts at dC 180.0 and 17 9.9 conirm th e ab sorp tion of
carb ony l g roup s. T h e sig nals at dC 94.8 and 99.8, com p ared
w ith literature data19 w ere attrib uted to C -8 and C -6 ,
resp ectiv ely , of th e lav onoids A ring . L ik ew ise, the sig nals at
dC 121.9, 106 .8 and 101.4 w ere attrib uted to C -3 ’, C -8’’ and
C -6 ’’ consistent w ith b ilav onoids b ond C -3 ’-C -8’’, b ecause
w h en f usion is C -3 ’-C -6 ’’, th e ch em ical sh if ts f or th e sam e
carb ons are 116 .7 , 93 .9 and 103 .5 , resp ectiv ely . T he b ond
C -3 ’-C -8’’ w as conirm ed b y th e H M BC correlations of
th e sig nal at 6 .3 1 ( s, H -6 ’’) , and 7 .08 ( d, J 9.0, H -5 ’) w ith
106 .8 ( C -8’’) .
T h e ab sence of carb on sig nals at dC 104.0 and 103 .4,
as in am entolav one (2) , and th e ch em ical sh if ts f or th e
m eth ox y carb ons at dC 6 0.5 and 6 0.6 and com p arison
w ith th e ch em ical sh if ts of isok aem p f eride19 sug g est th e
insertion of th ese g roup s at C -3 and C -3 ’’. T h is notion w as
conirm ed b y th e H M BC sp ectrum th roug h th e correlations
of th e m eth ox y h y drog ens w ith th e carb ons at dC 13 9.4
and 13 9.3 ( C -3 and C -3 ’’) . I t is f urth er sup p orted b y th e R O E SY sp ectrum th roug h th e correlations of th e m eth ox y
p rotons at C -3 and C -3 ’’ w ith th e h y drog ens H -6 ’ and
H -2’’’, resp ectiv ely . T h us, (1) dif f ers f rom am entolav one
b y h av ing p ositions C -3 and C -3 ’’ occup ied b y m eth ox y g roup s. By com p aring th e ch em ical sh if ts of m onom eric units of (1) w ith th e literature could b e ch aracterized
th em as units isok aem p f eride. T h eref ore, (1) is a new
b ilav onoid w ith union C -3 ’-C -8’’ of isok aem p f eride, nam ed
3 ’,8’’-b iisok aem p f eride. T h e data f or 1H , 13C , H M QC and
H M BC NM R are com p iled in T ab le 1. T h e k now n lav onoid
am entolav one (2) w as identiied b y sp ectral data analy sis
and com p arison w ith literature v alues.19 T h e structures
of 3 and 4 w ere identiied b y NM R as p atag onic acid14
and ( 4aR ,5 S,6 R ,8aR ) -5 -[ 2-( 2,5 -dih y dro-5 -m eth ox y
-2-ox of uran-3 -y l) eth y l] -3 ,4,4a,5 ,6 ,7 ,8,8a-octah y dro-5 ,6
,8a-trim eth y lnap h th alene-1-carb ox y lic acid, resp ectiv ely , b oth
diterp enes of th e clerodane ty p e.15 ,24,25 A ccording to th e
literature14 on sub stance 3 th e ch em ical sh if ts of C -2 is 27 .4
and C -7 is 27 .2. H M BC sp ectrum sh ow ed a correlation of
th e sig nal at 0.85 ( d, J 6 .7 H z, C H3-17 ) w ith th e sig nal at
27 .2, b eing th is sig nal assig ned to C -7 , and a correlation of th e sig nal at 6 .81 ( b r s H -3 ) w ith th e sig nal at 27 .4,
Pinto et al. 1821 Vol. 21, No. 10, 2010
w h ich w as assig ned to C -2. T h us, 13C NM R data of 3 w ere
uneq uiv ocally m ark ed.
T h e sp ectral data analy sis of 5 and 6 identiied th ese
com p ounds as 5 -caf f eoy lq uinic acid m eth y l ester26 and 3 ,5
-di-caf f eoy lq uinic acid,18 resp ectiv ely , b oth rep orted f or th e irst
tim e in th e f am ily Velloziaceae. L uteolin (7) w as identiied b y
sp ectral data analy sis and com p arison w ith literature v alues.19
T h e ef f ect of th e concentration of com p ound 1 on th e
induction of cy totox icity to h um an g liob lastom a G L -15
cells w as inv estig ated ( F ig ure 2) . I t w as f ound th at th e
m inim al cy totox ic concentration w as 20 μm ol L-1 as
com p ared to control g roup , k illing 46 .9% of cells af ter
7 2 h 3 ’,8’’-b iisok aem p f eride -induced cy totox icity w as
itted to eq uation 1:
V = 4.987 + { 115 .113 / [ 1 + 10 ( 1.991 log [ C ] - 2.919)] } ( 1)
( R2 = 0.926 2)
in w h ich V corresp onds to cell v iab ility norm alized to
data m easured under control conditions, and [ C ] is th e
3 ’,8’’-b iisok aem p f eride concentration. T h e calculated
ef f ectiv e concentration, w h ich k illed 5 0% ( E C5 0) of G L -15
cells af ter 7 2 h w as 3 6 .5 μm ol L-1.
T h is is im p ortant to state th at th e sam e inv estig ation
carried out w ith am entolav one sh ow ed th at it does not
p ossess any antitum oral activ ity ag ainst G L -15 cells until
6 00 μm ol L-1 ( data not sh ow n) . Since th e dif f erence b etw een
th ese com p ounds sh ow n in f orm ulas 1 and 2 ( F ig ure 1) is
th e p resence of tw o m eth ox y carb ons, th ese g roup s enh ance
th e induction of cy totox icity to G L -15 cells.
T h e m orp h olog y of cells treated w ith 1.5 μm ol L-1
3 ’,8’’-b iisok aem p f eride w as not m odif ied af ter 7 2 h,
w h en com p ared to th e neg ativ e control g roup . C h ang es
in cellular m orp h olog y w ere ob serv ed w h en cells w ere
treated w ith 20 μm ol L-1 3 ’,8’’-b iisok aem p f eride f or 7 2 h ,
som e cells sh ow ed retraction and deg radation of cy top lasm ,
oth ers b ecam e round and b ig . F urth erm ore, som e cells
detach ed f rom p lates. C ells treated w ith 200 μm ol L-1
3 ’,8’’-b iisok aem p f eride f or 7 2 h p resented an alm ost
T ab le 1 . NM R data f or 1a
Positions H M QC H M BC
1H 13C
2 - 15 8.1
3 - 13 9.4
4 - 180.0
5 - 16 2.9
6 6 .14 ( d, J 2.0) 99.8 C -5 , C -7 , C -10
7 - 16 6 .2
8 6 .19 ( d, J 2.0) 94.8 C -7 , C -9, C -10
9 - 15 8.4
10 - 105 .7
1’ - 122.7
2’ 8.16 ( d, J 2.0) 13 4.6 C -2, C -4’, C -6 ’, C -8’’
3 ’ - 121.9
4’ - 16 1.1
5 ’ 7 .08 ( d, J 9.0) 118.4 C -1’ , C -3 ’ , C -4’
6 ’ 8.08 ( dd, J 9.0; 2.0) 13 0.4 C -4’
2’’ - 15 7 .2
3 ’’ - 13 9.3
4’’ - 17 9.9
5 ’’ - 16 2.1
6 ’’ 6 .3 1 ( s) 101.4 C -5 ’’, C -7 ’’, C -8’’
7 ’’ - 16 7 .5
8’’ - 106 .8
9’’ - 15 5 .6
10’’ - 105 .2
1’’’ - 122.6
2’’’ 7 .7 8 ( d, J 8.0) 13 1.3 C -2’’, C -4’’’
3 ’’’ 6 .6 5 ( d, J 8.0) 116 .3 C -1’’’, C -4’’’
4’’’ - 16 1.4
5 ’’’ 6 .6 5 ( d, J 8.0) 116 .3 C -4’’’
6 ’’’ 7 .7 8 ( d, J 8.0) 13 1.3 C -2’, C -4’’’
3 -O C H3 3 .7 3 ( s) 6 0.5 C -3
3 ’’-O C H3 3 .7 8 ( s) 6 0.6 C -3 ’’
5 -O H 12.7 4 ( s)b
-5 ’’-O H 12.86 ( s)b
-a D ata ob tained at 5 00 M H z, C D
3O D ; J in H z and d in p p m ; b Values
ob tained in D M SO -d6.
F ig ure 2 . 3 ’,8’’-b iisok aem p f eride induced cy totox icity to h um an g liob lastom a G L -15 cells. G L -15 cells w ere ex p osed to 3 ’,8’’-b iiso-k aem p f eride 1.5 -200 μm ol L-1 f or 7 2 h , at 3 7 °C , and 5 % C O
2, in 96 -w ell
p lates ( n = 8 f or each concentration) . C ell v iab ility w as norm alized to data m easured under control conditions w ith out 3 ’,8’’-b iisok aem p f eride.
3 ’,8’’-Biisok aem p f eride, a C y totox ic Bilav onoid and O th er C h em ical C onstituentsJ. Braz. Chem. Soc.
1822
com p lete deg radation of cy top lasm , ch aracterizing cellular
death .
T em ozolom ide ( T M Z ) w as th e irst ch em oth erap eutic
ag ent ap p rov ed f or treatm ent of h ig h -g rade m alig nant
g liom as in m ore th an 20 y ears. I n p rim ary culture of
h um an g liob lastom a cells, dif f erences in T M Z cy totox icity
w as ob serv ed, th e indiv idualized E C5 0 af ter 7 2 h sh ow ed
v ariab ility b etw een p atients, 45 0-900 m m ol L-1.27 A lth oug h
relativ ely uncom m on, m alig nant g liom as are associated
w ith disp rop ortionately h ig h m orb idity and m ortality , and
desp ite op tim al treatm ent, th e m edian surv iv al is only 12 to
15 m onth s f or p atients w ith g liob lastom a.28 G liob lastom a
stem cells disp lay strong cap ab ility of tum or’s resistance to
T M Z .29 I n th is study , th e 3 ’,8’’-b iisok aem p f eride p resented
an E C5 0 of 3 6 .5 μm ol L-1 in G L -15 cells, a cy totox ic
concentration b eneath th at f ound f or T M Z .27
E xperimental
G eneral p rocedures
M elting p oints h av e not b een corrected. I R sp ectra w ere
recorded on a BO M E M -M B 100 sp ectrop h otom eter. 1H
( 5 00 M H z) and 13C ( 125 M H z) NM R sp ectra w ere recorded
on a VA R I A N-Sy stem sp ectrom eter using D Md6, SO
-C D3O D or C D C l3 w ith T M S as internal standard. H R E SI
m ass sp ectra w ere ob tained w ith a Brük er D altonics
U ltrO T O F -Q, Billerica, M A , sp ectrom eter using ( H2O ,
A r) , 20 eV f or M S and 45 eV f or M S/ M S in p ositiv e m ode.
C olum n ch rom atog rap h y w ith silica g el ( M erck 0.06 3 -0.20
m m ) and Sep h adex L H -20 ( Sig m a, U SA ) ; silica g el F 25 4 G ( Vetec) w as used f or p rep arativ e T L C , silica g el p lates
PF25 4 7 7 49 ( M erck ) w as used f or analy tical T L C ; w ith
v isualization under U V ( 25 4 and 3 6 6 nm ) , or ex p osure to iodine v ap or.
P lant material
T h e entire p lant of N anuza p licata ( M art.) L .B.Sm . &
A y ensu ( Velloziaceae) w as collected in M arch 2007 in th e sem i-arid north east reg ion of Brazil, city of Serra Branca
in Paraib a State. Vouch er sp ecim en ( A g ra et al.; 5 7 3 0)
is dep osited at th e H erb arium Prof . L auro Pires X av ier, U niv ersidade F ederal da Paraíb a, J oão Pessoa-PB, Brazil.
E x traction and is olation
T h e dry and p ulv erized p lant m aterial ( 2.0 k g ) w as ex tracted successiv ely w ith 95 % eth anol at room tem p erature. T h e solv ent w as rem ov ed under reduced
p ressure, ob taining th e crude eth anol ex tract ( 6 0 g) .
E ig h t g ram s of th is ex tract w ere sub m itted to g el
iltration th roug h Sep h adex L H 20, eluted w ith m eth anol
f ollow ed b y a m eth anol: ch lorof orm ( 1: 1) , resulting in
7 6 f ractions ( A 1-A 7 6 ) . T h e eluate f rom th e colum n
w as m onitored b y analy tical th in-lay er ch rom atog raph y
and sim ilar f ractions w ere com b ined. T h e g roup of
f ractions A 5 -A 7 ( 3 5 m g ) w as sub m itted to p rep arative
T L C w ith ch lorof orm : m eth anol ( 9: 1) , ob taining
1 ( 20 m g ) . T h e g roup of f ractions A 8-A 9 ( 25 m g )
w as recry stallized f rom acetone ob taining 2 ( 15 m g ) .
T h e f ractions A 10-A 13 ( 100 m g ) w ere ch rom atog rap h ed
on a silica g el colum n utilizing ch lorof orm : m eth anol as
th e m ob ile p h ase, w ith an increasing p olarity g radient,
ob taining 3 and 4 ( 10 m g ) , 5 ( 11 m g ) and 6 ( 12 m g ) ,
resp ectiv ely . T h e g roup of f ractions A 23 -A 28 w as
recry stallized f rom acetone, ob taining 7 ( 8 m g ) .
G lioblas toma cell cultures
E x p erim ents w ere carried out using h um an g liob lastom a
G L -15 cell line of clonal orig in, w h ich h as b een p rev iously
estab lish ed.3 0 G L -15 cell cultures w ere p rep ared as
describ ed p rev iously .3 1 C ells w ere g row n in a h um idiied
95 % air and 5 % C O2 atm osp h ere at 3 7 ºC , and th e culture
m edium w as rep laced th ree tim es a w eek . A t th e tim e of
th e ex p erim ent, conluent cells w ere try p sinized and p lated
in a 96 -w ell p late, at a inal density of 3 × 104 cells/ cm2.
E x p erim ents w ere initiated 7 2 h af ter p lating .
I n vitro evaluation of 3 ’,8 ’’- biis ok aemp f eride induced cy totox icity
3 ’,8’’-b iisok aem p f eride at concentrations b etw een
1.5 -200 m m ol L-1, and am entolav one at concentrations
b etw een 3 -6 00 m m ol L-1 w ere used to ex am ine its
cy totox ic ef f ects. E ig h t rep licates w ere used f or each
dose in a 96 -w ell p late. C ells w ere ex p osed to 1 f or 7 2 h .
C ells v iab ility w as assessed using 3 -( 4,5 -dim eth y lth
iazol-2-y l) -2,5 -dip h eny ltetrazolium b rom ide ( M T T ; Sig m a,
St. L ouis, M O ) . M T T w as dissolv ed at a concentration
of 5 m g L-1 in sterile p h osp h ate b uf f ered saline ( PBS) at
room tem p erature, and th e solution w as f urth er sterilized b y p assing th roug h a 0.2 m m ilter and stored at 4 °C in th e dark . T h e inal concentration of M T T added to each
w ell w as 1 m g L-1. A f ter 2 h of incub ation at 3 7 ºC , a
sam e v olum e of ly sis b uf f er w as added. L y sis b uf f er w as p rep ared as f ollow s: 20% ( m / v ) sodium dodecy l sulp h ate ( SD S) w as dissolv ed at 3 7 ºC in a solution of 5 0% ( v / v )
dim eth y lf orm am ide ( D M F ) and reag ent g rade w ater, p H 4.7 .
A f ter an ov ernig h t incub ation at room tem p erature, op tical
Pinto et al. 1823 Vol. 21, No. 10, 2010
norm alized to data m easured under control conditions,
w ith out 1.
P has e contras t micros cop y
C ell m orp h olog y w as ev aluated b y p h ase contrast m icroscop y using an inv erted m icroscop e E clip se T S100 ( Nik on, T ok y o, J ap an) . Ph otog rap h s w ere tak en b y a C oolp ix 43 00 dig ital cam era ( Nik on) attach ed to th e
m icroscop e. Nik on View v ersion 6 .1.0 w as used to transf er
im ag es f rom th e cam era to a com p uter to b e edited. T h e only p rocess used to edit im ag es w as to transf orm color
p h otog rap h s into h alf tone p ictures. A ruler w ith tick s ev ery
10 m m ( O ly m p us, T ok y o, J ap an) w as p h otog rap h ed under th e sam e conditions. A new lay er containing th e ruler w as
added to p ictures using Ph oto I m p ression 4.0 ( A rcSof t,
F rem ont, U SA ) .
Statis tical analy s is
T h e data f or th e concentration-resp onse curv e w ere analy zed using one-w ay A NO VA and g roup s w ere com p ared b y Student-New m an-K euls test. T h e data w ere
itted using nonlinear reg ression p erf orm ed w ith G rap h Pad
Prism sof tw are ( San D ieg o, U SA ) .
3 ’,8 ’’- biis ok aemp f eride (1)
Y ellow am orp h ous p ow der; I R ( K Br) ν
m ax/ cm
-1: 3 3 5 0,
16 5 1, 15 00, 13 5 7 , 1980; H R Mm/ z: 5 99.5 3 3 1 S-E SI
( M + H ) +, ( calc. f or C
3 2H23O12 5 99.5 3 89) , f ound 46 4.3 6 6 2
( 100% ) ; 1H and 13C NM R sp ectral data: see T ab le 1.
P atagonic acid (3)
W h ite am orp h ous p ow der; I R ( K Br) ν
m ax/ cm
-1: 3 43 3 ,
295 8, 17 5 1, 16 7 8, 145 8, 126 1; 1H NM R ( C D C l
3; 5 00M H z) :
d 1.7 0 ( m , 2H -1) , 1.45 ( m , 2H -2) , 6 .81 ( b r s, H -3 ) , 1.43 ( m ,
H -6 eq ) , 2.40 ( m , H -6 ax ) , 2.25 ( m , 2H -7 ) , 1.5 0 ( m , H -8) , 1.3 5 ( m , H -10) , 1.19 ( m , H -11eq ) , 2.40 ( m , H -11ax ) , 2,05 ( m , H -12eq ) , 2.20 ( m , H -12ax ) , 7 .06 ( b r s, H -14) , 4.7 9 ( b r s, 2H 15 ) , 0.85 ( d, 3 H 17 ) , 1.27 ( s, 3 H 19) , 0.7 5 ( s, 3 H
-20) ; NM R ( C D C l3; 5 00M H z) : d 17 .4 ( C H2-1) , 27 .4 ( C H2
-2) , 140.1 ( C H -3 ) , 141.1 ( C -4) , 3 7 .4 ( C -5 ) , 3 6 .0 ( C H2-6 ) ,
27 .2 ( C H2-7 ) , 3 6 .3 ( C H -8) , 3 8.7 ( C -9) , 46 .7 ( C H -10) , 3 5 .7
( C H2-11) , 19.0 ( C H2-12) , 13 5 .0 ( C -13 ) , 143 .4 ( C H -14) ,
7 0.1 ( C H2-15 ) , 17 4.3 ( C -16 ) , 15 .8 ( C H3-17 ) , 17 4.2 ( C -18) ,
20.5 ( C H3-19) , 18.1 ( C H3-20) .
S upplementary I nformation
Sup p lem entary data are av ailab le f ree of ch arg e at h ttp : / / j b cs.sb q .org .b r, as PD F ile.
A cknow ledg ments
T h e auth ors are g ratef ul to C NPq , F A PE SB and C A PE S
f or sch olarsh ip s and inancial sup p ort, as w ell as to V. C . de
O . C osta ( U F PB) f or th e NM R sp ectra, and N. P. L op es of
F aculdade de C iências F arm acêuticas-U SP-R ib eirão Preto
f or th e H R -E SI M S.
R eferences
1. Stannard, B. L .; Flora of the P ico das A lmas : Chap ada da D iamantina, W h itstab le L ith o L td: G reat Britain, 1995 . 2. Pinto, A . C .; E p if ânio, R . A .; Z och er, D . H . T .; Biochem. Sy s t.
E col. 2 0 0 4, 3 2, 5 97 .
3 . Pinto, A . C .; Patitucci, M . L .; Silv a, R . S.; Queiroz, P. P. S.; K elecon, A .; Tetrahedron 1 9 83, 3 9, 3 3 5 1.
4. Pinto, A . C .; Pinch in, R .; Prado, S. K .; P hy tochemis try 1 9 83,
2 2, 2017 .
5 . Branco, A .; Pinto A . C .; Braz-F ilh o, R .; A n. A cad. Bras . Cienc. 2 0 0 4, 7 6, 5 05 .
6 . Branco, A .; Pereira, A . S.; C ardoso, J . N.; A q uino-Neto, F . R .; Pinto, A . C .; Braz-F ilh o, R .; P hy tochem. A nal. 2 0 0 1, 1 2, 26 6 .
7 . Branco, A .; Pinto, A . C .; I f a, D . R .; Braz-F ilh o, R .; J. Braz. Chem. Soc. 2 0 0 2, 1 3, 3 18.
8. Salatino, A .; Salatino, M . L . F .; Santos, D . Y . A . C .; Patrício, M . C . B.; G enet. M ol. Biol. 2 0 0 0, 2 3, 93 1.
9. W illiam s, C . A .; H arb orne, J . B.; M enezes, N. L .; Biochem. Sy s t. E col. 1 9 9 1, 1 9, 483 .
10. R ieh l, C . A . S.; Pinto, A . C .; F ig ueroa-Villar, J . D ; N at. P rod. R es . 2 0 0 6, 2 0, 1225 .
11. R ieh l, C . A . S.; Pinto, A . C .; K aiser, C . R .; F ig ueroa-Villar, J . D .; C ruz, E . R .; Sp ectros c. Lett. 2 0 0 0, 3 3, 6 43 .
12. W illiam s, C . A .; H arb orne, J . B.; T om as-Barb eran, F . A .;
P hy tochemis try 1 9 87, 2 6, 25 5 3 .
13 . M ark h am , K . R .; Sh ep p ard, C .; G eig er, H .; P hy tochemis try 1 9 87, 2 6, 3 3 3 5 .
14. R iv era, A . P.; F aini, F .; C astillo, M .; J. N at. P rod. 1 9 88, 5 1, 15 5 .
15 . K rish na, V.; Sing h , P.; P hy tochemis try 1 9 9 9, 5 2, 13 41.
16 . Barron, D .; K aouadj i, M .; M ariotte, A . M .; Z . N aturf ors ch.
1 9 84, 3 9, 16 7 .
17 . T im m erm ann, B. N.; H of f m an, J . J .; J olad, S. D .; Sch ram , K . H .; K lenck , R . E .; Bates, R . B.; J. N at. P rod. 1 9 83, 4 6,
3 6 5 .
18. M eira, M .; D av id, J . M .; D av id, J . P.; A raúj o, S. V.; R eg is, T . L .; G iulietti, A . M .; Queiróz, L . P.;Q uim. N ova
2008, 3 1, 7 5 1.
19. A g raw al, P. K .; Carbons - 1 3 N M R of lavonoids, E lsev ier: New Y ork , 1986 .
3 ’,8’’-Biisok aem p f eride, a C y totox ic Bilav onoid and O th er C h em ical C onstituentsJ. Braz. Chem. Soc.
1824
21. K ang , S. S.; L ee, J . Y .; C h oi, Y . K .; Song , S. S.; K im , J . S.; J eon, S. J .; H an, Y . N.; Sonc, K . H .; H ana, B. H .; Bioorg. M ed. Chem. Lett. 2 0 0 5, 1 5, 3 5 88.
22. M atsuda, S.; H isam a, M .; Sh ib ay am a, H .; I tou, N.; I w ak i, M .;
Y ak ugak u Z as s hi 2 0 0 9 , 1 2 9 , 1113 . ( C oden: Y K K Z A J I SSN: 003 1-6 903 , A N 2009: 13 1486 9 C aPlus) .
23 . Valensin, S.; G h iron, C .; L am anna, C .; K rem er, A .; R ossi, M .; F erruzzi, P.; Niev o, M .; Bak k er, A .; BM C Cancer 2 0 0 9, 9, 196 .
24. Santos, A . G .; Perez, C . C .; T ininis, A . G .; Bolzani, V. S.; C av alh eiro, A . J .; Q uim. N ova 2 0 0 7, 3 0, 1100.
25 . A h m ad, V. U .; K h an, A .; F arooq , U .; K ousar, F .; K h an, S. S.; Naw az, S. A .; A b b asi, M . A .; C h oudh ary , M . I .; Chem. P harm.
Bul. 2 0 0 5, 5 3, 3 7 8.
26 . R um b ero-Sanch ez, A .; Vazq uez, P.; P hy tochemis try 1 9 9 1, 3 0, 3 11.
27 . Pédeb oscq , S.; L ’A zou, B.; L ig uoro, D .; Pom etan, J . P.; C am b ar, J .; E x p . Tox icol. P athol. 2 0 0 7 , 5 8, 247 .
28. W en, P. Y .; K esari, S.; N. E ngl. J. M ed. 2 0 0 8, 3 5 9, 492.
29. F u, Z .-G . J .; L iu, X .-M .; L iu, F .-R .; C h en, H .-L .; Sh i, J . C .-S.; Pang , H .-K .; C h en Ng , Z .-P.; Chin. M ed. J. 2 0 0 9, 1 2 2, 125 5 .
3 0. Bocch ini, V.; C asalone, R .; C ollini, P. ; R eb el, G .; L o C urto, F .; Cell Tis s ue R es . 1 9 9 1 , 2 6 5, 7 3.
3 1. Planch enault, T .; C osta, S. L .; F ag es, C .; R ich e, D .; C h
arriére-Bertrand, C .; Perzelov a, A .; Barlov atz-M eim on, G .; T ardy , M .
N euros ci. Lett. 2 0 0 1 , 2 9 9 , 140.
Submitted: D ecember 1 8 , 2 0 0 9
Su
pp
le
m
enta
ry
Inf
or
m
ati
on
J. Braz. Chem. Soc., Vol. 21, No. 10, S1-S17, 2010. Printed in Brazil - ©2010 Sociedade Brasileira de Química 0103 - 5053 $6.00+0.00
*e-mail: marcelosobral@ltf.ufpb.br
3’,8’’-Biisokaempferide, a Cytotoxic Bilavonoid, and Other Chemical Constituents
of Nanuza plicata (V elloz iaceae)
Meri Emili F. Pinto,a Marcelo Sobral da Silva,*,a Elisabete Schindler,b José Maria Barbosa Filho,a Ramon dos Santos El- Bachá,b Marianna V ieira S. C astello- Branco,a
Maria de Fatima A g raa and Josean Fechine T avaresa
aLaboratório de Tecnologia Farmacêutica, Univers idade Federal da P araíba, CP 5 0 0 9 ,
5 8 0 5 1 - 9 7 0 João P es s oa- P B, Brazil
bLaboratório de N euroq uímica e Biologia Celular ( LabN q ) , I ns tituto de Ciências da Saúde ( I CS) ,
Univers idade Federal da Bahia, 4 0 1 1 0 - 9 0 2 Salvador- BA , Brazil
3’,8’’-Biisokaempferide, a Cytotoxic Bilavonoid, and Other Chemical Constituents J. Braz. Chem. Soc.
2
Figure S2. 13C NMR spectrum (CD
3OD, 125 MHz) of the compound 1 isolated of N anuza p licata.
Figure S3. 13C NMR spectrum (CD
Pinto et al. 3 Vol. 21, No. 10, 2010
Figure S4. 13C NMR spectrum (CD
3OD, 125 MHz, dC 94-139) of the compound 1 isolated of N anuza p licata.
3’,8’’-Biisokaempferide, a Cytotoxic Bilavonoid, and Other Chemical Constituents J. Braz. Chem. Soc.
4
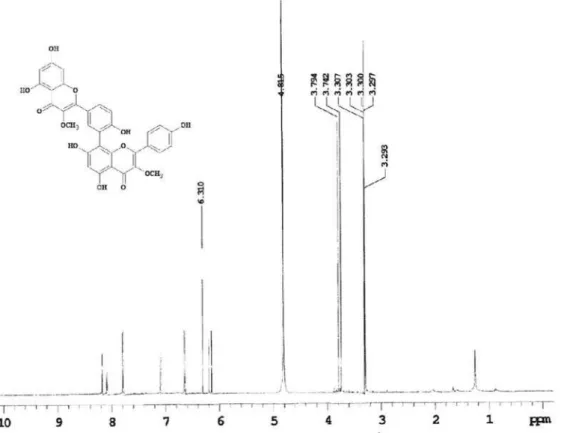
Figure S6. 1H NMR spectrum (CD
3OD, 500 MHz) of the compound 1 isolated of N anuza p licata.
Figure S7. 1H NMR spectrum (CD
Pinto et al. 5 Vol. 21, No. 10, 2010
Figure S8. 1H NMR spectrum (CD
3OD, 500 MHz, dH 6.1-7.1) of the compound 1 isolated of N anuza p licata.
3’,8’’-Biisokaempferide, a Cytotoxic Bilavonoid, and Other Chemical Constituents J. Braz. Chem. Soc.
6
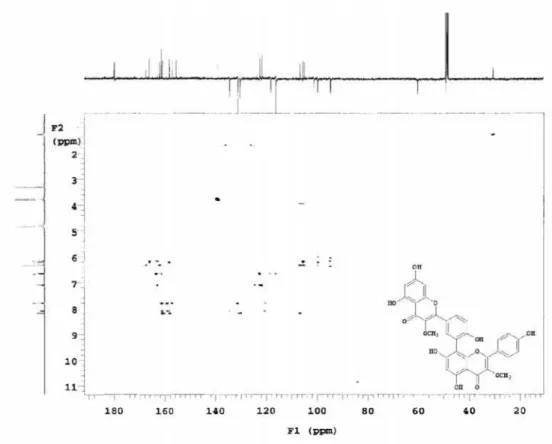
Figure S10. HMBC NMR experiment (CD3OD, 500×125 MHz, dC 158-168) of the compound 1 isolated of N anuza p licata.
Pinto et al. 7 Vol. 21, No. 10, 2010
Figure S12. HMBC NMR experiment (CD3OD, 500 x 125 MHz, dC 138-140) of the compound 1 isolated of N anuza p licata.
3’,8’’-Biisokaempferide, a Cytotoxic Bilavonoid, and Other Chemical Constituents J. Braz. Chem. Soc.
8
Figure S14. 13C NMR spectrum (CD
3OD, 125 MHz) of the compound 2 isolated of N anuza p licata.
Figure S15. 13C NMR spectrum (CD
Pinto et al. 9 Vol. 21, No. 10, 2010
Figure S16. 13C NMR spectrum (CD
3OD, 125 MHz, dC 95-135) of the compound 2 isolated of N anuza p licata.
Figure S17. 1H NMR spectrum (CD
3’,8’’-Biisokaempferide, a Cytotoxic Bilavonoid, and Other Chemical Constituents J. Braz. Chem. Soc.
10
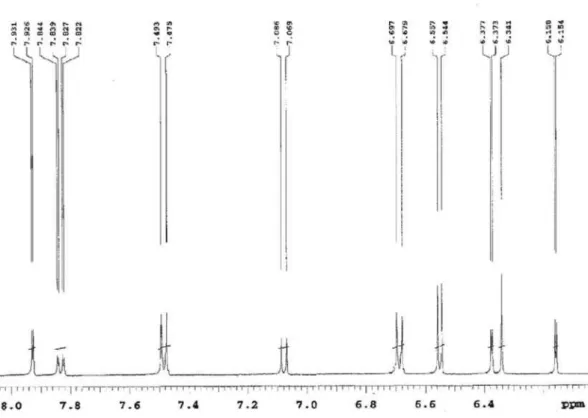
Figure S18. 1H NMR spectrum (CD
3OD, 500 MHz, dH 6.1-7.9) of the compound 2 isolated of N anuza p licata.
Pinto et al. 11 Vol. 21, No. 10, 2010
Figure S20. 13C NMR spectrum (CDCl
3, 125 MHzdC 102-174) of the compounds 3 and 4 isolated of N anuza p licata.
Figure S21. 13C NMR spectrum (CDCl
3’,8’’-Biisokaempferide, a Cytotoxic Bilavonoid, and Other Chemical Constituents J. Braz. Chem. Soc.
12
Figure S22. 1H NMR spectrum (CDCl
3, 500 MHz) of the compounds 3 and 4 isolated of N anuza p licata.
Figure S23. 1H NMR spectrum (CDCl
Pinto et al. 13 Vol. 21, No. 10, 2010
Figure S24. 1H NMR spectrum (CDCl
3, 500 MHz,dH 2.0-2.4) of the compounds 3 and 4 isolated of N anuza p licata.
Figure S25. 1H NMR spectrum (CDCl
3’,8’’-Biisokaempferide, a Cytotoxic Bilavonoid, and Other Chemical Constituents J. Braz. Chem. Soc.
14
Figure S26. HMQC NMR experiment (CDCl3, 500×125 MHz
,
dC 140-144) of the compounds 3 and 4 isolated of N anuza p licata.Pinto et al. 15 Vol. 21, No. 10, 2010
Figure S28. HMBC NMR experiment (CDCl3, 500 x 125 MHz, dC 100-145) of the compounds 3 and 4 isolated of N anuza p licata. * Correlation of
compounds 4.
3’,8’’-Biisokaempferide, a Cytotoxic Bilavonoid, and Other Chemical Constituents J. Braz. Chem. Soc.
16
Figure S30. 13C NMR spectrum (CD
3OD, 125 MHz) of the compound 7 isolated of N anuza p licata.
Figure S31. 13C NMR spectrum (CD
Pinto et al. 17 Vol. 21, No. 10, 2010
Figure S32. 1H NMR spectrum (CD
3OD, 500 MHz) of the compound 2 isolated of N anuza p licata.
Figure S33. 1H NMR spectrum (CD