Universidade de Lisboa
Faculdade de Ciências
Departamento de Biologia Vegetal
Development of nanoformulations of pyrazinoic
acid prodrugs for treatment of Mycobacterium
avium infections
Mariana Cordeiro Matoso
Mestrado em Microbiologia Aplicada
2012
Universidade de Lisboa
Faculdade de Ciências
Departamento de Biologia Vegetal
Development of nanoformulations of pyrazinoic
acid prodrugs for treatment of Mycobacterium
avium infections
Mariana Cordeiro Matoso
Dissertação orientada por Doutora Manuela Gaspar (FFUL) e
Prof. Doutora Sandra Chaves (FCUL)
Mestrado em Microbiologia Aplicada
2012
Development of nanoformulations of pyrazinoic
acid prodrugs for treatment of Mycobacterium
avium infections
Mariana Cordeiro Matoso
Master Thesis
2012
This thesis was fully performed at the Departamento de Farmácia
Galénica e Tecnologia Farmacêutica of the Faculty of Pharmacy
(University of Lisbon), Campus do Lumiar, under the direct
supervision of Doutora Manuela Gaspar and Prof. Doutor Luís
Constantino.
I
Acknowledgments
A realização desta Dissertação de Mestrado só foi possível graças à colaboração e ao contributo, de forma directa ou indirecta, de várias pessoas e instituições, às quais gostaria de exprimir algumas palavras de agradecimento e profundo reconhecimento, em particular:
À Doutora Manuela Gaspar pela disponibilidade manifestada para orientar este trabalho. Agradeço todo o apoio, todas as horas perdidas de sono e todo o emaranhado de pensamentos que tornaram possível a obtenção de boas soluções para alguns pequenos contratempos que foram surgindo durante a realização deste trabalho e que, por certo, causaram alguns cabelos brancos. Por todos os conhecimentos que me foram transmitidos e que me ajudaram a tornar numa pequena adulta e por toda a amizade que foi sendo construída durante este último ano, um muito obrigada.
Ao Prof. Doutor Luís Constantino pela oportunidade que me deu em poder trabalhar neste projecto bem como toda a disponibilidade concedida enquanto co-orientador deste trabalho. Por todas as discussões científicas que protagonizámos em conjunto com a Doutora Manuela Gaspar e a Prof. Doutora Emília Valente e por todas as criticas que daí surgiram e que me guiaram no bom caminho. Gostaria ainda de expressar o meu agradecimento pelo fornecimento dos pró-fármacos utilizados durante esta dissertação.
Á Prof. Doutora Emília Valente agradeço a disponibilidade para as discussões científicas realizadas e todas as críticas relativas ao trabalho.
À Doutora Eugénia Cruz, investigadora do grupo Nanomedicine & Drug Delivery Systems da Faculdade de Farmácia da Universidade de Lisboa, pioneira na tecnologia de lipossomas em Portugal, pela oportunidade concedida em poder trabalhar no laboratório do Campus do Lumiar.
À Prof. Doutora Sandra Chaves por ter aceite ser minha orientadora da Faculdade de Ciências, por toda a disponibilidade e pela revisão critica desta tese.
À Carla Eleutério pela paciência que teve em me educar e me ensinar a funcionar com o HPLC. Por todo o carinho e amizade com que me recebeu e me aturou alguns devaneios e crises existenciais.
À Alexandra Borges por ter cuidado tão bem dos “meus” ratinhos e por toda a alegria e boa disposição que tornou os dias menos bons em dias excepcionais.
À Susana Calado e à Joana Pereira de Almeida pelo apoio e amizade que criámos e que tão bem me fez à saúde e por todos aqueles momentos que às vezes parecem pequenos mas que se tornam enormes.
II
Aos colegas e amigos Filipa Fontes e Rui Lopes por toda a amizade e apoio demonstrados.
Às investigadoras e amigas Sandra Simões e Manuela Colla obrigada pela disponibilidade e apoio prestados quando necessário.
Aos amigos de Sintra, Bruno, FT, Jises, Sandro, Sheila, Guida e Rute, por toda a preocupação e pelas cafezadas tão boas que me permitiram desanuviar das semanas de trabalho.
Ao meu namorado, João, um muito obrigada pelas longas horas de atenção relativamente às minhas dúvidas, inquietações, desânimos e subvalorizações. Por todo o apoio, confiança, valorização do meu trabalho e da minha pessoa e por toda a coragem que me deu para ultrapassar a culpa pelo tempo que a cada dia lhe subtraía.
Aos meus pais, Lina e João, e à minha mana Madalena, por todos os conselhos e incentivos que me deram e que me permitiram fazer mais e melhor nunca desistindo perante os vários obstáculos e muros que se colocaram à minha frente. Obrigada!
Igualmente expresso o meu agradecimento à Fundação para a Ciência e Tecnologia (FCT) que através do projecto A new life for old antimycobacterial drugs: development of prodrugs of pyrazinoic acid activated by mycobacterial esterases as a way to circumvent resistance to pyrazinamide, PTDC/SAU-FCF/101950/2008, contribuiu para o suporte financeiro da presente dissertação.
III
Communications in Scientific Meetings
Stable Prodrugs Liposomes for Treatment of Mycobacterial Infections, Matoso M., Eleutério C.,
Constantino L., Cruz M.E.M., Gaspar M.M. 3rd iMed.UL Post--Graduate Students Meeting, Faculdade de Fármacia Universidade de Lisboa, Lisboa, Portugal, p60
IV
Resumo
As micobactérias são bactérias Gram+ cujo diverso número de espécies actualmente descritas (cerca de 130) partilham as mesmas características no que diz respeito à espessura da parede celular e à sua composição única que as torna uma forte barreira à permeabilidade de antibióticos. Algumas destas micobactérias têm um papel preponderante como agentes patogénicos em indivíduos imunocompetentes como acontece com Mycobacterium tuberculosis, Mycobacterium leprae and Mycobacterium ulcerans, os agentes causadores da tuberculose (TB), lepra e úlcera de Buruli, respectivamente. A grande maioria das outras espécies de micobactérias está presente no ambiente como microrganismos saprófitas, podendo alguns ser considerados patogénicos oportunistas e causadores de morte em indivíduos imunocomprometidos. Deste grupo de micobactérias ambientais oportunistas faz parte o complexo Mycobacterium avium (MAC), composto por M. avium, subespécie avium, paratuberculosis, silvaticum, e M. intracellulare, responsável por uma grande percentagem de doenças micobacterianas não tuberculosas (NTM). As infecções provocadas por este complexo são adquiridas através do ambiente, especialmente pela água, solo e alimentos. Apesar da infecção poder ocorrer por inalação a principal via de infecção, especialmente em indivíduos imunocomprometidos, é o tracto gastrointestinal, já que um sistema imunitário imunocompetente consegue controlar estas micobactérias.
Os problemas associados aos tratamentos contra M. avium e M. tuberculosis são provocados, entre outras causas, pela incapacidade dos antibióticos atravessarem a parede celular complexa das micobactérias, atingindo concentrações sub-terapêuticas no interior dos macrófagos, local de proliferação das micobactérias. A necessidade de ultrapassar esta desvantagem utilizando doses mais elevadas dá origem ao aparecimento de efeitos tóxicos. Uma vez que os esquemas de tratamento destas infecções são muito longos a adesão á terapia é muitas vezes quebrada contribuindo para o desenvolvimento de resistências à grande maioria dos antibióticos.
A pirazinamida (PZA) além de ser um antibiótico de primeira linha no tratamento da TB é um pró-fármaco que requer activação pela enzima pirazinamidase (PZase) para ser transformado em ácido pirazinóico (POA), a sua forma activa.Esta enzima é sintetizada pela própria micobactéria que hidrolisa o pró-fármaco apenas dentro desta. No entanto, esta activação está dependente apenas de uma enzima tendo sido observada a emergência de estirpes resistentes à PZA. A origem destas resistências poderão ser devidas a mutações no gene que codifica a PZase podendo não ocorrer a formação do POA uma vez que a enzima não reconhece o seu substacto. De modo a ultrapassar estes problemas, têm sido investigadas formas alternativas ao uso da PZA. Assim, a síntese de novos pró-fármacos que possam ser activados por esterases micobacterianas e que, em virtude da sua elevada abundância, possam contornar o desenvolvimento de resistências à PZA constitui uma excelente estratégia terapêutica.
Nesta linha de investigação, novos pró-fármacos de POA foram investigados por diversos autores tendo demonstrado actividade in vitro superior à PZA contra estirpes de M.
V
tuberculosis, M. avium e M. kansasii. No entanto, estes compostos apresentaram estabilidades reduzidas na presença de fluidos biológicos. Recentemente, no grupo de Química Medicinal da Faculdade de Farmácia da Universidade de Lisboa, foram sintetizados pró-fármacos de amidas e ésteres de POA, com cadeias alcóxido de diferentes tamanhos. Os ésteres de POA apresentaram elevada estabilidade em plasma, particularmente os de cadeia alcóxido longa. Para além disso, estes pró-fármacos são facilmente activados pelas esterases micobacterianas. Estudos in vitro demonstraram que estes ésteres são activos contra estirpes sensíveis de M. tuberculosis em concentrações 10 vezes mais baixas do que as necessárias para a PZA.
Na presente dissertação foram desenvolvidas formulações lipossomais de dois ésteres de POA e o seu efeito terapêutico avaliado num modelo murino infectado com uma estirpe de M. avium. A associação destes ésteres aos lipossomas é baseada no facto destes sistemas lipídicos conseguirem contornar os problemas de solubilidade associados a estas moléculas e ainda no facto de, após administração parentérica, poderem atingir o fígado e o baço, os principais orgãos infectados por M. avium.
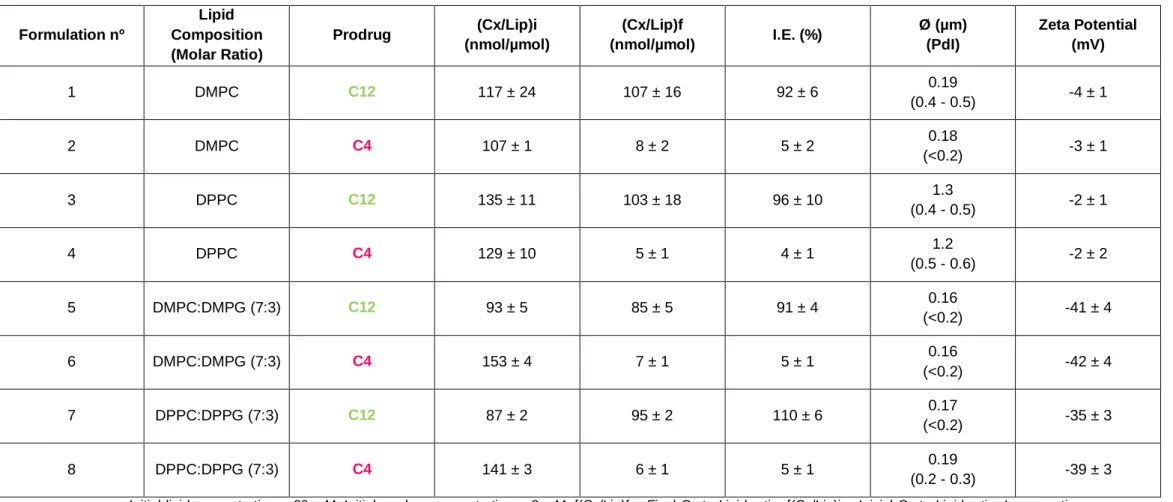
Na primeira parte desta tese foram desenvolvidas e caracterizadas formulações lipossomais de dois ésteres de POA contendo 4 e 12 carbonos na cadeia alquílica linear, designados por C4 e C12. Todas as formulações lipossomais de C12 apresentaram eficácias de incorporação superiores a 90%. No entanto, para as formulações de C4 os valores obtidos foram inferiores a 6% o que pode ser explicado pelo maior carácter lipofílico do C12 em comparação com o C4.
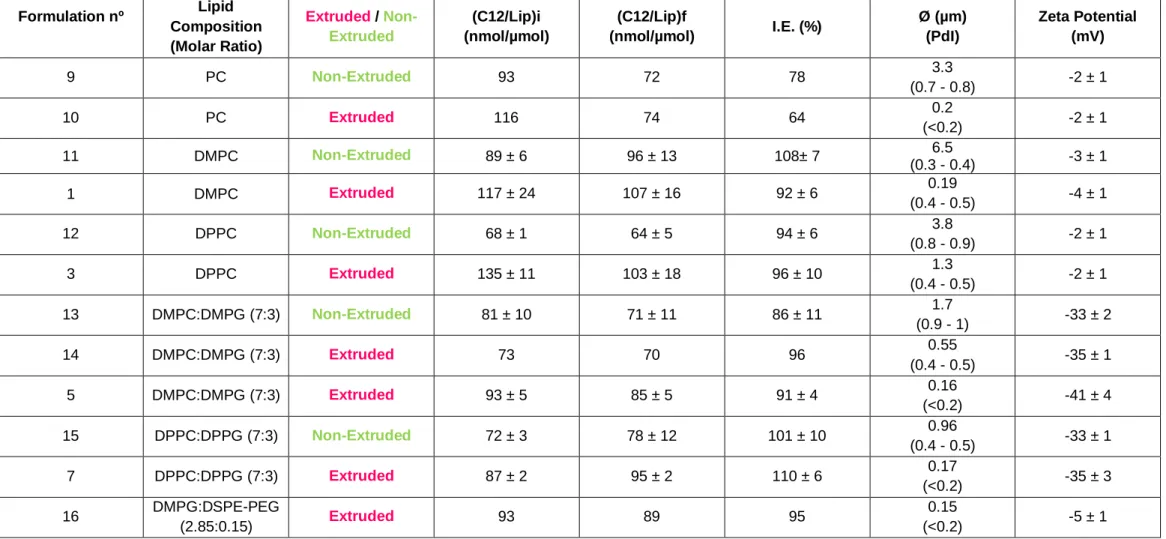
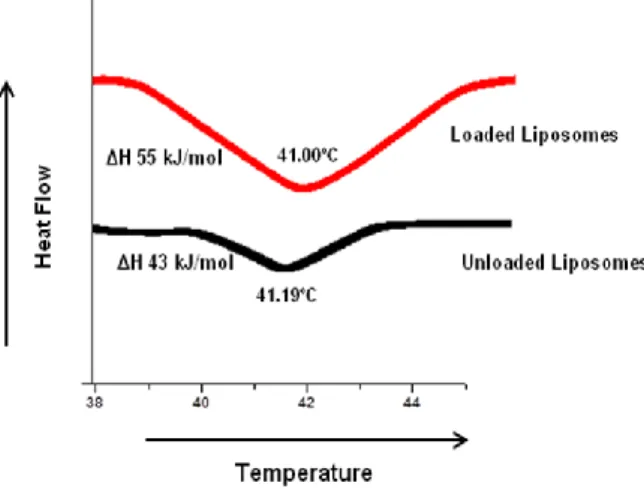
Com base nestes resultados, o pró-fármaco C12 foi seleccionado para todos os estudos efectuados a posteriori. Sendo assim, foram preparadas dois tipos de formulações lipossomais: extrusadas e não extrusadas. Os resultados obtidos indicam que a razão final entre o C12 incorporado e o lípido foi superior a 71 nmol/µmol de lípido para todas as suspensões, independentemente do diâmetro e da composição lipídica, o que apoia a hipótese do pró-fármaco estar incorporado na bicamada lipídica. Para fortalecer esta afirmação, foram realizados estudos de calorimetria com lipossomas vazios e com C12 incorporado. Não se observaram alterações nas temperaturas de transição de fase para as duas formulações. No entanto, o aumento de entalpia registado na formulação lipossomal com C12 é o resultado da interacção deste pró-fármaco com as cadeias lipídicas. Estas observações estão relacionadas com o carácter lipofílico do C12, contribuindo para a rigidez do sistema lipossomal e confirmando a sua localização na bicamada lipídica.
Na segunda parte deste trabalho foi realizado um estudo sistemático da estabilidade das formulações de C12 na presença de tampão HEPES pH 7.4 e de plasma humano, avaliando a velocidade de hidrólise de C12 e a respectiva formação de POA. Assim, a quantificação destas duas moléculas foi realizada por HPLC, tendo-se procedido previamente à optimização desta metodologia. A estabilidade em tampão foi realizada em duas condições distintas: temperatura ambiente e 37ºC. A evolução do diâmetro das vesículas foi avaliada durante um mês, para garantir a homogeneidade dos lipossomas aquando da sua utilização,
VI
não se verificando alterações nestas propriedades para as formulações em estudo. A realização da estabilidade em tampão a 37ºC pretendeu avaliar se a percentagem de C12 incorporado se mantém ao longo de 24 horas. Os resultados obtidos demonstraram uma elevada estabilidade de todos as suspensões relativamente ao pró-fármaco incorporado. Para uma das formulações testadas (DMPC:DSPE-PEG) foi, ainda, analisada a concentração de C12 e a possível formação de POA, tendo-se verificando que o pró-fármaco incorporado não se encontrava na forma hidrolisada. A estabilidade das formulações na presença de plasma humano foi também realizada a 37ºC a fim de avaliar a velocidade de hidrólise do pró-fármaco, na forma livre e lipossomal, em função da composição lipidica, e de modo a estimar o seu comportamento in vivo. Os resultados obtidos indicaram que a hidrólise do C12 foi influenciada principalmente pelo diâmetro médio e rigidez das formulações testadas. De facto, os tempos de semi-vida mais elevados foram obtidos para os lipossomas preparados com fosfolípidos neutros e não submetidos a extrusão. A existência de um elevado número de bicamadas lipídicas que caracterizam estas vesículas está na origem destes resultados. A incubação de C12 na forma livre apresentou uma estabilidade superior comparativamente a algumas formulações, particularmente para as vesículas extrusadas. No entanto, esta estabilidade foi dependente da concentração inicial do pró-fármaco uma vez que, utilizando a dose terapêutica usada no modelo animal infectado com M. avium testado neste trabalho, a velocidade de hidrólise do C12 foi mais lenta nos lipossomas extrusados do que na forma livre.
Embora na literatura esteja descrito que as estirpes de M. avium são intrinsecamente resistentes à PZA pretendeu-se avaliar se era possível com este pró-fármaco ultrapassar estas desvantagens tendo em consideração os resultados promissores obtidos in vitro para os ésteres de POA, contra a estirpe de M. tuberculosis. Foi a primeira vez que lipossomas deste pró-fármaco foram testados num modelo murino infectado com M. avium. O estudo preliminar permitiu demonstrar que após administração i.v. do inóculo os principais orgãos infectados são o fígado e o baço. Tendo em consideração estes resultados foi avaliado o efeito terapêutico das diferentes formulações em estudo através da contagem de unidades formadoras de colónias (CFU) nestes dois orgãos. Paralelamente às formulações de C12 foi também avaliada a actividade terapêutica de POA e PZA na forma livre. Os grupos de animais infectados e não tratados evidenciaram valores de carga bacteriana mais elevados. O maior efeito terapêutico foi observado para os grupos de animais tratados com C12 nas formas livre ou incorporado em lipossomas extrusados, particulamente no fígado. Tendo em consideração que a estirpe de M. avium utilizada neste modelo apresenta uma resistência superior à maioria dos antibióticos comparativamente a algumas estirpes de M. tuberculosis os resultados obtidos são bastante promissores.
Palavras-chave
VII
Abstract
Pyrazinamide is a first line agent for tuberculosis treatment. Being a prodrug requires activation by Mycobacterium enzyme pyrazinamidase to be converted into its active form, pyrazinoic acid (POA). To overcome the emergence of resistant strains the synthesis of prodrugs, esters of POA, which may be transformed in its active form, through mycobacterial esterases were performed. Their highabundance contributes to a reduction in the emergence of resistances.
In the present thesis, the development of liposomal formulations of two POA esters, with different alkoxy chain lengths, and their therapeutic potential in an in vivo model infected with Mycobacterium avium were performed. The association of POA esters to liposomes was based on the ability of these lipidic systems to circumvent some solubility problems associated to these molecules and, after parenteral administration, they can passively target liver and spleen, the main affected organs in M. avium infections.
The incorporation parameters for these two prodrugs were dependent on their chain length. Selecting the prodrug with higher incorporation efficiencies, in vitro and in vivo studies were performed. A systematic study was performed in the presence of HEPES buffer pH 7.4 and biological fluids. In the buffer medium all liposomal formulations evidenced high stability in terms of percentage of incorporated prodrug and preservation of non-hydrolyzed form. In human plasma, the prodrug hydrolysis rate was mainly influenced by mean size and rigidity of liposomal formulations: higher half-lives were obtained for neutral and non-extruded liposomes. In vivo studies represent the first biological evaluation and comparison of new synthesized esters of POA either in free or in liposomal forms. Extruded liposomes, although in in vitro stability studies have shown a higher prodrug hydrolysis rate, leading to lower CFU/g of liver than large sized vesicles. The use of other treatment schedules and mycobacterial strains should be further considered.
Keywords
VIII
List of Contents
Acknowledgments……….…....I
Communications in Scientific Meetings………...III
Resumo………...IV
Abstract………...VII
List of Contents……….VIII
List of Figures……….……..X
List of Tables………...…XII
List of Abbreviations………..………….XIV
1. Introduction ... - 1 -
1.1. Mycobacterial Infections... - 1 -
1.1.1. Nontuberculous Mycobacterial Diseases – The particular case of MAC ... - 1 -
1.2. Conventional treatment of mycobacterial infections ... - 2 -
1.3. Strategies to overcome antibiotic resistance ... - 3 -
1.3.1. Pyrazinamide ... - 3 -
1.3.2. Pyrazinoic Acid Prodrugs ... - 4 -
1.3.3. Liposomes ... - 5 -
1.4. Mycobacterial murine models of infection ... - 6 -
1.5. Objectives of the thesis ... - 7 -
2. Materials and Methods ... - 8 -
2.1. Materials ... - 8 -
2.1.1. Chemical products ... - 8 -
2.1.2. Animals ... - 8 -
2.1.3. Mycobacterium avium strain ... - 8 -
2.2. Methods ... - 8 -
2.2.1. Preparation of prodrug liposomal formulations ... - 8 -
2.2.1.1. Characterization of prodrug liposomal formulations ... - 9 -
2.2.1.1.1. Liposomal size measurements ... - 9 -
2.2.1.1.2. Zeta Potential Determination ... - 10 -
2.2.1.1.3. Prodrugs quantification ... - 10 -
2.2.1.1.4. Phospholipid quantification ... - 10 -
2.2.2. Differential scanning calorimetry (DSC) studies ... - 11 -
2.2.3. HPLC System ... - 11 -
IX
2.2.5. Stability of C12 formulations in presence of human plasma ... - 12 -
2.2.5.1. Half-Life quantification of C12... - 12 -
2.2.5.2. Stability of liposome structure in presence of human plasma ... - 12 -
2.2.6. Murine model ... - 13 -
2.2.6.1. Mycobacterium avium inocula quantification ... - 13 -
2.2.6.2. In vivo evolution of mycobacterial infection ... - 13 -
2.2.6.3. Biological evaluation of antimycobacterial formulations ... - 13 -
2.2.6.4. Evaluation of M. avium growth in mice ... - 14 -
2.3. Statistical analysis ... - 14 -
3. Results and discussion ... - 15 -
3.1. Physicochemical properties of esters of POA ... - 15 -
3.2. Incorporation of C12 and C4 in liposomes ... - 16 -
3.3. Optimization of HPLC procedures for quantification of C12 and POA ... - 21 -
3.4. Stability of C12 formulations... - 23 -
3.4.1. Stability of C12 formulations in HEPES buffer at room temperature ... - 23 -
3.4.2. Stability of C12 formulations in HEPES buffer at 37ºC ... - 24 -
3.4.3. Stability of C12 formulations in human plasma at 37ºC ... - 25 -
3.4.3.1. Stability of C12 in the free form ... - 26 -
3.4.3.2. Stability of C12 liposomes – influence of lipid composition ... - 28 -
A) C12 incorporated in PC liposomes ... - 28 - B) C12 incorporated in DMPC liposomes ... - 29 - C) C12 incorporated in DMPC:DMPG liposomes ... - 31 - D) C12 incorporated in DPPC ... - 32 - E) C12 incorporated in DPPC:DPPG ... - 34 - F) C12 incorporated in DMPC:DSPE-PEG ... - 35 -
3.4.3.3. Stability of C12 formulations – Influence of C12 concentration ... - 37 -
3.4.3.4. Stability of liposome structure in presence of human plasma ... - 39 -
3.5. Mycobacterium avium murine model of infection ... - 40 -
3.5.1. Biological evaluation of antimycobacterial formulations ... - 40 -
3.5.2. In vivo evolution of mycobacterial infection ... - 40 -
3.5.3. Influence of antimycobacterial formulations on M. avium murine model ... - 41 -
4. Conclusions and Future Perspectives ... - 45 -
X
List of Figures
Figure 1. Action sites of the principal anti-TB drugs……….……2
Figure 2. Zhang hypothesis for the mode of action of PZA……….…4
Figure 3. Cross section view of a liposome structure………..5
Figure 4. Schematic representation of the experimental M. avium murine model of
infection……….…….14
Figure 5. DSC thermograms. Influence of C12 on thermotropic behavior of DPPC
liposomes………...20
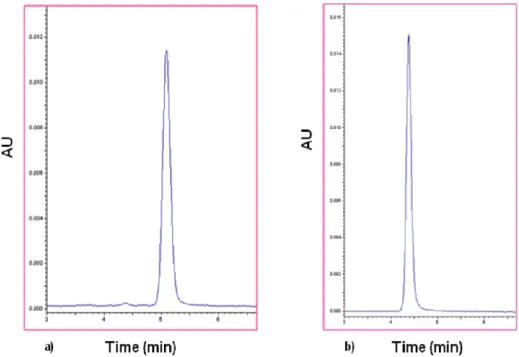
Figure 6. Typical chromatogram of a C12 (a) and POA (b) freshly prepared solutions………...21
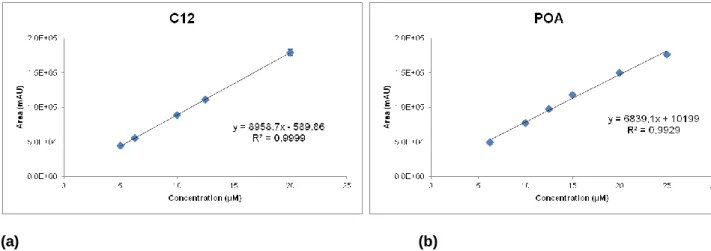
Figure 7. Calibration curves for C12 (a) and for POA (b)……….……….……22
Figure 8. C12 hydrolysis over time………...23
Figure 9. POA formation over time due to C12 hydrolysis………23
Figure 10. Stability on storage of C12 formulations: variation of mean size of C12 liposomes
during one month at room temperature……….24
Figure 11. Stability in buffer, at 37ºC, of C12 formulations: influence of lipid
composition………..…….24
Figure 12. Concentration of C12 and POA after incubation in buffer at 37ºC..……….25
Figure 13. Hydrolysis of C12 in free form and formation of POA in the presence of human
plasma 50% (v/v)……….…….26
Figure 14. Hydrolysis of C12 in the free form in the presence of plasma for an initial
concentration of 60 µM………26
Figure 15. Hydrolysis of C12 incorporated in PC liposomes and formation of POA in the
XI
Figure 16. Hydrolysis of C12 incorporated in DMPC liposomes and formation of POA in the
presence of human plasma 50% (v/v)………...30
Figure 17. Hydrolysis of C12 incorporated in DMPC:DMPG (7:3) liposomes and formation of
POA in the presence of human plasma 50% (v/v)………..31
Figure 18. Hydrolysis of C12 incorporated in DPPC liposomes and formation of POA in the
presence of human plasma 50% (v/v) ……….…….33
Figure 19. Hydrolysis of C12 incorporated in DPPC:DPPG (7:3) non-extruded liposomes and
formation of POA in the presence of human plasma 50% (v/v)………..…………..34
Figure 20. Hydrolysis of C12 incorporated in DMPC:DSPE-PEG (2.85:0.15) extruded liposomes
and formation of POA in the presence of human plasma 50% (v/v)………. …..35
Figure 21. Half-life of C12 formulations after incubation in human plasma 50% (v/v)...36
Figure 22. Hydrolysis of C12 and formation of POA after incubation with human plasma 50%
(v/v)……….38
Figure 23. Absorbance at 420 nm of eluted C12 DMPC:DMPG extruded liposomes following
their application on the top of a Sephadex G200 column ……….…39
Figure 24. Evolution of CFU per g of organ in liver, spleen and lung of BALB/c mice 15 days
after infection induction ………...…41
Figure 25. Evolution of M. avium infection in mice. ………...………42
Figure 26. . Evolution of M. avium infection in mice. Influence of administered formulations on
XII
List of Tables
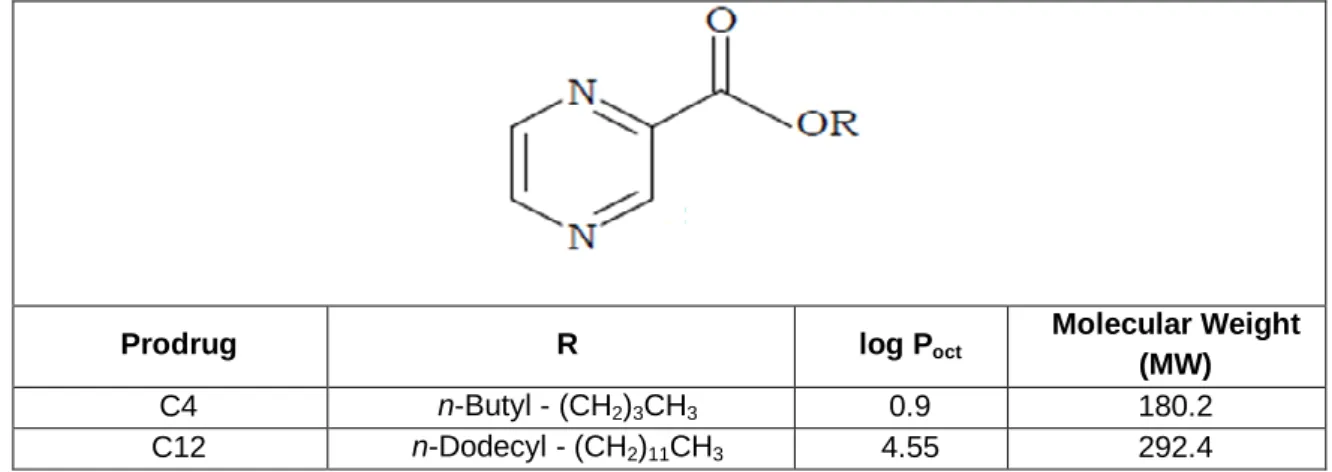
Table 1. Structure and physicochemical properties of C4 and C12……….15
Table 2. Physicochemical characterization of C12 and C4 extruded liposomes. Influence of
alkoxy chain length of prodrugs on incorporation parameters………...…………...17
Table 3. Physicochemical characterization of extruded and non-extruded C12 liposomal
formulations………...19
Table 4. Detection and quantification limits for C12 and POA according to the respective
standards for each calibration curve………..22
Table 5. . kobs and half-life of free C12 in the presence of human plasma. Influence of initial
concentration……….27
Table 6. kobs and half-life of free C12 in the presence of human plasma. Initial concentration: 60
µM………...…27
Table 7. kobs and half-life of C12 incorporated in PC liposomes in the presence of human
plasma: influence of mean vesicle size ………29
Table 8. kobs and half-life of C12 incorporated in DMPC liposomes in the presence of human
plasma: influence of mean vesicle size ………..………30
Table 9. . kobs and half-life of C12 incorporated in DMPC:DMPG (7:3) liposomes in the presence
of human plasma: influence of mean vesicle size……….………..32
Table 10. kobs and half-life of C12 incorporated in DPPC liposomes in the presence of human
plasma: influence of mean vesicle size……….33
Table 11. kobs and half-life of C12 incorporated in DPPC:DPPG (7:3) non-extruded liposomes in
the presence of human plasma………..…34
Table 12. kobs and half-life of C12 incorporated in DMPC:DSPE-PEG (2.85:0.15) liposomes in
the presence of human plasma………..36
Table 13. kobs and half-life of C12 in free form and incorporated in DMPC:DMPG (7:3) extruded
XIII
Table 14. Stability of liposome structure in the presence of human plasma - Mean size of
liposomes at time zero and 1 and 3h post-incubation following their application on the top of a Sephadex G200 column………..40
Table 15. C12 liposomal formulations used for the treatment of M. avium murine model of
XIV
List of Abbreviations
Ø Mean size
[(Cx/Lip)f Final Cx to Lipid ratio [(Cx/Lip)i Inicial Cx to Lipid ratio
ΔH Enthalpy variation
ACN Acetonitrile
AIDS Acquired immune deficiency syndrome ATCC American Type Culture Collection
CFU Colony forming units
D.L. Detection Limit
DMPC Dimyristoyl Phosphatidylcholine DMPG Dimyristoyl Phosphatidylglycerol DPPC Dipalmitoyl Phosphatidylcholine DPPG Dipalmitoyl Phosphatidylglycerol DSC Differential Scanning Calorimetry
DSMZ Deutsche Sammlung von Mikroorganismen und Zellkulturen DSPE Distearoyl Phosphatidyl Ethanolamine
ETB Ethambutol
h hour
HIV Human immunodeficiency virus
HPLC High Performance Liquid Chromatography I.E. Incorporation efficiency
INH Isoniazid
i.v. Intravenous
kobs First order rate constant
Lip Lipid
ln neperian logarithm
Ltd Limited
XV
M. avium Mycobacterium avium
M. tuberculosis Mycobacterium tuberculosis
MAC Mycobacterium avium complex
min minute
MPS Mononuclear phagocytic system NAD Nicotinamide adenine dinucleotide non-MDR-TB non-Multi-Drug-Resistant-Tuberculosis NTM Nontuberculous mycobacterial
OADC Oleic acid-albumin-dextrose-catalase
PC Egg Phosphatidylcholine
PdI Polydispersity index
PEG Polyethyleneglycol
POA Pyrazinoic Acid
PZA Pyrazinamide PZase Pyrazinamidase RIF Rifampicin RT Retention time s second S.D Standard deviation t1/2 Half-life time
Tc Phase transition temperature
TB Tuberculosis
UK United Kingdom
- 1 -
1. Introduction
1.1.
Mycobacterial Infections
Mycobacteria are Gram positive, nonspore-forming, aerobic bacteria. They are considered facultative intracellular microorganisms presenting intracellular and extracellular multiplication phases. Macrophages, resident phagocytes in different organs, are the main cells that engulf invading microorganisms. Some pathogens that are internalized by macrophages are transferred to lysosomal organelles followed by degradation. However, pathogenic mycobacteria evade innate immunity by manipulating host, ensuring long-term survival and proliferation that can occur in the phagosome (Gaspar et al., 2008a).
There are now over 130 known species of mycobacteria. However, only a few play an important role as pathogenic agents for immunocompetent human hosts, namely Mycobacterium tuberculosis, Mycobacterium leprae and Mycobacterium ulcerans, the causative agents of tuberculosis (TB), leprosy and Buruli ulcer, respectively. The majority of other Mycobacterium species are present in the environment as saprophytes, some of which can be opportunistic and often deadly pathogens for immunocompromised human hosts. From this group of environmental opportunistic mycobacteria, those from Mycobacterium avium complex (MAC) are responsible for a large percentage of nontuberculous mycobacterial (NTM) diseases (Reed et al., 2006).
1.1.1. Nontuberculous Mycobacterial Diseases – The particular case of MAC
NTM diseases are acquired from environmental (water, soil) reservoirs and are not transmitted between humans or between animals and humans. NTM infection progression to clinical disease requires one or more predisposing host conditions. NTM species that cause pulmonary disease vary by geographic region being MAC the predominant pathogen (Cook, 2010). In pre-AIDS (acquired immune deficiency syndrome) era, MAC, predominantly caused pulmonary mycobacteriosis, that were difficult to distinguish radiologically from tuberculosis and were rarely associated with extrapulmonary sites or disseminated diseases (Wolinsky, 1979). Disseminated MAC became a frequent diagnosis in late-stage HIV (human immunodeficiency virus) infected patients, usually occurring in patients with CD4+ T-cell counts below 50/mm3. Food-stuffs and water are the source of these organisms and the portal of entry in hosts is likely to be the gastrointestinal tract. The syndrome is characterized by fever, shills, sweats, malaise, weight loss, abdominal pain, and diarrhea (Cynamon and DeStefano, 1999). MAC refers to a group of two closely related environmental mycobacteria: M.avium and M. intracellulare (Cosma et al., 2003). Unlike TB, MAC disease is not required to be reported to public health entities in most countries, and therefore, precise incidence and prevalence data are not available (Gaspar et al., 2008a).
- 2 -
1.2.
Conventional treatment of mycobacterial infections
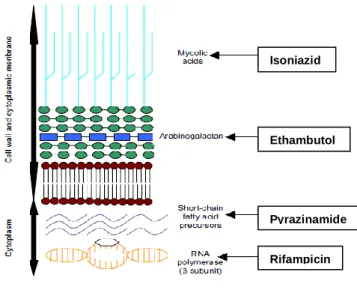
Mycobacterial species share a characteristic thick cell wall, with a unique composition that confers an exceptionally strong barrier to antibiotics, leading to resistances to a wide variety of antimycobacterial agents. The lipid content of this cell wall gives rise to the common characteristics of the Mycobacterium genus, which is the ability to retain basic dyes in presence of an acid-alcohol solution (Cosma et al., 2003). The cell wall of mycobacteria is composed of four layers: the first consists of peptidoglycan and the other three of a variety of soluble proteins, carbohydrates, lipids and insoluble macromolecular components like arabinogalactan, peptidoglycan and mycolic acid. The outer lipid barrier is extremely hydrophobic due to the covalent bond between molecules of mycolic acid and arabinogalactan (Gaspar et al., 2008a). The thick cell wall of mycobacterial species is in the origin of the high difficulty on the treatment of mycobacterial infections. The most effective drugs to control M. tuberculosis infection are Isoniazid (INH), Ethambutol (ETB), Pyrazinamide (PZA) and Rifampicin (RIF). These drugs are first line therapeutic drugs and they represent the basis of tuberculosis therapeutic regiments. In Figure 1 are shown their action sites in mycobacterial membrane: RIF inhibits bacterial RNA synthesis by binding to the β-subunit of bacterial DNA-dependent RNA polymerase and blocking the initiation chain formation in RNA synthesis; PZA is converted to Pyrazinoic Acid (POA) and by decreasing the pH inside the mycobacterial cell prevents the microorganism growth. PZA may also function as an antimetabolite of nicotinamide and interferes with the synthesis of nicotinamide adenine dinucleotide (NAD), inhibiting the synthesis of short-chain, fatty-acid precursors; ETB inhibits mycobacterial arabinosyl transferases involved in the polymerization of D-arabinofuranose to arabinoglycan, an essential cell wall component; INH is a prodrug activated by the mycobacterial catalase-peroxidase enzyme KatG inhibiting the synthesis of mycolic acids, an essential component of mycobacterial cell walls (du Toit et al., 2006).
Figure 1. Action sites of the principal anti-TB drugs. Adapted from du Toit et al., (2006)
Pyrazinamide Isoniazid
Ethambutol
- 3 -
In contrast with TB therapy, which is nearly uniformly effective when correctly administered to non-Multi-Drug-Resistant-TB (non-MDR-TB), the therapy of MAC infections has historically been less efficient and there are no standardized treatments (French et al., 1997; Georgiev, 1994; Nuermberger and Grosset, 2004). Therapeutic regimens include drugs such as clarithromycin, RIF, Amikacin, Ciprofloxacin, ETB, Azithromycin, Rifabutin. However, an increased number of mycobacterial strains have shown to be resistant to the available drugs. A combinatory therapy for the treatment of these infections is recommended since monotherapy gives rise to an enhanced occurrence of drug resistance and clinical failure.
1.3.
Strategies to overcome antibiotic resistance
In an attempt to overlap the emergence of resistance strains to the available first-line drugs, a viable strategy may be achieved by synthesis of new molecules or chemical modification of already available ones. The design of new molecules such as prodrugs, entities that need to undergo an enzymatic and/or chemical transformation in vivo to release the active parent drug constitutes a good alternative. The development of prodrugs has become an established tool for improving physicochemical, biopharmaceutical or pharmacokinetic properties of pharmacologically active molecules and represent an increased tendency in pharmaceutical industry (Rautio et al., 2008). In particular, the prodrug approach via activation inside mycobacterial cells is a relevant alternative especially considering the problematic penetration of drugs into these cells (Valente et al., 2011).
1.3.1. Pyrazinamide
PZA, a first line agent for the treatment of TB is also a prodrug. PZA plays an important role in shortening TB therapy. This ability is related to its activity against a population of semi-dormant mycobacteria residing in an acidic pH environment and that are not killed by other TB drugs (Wade and Zhang, 2004; Zhang and Mitchison, 2003). In fact in vitro tests revealed a lower activity in normal culture conditions close to neutral pH, while higher activity is observed in acidic media (Wade and Zhang, 2004).
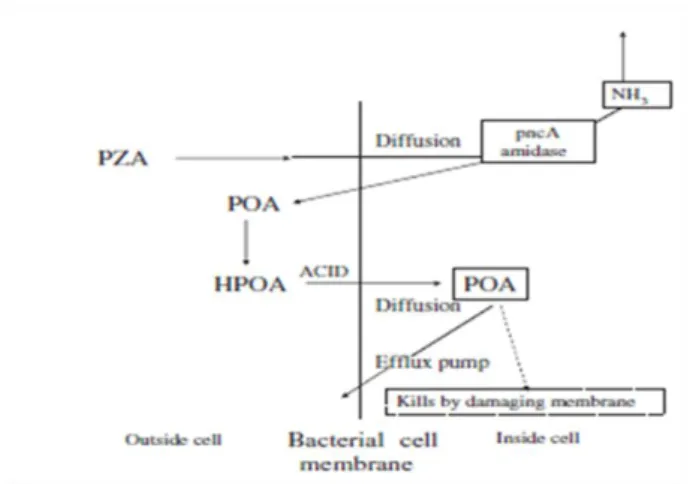
In order to have antimycobacterial activity, PZA requires activation by the Mycobacterium enzyme pyrazinamidase (PZase) to be transformed into its active form, the pyrazinoic acid (POA). This enzyme conveniently activates the drug only inside the bacteria (Pires, 2011). The hypothesis for the mode of action of PZA according to Zhang and Mitchison, (2003) is shown in Figure 2. PZA diffuses into the bacterial cell being then deaminated to form POA. This molecule can only leave the cell again when it is excreted by an inefficient mycobacterial efflux pump requiring energy (Mitchison and Fourie, 2010; Zhang et al., 2003).
- 4 - Figure 2. Zhang hypothesis for the mode of action of PZA. Adapted from Mitchison and Fourie, (2010)
However, since the activation of PZA is dependent on only one enzyme, it has been observed the emergence of resistant strains to this prodrug. As reviewed by Zhang and Mitchison, (2003) the acquired PZA resistance in M. tuberculosis occurs by pncA gene mutation (Raynaud et al., 1999).In other mycobacteria infections, in particular by M. avium, the natural resistance to PZA may be due to an efficient POA efflux mechanism (Zhang and Mitchison, 2003). On the other hand the administration of POA, the active form of PZA, is not recommended for treatment of mycobacteriosis due to its poor absorption and significantly serum binding (Konno et al., 1967).
1.3.2. Pyrazinoic Acid Prodrugs
To overcome the already mentioned drawbacks, the synthesis of new POA prodrugs were conducted by several authors (Cynamon et al., 1992; Cynamon et al., 1995; Fernandes et al., 2010; Yamamoto et al., 1995). Cynamon et al., (1992) prepared series of POA esters and evaluated their activity in vitro. Compared with PZA some compounds displayed higher activity than PZA against M. tuberculosis, M. avium and M. kansasii (Cynamon et al., 1995). However, these new compounds had lower stability in presence of biological fluids (Bergmann et al., 1996).
Recently Simões et al. (2009) have synthesized series of pyrazinoic lipophilic prodrugs esters with different alkoxy groups, and amide (Simões, 2005; Simões et al., 2009; Valente et al., 2011). They observed that lipophilic esters with long alkoxy chains were more resistant to plasma hydrolysis than the short chain esters (Simões et al., 2009). The selection of long chain esters over amide prodrugs was based on the fact that lipophilic esters are easily activated by mycobacterial esterases. This may contribute to a reduction in resistances to these prodrugs. These esters were active in concentrations 10-fold lower than those needed for PZA to kill sensitive M. tuberculosis in in vitro tests. On the other hand, it seems that amide prodrugs cannot easily be activated by mycobacterial enzymes and thus have low in vitro activity (Simões et al., 2009).
- 5 -
Besides the development of prodrugs with high in vitro activity, when applied to in vivo, their pharmacological performance will be dependent on several factors, such as clearance or metabolization after administration; distribution to infected sites, possible binding to plasmatic proteins and the toxicity resulting from localization in non-affected sites, restricting the amount of drug that can be administered (Gaspar et al., 2008a). A good strategy to overcome some of these possible drawbacks could be the association of therapeutically promising molecules to drug delivery systems. Ideally, these delivery systems should allow a preferential targeting of a specific compound within a therapeutic concentration range at the affected sites; should be non-toxic and non-immunogenic; should be biodegradable or easily excreted after exerting its effect; and should be cheap and stable upon storage (Gaspar et al., 2008a). One of the most extensively studied drug delivery systems are liposomes.
1.3.3. Liposomes
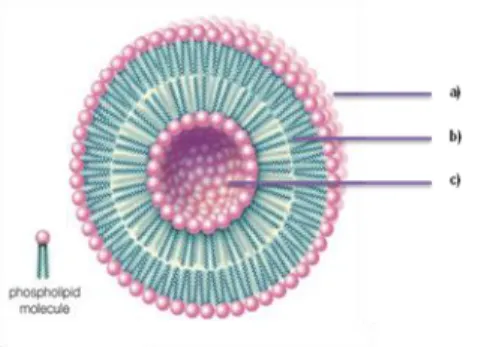
Liposomes, by definition, are submicron lipidic particles consisting of one or more concentric lipid bilayers, separated by aqueous compartments (Gaspar et al., 2008a). It has been more than 4 decades since the first report of the successful preparation of liposomes by Bangham et al., (1965). There are several types of liposomes according to their lipid compositions, number of lipid bilayers, superficial charges and mean sizes, ranging from few nanometers to several microns. These liposomes can also be prepared using different methods (Crommelin et al., 1994). Liposomes have been widely used for targeted drug delivery due to their structural versatility, biodegradability, innocuous nature and resemblance to biological membranes (Gregoriadis and Ryman, 1972). They can entrap drugs of different sizes and solubility properties since the water-soluble compounds will be encapsulated in the aqueous spaces while lipid-soluble ones will be incorporated in the lipid bilayer (Cruz et al., 2009; Torchilin, 2005). In Figure 3 is shown the schematic structure of a liposome.
Figure 3. Cross section view of a liposome structure: (a) Hydrophilic head; (b) Hydrophobic tail; (c)
Internal aqueous space.
(Available at 22-08-2012 in http://www.britannica.com/EBchecked/media/92244/Phospholipids-can-be-used-to-form-artificial-structures-called-liposomes )
Liposomes can be prepared according to the physicochemical properties of the molecule to be incorporated and in order to reach the target either for therapy or for diagnosis (Cruz et al., 2009). The structural and functional diversity of liposomes has been explored for the design of
- 6 -
drug carrier systems in particular for the treatment of mycobacterial infections (Fielding and Lasic, 1999). After intravenous (i.v.) administration, some liposomes, depending on their properties, are rapidly removed from blood circulation by the phagocytic cells of the mononuclear phagocyte system (MPS), particularly by macrophages in liver and spleen. This natural tendency has been exploited for treatment of infectious diseases localized in these organs (Gaspar et al., 2008a). Intracellular infections caused by M. avium and M. tuberculosis may be envisioned as a preferential target for liposomes The therapeutic advantages by incorporating antibiotics in liposomes over the free respective molecules has been demonstrated in several animal models of infection (Fielding and Lasic, 1999; Gaspar et al., 2008a; Pinto-Alphandary et al., 2000). This superior therapeutic effect may be explained by the different biodistribution profile of liposomal formulations in comparison with the free antibiotic (Allen and Hansen, 1991; Allen and Stuart, 1999; Bakker-Woudenberg et al., 1993; Gaspar et al., 2008b). However, the use of these type of liposomes, that are rapidly cleared from bloodstream, may not be the right choice when other organs than liver and spleen are the target. For inflammation and tumor pathological situations, that are not localized in liver and spleen, the use of other type of liposomes with longer blood circulation half-lives represent better solutions. Thus, the selection of lipid components is essential for the design of a liposomal formulation. One successful hypothesis has been achieved by including in the lipid composition the polyethyleneglycol (PEG), a polymer covalently linked to a phospholipid. The presence of PEG at liposome surface is able to reduce plasmatic proteins adsorption and consequently to decrease MPS clearance (Allen and Cullis, 2004; Torchilin, 2005). The increased circulation time of liposomes has improved the delivery of therapeutic molecules to infections localized in other organs than liver and spleen or even to inflammation and tumor sites (Bakker-Woudenberg et al., 1993; Corvo et al., 2002; Gaspar et al., 1996; Gaspar et al., 2007; Liu et al., 2006).
Therefore, the incorporation of esters of POA within liposomes appears as a good strategy to enhance their in vivo antimycobacterial properties.
1.4.
Mycobacterial murine models of infection
During the past years various animal models of MAC infection have been explored, namely pigs, sheep, fowl, rabbits, goats, mice and guinea-pigs. However, the mouse is the most widely used model of infection to evaluate the chemotherapeutic effect of antimycobacterial drugs (Cynamon and DeStefano, 1999). Mice require small laboratory space, their purchase and maintenance are relatively inexpensive and handling of these animals is easy if performed by trained personal.
The induction of the infection may be perforrmed by a variety of routes including intraperitoneal, intranasal, oral, intrarectal, aerogenic and i.v. The i.v. route is the most commonly used as provides a consistent disseminated disease model with viable organisms recovered from spleen, liver, blood, lung and lymph nodes. Usually for i.v. induction, mice are infected in the tail vein. Each experiment consists of an early control group that is sacrificed at
- 7 -
the beginning of treatment and a later control that is sacrificed at the end of treatment schedule, together with all treated groups. Primary cultures of mycobacteria species to be used for infection may be obtained from clinical isolates or from bioresource centers such as American Type Culture Collection, (ATCC) or from German Collection of Microorganisms and Cell Culture.
1.5.
Objectives of the thesis
The main objectives in the present work were the development and characterization of liposomal formulations of prodrugs of pyrazinoic acid (POA), particularly esters of POA, that in previous in vitro tests have demonstrated to be active against M. tuberculosis (Simões et al., 2009). The possible therapeutic effect of some of these liposomal formulations was tested in a preliminary in vivo model infected with M. avium.
In order to fulfill the objectives of the present master thesis the work was executed according to the following activities:
Development of best liposome methodologies for incorporating POA prodrugs in liposomes. The influence of lipid composition, mean size, superficial charge and of the physicochemical properties of POA prodrugs on incorporation parameters were studied.
Optimization of HPLC methodologies for evaluating the stability of the prodrug in buffer and biological media. The comparison of chemical stability of the prodrug in free and liposomal forms was performed.
Establishment of a M. avium model of infection that may be suitable for testing the chemotherapeutic effect of POA prodrugs was carried out. Microbiological techniques were used.
- 8 -
2. Materials and Methods
2.1.
Materials
2.1.1. Chemical products
The prodrugs used C4 and C12 (esters of pyrazinoic acid) were previously synthesized by the Medicinal Chemistry group of the Faculty of Pharmacy of the University of Lisbon according to Simões et al., (2009). The following pure phospholipids were purchased from Avanti Polar Lipids (USA): Dimyristoyl Phosphatidylcholine (DMPC), Dimyristoyl Phosphatidylglycerol (DMPG), Dipalmitoyl Phosphatidylcholine (DPPC), Dipalmitoyl Phosphatidylglycerol (DPPG), egg Phosphatidylcholine (PC) and Distearoyl Phosphatidyl Ethanolamine covalently linked to Polyethyleneglycol (DSPE-PEG). Deionized water (Milli-Q system; Millipore, Japan) was used for the preparation of all experimental solutions. Middlebrook 7H9 broth and 7H10 agar, and Bacto Middlebrook albumin-dextrose-catalase and oleic acid-albumin-dextrose-catalase (OADC) enrichments were obtained from Difco Laboratories (USA). Nuclepore Track-Etch Membranes were purchased from Whatman Ltd, USA. Kolliphor ELP from BASF, Germany, was kindly supplied by DS Produtos Químicos, Lda, Portugal. All other reagents were of analytical grade.
2.1.2. Animals
Male BALB/c mice (5 to 7 weeks old, weight 25-30 g) were obtained from the Gulbenkian Institute of Science (Portugal). The animals were kept under hygienic conditions, fed commercial chow, and given acidified drinking water ad libitum. All the experimental procedures were carried out with the permission of the local laboratory animal committee.
2.1.3. Mycobacterium avium strain
The bacterial strain used for the mouse model was M. avium 44157 from DSMZ depository (Deutsche Sammlung von Mikroorganismen und Zellkulturen) and was kindly provided by Unidade dos Retrovírus e Infecções Associadas of de Faculty of Pharmacy of the University of Lisbon.
2.2.
Methods
2.2.1. Preparation of prodrug liposomal formulations
Multilamellar vesicles composed of selected phospholipids were prepared as previously described (Gaspar et al., 2000). The phospholipids and each prodrug used, in a molar ration of 1:10, were previously solubilized in chloroform and dried by rotary evaporation
- 9 -
(Buchi, Switzerland) under nitrogen stream, to eliminate the organic solvent, until the formation of a thin lipid film. The film was dispersed in deionized water, frozen and lyophilized (Edwards, USA) overnight. Rehydration of lyophilized powder was made in two steps of 30 minutes (min) each, at a temperature above the phase transition temperature (Tc) of the correspondent lipid
mixture. First rehydration was done in a volume of 2/10 of the final volume with 150 mM NaCl in 10 mM HEPES buffer, pH 7.4 (HEPES buffer, pH 7.4). After 30 min, rehydration was completed with the same buffer. In order to reduce and homogenize the diameters of prodrug liposomes, some formulations were submitted to an extrusion technique using an Extruder device (Lipex Biomembranes Inc., Canada). Liposomal suspensions were sequentially filtered through polycarbonate membranes of different porosities (0.8, 0.6, 0.4 and 0.2 µm) using a nitrogen pressure ranging from 100 to 500 lb/in2 , until an average vesicle size between 0.1 – 0.2 µm was achieved. The separation of non-incorporated prodrug from liposomes was performed by ultracentrifugation (300 000 g for 2 hour (h) at 17ºC in a Beckman LM-80 ultracentrifuge (Beckman Instruments, USA). The obtained pellet was ressuspended in the desired volume of HEPES buffer, pH 7.4.
2.2.1.1. Characterization of prodrug liposomal formulations
Prodrug liposomal formulations were characterized in terms of lipid composition, lipid and prodrug concentration, zeta potential, mean vesicle size and polydispersity index. The following abbreviations and equations were used to determine these incorporation parameters:
(
equation 1)2.2.1.1.1. Liposomal size measurements
Liposome mean diameter was determined by dynamic light scattering in a hydrodynamic sizing system (Zetasizer Nano S, Malvern Instruments, UK). This technique was based on Brownian motion and was related to the size of the particles. For viscosity and refractive index, the values of pure water were used. As a measure of particle size distribution of the dispersion, the system reports the polydispersity index (PdI). The index ranges from 0.0 for an entirely monodisperse sample up to 1.0 for a polydisperse suspension. To determine the
- 10 -
mean diameter and PdI of liposomal preparations, a dilution to a final lipid concentration of 0.3 mM in HEPES buffer, pH 7.4 was previously performed, and samples were placed in an appropriate polycarbonate cell. To ensure that appropriate mean diameter and PdI were achieved, besides the measurements done for final liposomal preparations, these parameters were also determined during the extrusion procedure.
2.2.1.1.2. Zeta Potential Determination
Zeta potential of liposomal formulations was measured in a hydrodynamic sizing system (Zetasizer Nano Z, Malvern Instruments, UK). Zeta potential is defined as an electric potential between the membrane surface and the ionic dispersion medium. This method determines how fast a particle moves in a liquid when an electrical field is applied. For viscosity and refractive index, the values of pure water were used.
Before determination of the zeta potential of liposomal formulations, an initial check of the apparatus was made with a standard of a known zeta potential value (standard DTS5050, Malvern Instruments, Ltd., UK). Dilutions of liposomal formulations were made in HEPES buffer, pH 7.4 for a final lipid concentration of 0.3 mM. Samples were slowly introduced into a clear disposable zeta cell with a syringe to avoid air bubbles. The zeta potential of samples at a temperature of 25ºC was recorded. Three independent dilutions were prepared for each liposomal formulation under study.
2.2.1.1.3. Prodrugs quantification
The prodrugs were quantified spectrophotometrically at 267 nm after disruption of the liposomes with ethanol. Briefly, samples containing an amount of prodrug between 6.25 and 31.25 µM were pipetted in triplicate into 1.5 mL tubes and completed to 1 mL with ethanol. In parallel, a calibration curve using a 2.5 mM stock prodrug solution and standards ranging from 6.25 to 31.25 µM were used. All tubes were shaken for 10 min and absorbances at 267 nm were recorded in a UV 160 Spectrophotometer (Shimadzu, Japan).
The amount of prodrug in samples was obtained using a linear regression. The calibration curve was linear up to absorbance values of at least 0.364.
2.2.1.1.4. Phospholipid quantification
The method for phospholipid quantification was based on the colorimetric determination of PO4
according to Rouser et al., (1970). Briefly, samples in triplicate containing a phosphate amount between 20 and 80 nmol (sample volume less than 100 µL) were pipetted into 15 mL glass tubes. In parallel, a calibration curve with phosphate amounts ranging from 20 to 80 nmol was prepared using a 0.5 mM phosphate stock solution. All samples were then heated (180ºC) in a heating block until their dryness. After cooling, 0.3 mL of perchloric acid (70-72%) was added to all tubes. Marbles were placed on the top of all tubes and heated at 180ºC for 1 h to convert all the organic lipid phosphate to inorganic form. After cooling samples to room
- 11 -
temperature, 1 mL of distilled water, 0.4 mL of hexa ammonium heptamolybate solution [1.25% (w/v)], followed by 0.4 mL of ascorbic acid solution [5% (w/v)] were added to all glass tubes. All tubes were heated in a boiling water bath for 5 min. During heating, the inorganic phosphate is converted to phosphomolybdic acid due to reduction of ascorbic acid and a blue color is developed. After cooling, the absorbance at 797 nm of all samples was recorded in a UV 160 Spectrophotometer (Shimadzu, Japan) against the blank of the calibration curve. The amount of phosphate in samples was obtained using a linear regression. The calibration curve was linear up to absorbance values of at least 1.000.
2.2.2. Differential scanning calorimetry (DSC) studies
The phase transition behavior of POA esters in liposomal formulations was performed in a calorimeter DSC Q200 (TA Instruments, USA). Approximately 10 µL of liposomal formulations (lipid concentration ca. 60 mM) were accurately measured into aluminum pans which were hermetically sealed and then measured against an empty reference pan. The pans were heated and the thermograms were recorded at a temperature ranging from 15 to 65ºC at a heating rate of 3ºC/min.
2.2.3. HPLC System
All stability studies were made using C12. The stability of C12 was evaluated by HPLC following an optimization of best conditions for quantifying the prodrug C12 and the corresponding hydrolysis product, the POA, according to Simoes et al., (2009). The Beckman System Gold HPLC system consisted of a 126 Pump Direct Control, the model detector 166 from Beckman Instruments, Inc and the Midas Spark 1.1 autoinjector. The wavelenght of the detector was set at 267 nm. The analytical column was a LiChroCart® 125-4 Purospher Star RP-8 (5 µm) (Merck, Germany). The system was attached to a computer with the software, Katarat 7.7 for integration and treatment of chromatograms. The mobile phase, in an isocratic solvent system, consisted in 75% of acetonitrile (ACN) for C12 and in 2% for POA in KH2PO4/H3PO4 25 mM, (phosphate buffer, pH 2.0) with a flow rate of 1 mL/min. Calibration
curves for each compound, C12 and POA, were constructed with different standards ranging from 5 to 25 µM. The limits of detection and quantification were calculated based on the different standards used. All the quantifications were performed using calibration curves constructed freshly prepared C12 and POA solutions.
2.2.4. Stability of C12 formulations in presence of HEPES buffer
The stability of C12 liposomes in HEPES buffer was evaluated by incubating the formulations at 37ºC. At defined times, aliquots of suspensions were taken and applied onto the top of a PD10 column to separate non incorporated C12. The fraction correspondent to liposomes was analyzed for C12 and lipid contents. The mean size and superficial charge were also analyzed along the incubation period. Different lipid compositions were characterized (DMPC, DMPC:DSPE-PEG (2.85:0.15), DMPC:DMPG (7:3)). The stability was defined as the
- 12 -
ratio in percentage between C12 to lipid ratio at each studied time and the C12 to lipid ration at time zero as shown in the following equation:
(equation 2)
,where tx are the ratios obtained at 0.5, 1, 4 and 24 h after incubation and t0 is the ratio at time zero.
2.2.5. Stability of C12 formulations in presence of human plasma
The stability in plasma of C12 in free and liposomal forms was evaluated by incubating the formulations in the presence of plasma diluted with HEPES buffer, pH 7.4 (50% v/v). The hydrolysis of C12 and the formation of POA were determined by HPLC. At defined times 100 µL of the reaction medium were 10 times diluted in ACN or in phosphate buffer, pH 2.0, respectively for C12 and POA. Samples were centrifuged (Bench Centrifuge 202 MK, Sigma, Germany) for 15 min, at 13 000 g at 17ºC, and the supernatant was filtrated with a 0.2 µm filter and injected onto the HPLC system.
2.2.5.1. Half-Life quantification of C12
Apparent pseudo first-order kinetics and rate constants were determined, according to Gupta et al., (2009), by using initial rates of hydrolysis. The apparent pseudo first-order hydrolysis rate constants of C12 at 37ºC were determined by plotting the logarithm of C12 concentration as a function of time according to the equation:
(equation 3)
The hydrolysis half-live (in min) was then calculated by the equation:
(equation 4)
2.2.5.2. Stability of liposome structure in presence of human plasma
The evaluation of liposome structure in presence of human plasma was performed by incubating liposomal formulations at 37ºC. At defined times aliquots of the suspension were taken and applied onto the top of a Sephadex G200 column to separate liposomes from human plasma. The fraction correspondent to liposomes was analyzed in terms of turbidity and recorded by spectrophotometry at 420 nm. The mean size was also analyzed along the
- 13 -
incubation period. A single lipid composition was tested – DMPC:DMPG (7:3) extruded liposomes.
2.2.6. Murine model
2.2.6.1. Mycobacterium avium inocula quantification
Inocula were prepared as previously described (Silva et al., 1987). Briefly, transparent colonies of M. avium DSMZ 44157 were subcultured in Middlebrook 7H9 broth with albumin-dextrose-catalase supplement and 0.04% Tween 80 (w/v) and allowed to grow at 37ºC on an orbital shaker for 2 weeks. The bacteria were harvested by centrifugation (2 000 g, 10 min) in a GPR Beckman centrifuge (Beckman Instruments, USA), suspended in a small volume of saline with 0.04% Tween 80 (w/v), sonicated at low energy for 90 second (s) in a bath-type sonicator (Bandelin Sonorex RK156, Germany) to disrupt bacterial clumps, diluted in the same medium to an optical density of 0.8 at 600 nm and stored frozen at -70ºC until use. When needed, aliquots were diluted to the desired concentration, and inoculated.
2.2.6.2. In vivo evolution of mycobacterial infection
BALB/c mice were injected intravenously with 5x105 colony forming units (CFU) of M. avium DSMZ 44157. Fifteen days after infection, mice were sacrificed and their livers, spleens and lungs were aseptically removed and homogenized. Homogenates were suspended and serially diluted in 0.04% Tween 80 (w/v) and plated onto Middlebrook 7H10 agar medium enriched with OADC for CFU counting after incubation at 37ºC for 10 to 15 days (Gaspar et al., 2000).
2.2.6.3. Biological evaluation of antimycobacterial formulations
A murine model of M.avium infection previously described (Pedrosa et al., 1994) was used. Each animal was infected by i.v. injection in a lateral tail vein with 1x106 CFU per mouse in 200 µL of a M. avium suspension.
The therapeutic treatment started 2 weeks after the infection. On each week, treated animals received three i.v. injections in a lateral tail vein during 2 weeks. The formulations under study were C12 in the free form (solubilized in HEPES buffer pH 7.4 and Kolliphor ELP 5% (w/v)) or incorporated in DMPC:DMPG (7:3) extruded and non-extruded liposomes. POA and PZA previously solubilized in HEPES buffer pH 7.4 were also tested. The administered dose was 12mg/kg of body weight related to POA.
- 14 - 2.2.6.4. Evaluation of M. avium growth in mice
Two days after the last treatment mice were killed by cervical dislocation and livers and spleens were removed according to the method described in 2.2.6.2.
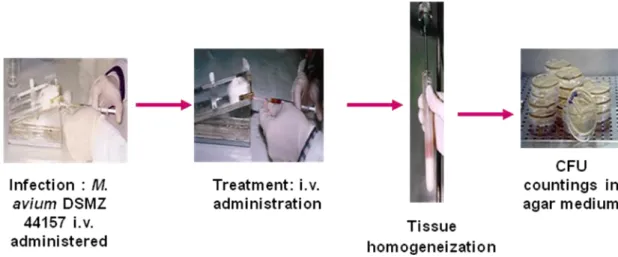
In Figure 4 a schematic representation of M. avium murine model of infection used in this work is shown.
Figure 4. Schematic representation of the experimental M. avium murine model of infection
2.3.
Statistical analysis
Data presented are expressed as mean (±) and standard deviation (S.D.). Statistical analysis was performed using Single Factor ANOVA. The acceptable probability for a significant difference between mean values was p<0.05 (Zar, 2009).
- 15 -
3. Results and discussion
3.1.
Physicochemical properties of esters of POA
In previous work, developed by Simões et al. (2009) series of esters of POA, with a linear alkoxy chain, ranging from 4 to 16 carbon atoms have been synthesized and stability studies performed either in plasma or in macrophage cell cultures have demonstrated to be good candidates as antituberculous drugs. Two of those compounds were selected for the present work to be incorporated in liposomes: the esters containing 4 and 12 carbons linear chain length (C4 and C12, respectively). The rational for associating these two esters of POA to liposomes was based on the fact that these lipid systems are able to circumvent some solubility problems associated to these molecules. As widely described in literature after parenteral administration liposomes can passively target their incorporated material to infected cells in liver and spleen as is the case of M. avium infections (Cruz et al., 2009; Gaspar et al., 2008a).
C12 and C4 are two prodrugs with different properties, which may influence their incorporation in liposomes as well as their retention. In Table 1 are shown some physicochemical properties of these two low molecular weight prodrugs, in particular the log octanol/water partition coefficient (log Poct.). The
increase on the carbon linear chain resulted on a concomitant enhancement on the log Poct from 0.9 to
4.55 for C4 and C12, respectively. The log Poct is a measure of the equilibrium concentration of a solute
between two immiscible phases: octanol and water. It may predict the potential for partitioning into hydrophobic compartments such as lipid bilayers and hydrophilic compartments such as the aqueous core of liposomes (Ribeiro et al., 2010). Generally, we can say that molecules with a log Poct higher than
1 present a lipophilic character while solutes with a log Poct below 1 are considered to be hydrophilic.
Moreover, log Poct > 5 correspond to high lipophilic compounds (Allen and Stuart, 1999;
Defrise-Quertain et al., 1984).
Table 1. Structure and physicochemical properties of C4 and C12
Data from Simões et al., (2009)
Prodrug R log Poct
Molecular Weight (MW)
C4 n-Butyl - (CH2)3CH3 0.9 180.2
- 16 -
3.2.
Incorporation of C12 and C4 in liposomes
Due to the hydrophobic properties of C12 and C4, the liposome method for incorporating these molecules in liposomes was the lipid film method, where both phospholipids and prodrugs were solubilized in chloroform, submitted to an evaporation step, rehydrated with water, lyophilized overnight and then rehydrated with a suitable buffer. This methodology has been widely used for incorporating low molecular weight molecules of hydrophobic properties (Carvalheiro et al., 2009; Constantino et al., 1993; Gaspar et al., 2000)
As described in materials and methods, two types of liposomes were used in the present work: extruded and non-extruded. This technique constitutes one of the best methodologies to reduce and homogenize the mean size of liposomes as it is suitable for the preparation of lipid systems in a scale ranging from one to hundreds of mL (Cruz et al., 2009).
The influence of different lipid compositions on incorporation parameters was studied. Taking this into consideration, the influence of phase transition temperature (Tc)* of the phospholipids as well
as the presence of negatively charged phospholipids in the lipid composition on the incorporation efficiencies (I.E.) was compared for both prodrugs. The phospholipids tested were Dimiristoyl Phosphatidylcholine (DMPC), Dimiristoyl Phosphatidylglycerol (DMPG) with a Tc of +23ºC and
Dipalmitoyl Phosphatidylcholine (DPPC) and Dipalmitoyl Phosphatidylglycerol (DPPG) with a Tc of
+41ºC. Liposomal formulations of C4 and C12 were prepared with neutral phospholipids (DMPC or DPPC) and lipid mixtures containing also negatively charged phospholipids (DMPG or DPPG). In addition, zeta potential, mean vesicle sizes and PdI were determined for all liposomal formulations.
In Table 2 are shown the physicochemical properties of extruded C12 and C4 liposomal formulations.
17 Table 2. Physicochemical characterization of C12 and C4 extruded liposomes. Influence of alkoxy chain length of prodrugs on incorporation parameters
Formulation nº Lipid Composition (Molar Ratio) Prodrug (Cx/Lip)i (nmol/µmol) (Cx/Lip)f (nmol/µmol) I.E. (%) Ø (µm) (PdI) Zeta Potential (mV) 1 DMPC C12 117 ± 24 107 ± 16 92 ± 6 0.19 (0.4 - 0.5) -4 ± 1 2 DMPC C4 107 ± 1 8 ± 2 5 ± 2 0.18 (<0.2) -3 ± 1 3 DPPC C12 135 ± 11 103 ± 18 96 ± 10 1.3 (0.4 - 0.5) -2 ± 1 4 DPPC C4 129 ± 10 5 ± 1 4 ± 1 1.2 (0.5 - 0.6) -2 ± 2 5 DMPC:DMPG (7:3) C12 93 ± 5 85 ± 5 91 ± 4 0.16 (<0.2) -41 ± 4 6 DMPC:DMPG (7:3) C4 153 ± 4 7 ± 1 5 ± 1 0.16 (<0.2) -42 ± 4 7 DPPC:DPPG (7:3) C12 87 ± 2 95 ± 2 110 ± 6 0.17 (<0.2) -35 ± 3 8 DPPC:DPPG (7:3) C4 141 ± 3 6 ± 1 5 ± 1 0.19 (0.2 - 0.3) -39 ± 3 Initial lipid concentration – 20 mM; Initial prodrug concentration – 2 mM; [(Cx/Lip)f – Final Cx to Lipid ratio; [(Cx/Lip)i – Inicial Cx to Lipid ratio; Incorporation Efficiency I.E. (%) - [(Cx/Lip)f / (Cx/Lip)i)] * 100; Ø – mean size of liposomes; PdI – Polydispersity index.. Values correspond to mean ± S.D. of at least three independent preparations