Crystal Chemistry and Struture of the
Orthorhombi (Fe,Mn)(Ta,Nb)
2 O
6
Family of Compounds
C.A. dos Santos
, L.I. Zawislak, E.J.Kinast, V. Antonietti,and J.B.M. daCunha
Institutode Fsia, UniversidadeFederaldoRioGrandedoSul,
CampusdoVale,C.P.15051, 91501-970PortoAlegre,RS,Brazil
Reeivedon19July,2001
Inthispaperwereviewtherystalhemistryandstruturedatafromtheliteratureandourresults
reportedinthelastdeadefornaturalandsynthetisamplesofthetantalite-olumbiteseries.
Order-disordertransitionsandoxidationreationsontheixiolite-olumbite-wodginitesystemarereported
for oneBrazilian sample. It is shownthat reports on thesesubjets are sometimes dubious and
inomplete. Inadditionto thereationatmosphere(air orvauum),order-disorderandoxidation
are strongly dependent on the physial state of the samples (powdered or rystal). Struture
andhemialompositionhavebeeninvestigatedinourlaboratoryusingX-raydiration(XRD),
eletronprobemiroanalysis(EPMA)andMossbauerspetrosopy(MS).
I Introdution
The(Fe,Mn)(Ta,Nb)
2 O
6
family ofompounds
enom-passestwodierentsolidsolutions. One,orthorhombi,
isnamedtantalite-olumbiteandtheotherone,
tetrag-onal,isnamedtapiolite-mossite[1-9℄.Besidestheir
eo-nomialimportane(theyarethemajorsoureof
tanta-lum)thesematerialspresentinterestingphysial
prop-erties whih havemotivated investigations reported in
thelasttwodeades[10-18℄.
For long time and even in some modern reports,
these solid solutions were onsidered ontinua. In
suhsenerybothsolidsolutionswouldhaveFeTa
2 O
6 ,
FeNb
2 O
6
, MnTa
2 O
6
and MnNb
2 O
6
as end-members.
Theseompoundswereproposedwithdierent
denom-inations for eah series.[19℄ The orthorhombi series
would be omprised of ferrotantalite (FeTa
2 O
6 ),
fer-roolumbite(FeNb
2 O
6
),manganotantalite(MnTa
2 O
6 )
and manganoolumbite (MnNb
2 O
6
). On the other
hand the tetragonal series would inlude
ferrotapio-lite (FeTa
2 O
6
), ferromossite (FeNb
2 O
6
),
manganota-piolite (MnTa
2 O
6
) and manganomossite (MnNb
2 O
6 ).
However, it is now well established that eah series
has limited solubility[19-26℄. For example, longtime
agoHuthinson[20℄hasquestionedtheexisteneofthe
mineral mossite (tetragonal(Fe,Mn)Nb
2 O
6
) and sine
then none suh natural sample hasbeen reported.[26℄
In spite of the fat that Aruga et al.[27℄ have
de-sribedasynthetiFeNb
2 O
6
samplewithadisordered
ferromossite struture, today the tetragonal series is
simply named tapiolite, whose Mn and Nb
solubil-ity are limited to less than 50 at.%. Although the
orthorhombi series ontinue to be named
tantalite-olumbite,[26℄ single-phase speies of tantalite
(or-thorhombi FeTa
2 O
6
) have not yet been reported as
naturalourreneorassyntheti one.
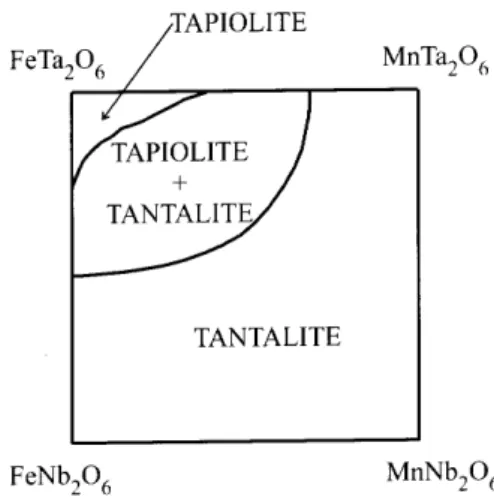
Figure1. Skethofthe ompositional eld for theAB
2 O
6
system.
Ashematiompositionaleldfornaturalsamples,
basedonareentpaperbyWiseandCerny,[26℄is
pre-sented in Fig. 1. Clark and Fejer[24℄ suggested that
the rystallization historyfor both syntheti and
nat-uralsamples. Thisisevidentfromresultsreported for
syntheti samples. Moreauand Tramasure[5℄reported
asolubilitylimitof15at.%Nbforthetapiolitesolid
so-lution, whiledosSantosandOliveira[12℄extendedthis
limitto45at. %Nb.
In addition to this question about the
solubil-ity limit, the orthorhombi series has been the
sub-jet of several investigations (for a review, see[8, 22,
23℄)onorder-disorderphenomenaonneting
tantalite-olumbiteandthemineralswodginite[1℄andixiolite.[2℄
From the strutural point of view it is sure that an
order-disorder transition an transform ixiolite into
tantalite-olumbiteorintowodginite. However,aswill
bedisussed below,it isnot yet knownifthe
alterna-tive transformations are governed by geohemial, or
simplybyrystallohemialfators.
Interest in these materials has reently inreased
motivated by its magneti properties. In tetragonal
FeTa
2 O
6
thesublattie formed bythe iron atoms has
the same symmetry asthe Ni sublattie in the
body-enteredtetragonalK
2 NiF
4
ompound,thewell-known
two-dimensional Heisenberg antiferromagnet. As
ex-peted,themagnetibehaviorofFeTa
2 O
6
isanalogous
tothatoftheK
2 NiF
4
. Forexample,inbothompounds
the suseptibility has a broad maximum and falls o
slowlyin theparamagneti region, aharateristi
as-soiated with planar magnets.[10, 13, 28, 29℄ Another
interesting point is that while FeTa
2 O
6
presents
uni-axial anisotropy on the basal plane, the isomorphous
ompoundCoTa
2 O
6
presentsaveryomplextwo-one
axishelialspinstruture[30℄with omponents onthe
basal planeaswellasonthe-diretion. Suhfeatures
suggestthat Fe
x Co
1 x Ta
2 O
6
ouldpresentompeting
anisotropies.
The systems with ompeting anisotropies lead to
a tetraritial phase transition, [31-33℄ whose
experi-mental onrmationhasbeenreportedforseveral
ma-terials, betweenthem K
2 Co
x Fe
1 x F
4
.[34, 35℄Keeping
in mindthesimilaritiesbetweenK
2 NiF
4
andFeTa
2 O
6
ompounds, it is expeted Fe
x Co
1 x Ta
2 O
6
to present
somekindofompetinganisotropiesbehavior. Indeed,
thesemixedoxideshavebeenreentlyprepared,[14,15℄
but the ompeting anisotropies behavior was not
ob-servedin thepreliminarymagnetimeasurements
per-formed.
In this paper wereview the rystal hemistry and
struture data from the literature and our results
re-ported in the last deade for natural and syntheti
samples of the tantalite-olumbite series. Struture
and hemial omposition have been investigated in
trosopy(MS) measurements.
II Crystal hemistry and
stru-ture
There is no doubt that the ompounds
(Fe,Mn)(Ta,Nb)
2 O
6
,with dierentFe/Mnand Ta/Nb
ratios,arethemorerepresentativeoftheAB
2 O
6 series.
However,thesematerialsanhaveminor substitutions
of Mg, Sn, W and Ti[8, 19, 22℄ and an also be
syn-thesized with A=Ni,[18, 36℄ Co,[14, 15, 37, 38℄ Cu,
[17,39-41℄Zn[41℄ andB=Sb.[30℄
0
10 20 30 40 50 60 70 80
0
10
20
30
40
50
CPRM
samples
7
9
Nb
2
O
5
%Ta
2
O
5
Figure 2. Nb2O5 vs Ta2O5 onentration for Brazilian
tantalite-olumbitesamples.
4
6
8
10 12 14 16 18
0
2
4
6
8
10
12
14
16
Natural
samples
%FeO
%MnO
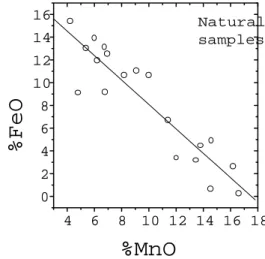
Figure3. FeOvsMnO onentration for naturalsamples,
takenfrom[11,22,41-44℄.
Fig. 2andFig. 3illustratetheorrelationbetween
NbandTaontentsandbetweenFeandMnfor,
respe-tively, twentypowder samplessupplied by the
Table1: Meanhemialomposition(wt.%)basedon6oxygens,from EPMAmeasurements.
Sample FeO MnO Ta
2 O
5 Nb
2 O
5 TiO
2 WO
3
TOTAL
Fe
0:1 Mn
0:9 Ta
1:8 Nb
0:2 O
6
1.67 12.87 78.69 5.61 0.21 | 99.05
Fe
0:1 Mn
0:8 Ta
1:7 Nb
0:3 O
6
1.54 12.77 77.62 6.38 0.39 0.29 98.99
Fe
0:6 Mn
0:4 Ta
1:3 Nb
0:7 O
6
8.87 6.85 59.12 21.95 1.59 | 98.38
authors.[11,22,41-44℄The samples labeled 7 and 9 in
Fig. 2,learlyoutofthelineartting,areSn-and
Ti-rihsamples(7: 1.9%SnO
2
/20%TiO
2
;9: 7.9%SnO
2
/ 23.3%TiO
2
). Therefore, these samples present low
Nb+Taonentration('64and42wt.%,respetively)
asompared to themean onentrationof the CPRM
olletion(' 73wt.%).
Yet,thestrongorrelationbetweenFeandMn
on-tents and between Ta and Nb ones an be niely
ap-preiated by sanning rystal samples with EPMA.
Fig. 4 shows the amount of Ta and Nb, in atoms
per formula unit (apfu), measured along a line 2
mmlongintheBrazilianFe
0:1 Mn
0:9 Ta
1:8 Nb
0:2 O
6
sam-ple (see Table 1 for the omplete hemial
ompo-sition). Ta-rih zones orrespond to Nb-poor ones
and vie versa, with Ta+Nb'2.0 apfu. A similar
re-sult for Fe and Mn is illustrated in Fig. 5 for the
BrazilianFe
0:6 Mn
0:4 Ta
1:3 Nb
0:7 O
6
sample. Inthis ase
Fe+Mn'1.0apfu.
0.0
0.5
1.0
1.5
2.0
0.0
0.4
0.8
1.2
1.6
2.0
Fe
0.1
Mn
09
Ta
1.8
Nb
0.2
O
6
Nb
Ta
Number of cations
Scan distance (x10
3
m
m)
Figure 4. Nb and Ta miroprobe sans for the Brazilian
Fe0:12Mn0:83Ti0:01Ta1:78Nb0:24O6sample.
0
100 200 300 400 500 600
0.40
0.45
0.50
0.55
0.60
Fe
Number of cations
Scan distance ( mm)
0.30
0.35
0.40
0.45
0.50
0.55
0.60
Fe
0.6
Mn
0.4
Ta
1.3
Nb
0.7
O
6
Mn
Figure 5. Mn and Fe miroprobe sans for the Brazilian
Fe0:57Mn0:38Ti0:10Ta1:26Nb0:68O6sample.
10
20
30
40
50
60
70
Fe(Ta
0.4
Nb
0.6
)
2
O
6
Intensity (arb.
2-Theta (deg.)
Figure 6. Representative part of the room-temperature
X-ray powder diration pattern for the syntheti
Fe(Ta
0:4 Nb
0:6 )
2 O
6
sample. Openirlesrepresentobserved
data. The solidline represents the alulated pattern
ob-tained withthe Rietveldrenement. The lower traeis a
plot of the residual spetrum, observed minus alulated
intensities.
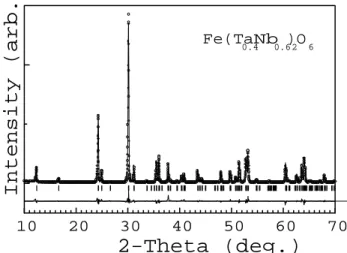
A typial XRD pattern is shown in Fig. 6 for
a syntheti ferroolumbite sample, with omposition
Fe(Ta
0:4 Nb
0:6 )
2 O
6
. It waspreparedby mixing
appro-priate amounts of powdered Fe, Fe
2 O
3 , Ta
2 O
5 and
Nb
2 O
5
. Pellets of the mixture were heated under a
nitrogenowat1320K.After24hitwasooledat15
K/h. New pellets were again submitted to the same
heat treatment for 24 h, followed by the same slow
ooling. Hereafterthis syntheti samplewill bealled
Fe(Nb,Ta)
2 O
6
. Thepatternwasindexed[45,46℄to the
spae groupPbn withrystallographiparametersas
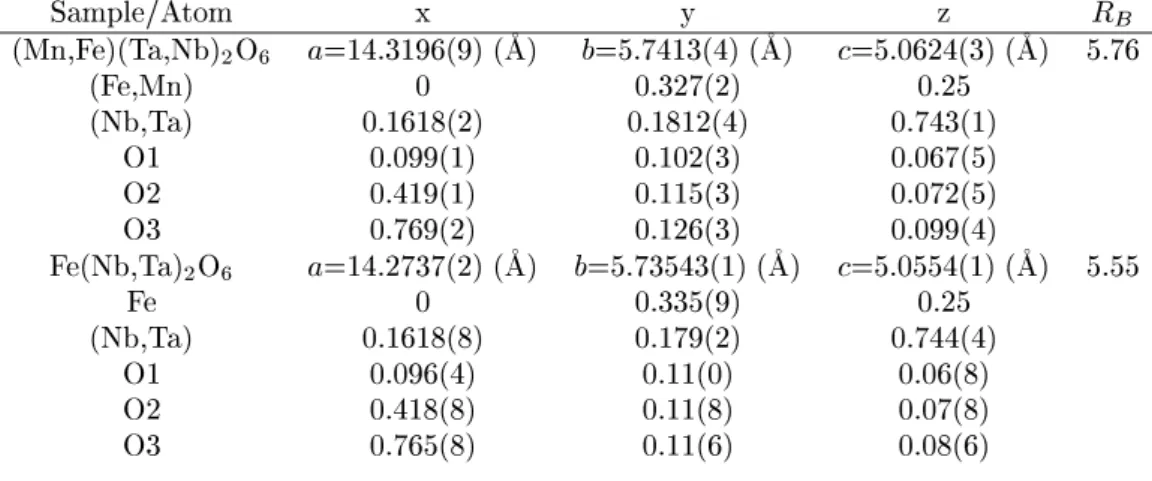
stru-Table 2: Atomiparameters for Mn 0:88 Fe 0:09 Ta 1:72 Nb 0:28 O 6
and Fe(Ta
0:4 Nb 0:6 ) 2 O 6
. Spae groupPbn. R
B = 100 P jI obs I al j= P I obs .
Sample/Atom x y z R
B (Mn,Fe)(Ta,Nb) 2 O 6 a=14.3196(9)(
A) b=5.7413(4)(
A) =5.0624(3)(
A) 5.76
(Fe,Mn) 0 0.327(2) 0.25
(Nb,Ta) 0.1618(2) 0.1812(4) 0.743(1)
O1 0.099(1) 0.102(3) 0.067(5)
O2 0.419(1) 0.115(3) 0.072(5)
O3 0.769(2) 0.126(3) 0.099(4)
Fe(Nb,Ta) 2 O 6 a=14.2737(2)(
A) b=5.73543(1)(
A) =5.0554(1)(
A) 5.55
Fe 0 0.335(9) 0.25
(Nb,Ta) 0.1618(8) 0.179(2) 0.744(4)
O1 0.096(4) 0.11(0) 0.06(8)
O2 0.418(8) 0.11(8) 0.07(8)
O3 0.765(8) 0.11(6) 0.08(6)
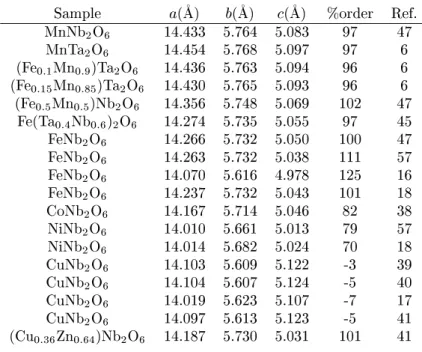
Table3: Cellunitparametersfornaturaltantalite-olumbitesamples.
Sample a( A) b( A) (
A) %order Ref.
(Fe 0:01 Mn 0:97 )(Ta 0:64 Nb 0:36 ) 2 O 6
14.413 5.760 5.084 101 22
(Fe 0:09 Mn 0:88 )(Ta 0:86 Nb 0:14 ) 2 O 6
14.320 5.741 5.062 101 45
(Fe 0:19 Mn 0:79 )(Ta 0:61 Nb 0:39 ) 2 O 6
14.300 5.741 5.124 38 42
(Fe 0:23 Mn 0:72 )(Ta 0:61 Nb 0:39 ) 2 O 6
14.288 5.753 5.167 -5 44
(Fe 0:38 Mn 0:62 )(Ta 0:09 Nb 0:91 ) 2 O 6
14.311 5.742 5.101 62 42
(Fe 0:46 Mn 0:53 )(Ta 0:28 Nb 0:72 ) 2 O 6
14.295 5.729 5.113 47 42
(Fe 0:54 Mn 0:42 )(Ta 0:15 Nb 0:85 ) 2 O 6
14.400 5.775 5.092 90 43
(Fe 0:63 Mn 0:41 )(Ta 0:20 Nb 0:78 ) 2 O 6
14.258 5.731 5.086 65 42
(Fe 0:64 Mn 0:34 )(Ta 0:24 Nb 0:73 ) 2 O 6
14.189 5.727 5.120 18 11
(Fe 0:68 Mn 0:28 )(Ta 0:25 Nb 0:75 ) 2 O 6
14.221 5.727 5.102 42 11
ture along the a and diretions are shown in Figs.
7(a)and7(b),respetively. SuhorthorhombiAB
2 O
6
strutureanbederivedfromahexagonallosed
pak-ing of oxygen ions. Within the b- plane half of the
oxygen otahedra are oupied by metal ions (A or
B) forming zigzag hains of edge-sharing MO
6
ota-hedra running alongthe -axis. Within the a-b plane
the layers are staked up in an order
A-B-B-A-B-B-A. The threeindependent oxygen atomsare all
three-oordinated: O1bridgingoneAand twoBatoms,O2
bridgingtwoAatomsandoneBatomandO3bridging
three Batoms.
Asexpeted forsolid solution,theunit ell
dimen-sions would obey the Vegard's law. This means, the
ationioniradiiontheAand Bsites. Asan
illustra-tionwepresentin Table3theellunit parametersfor
some natural tantalite-olumbite samples with
dier-entFe/Mn andTa/Nbratios. Thebandparameters
displaysattered data asa funtion of both A and B
sites. The a parameter data are verysattered when
plotted against the B site ontent. Only ation
sub-stitution in the A site obeys reasonably the Vegard's
law. There are several fators ontributing for suh a
behavior. Theonlyinvestigatedinsomeextentwillbe
disussedin setionIII andonernsto order-disorder
transition. Other interveningfatorsareminor
substi-tutionsofTi, Snand W,aswellastheunontrollable
Figure7.Coordinationpolyhedronhainsstakedalong(a)
a-axisand(b)-axisoftheolumbitestruture.
Forsyntheti samplespreparedwiththesame
pro-edure,irrespetiveofthespeialkindofroute,theV
e-gard'slawisfairlyillustratedinTable4andFig. 8for
theFe
x Mn
1 x Nb
2 O
6 andFe
x Mn
1 x Ta
2 O
6
series.[6,47℄
These results suggest that Nb!Ta substitution has
minoreetontheelldimensions,whileFe!Mn
sub-stitutionstronglyaetstheserystallographi
param-eters. As shown in Fig. 9for ANb
2 O
6
syntheti
sam-ples, with A=Mn, [47℄ Fe, [47℄ Co, [38℄ and Ni,[36℄
the ell unit dimensions vary linearly with the ioni
radii(Mn 2+
=0.80,Fe 2+
=0.76,Co 2+
=0.74,Ni 2+
=0.72)
in agreementwith theVegard's law. However, allthe
CuNb
2 O
6
samples reported [17,39-41℄ are learly out
of the Vegard's lawtting. Suh a behaviorould be
explainedbyastrongJahn-Tellereet.
Another way to summarize the rystallographial
data from the olumbite-tantalite series is by
plot-ting the against the a parameter. Indeed, the
a-plot[42, 43, 48, 49℄ has been onsidered the most
ap-propriate proedure beause it enables us to onnet
rystallographialdata to ation ordering features, as
14.3
14.4
14.5
FeMnNb
FeMnTa
Columbite
Synthetic samples
a (Angstron)
5.72
5.76
b (Angstron)
0
20
40
60
80
100
5.04
5.08
5.12
c (Angstron)
Fe concentration (mol %)
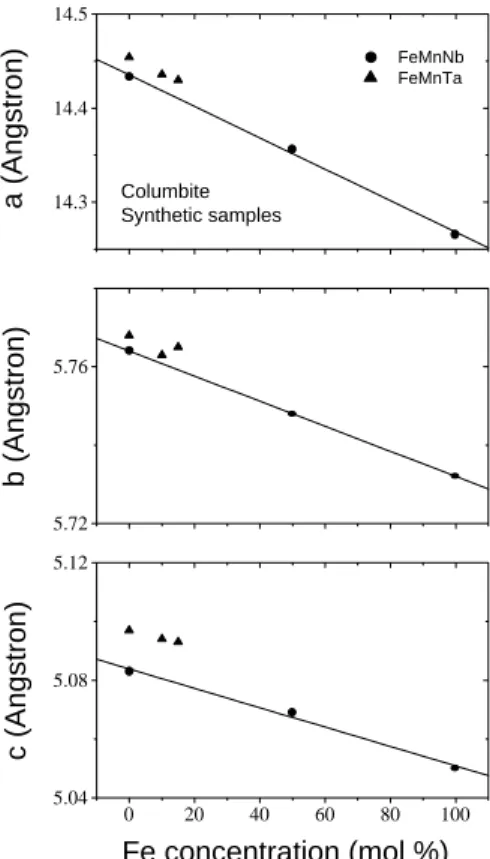
Figure8. CellunitparametersasafuntionofFe
onen-tration for synthetiolumbitesamples. Datafrom Table
4.
14.0
14.1
14.2
14.3
14.4
14.5
Columbite
Synthetic samples
a (Angstron)
5.64
5.68
5.72
5.76
b (Angstron)
25
26
27
28
5.00
5.04
5.08
5.12
c (Angstron)
A site atomic number
Table4: Cellunitparametersforsyntheti tantalite-olumbitesamples.
Sample a(
A) b(
A) (
A) %order Ref.
MnNb
2 O
6
14.433 5.764 5.083 97 47
MnTa
2 O
6
14.454 5.768 5.097 97 6
(Fe
0:1 Mn
0:9 )Ta
2 O
6
14.436 5.763 5.094 96 6
(Fe
0:15 Mn
0:85 )Ta
2 O
6
14.430 5.765 5.093 96 6
(Fe
0:5 Mn
0:5 )Nb
2 O
6
14.356 5.748 5.069 102 47
Fe(Ta
0:4 Nb
0:6 )
2 O
6
14.274 5.735 5.055 97 45
FeNb
2 O
6
14.266 5.732 5.050 100 47
FeNb
2 O
6
14.263 5.732 5.038 111 57
FeNb
2 O
6
14.070 5.616 4.978 125 16
FeNb
2 O
6
14.237 5.732 5.043 101 18
CoNb
2 O
6
14.167 5.714 5.046 82 38
NiNb
2 O
6
14.010 5.661 5.013 79 57
NiNb
2 O
6
14.014 5.682 5.024 70 18
CuNb
2 O
6
14.103 5.609 5.122 -3 39
CuNb
2 O
6
14.104 5.607 5.124 -5 40
CuNb
2 O
6
14.019 5.623 5.107 -7 17
CuNb
2 O
6
14.097 5.613 5.123 -5 41
(Cu
0:36 Zn
0:64 )Nb
2 O
6
14.187 5.730 5.031 101 41
III Cation ordering on the
ixiolite-olumbite-wodginite
system
Ixiolite, with general formula MO
2
(M=Mn, Sn, Fe,
Ta, Nb, Ti), is a olumbite substruture.[2℄ Due to
this relationship, disordered olumbite was formerly
alled pseudo-ixiolite. Todaythe inappropriatenessof
this denomination is well established,[22℄ but
imme-diately after its suggestion a vivid debate was
estab-lished ontheliteraturetodistinguishtrue-ixiolitefrom
pseudo-ixiolite. Nikel et al.[2 ℄ suggested that
ixio-lite reveal olovotantalite XRD pattern upon heating,
while Grieetal.[22 ℄ proposed thattheobtained
om-poundis wodginite. Infat,olovotantaliteisidentied
with orthorhombi wodginite.[2, 5, 50℄ On the other
hand,disorderedolumbiteyieldsanorderedolumbite
pattern[2,8,22℄uponheating.
Wodginite, with the general formula ABC
2 O
8 (A
= Mn, Fe 2+
; B = Sn, Ti, Fe 3+
, Ta; C = Ta, Nb),
rystallizesinthemonolinispaegroupC2/.[23,51℄
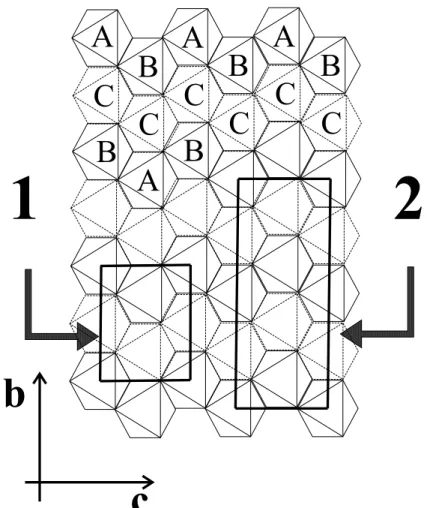
Idealized setions of these strutures, projeted along
the a-axis, are outlined in Fig. 10. TheA and B
o-tahedra in Fig. 10 refer to the wodginite struture.
ForolumbitebothotahedraorrespondtotheAsite,
oupied by Fe and Mn ations, while theC sites for
wodginiteorrespondtotheBsitesforolumbite,
ou-piedbyTaandNbations. Inthewodginitestruture,
onelayerofCationsfollowseahlayerofA+Bations.
Intheolumbitestruture, layersof A ationsat x=0
and 1/2, are separated by two layers of B ations at
x=1/6and2/6. Previousstrutureinvestigations[8,23℄
haveshownthat olumbiteand wodginite anbe
on-sideredasdierentsuperstruturesof the-PbO
2 -like
strutureofixiolite.
Order-disorder phenomena onneting
these minerals have been extensively
investi-gated.[8,11,22,23,25,47, 52-54℄ In the ontext of the
presentdisussion, anordered olumbite meansall Fe
andMnoupyingtheA site,whileallTaand Nb
o-upy theBsite. It doesnotmeansorder betweenMn
andFeonAsites,norbetweenTaandNbonBsites;a
similarapproahis appliedtothewodginitestruture.
CationorderinginMO
2
strutureispossibleiftheunit
ell size is inreased.[48℄ To satisfy symmetry
restri-tionsonthisproess,twoalternativesarepossible(see
Fig. 11). IntherstonetheMO
2
ellistripled, given
theAB
2 O
6
struture. Intheother alternativetheell
Figure 10. Idealized setion of the -PbO2 strutureprojetedalong the a-axis. A, B and C referto the ations onthe
general formula, ABC2O8,for wodginite. Oupiedotaedra inthe upperlevel are shown infull lines; thoseoupied in
thenextleveldownareshowninbrokenlines. Theolumbiteandixioliteells arerepresentedbytheretangle (1), while
retangle (2)represents thewodginite ell. Ixiolite ellis onedoublelayerdeep,wodginite istwodoublelayersdeep,and
olumbiteisthreedoublelayersdeep.
Figure11. Orientationandvolumerelationshipsamong
ix-Therefore, fully disordered AB
2 O
6
and ABC
2 O
8
ompounds an be misidentied as MO
2
ompounds.
Thisfat hasproduedonsiderableexperimental
mis-takes and misoneptions, as reported during the
last three deades. For instane, heating
experi-ments have been used to distinguish these
materi-als,withthegenerallyaeptedassumption that
heat-ing the mineral ixiolite reveals awodginite XRD
pat-tern, whereasheating the disordered olumbite yields
an ordered olumbite pattern haraterized by its
superstruture reetions.[2℄ However, alulation of
a theoretial powder diration pattern of fully
or-dered manganoolumbite, MnNb
2 O
6
, yields only low
intensities for the superstruture reetions, namely
I
200 /I
311
=0.096andI
110 /I
311
=0.030.[11℄Thus,itis
dif-ulttodistinguishbetweenMO
2
andpartiallyordered
AB
2 O
6
by means of their XRD patterns, even when
donebyomplexandtime-onsumingproeduresbased
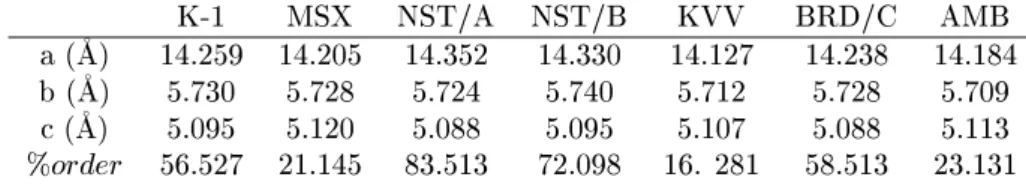
Table5: Cellunit parametersforas-olletedixiolitesamples. TakenfromCernyetal.[50 ℄
K-1 MSX NST/A NST/B KVV BRD/C AMB
a(
A) 14.259 14.205 14.352 14.330 14.127 14.238 14.184
b(
A) 5.730 5.728 5.724 5.740 5.712 5.728 5.709
(
A) 5.095 5.120 5.088 5.095 5.107 5.088 5.113
%order 56.527 21.145 83.513 72.098 16. 281 58.513 23.131
Table6: Cellunit parametersforheated(1000 Æ
C,16hoursin air)ixiolitesamples. Takenfrom Cernyetal.[50℄
K-1 MSX NST/A NST/B KVV BRD/C AMB
a(
A) 14.284 14.261 14.378 14.370 14.233 14.269 14.255
b (
A) 5.733 5.731 5.742 5.748 5.720 5.722 5.713
(
A) 5.072 5.072 5.081 5.074 5.056 5.060 5.065
%order 83.67 78.62 95.80 100.64 87.548 91.676 83.898
14.1
14.2
14.3
14.4
5.06
5.07
5.08
5.09
5.10
5.11
5.12
96
83
ordered
disordered
101
88
56
21
16
c (Angstron)
a (Angstron)
Figure 12. -a plot for natural olumbite samples. The
numbernearthesymbolsaretherespetiveEOP.Triangles
representtheorderedones. DatafromTables5and6.
Other riteria havebeen tentatively establishedto
estimate the degree of ation order. In his PhD
the-sis,Erithasderivedthefollowingequationforthe
or-thorhombi(Fe,Mn)(Ta,Nb)
2 O
6
system:[54℄
perentorder=1727 941:6( 0:2329a); (1)
where a and arethe lattieparameters. TheErit's
orderparameter(EOP)hasbeenobtainedfromthe
so-alled a- plot,mainly for samplessubmittedto
heat-ing experiments. Asan illustrationletus examinethe
data summarizedin Tables5and6,takenfromCerny
et al.[50 ℄ The as-olleted samples (Table 5) were
in-dexedtotheixiolitestruturewithaverageellunit
pa-rametera'4.747
A. However,foromparisonpurposes
with the ordered olumbite phase this parameter was
tripled. Fig. 12 displaysthea- plotforTables5and
EOP. As an be seen, the disordered samples
(trian-gles)presenthigherandloweraparameters,andlow
EOP. Nevertheless someixiolite samples display EOP
(e.g. 83%) similar to those alulated for olumbite
(e.g. 96%). Therefore, even some learly disordered
samples(ixiolite)anbemisidentied withanordered
one(olumbite).
Thea- plotshown in Fig. 13summarizes the
ob-tainedresultsforsynthetisamples(seeTable4). The
ferroolumbite and manganoolumbite samples follow
thehighEOPline(EOP'96%-125%),aswasobserved
forhighlyorderednaturalsamples. TheCuNb
2 O
6
sam-ples, prepared at dierent laboratories, [17,39-41℄but
using similar routes, present anomalous EOP values
(between -3% and -7%). Aordingly, in the a- plot
theyfollow thelowEOP line. However, itis
interest-ingtonotethatthesampleCu
0:36 Zn
0:64 Nb
2 O
6
presents
EOP=101%,typialofahighlyorderedolumbite. The
available data do not enable us to eluidate this
ap-parent ontradition. It is not possible to onlude
if CuNb
2 O
6
is a disordered sample, or if it is simply
a quite distorted one, as previously suggested. It is
interesting to point out that the parameters for all
CuNb
2 O
6
samplesarehigherthan5.107
Aandthatfor
Cu
0:36 Zn
0:64 Nb
2 O
6
thisparameterbelongstotherange
oftheorderedsamples,i.e. between4.978and5.097
A.
Theelongationoftheparameterisnotorrelatedwith
theioni radii of theonerned ations(r
Cu
2+ =0:72
A; r
Zn
2+ = 0:82
14.0 14.1 14.2 14.3 14.4 14.5
4.95
5.00
5.05
5.10
5.15
96
125
Mn
Fe
Co
Ni
Cu
Columbite
Synthetic samples
c (Angstron)
a (Angstron)
Figure 13. -a plot for synthetiolumbite samples. The
numbernearthesymbolsaretherespetiveEOP.Datafrom
Table4.
Anotherwayto evaluateation order in
tantalite-olumbite speies an be done by using MS,
be-ause it permits to easily dierentiate between
ixi-olite and tantalite-olumbite (or wodginite), as long
as hyperne interations are very sensitive to hange
of the near-nearest environment. To the best of
our knowledge Wenger et al.[11 ℄ were the rst to
report MS results onerning ation distribution in
partially ordered olumbite samples. Afterward
we published[45, 52℄ MS studies of ation
order-ing on anatural manganoolumbite with omposition
Mn
0:88 Fe
0:09 (Ta
0:86 Nb
0:14 )
2 O
6
,inthefollowingnamed
simply MnFeTa
2 O
6
. A similar study has been
pub-lished for olumbite-tantalite samples from San Luis
pegmatite(Argentina). Other authors [45,52,55℄ have
proposedthefollowingparametertoquantifytheation
orderperentage
x(%)= 50+1:515A
A
; (2)
where A
A
is the relative Mossbauer spetral area
at-tributedto theAsiteoftheAB
2 O
6
struture.
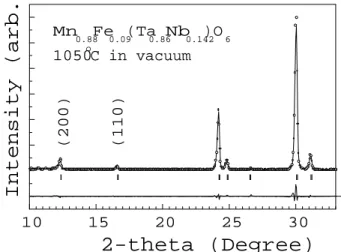
Fig. 14 displays part of the XRD pattern for
MnFeTa
2 O
6
heated in vauum at 1050 Æ
C. This
pat-tern is quite similar to that for the as-olleted
sam-ple (Table 2), but shows a small inrease in the
rel-ative intensity of the superstruture reetions. That
is,I
200 /I
311
=0.08and I
110 /I
311
=0.03,whileforthe
as-olleted sample, I
200 /I
311
=0.05 and I
110 /I
311 =0.02.
Thus, it appears that it is not appropriate to use
su-perstruture reetions to evaluate ation ordering in
this ase. TheEOP, whose valuesfor theas-olleted
andorderedsampleare,respetively,68%and93%[45℄
10
15
20
25
30
(110)
(200)
Mn
0.88
Fe
0.09
(Ta
0.86
Nb
0.14
)
2
O
6
1050
o
C in vacuum
Intensity (arb.
2-theta (Degree)
Figure14. Representativepartoftheroom-temperature
X-raypowderdirationpatternforthenatural
Mn0:88Fe0:09Ta1:72Nb0:28O6 sample heated-treated in
va-uum. Openirles,solidlineandthelowertraeasdened
inFig. 6.
-1
0
1
2
3
1 %
(a)
Absorption (%)
Velocity (mm/s)
1 %
(b)
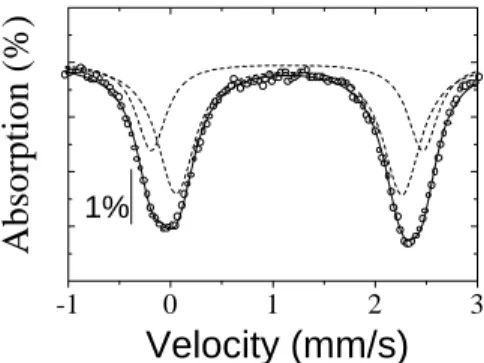
Figure 15. Mossbauer spetrataken at300 Kfor: (a)
as-olletednaturalsample;(b)powderednaturalsample
heat-treated invauum. Open irles represent observed data.
Solidlinesrepresentthealulatedspetrabasedona
least-squares proedure. Smashed linesrepresentthe alulated
subspetra.
The MS spetra taken at room temperature for
these samples are shown in Fig. 15. The
spe-trum for the as-olleted sample, displayed in Fig.
15(a), was tted to two doublets (see Table 7)
with 57
Fe hyperne parameters harateristi of Fe 2+
high spin in otahedral oordination. One doublet
should be attributed to Fe 2+
in FeO
6
otahedra,
while the other one should be attributed to Fe 2+
in TaO
6
otahedra. Our results are quite similar
struture renement these authors suggestedthat the
FeO
6
otahedraaremoredistortedthantheNbO
6 ones,
andattributedtheinnerdoublettoFe 2+
intheformer
otahedra,while theouteronewas attributed to Fe 2+
in NbO
6
otahedra. This same site assignment was
adopted byAugsburgeretal.[55℄forsimilarsamples.
As it is known, is proportional to the eletri
eld gradient (EFG) tensor, whih has ontributions
from theexternal ligandharges (lattie ontribution)
and from the valene eletrons(valene ontribution).
Ontheotherhand,thevaleneontributionanbe
di-videdintotwoontributions: onefromtherystaleld
(CF ontribution) and other from the metal orbitals
(MO ontribution). For Fe 2+
high spin the CF
on-tribution dominatesthe valene ontribution whih is
very temperature dependent. For several materials it
anbedemonstrated[56℄thatastheotahedral
distor-tion inreases,the quadrupole splitting dereases. On
theontrary,forFe 3+
mineralsthevaleneontribution
is almost nulland is fully due to thelattie
ontri-bution. In this ase inreases with the otahedral
distortion andislesstemperaturedependent.
-1
0
1
2
3
1%
Velocity (mm/s)
Absorption (%)
Figure 16. Mossbauer spetrum taken at 80Kfor the
as-olletednaturalsample. Openirlesandsmashedlinesas
denedinFig. 15.
Nevertheless, site assignments following the above
reasoningforFe 2+
highspinis notsostraightforward.
The above assignment is ertainly orret, sine the
spetrum for the heated-in-vauum sample was tted
toonlyonedoubletwithhyperneparameters(seeT
a-ble 7) almost the same as those used to t the inner
quadrupolar doublet. The disappearing of the outer
quadrupolar doubletis alear indiationof ation
or-deringandenablesustoattributetheinner doubletto
Fe 2+
inFeO
6
otahedra. However,toorrelate
otahe-dradistortionandvalues,lowtemperatureMS
mea-surementsshould beperformed. Siteswith low
distor-tionsshow valuesmorestronglydependenton
tem-perature than those ones with high distortions. Figs.
Kthe inner quadrupole splitting inreases 42% (from
1.55mm/sto2.21mm/s),whiletheouteroneinreases
18% (from 2.26 mm/s to 2.66 mm/s). Suh a
ther-mal evolutionindiates that the outer doublet should
beattributedtothemoredistortedotahedra,inlear
ontraditionwiththepreviousndings.[11,55℄
IV Cation orderingversus
oxida-tion of olumbite
Heating experiments are, by far, the main soure of
dataon thestudy oforder-disorderphenomena in the
ixiolite-olumbite-wodginite system. Tointrodue the
present disussion, it is interesting to highlight some
statements found in the literature: `When ixiolite is
heatedinair,severalhangesourinthex-ray
dira-tionpattern.'[2℄`Disorderedmembersofthe
olumbite-tantalite group, (...), develop an ordered struture on
heating at about 1000 Æ
C (...).'[19℄ ` Pseudo-ixiolite,
(...), is a omplex Nb-Ta-oxide similar to ixiolite but
withthedierenethatitsstruturemaybeinvertedby
heating(probablybyorderingoftheations)togivean
XRDpatternsimilartoolumbite.'[49℄`Heatingof
sam-plesinexessof950 Æ
Chasbeenshowntoindueation
order(...).'[54℄Suhdesriptionsofheatingexperiments
arelearlyinomplete. Someofthemdonotspeifythe
atmosphere,andobviouslyheatinginair,inareduing
atmosphere,orinvauumwillertainlyprodue
dier-ent reations. Also, heating a powdered or a rystal
sample in air will produe dierent oxidation eets,
beauseforthelatter onetheareaavailable for
oxida-tionis smaller. Therefore, these kindof experimental
onditionsneedtobeinformed. Reentlyitwasstated
thatheatingoarserystalfragmentsinairat1000 Æ
C
for16h,induesationorderinolumbiteandresultsin
nosigniantoxidationof bulkFe 2+
,asmonitoredby
unit-ellparameters.[54℄ Wehaveinvestigatedthese
is-suesperformingheatingexperimentsonthreedierent
samples:[45, 52, 53℄ (i) rystal fragment of the
natu-ralmanganoolumbite,Mn
0:88 Fe
0:09 (Ta
0:86 Nb
0:14 )
2 O
6 ;
(ii)powderedspeimenofthemanganoolumbite;(iii)
powder syntheti ferroolumbite, Fe(Ta
0:4 Nb
0:6 )
2 O
6 .
Sample(i)washeatedinair(theobtainedsamplewas
labeledNCHair). Part ofthesample(ii)wasusedfor
the heating experiment in air (label PNH air), while
the other part (label PNH va) was pelleted and
en-apsulatedinquartzampoulesundervauum(p'10 5
Pa). Sample(iii)washeatedinair(labelPSHair). All
thesampleswereheatedat1320Kfor48hand
subse-quently slowly ooledat 15 K/h. This oolingrate is
adequateforationordering,e.g.,allFe+Mnin siteA
Table7: Hyperneparametersforallmeasurementsreportedinthepresentwork. As-oll,PNHva,PNHair,NCH
air,as-prepandPSHairstandfor,respetively: untreatednaturalsample;powderednaturalsampleheat-treatedin
vauum;naturalsampleheat-treatedinair;rystalfragmentofthenaturalsampleheat-treatedinair;as-prepared
synthetisampleandpowderedsynthetisampleheat-treatedinair. isthequadrupolesplittingattheironsites;
Æistheisomershiftrelativeto -Fe; 0:01isthelinewidthathalf-height;Ais thesite oupany,givenbythe
relativespetralarea. Unertaintiesonthelast guresarereportedinbrakets.
Sample Temp Æ A Site
(K) (mm/s) (mm/s) (mm/s) (%) assignment
As-oll 300 1.55(5) 1.13(3) 0.40 71(4) FeO
6
2.26(7) 1.09(3) 0.47 29(1) TaO
6
80 2.21(7) 1.27(4) 0.44 66(3) FeO
6
2.66(8) 1.25(4) 0.35 34(2) TaO
6
PNHva. 300 1.58(5) 1.14(3) 0.32 100 FeO
6
80 2.29(7) 1.27(4) 0.34 100 FeO
6
PNHair 300 0.52(2) 0.40(1) 0.31 78(4) FeO
6
0.34(1) 0.39(1) 0.33 22(1) TaO
6
80 0.59(2) 0.52(2) 0.30 52(3) FeO
6
0.40(1) 0.49(1) 0.28 48(2) TaO
6
NCHair 300 0.55(2) 0.44(1) 0.44 29(1) FeO
6
0.32(1) 0.43(1) 0.41 25(1) TaO
6
1.51(4) 1.13(3) 0.47 41(2) FeO
6
2.26(7) 1.14(3) 0.43 5(2) TaO
6
80 0.61(2) 0.54(2) 0.33 28(1) FeO
6
0.43(1) 0.52(2) 0.30 24(1) TaO
6
2.17(6) 1.26(4) 0.38 26(1) FeO
6
2.59(8) 1.27(4) 0.37 22(1) TaO
6
As-prep 300 1.78(5) 1.15(3) 0.32 100 FeO
6
80 2.51(8) 1.27(4) 0.32 100 FeO
6
PSHair 300 0.37(1) 0.40(1) 0.30 100 FeO
6
80 0.39(1) 0.51(2) 0.30 100 FeO
6
RepresentativepartsoftheXRDpatternsforallthe
samplesareshownin Fig. 17. Asummaryofthe
rys-tallographi parameters is displayed in Table 8. The
PNHva samplewasdisussedabove. TheXRD
pat-ternforthePNHairsamplewasttedtothreephases.
The majorone was indexed to thespae group C2/,
with lattie parameters (see Table 8) similar to those
reportedforwodginitefromRwanda.[4℄Additionallow
intensityreetionsanbeattributedtomonolini
ix-iolite with spae group P2/ and lattie parameters
quite lose to those reported by Roth and Waring,[3℄
and to monolini (Nb,Ta)
2 O
5
with spae group P2
andlattieparameterssimilarto thosereportedinthe
JCPDS le37-1468. Thepattern attributedto
wodgi-nite(MnFeTa
2 O
8
)iswellharaterizedbytheexistene
ofstrongpeaksatd'2:95
A(2'30:45 Æ
)andd'2:98
A(2'29:98 Æ
)orrespondingto(221)and(221)
ree-tions,respetively.
Therefore, heating the present powdered
transformsitintoamonoliniompoundquitesimilar
to MnFeTa
2 O
8
, with minor reetions from a
om-pound similar to ixiolite and the oxide (Ta,Nb)
2 O
5 .
The present result onrms the data of Moreau and
Tramasure.[5℄Theseauthorshavesynthesizeda
wodgi-nite from thereationMn
2 O
3 +Ta
2 O
5
at1040 Æ
C,for
95 h in air, suggesting that Mn plays an important
role onthetantalite! wodginitetransformation. We
havealsoreproduedearlyresultsreportedbyGouder
deBeauregardetal.,[7℄demonstratingthatupon
heat-ing in air,(Mn,Fe)Ta
2 O
6
showsa XRD pattern quite
similar to that of MnFeTa
2 O
8
. Similar results have
also been obtained by Turnok,[6℄ who showed that
whensynthesizedin air,MnFeTa
2 O
8
anbeformedin
asmallompositionrange,justwhilethereissuÆient
MntolltheAsiteoftheABC
2 O
8
struture,butdoes
notformintheabseneofFe 3+
. Thatistosay,partof
the Fe atoms should pertains to the Bsite, while the
Table8: Crystallographiparametersobtainedin thepresentwork. Thesamplelabelsarethesameasdened in
Table7. Numbersin braketsdesignate1onthelastdeimalgiven. R
B
asdened inTable2.
Sample S.g. a(
A) b (
A) (
A) (
Æ
) R
B
PNHva Pbn 14.3196(9) 5.7413(4) 5.0624(3) 90 5.76
PNHair C2/ 8.779(4) 12.145(2) 5.089(1) 89.37 4.01
P2/ 4.682(9) 5.647(28) 5.030(18) 90.14 2.13
P2 20.429(2) 3.825(1) 19.461(3) 115.49 3.86
NCH air Pbn 14.3487(9) 5.7459(5) 5.0779(3) 90 1.22
C2/ 9.0856(16) 12.0672(9) 5.0470(8) 88.29 2.34
P2/ 4.7238(9) 5.6205(9) 5.0143(20) 90.81 1.24
P2 20.4834(8) 3.8230(2) 19.4597(9) 115.38 1.57
As-prep Pbn 14.2737(2) 5.7354(1) 5.0554(1) 90 5.55
PSHair P2/ 4.6493(8) 5.6221(7) 5.0089(7) 90.20 1.76
P2 20.4022(27) 3.8322(3) 19.4007(16) 115.23 2.57
0.0
0.4
0.8
(200)
Pbcn
(131)
Pbcn
PNH vac
Normalized intensity
0.0
0.4
0.8
(040)
C2c
(221)
C2c
(-221)
C2c
PNH air
0.0
0.4
0.8
NCH air
28
29
30
31
0.0
0.4
0.8
(121)
P2c
(200)
P2
(180)
P2
PSH air
2-Theta (Degr
Figure17. RepresentativepartsoftheX-raypowder
dira-tionpatternsfor allthe heated-treatedsamples: powdered
natural heated in vauum (PNH va), powdered natural
heatedinair(PNHair),fragmentsofnaturalrystalheated
inair(NCHair)andpowderedsynthetiheatedinair(PSH
air). Open irles and solid lines as dened in Figure 6.
Tantalite,wodginite,ixioliteand(Nb,Ta)
2 O
5
reetionsare
indiated,respetively,byPbn,C2,P2andP2.
It is interestingto pointout that although the
re-sults from Gouder de Beauregard et al.[7 ℄ are more
theGraham and Thornber[8℄ eort to re-examine the
(Mn,Fe)Ta
2 O
6
!MnFeTa
2 O
8
transformation. Yet
re-ent papers[11, 19, 25, 48, 51, 54℄ have asually
dis-missed this subjet, although theyhave disussed the
related subjet (Mn,Fe,Ta)O
2
! MnFeTa
2 O
8
transi-tion.
Dierently, for the NCH air the reetions
at-tributedtoMnFeTa
2 O
8
arelearlylessintenseas
om-paredto those observed forPNH air(Fig. 17). Note,
forinstane,thestrongdereaseof(221)reetion. F
ol-lowingtherenementproedureitanbeseenthatthe
eet ofheatingarystal sampleisto produea
mix-ture of four phases; that one in large amount (label
Pbn)is theordered(Mn,Fe)Ta
2 O
6
. Minorreetions
were indexed to MnFeTa
2 O
8
, (Mn,Fe)(Ta,Nb)O
4 and
(Ta,Nb)
2 O
5
. Theformer arrives from ation ordering
inthebulkportionofthesample,whiletheminor
on-tributionresultfrom thenear-surfaeoxidation.
The syntheti Fe(Nb,Ta)
2 O
6
was used as a rst
hekfortheroleplayedbyMn. TheXRD patternfor
PSHairwasindexedto amixtureof twophases. The
majoroneismonoliniixiolite,withspaegroupP2/
andlattieparameterssimilarto thosereportedabove
forthe PNHair sample. The phase withweak
ree-tions (label P2) is the monolini oxide (Nb,Ta)
2 O
5 ,
also reported above for PNH air sample. Therefore,
supposingationonservationduringtheoxidation
pro-essandtakingintoaounttheXRDresults,the
om-pound Fe(Nb
0:6 Ta
0:4 )O
4
,hereafterFe(Nb,Ta)O
4 , with
struturesimilartoixiolite isthemain produt
result-ingfrom the oxidationofthe syntheti ferroolumbite
Fe(Nb,Ta)
2 O
6 .
To improve our interpretation we have performed
MS measurements of the samples analyzed by XRD.
Fig. 18 showsthespetratakenat 300KforPNHair
ontribution forthePNHairspetrum, whih was
t-ted to two doublets with = 0.52 mm/s and =
0.34mm/s (seeTable7), attributed to highspinFe 3+
in otahedral oordination. As disussedabove, there
aretwodierent sitesfor Fe 3+
in thewodginite
stru-ture: one of them is the B site and the other is the
C site. Bearingin mind the great similarity between
theouter doubletandthat reported( =0.54mm/s,
Æ=0.39mm/s)forasyntheti Fe 3+
-rih wodginite,[51℄
we have attributed the larger quadrupole splitting to
Fe 3+
at FeO
6
otahedra(site B) andthe smallestone
to Fe 3+
at TaO
6
otahedra(site C). As disussed
be-low, the smallest quadrupole is also quite similar to
that attributed to ixiolite-like Fe(Nb,Ta)O
4
, so that
it is reasonableto suppose amixture of ontributions
fromwodginitesiteCandFe(Nb,Ta)O
4
. Thisis
onsis-tent with the XRD result, suggestingthe tantalite !
wodginite transformation, i.e., (Fe 2+
,Mn)(Ta,Nb)
2 O
6
!MnFe 3+
(Ta,Nb)
2 O
8 .
-1
0
1
2
3
1%
NCH air
Velocity (mm/s)
Absorption (%)
1%
PNH air
Figure18. Mossbauer spetratakenat 300Kfor PNHair
andNCHair(labels denedinFig. 17). Openirlesand
smashedlinesasdenedinFig. 15.
TheXRDresultshaveshownthattheeetof
heat-inginairarystalfragmentofthe(Mn,Fe)Ta
2 O
6
sam-ple is to produea mixture of ordered (Mn,Fe)Ta
2 O
6
and MnFeTa
2 O
8
, with minor reetions indexed to
(Fe,Mn)(Ta,Nb)O
4
and (Nb,Ta)
2 O
5
. Consequently
omplexMossbauerspetraareexpetedfor thiskind
trum for the NCH air sample learly ontains
ontri-bution from Fe 2+
and Fe 3+
ions. This spetrum was
ttedtofourdoublets: =0.55mm/s,=0.32mm/s,
=1.51mm/s and =2.26mm/s. The site
assign-mentfollowedthatusedbeforefortheas-olletedand
PNH air samples. Thus, =0.55 mm/s and =1.51
mm/s are attributed, respetively, to Fe 3+
and Fe 2+
at FeO
6
otahedra, while =0.32 mm/s and =2.26
mm/sareattributed,respetively,toFe 3+
andFe 2+
at
TaO
6
otahedra.
-3 -2 -1 0
1
2
3
1%
(a)
Velocity (mm/s)
Absorption (%)
-1
0
1
1%
(b)
Figure 19. Mossbauer spetrataken at300 Kfor: (a)
as-prepared Fe(Ta
0:4 Nb
0:6 )
2 O
6
sample; (b) syntheti sample
heatedinair. Openirlesandsmashedlinesasdenedin
Fig. 15.
Tovalidate someof thehyperneparametersused
to t the natural samples, we have submitted the
syntheti Fe(Nb
0:6 Ta
0:4 )
2 O
6
sample to similar
stud-ies. TheMossbauerspetrumatroomtemperaturefor
the as-prepared sample is shown in Fig. 19(a). The
existene of only one narrow doublet is indiative of
high degree ation ordering. It was tted (see Table
7) to =1.78 mm/s and Æ=1.15mm/s. These
hyper-ne parameters are lose to those early reported for
FeNb
2 O
6
.[16,57℄Wehavealsomeasuredthissampleat
85K.[45,53℄Therelativeinreasingofthequadrupole
splittingfrom300to85Kisremarkablysimilartothat
reported forFeNb
2 O
6
.[16℄ Thespetrummeasured at
300Kforthesynthetisampleheatedin air,displayed
in Fig. 19(b), was tted to a doublet attributed to
Fe 3+
similar resultsforasynthetiFeNbO
4
sample.[58℄
Now,itisinterestingtoevaluatetheonsisteneof
our Mossbauer measurements. Forthe Mn-rih
natu-ralsample(Mn,Fe)Ta
2 O
6
submittedtoationordering
we have observed just one quadrupole doublet, with
=1.58 mm/s. This quadrupole splitting is smaller
than that reported for FeNb
2 O
6
(=1.70 mm/s).[16℄
These distint values for should be due to distint
Mn/FeandTa/Nbratioonentrations. This
hypothe-sisanbeanalyzedbyonsideringtheresultsfromthe
syntheti Fe(Nb
0:6 Ta
0:4 )
2 O
6
, for whih we have
mea-sured =1.78 mm/s. By omparing these hyperne
parametersit isreasonabletoasribeasmalleet to
the homopolar substitution Ta $ Nb. The situation
is quite dierent when FeNb
2 O
6
and (Mn,Fe)Ta
2 O
6
are ompared. In thisasethe measurementsindiate
that thehomopolarsubstitutionMn$Feismore
ee-tivein hangingthe hyperneparameters(1.70mm/s
versus 1.58 mm/s) than the Ta$Nb one(1.70 mm/s
versus 1.78 mm/s). Considering the interdependene
between quadrupole splitting and distortions of the
metal-oxygen otahedra, and taking into aount the
ioni radii, these results seem to be onsistent, sine
(R
Mn 2+=R
Fe
2+) ' 1.08, while (R
Nb 5+=R
Ta
5+) ' 1.01.
Therefore, onrmingpreviousstruturerenementin
similar samples[11℄the eet ofthehomopolar
substi-tution Mn$Fe onthe distortions ofthe metal-oxygen
oathedra (MO
6
) is strongest than that produed by
theTa$Nbone.
Conerning the PSH air sample a similar senery
an be obtained. For this sample we have obtained
=0.37mm/s,adoubletattributedtothe
orthorhom-bi ompound Fe(Nb,Ta)O
4
. For thetetragonal
om-pound FeTaO
4
, with the rutile struture, it was
re-ported =0.53 mm/s.[59℄ As the homopolar
substi-tution Ta$Nb presents a minor eet on the
hyper-neparametersoftheseompounds(seetheresultsfor
FeNbO
4
[58℄),thereportedresultsstronglysuggestthat
the hyperne parameters are more dependent on the
rystalstruture. Thus,ifforolumbite(orthorhombi
FeNb
2 O
6
) ' 1.70 mm/s, and for tapiolite
(tetrag-onal FeTa
2 O
6
) '3.0 mm/s[10, 28℄, it appears
rea-sonable that forixiolite-likeFe(Nb,Ta)O
4
shouldbe
smaller than for rutile FeTaO
4
. This beomes
onsis-tent ifwe take into aount the interrelation between
rystal strutures. That is to say, the substrutures
(ixiolite andrutile)preservetheinterrelationobserved
The orthorhombi (Fe,Mn)(Ta,Nb)
2 O
6
series presents
ontinuoussolidsolutionsontheFeNb
2 O
6
-MnNb
2 O
6
andontheMnNb
2 O
6
- MnTa
2 O
6
boundaries,buthas
solubilitylimitforFeTa
2 O
6
lessthan50%. Inanyase
theVegard's law isobeyed, mainly for syntheti
sam-ples. Order-disorderphenomenahavebeenextensively
investigatedin thissystem,andanillustrative
system-ati have been obtained in terms of the - and a-ell
parameters. The more disordered samples are those
withthehigher/aratios. Contrary,themoreordered
onesarethosewiththesmallest/aratios.
Heating in air a partially ordered tantalite yields
dierentresultsdepending onthe form ofthe sample.
Forapowdersample,thetantalite!wodginite
trans-formationhasbeenobserved,inadditiontominor
on-tribution from ixiolite-likeFeTaO
4
and Ta
2 O
5
. For a
rystal fragment, the heat treatment in air produed
a mixture of four phases: that one in large amount
isthe orderedtantalite, whileminor ontributions are
attributed towodginite,FeTaO
4
andTa
2 O
5
. The
for-merarrivesfrom ationorderinginthebulkportionof
thesample, while the minor ontributions resultfrom
the near-surfae oxidation. The Mn ontent as well
asthe oxidant atmosphere appears to play an
impor-tantroleonthetantalite!wodginitetransformation.
Thesameheattreatmentappliedtoapowdersyntheti
ferroolumbite indues a dierent reation: the
sam-pleistransformedinto FeNbO
4
withminor amountof
Nb
2 O
5 .
Aknowledgments
Wewould liketo thankDr. M.Adusumilli (UNB,
Brazil)andCompanyforResearhofMineralResoures
(CPRM) for providing us with the natural samples;
to Dr. J.-Y. Henri (DRFMC/CENG, Grenoble) for
providing uswith thesyntheti ferroolumbite; toDr.
M.A.Z.VasonellosfortheEPMA analyses. Valuable
disussions with Prof. F. C. Zawislak are gratefully
aknowledged. This work was supported in part by
theBrazilian ageniesCAPES,CNPq,FAPERGSand
FINEP.
Referenes
[1℄ E.H. Nikel, J.F. Rowland and R.C. MAdam, Can.
Mineral.7,390(1963).
[2℄ E.H.Nikel,J.F.RowlandandR.C.MAdam,Am.
Min-eral.48,961(1963).
[4℄ P. Bourguignon and J. Mlon,Ann. So.Gol. Belgique
88,291(1965).
[5℄ J.Moreauand G.Tramasure, Ann.So.Gol.Belgique
88,301(1965).
[6℄ A.C.Turnok,Can.Mineral.8,461(1966).
[7℄ C. G.Gouder deBeauregard, J.Dubois and P.
Bour-guignon,Ann.So.Gol.Belgique90,501(1967).
[8℄ J.GrahamandM.R.Thornber,Am.Mineral.29,1026
(1974).
[9℄ M.S. Adusumilli, Contribution to the Mineralogy of
NiobotantalitesfromtheNortheastProvine,PhD
The-sis, Universidade Federal de Minas Gerais, Brazil. (in
Portuguese),1976.
[10℄ S.M.Eiher,J.E.GreedanandK.J.LushingtonJ.Sol.
StateChem.62,220(1986).
[11℄ M.Wenger,T.ArmbrusterandC.A.Geiger,Am.
Min-eral.76,1897(1991).
[12℄ C.A.dosSantosandJ.Oliveira,SolidStateCommun.
82,89(1992).
[13℄ R.K. Kremerand J.E. Greedan, J. Sol. State Chem.
73,579(1988).
[14℄ V.D. Mello, L.I. Zawislak, J.B. Marimon da Cunha,
E.J. Kinast, J.B. Soares, C.A. dos Santos, J. Magn.
Magn.Mater.196-197,846(1999).
[15℄ V. Antonietti, E.J. Kinast, L.I. Zawislak, J.B.M. da
CunhaandC.A.dosSantos, J.Phys.Chem.Solids 62,
1239(2001).
[16℄ M. Eibshtz, U.Ganiel andS.Shtrikman,Phys.Rev.
156,259(1967).
[17℄ M.G.B. Drew, R.J. Hobson and V.T. Padayathy, J.
Mater.Chem.3,889(1993).
[18℄ C. Heid, H. Weitzel, F. Bourdarot, R. Calenzuk,T.
VogtandH. Fuess,J. Phys.:Condens.Matter8, 10609
(1996).
[19℄ P.CernyandT.S.Erit,Bull.Minral.108,499(1985).
[20℄ R.W.Huthison.Am.Mineral.40,432(1955).
[21℄ C.Hutton.Am.Mineral.44,9(1959).
[22℄ J.D. Grie,R.B.Ferguson andF.C. Hawthorne, Can.
Mineral.14,540(1976).
[23℄ R.B.Ferguson,F.C. Hawthorne andJ.D. Grie, Can.
Mineral.14,550(1976).
[24℄ A.M.Clark,E.E.Fejer.Mineral.Mag.42,477(1978).
[25℄ T.S. Erit, F.C. Hawthorne and P. Cerny,Can.
Min-eral.30,597(1992).
[26℄ M.A.WiseandP.Cerny,Can.Mineral.34,631(1996).
[27℄ A.Aruga,E.Tokizakik,I.NakaiandYSugitami,Ata
Crystallogr.C41,663(1985).
[28℄ L.I.Zawislak,J.B.M.daCunha,A.VasquezandC.A.
dosSantos,SolidStateCommun.94,345(1995).
[29℄ L.I.Zawislak,G.L.F.Fraga,J.B. MarimondaCunha,
D. Shmitt, A.S. Carrio, C.A. dos Santos, J. Phys.:
Condens.Matter9,2295(1997).
[30℄ J.N.Reimers, J.E.Greedan,C.V. Stager, R.Kremer,
[31℄ M.E. Fisher, D.R. Nelson, Phys. Rev.Lett. 32, 1350
(1974).
[32℄ A.Aharony,Phys.Rev.Lett.34,590(1975).
[33℄ A. Aharony, S. Fishman, Phys. Rev. Lett. 37, 1587
(1976).
[34℄ H. Kadowaki, H. Yoshizawa, M. Itoh, I. Yamada, J.
Phys.So.Jpn.59,713(1990).
[35℄ W.A.H.M. Vlak, E. Frikkee, A.F.M. Arts, H.W. de
Wijn,J.Phys.C:SolidStatePhys.16,L1015(1983).
[36℄ I.Yaeger,A.H.MorrishandB.M.Wanklyn,Phys.Rev.
B15,1465(1977).
[37℄ H.Weitzel.Z.Kristallogr.144,238(1976).
[38℄ T.Hanawa,M.IshikawaandK.Miyatani.J.Phys.So.
Japan61,4287 (1992).
[39℄ B. Kratzheller and R. Gruehn, J. Alloys and
Com-pounds183,75(1992).
[40℄ M. SatoandY Hama,J.SolidStateChem.118, 193
(1995).
[41℄ J.Norwig,H.Weitzel,H.Paulus,G.Lautenshlger,J.
Rodriguez-CarvajalandH.Fuess, J.SolidStateChem.
115,476(1995).
[42℄ P.Cerny,W.L.Roberts, T.S.Erit andR.Chapman,
Am.Mineral.70,1044(1985).
[43℄ M.N.SpildeandC.K. Shearer,Can. Mineral. 30,719
(1992).
[44℄ T.Mulja,A.E. Williams-Jones,R.F.Martinand S.A.
Wood,Am.Mineral.81,146(1996).
[45℄ C.A.dosSantos,L.I.Zawislak,V.Antonietti,E.J.
Ki-nast andJ.B.M.daCunha,J.Phys.: Condens. Matter
11,7021(1999).
[46℄ E.J. Kinast. Strutural renement with the Rietveld
method: implementation and essays with the program
Fullprof.MSdissertation(inPortuguese).Institutode
Fsia-UFRGS,2000.
[47℄ M.A.Wise,A.C.TurnokandP.Cerny,NeuesJahrb.
Mineral.H8,372(1985).
[48℄ P.Cerny,T.S.EritandM.A.Wise,Can.Mineral.30,
587(1992).
[49℄ P. Cerny and A.C. Turnok, Can. Mineral. 10, 755
(1971).
[50℄ P. Cerny, T.S. Erit, M.A. Wise, R. Chapman and
H.M.Buk,Can.Mineral.36,547(1998).
[51℄ T.S. Erit, P. Cerny, F.C. Hawthorne and C.A.
M-Cammon,Can.Mineral.30,613(1992).
[52℄ L.I. Zawislak, V. Antonietti, J.B.M. da Cunha and
C.A.dosSantosSolidStateCommun.101,767(1997).
[53℄ V. Antonietti, Order-disorder transformations on the
ixiolite-olumbite-wodginitesystem.MSdissertation(in
Portuguese).InstitutodeFsia-UFRGS,2000.
[54℄ T.S. Erit, M.A.R.Wise andP.Cerny. Am.Mineral.
80,613(1995).
tionforInorganiChemistsandGeohemists,
MGraw-Hill,London(1973).
[57℄ I. Yaeger, A.H. Morrish, C. Boumford, C.P. Wong,
B.M. Wanklyn and B.J. Garrard. Solid State Comm.
28,651(1978).
134,253(1997).
[59℄ D.N. Astrov, N.A. Kryukova, R.B. Zorin, V.A.
Makarov, R.P. Ozerov, F.A. Rozhdestrvenskii, V.P.
Smirnov, A.M. Turhaninov and N.V. Fadeeva. Sov.