w ww . e l s e v i e r . c o m / l o c a t e / b j p
Original
Article
Schinus
terebinthifolius
:
phenolic
constituents
and
in
vitro
antioxidant,
antiproliferative
and
in
vivo
anti-inflammatory
activities
Marciane
M.
da
Silva
a,
Edna
K.K.
Iriguchi
b,
Candida
Aparecida
L.
Kassuya
b,
Maria
do
Carmo
Vieira
c,
Mary
Ann
Foglio
d,
João
Ernesto
de
Carvalho
d,
Ana
Lúcia
T.G.
Ruiz
d,
Kely
de
P.
Souza
a,
Anelise
S.N.
Formagio
a,b,∗aFaculdadedeCiênciasBiológicaseAmbientais,UniversidadeFederaldaGrandeDourados,Dourados,MS,Brazil bFaculdadedeCiênciasdaSaúde,UniversidadeFederaldaGrandeDourados,Dourados,MS,Brazil
cFaculdadedeCiênciasAgrárias,UniversidadeFederaldaGrandeDourados,Dourados,MS,Brazil
dCentroPluridisciplinardePesquisasQuímicas,BiológicaseAgrícolas,UniversidadeEstadualdeCampinas,Campinas,SP,Brazil
a
r
t
i
c
l
e
i
n
f
o
Articlehistory:
Received2August2016
Accepted28December2016
Availableonline28April2017
Keywords:
Anacardiaceae
Biologicalactivity
Flavonoids Galloyl
Pinkpepper
a
b
s
t
r
a
c
t
SchinusterebinthifoliusRaddi,Anacardiaceae,nativetoBrazil,isreferredtoas“pimento-rosa”andisused
totreatinflammatorydiseaseinfolkmedicine.Studieshavereportedimportantpharmacological
prop-erties,buttheseeffectshavestillnotbeenfullyexploited.Thisstudyreportsthatthecrudeextractand
isolatedcompoundsofS.terebinthifolius(leaves)haveinvitroantioxidant,antiproliferative,andinvivo
anti-inflammatoryactivities.Thesampleswereevaluatedforantioxidantactivityusing2,
2-diphenyl-1-picrylhydrazyl,-carotene/linoleicacidand2,2′-azino-bis-(3-ethylbenzothiazoline)-6-sulphonicacid
reagents.Theanti-inflammatoryeffectswereassayedagainstacarrageenan-inducedpawoedemamodel
inmicetotestdosesof10,100and300mg/kgatdifferenttimepointsinadditiontomyeloperoxidase
activityanalysis.Theantiproliferativeactivitywasevaluatedusingtenhumantumourcelllines.Two
derivativesofgallicacidandfourflavonoidswereisolatedandexhibitedconsiderableantioxidantactivity.
Theextractanditscompoundsshowedselectivitytowardsovariancancercells,withgrowthinhibitory
activityvaluesrangingfrom1.9to6.5g/ml.Sampleextractsandmethylgallatesignificantlyinhibited
carrageenan-inducedoedemainthemicepawoedemaexperimentalmodel.Thecalculatedtopological
polarsurfaceareaformethylgallate(86.98 ˚A2)showedgoodintestinalabsorption.Theeffectsreported
hereinareberelatedtothepresenceofflavonoidsandthegalloylphenolicderivativecontent.
©2017PublishedbyElsevierEditoraLtda.onbehalfofSociedadeBrasileiradeFarmacognosia.Thisis
anopenaccessarticleundertheCCBY-NC-NDlicense(http://creativecommons.org/licenses/by-nc-nd/
4.0/).
Introduction
Schinusterebinthifolius Raddi,Anacardiaceae,isan evergreen
shrub that grows in South and Central America. In Brazil,
it is popularly known as “pimenta-rosa”, “aroeira-vermelha”,
“aroeira-pimenteira”, “aroeira-da-praia”, “aroeira-negra” and/or “aroeira-de-minas”andusedinfolkmedicinefortreatmentof sev-eralhealthdisordersaswellanti-inflammatoryprocesses(Morton, 1978;Gazzaneoetal.,2005).Itsbiologicalapplicationshavebeen describedsincethefirsteditionoftheBrazilianPharmacopoeia,
published in 1926. A Brazilian gel-based aqueous bark extract
ofS.terebinthifoliushasbeenmarketedsince1999forthe treat-ment ofvaginitis and cervical vaginitis(Leiteet al.,2011).The
∗ Correspondingauthor.
E-mail:aneliseformagio@ufgd.edu.br(A.S.N.Formagio).
Brazilian Pharmacopoeia recommends the decoction of S.
tere-binthifoliusforuseasanaturalanti-inflammatoryagent(Santosand Amorim, 2002).Pharmacological studies withextractsobtained from leaveshave reported antioxidant,anti-allergic, antimicro-bial,anti-inflammatory,antiulcerandantiadherentpropertiesas wellaswound-healingproperties(CasteloBrancoNetodeetal., 2006;Carvalher-Machadoetal.,2008;Gomesetal.,2010;Johann etal.,2010;Carvalhoetal.,2013;Barbierietal.,2014;Ulianaetal.,
2016).Chemicalstudiesshowedthatpolyphenolicandflavonoid
aremajorconstituentsoftheextractsofS.terebinthifoliusleaves (Farag,2008;El-Massryetal.,2009;Santanaetal.,2012).
Investigationsbyourresearchgroupshowthattheessential
oilofS.terebinthifoliusfruitscontainsapredominanceof monoter-penes,with-pineneasthemajorconstituent.Thisoilwaseffective
againstpersistentinflammationcausedbyCompleteFreund
Adju-vant(CFA)oracuteinflammationinducedbycarrageenaninthe
paw or in pouches (Formagio et al., 2011). In another study,
http://dx.doi.org/10.1016/j.bjp.2016.12.007
0102-695X/©2017PublishedbyElsevierEditoraLtda.onbehalfofSociedadeBrasileiradeFarmacognosia.ThisisanopenaccessarticleundertheCCBY-NC-NDlicense
theextractof theleaveshasanti-inflammatory, immunomodu-latory,chemopreventive,antigenotoxicandantimutageniceffects owingtophenolandflavonoidcompounds(Fedel-Miyasatoetal., 2014a,b).
Theoxidativedamageinducedincellsandtissuesisrelatedto theaetiologyofvariousdiseasesincludinginflammatoryand can-cerdiseases.Thus,thenaturalproductsarecandidatesforthese
testsbecausetheinsertionoffoodcompoundsor
phytopharma-ceuticalsmaybeanimportantalternativetotreatinflammation
andthepreventionofcancer.Althoughcancerisaspecificdisease, ithasbeenonlyslightlydefinedintermsoftraditionalmedicine. Recentcontributionstothearmamentofchemotherapeuticagents, inalliancewithnaturalproductsapprovedasdrugsinthis30-year timeframe,includepaclitaxel(Taxol®),isolatedfromTaxus brev-ifolia;thealkaloidsvincristineandvinblastinefromCatharanthus roseus;camptothecinandderivativesfromCamptothecaacuminata;
combretastatinfromCombretum caffrum(Newman etal., 2005;
Newmanand Gragg,2012); andcurcumin fromtherhizomeof Curcuma longa (Aggarwal and Bharti,2003)in addition to syn-theticderivativesorcombinationsofagentssuchasflavopiridol androscovitine(Newmanetal.,2002;DanceyandSausville,2003), justifyingtheimportanceofthesearchforcancertherapy.Even ifS. terebinthifolius hasbeenproposed asa folk remedyin the treatmentofinflammation,morestudiesmustbereported. There-fore,weevaluatedtheinvitroantioxidant,antiproliferative and
in vivo anti-inflammatory activities of methanolic extracts and
compoundsisolatedfromS.terebinthifolius(leaves).Asa comple-ment,acomputationalstudyforpredictingtheADMEpropertiesof
compoundswasperformedbydeterminingthelipophilicity,
topo-logicalpolarsurfacearea(TPSA),absorption(%ABS)andsimple moleculardescriptorsusingLipinski’srule.
Materialsandmethods
Plantmaterial
The leaves of Schinus terebinthifolius Raddi, Anacardiaceae,
werecollectedattheMedicinalPlantsGardenofFederal Univer-sityofGrandeDourados(22◦11′43.7′′S,54◦56′08.5′′Wand430m)
in November 2014. A voucher specimen was deposited in the
HerbariumoftheUFGDunderthenumberDDMS4600andwas
identifiedbyDr.MariadoCarmoVieira.Authorizationfor
acces-singand studying samples from the Brazilian genetic heritage
site wasobtainedfromtheBrazilian governmentthrough
Con-selhoNacionaldeDesenvolvimentoCiêntíficoeTecnológico(CNPq
authorizationno.010220/2015-1–CNPq/CGEN/MMA).
Extraction,fractionationandidentificationprocedures
Leaves(860g) weredriedand extractedbymacerationwith
methanol, filtered, concentrated under reduced pressure and
lyophilized to yield the methanolic extract (MEST) (42.7g).
The MEST (30g) was partitioned with hexane, chloroform and
ethyl acetate.The chloroform fraction(1.8g) was submittedto
columnchromatography(CC)silicagel,yieldingsitosterol-3-O- -glucopyranoside(64mg).Fractionationofpartoftheethylacetate
fraction(6.2g) by CCin silica gel wasperformed using a
mix-tureofhexane/EtOAcandEtOAc/MeOH,inincreasingpolarity,to
affordcompounds1(25.4mg),2(19.4mg)and3(12.2mg).The
hydromethanolicfraction(13g)waspurifiedbysuccessiveCCon SephadexLH-20usingH2O,H2O/MeOH7:3–3:7,andMeOHas
elu-ents,yieldingcompounds4(22.8mg),5(17.8mg)and6(26mg). The1Hand13CNMRspectrawerecollectedusingaVarianMercury
PlusBBspectrometeroperatingat300MHzand75.5MHzusing
CD3ODasthesolventandtetramethylsilane(TMS)astheinternal
standard.
HPLCanalysis
TheLCdatawithMESTincludecaffeicacid(Rt=14.78min),p -coumaricacid(Rt=25.82min),luteolin(Rt=62.08min),quercetin (Rt=64.62min)andapigenindata(Rt=66.79min).Thesedatawere
described in a previous report by our research group (
Fedel-Miyasatoetal.,2014a).
Invivoanti-inflammatoryactivity
Animals
Male Swiss mice (25–35g) were used for in vivo
anti-inflammatoryevaluationandwereprovidedbytheUniversidade
Federalda GrandeDourados.The micewerekeptundera 12h
light-darkcyclewithcontrolledhumidity(60–80%)and
temper-ature(22±1◦C).Twohoursbeforetheexperiments,theanimals
wereplacedinthelaboratoryandwereusedonlyoncefor experi-ments(n=5/group).Allexperimentalprocedureswereperformed inaccordancewiththeU.S.NationalInstituteofHealthandwere approvedbytheethicscommitteeforresearchonlaboratory ani-malsoftheUFGD(Nbr.005/2010).
Carrageenan-inducedpawoedema
Five groups of Swiss mice were orally treated with MEST
(100 and 300mg/kg) and 2 (100 and 300mg/kg) as well as a
vehicle.Twogroups weretreatedintraplantarly with2 (10and
100mg/kg).Onegroupofmicewastreatedsubcutaneouslywithan anti-inflammatorypositivecontroldrugdexamethasone(1mg/kg). After1h,theanimalsreceivedanintraplantarinjection(50l)ofa solutionofcarrageenan(300g/paw,dilutedinsterile0.9%saline) intotherighthindpaw.Thecontralateralpawreceivedonlysaline andwasusedasacontrol.
Theoedemawasthedifferenceinthicknessofbothpawsusing
adigitalmicrometre(DIGIMESS110-284)atseveraltimepoints
(0.5,1,2,and4h)aftercarrageenaninjection. Theresultswere expressedinm(Kassuyaetal.,2009).
Myeloperoxidaseactivity
Myeloperoxidase (MPO) activity was measured in the paw
after 6h to evaluate indirect neutrophil migration to this tis-sue(De Young et al., 1989).The paw tissue washomogenized
in 5%(w/v) 80mM phosphatebufferat pH5.4 containing0.5%
ofhexadecyltrimethylammoniumbromide.Thehomogenatewas
centrifugedat3200×gand4◦Cfor20min.Thirtymicrolitersof
each supernatant was mixed with100l of 80mM phosphate
buffer,85lof0.22Mphosphatebufferand15lof0.017%H2O2
ina96-wellplate.Thereactionwasinitiatedwith20lof
3,3,3-tetramethylbenzidine(dissolvedinN,N-dimethylformamide).The
platewasmaintainedat37◦Cfor3min,afterwhichthereaction
wasinterruptedbyadding30lof1.46Msodiumacetate(pH3.0).
Theenzymaticactivitywasdeterminedbymeasuringtheoptical
densityat630nmandwasexpressedasmOD/mgofprotein.
Invitroantiproliferativeactivity
MESTandothercompoundswereassessedinthefollowingten
humantumourcelllinesfromvarioustissues,kindlyprovidedby theNationalCancerInstitute(Frederick,MA,USA):U251(glioma,
CNS),MCF-7(breast),NCI-ADR/RES(ovarianexpressingthe
K-562(leukaemia)andHaCaT(human keratinocytes,
immortal-ized non-tumouralcell). The test results were measuredusing
thecolorimetricsulphorhodamineBmethod,accordingtotheNCI
standard protocol, and doxorubicin (0.025–25g/ml) wasused
aspositivecontrol(Monksetal.,1991).Assayswereperformed
ina 96-wellplateusing fourconcentrationsproduced bya
10-folddilution(0.25–250g/ml).Theactivitywasdeducedfromthe
concentrationresponse,and GI50 parameters(growthinhibitory
activity)werecalculated.
Invitroantioxidantactivity
DPPHradicalscavengingassay
SamplestocksolutionsofMEST(1.0mg/ml)andcompounds1–6
(0.1mg/ml)weredilutedtofinalconcentrationsof300,200,125,
50,25,10and5g/mlinmethanol.Sampleswereaddedto3ml
of methanolic DPPH (2, 2-diphenyl-1-picrylhydrazyl) (0.1mM)
andwereprepared daily,shaken,and leftatroomtemperature
in the dark for 30min. Absorbance was measured at 517nm
againsta blank containingallreagents exceptthe testsamples
(Brand-Williams et al.,1995).Assayswerecarried outin tripli-cate.ThepercentageofinhibitionofDPPH(I%)wascalculatedusing thefollowingequation:I%=(Ablank−Asample/Ablank)×100;theIC50
concentration,indicating50%inhibitionofDPPH,wasplottedina graphofI%versussampleconcentration.
ˇ-Carotene/linoleicacidassay
A-carotene-chloroformsolution(1ml)wasmixedwith20mg linoleicacidand0.2gTween40®,withsubsequentevaporationof thechloroform.Distilledwater(50ml)wasslowlyaddedwith vig-orousagitationtoformanemulsion.Emulsionaliquots(5ml)were
transferredwith0.2ml of extracts(1mg/ml)and isolated
com-pounds (0.1mg/ml) at different concentrations (10–200g/ml).
Control samples contained all reagents except the test
sam-ples (Jayaprakasha et al., 2001). An emulsion was added to
each tube, and the absorbance was read at 470nm for zero
time. Tubes were placed in a water bath at 50◦C, and
oxida-tionwasmonitoredbyabsorbanceat15-minintervalsuntilthe
colourof-caroteneinthecontrolsampledisappeared(105min).
The analyseswere performed in triplicate. Antioxidantactivity
(AA)wascalculated as the %inhibition relative tothe control: %AA=[1−(AsampleT0−AsampleT105)/(AcontrolT0−AcontrolT105)]×100.
ABTSradicalscavengingassay
MESTandcompoundstocksolutions(1mg/ml)weredilutedto
finalconcentrationsof250–5g/ml.Briefly,7mMof2,2′
-azino-bis-(3-ethylbenzothiazoline)-6-sulphonicacid(ABTS)and140mM
potassiumpersulphateweremixedandkeptinthedarkfor16h
atambienttemperature.Thereafter,3mlofABTS•+solutionwas
addedto30lsampleswithvaryingconcentrations.After5min,
theabsorbancewasmeasuredat734nmusinga
spectrophotome-ter(Djeridaneet al.,2006).TheABTS•+ scavengingactivitywas
calculatedusingthefollowingequation:ABTSradicalscavenging activity(%)=(Ablank−Asample/Ablank)×100.
InsilicostudyandLipinski’sruleoffive
Anin silico computational studyof the isolated compounds
(1–6)wasperformedbythedeterminationof Lipinski’s
param-eters, topological polar surface area (TPSA) and percentage of
absorption(%ABS)(Lipinskietal.,1997).Calculationswere
per-formed using the “Molinspiration online property calculation
toolkit” (http://www.molinspiration.com) (Ertl, 2014). The per-centageofabsorptionwasestimatedusingthefollowingequation: %ABS=109−[0.345×TPSA](Lipinskietal.,1997).
Lipinski’s“ruleoffive”(Zhaoetal.,2006)wasusedto evalu-atethedrug-likecharacterofthosecompounds.Generally,orally bioavailabledrugsfollowtheserules:theyhavefewerthanfive
hydrogenbonddonors,nomorethantenhydrogenbondacceptors,
amolecularweightbelow500Da,andanoctanol–waterpartition
coefficientlogPofnomorethan5.
Statisticalanalysis
All data are presented as the mean±S.E.M. The difference
betweengroupswasevaluatedbyanalysesofvariance(one-way
ANOVA)followedbytheTukeyorStudent–Newman–Keulstest.
Thenumberofanimalspergroupisindicatedinthelegends. Sta-tisticaldifferenceswereconsideredsignificantatp<0.05.
Resultsanddiscussion
In the present study, we characterized seven previously
known compounds, including one steroid, sitosterol-3-O-
-glucopyranoside; two gallic acid derivatives, 1,2,3,4,6-penta-O -galloyl--glucopyranoside(1)andmethylgallate(2);andthefour followingflavonoids:robustaflavone(3),quercetin(4),quercetrin (5)andluteolin(6)fromS.terebinthifolius.Thestructuresof
com-poundswereelucidatedusing1Dand2DNMRspectraldataand
acomparisonof1Hand13CNMRreporteddata(Agrawal,1989;
Carvalher-Machadoetal.,2008;Ceruksetal.,2007).Thequercetin derivativesofthesugarunitandhydrogen,aswellastheposition oftheirlinkagetotheaglycone,weredeterminedusinga combi-nationof2DNMRexperiments.Tothebestofourknowledge,this studyisthethirdphytochemistrystudyofS.terebinthifoliusleaves andthefirstreportofluteolinfrommethanolicextract,conducted duringthepreviousHPLCdatastudy.
The antioxidant activity was initially evaluated for S. tere-binthifoliusMEST.Theresultsshowedthatthesamplepresented
potent antioxidant activity when tested against DPPH (IC50
12.32±1.50g/ml), -carotene/linoleic acid(AA=70.44±0.88%) and ABTS (AA=86.94±2.04%) (Table 1). Fractionation of these extractsbysolventpartitionandpurificationbyachromatographic
columnprovidedsixphenoliccompounds.Theresultsshowedthat
phenolics(2),hydrolysedtannins(1)andflavonoids(4and6)were activeinallassays,comparabletothenaturalantioxidant ascor-bicacid(Table1).Methylgallate(2)and quercetin(4)are also
considered natural antioxidants. Anacardiaceaespecies showed
Table1
Antioxidant assays of MEST and phenolic compounds (2–7) from Schinus
terebinthifolius.
Sample DPPHIC50a(95% confidencelimit)
-Carotene/linoleic acidAA(%)
ABTSAA(%)
MEST 12.32±1.50c (11.01–14.86)
70.44±0.88b 86.94±2.04ab
1 3.03±0.89a (2.54–3.10)
87.57±2.28a 90.41±1.84a
2 5.54±1.55b (5.30–5.78)
86.26±2.84a 77.65±1.06c
3 19.44±1.89d (18.64–20.11)
68.92±0.50b 65.72±0.83d
4 4.03±1.57ab (3.88–4.12)
84.40±2.13a 83.07±3.34b
5 20.43±0.54d (18.10–21.66)
62.18±0.64c 63.21±1.55d
6 4.57±2.43ab (3.35–5.08)
86.36±2.18a 85.32±1.32b
Ascorbicacid 3.99±0.36ab (14.3–20.1)
88.37±1.91a 87.32±1.33ab
Values are expressed as the mean±SD (n=3); n.d.= not determined; aIC50=concentration resulting in 50% inhibition of DPPH, derived from the
graphofI%(inhibition percentage)versus concentrationing/ml(MEST and ascorbicacid) andM (2–7compounds).%AA=antioxidant activity,evaluated usingthe-carotene/linoleicacidandABTSmethods.Differentsuperscriptletters indicatestatisticallysignificantdifferencesineachline(p<0.05)usingtheTukey test.
andHarpephyllumcaffrum(Moyoetal.,2010).Thesecompounds exhibitedasimilarscavengingactivityasourextract,andthis
activ-itywasattributedtovariouscompoundsfoundinthesespecies,
includingphenolicacids,flavonoidsandtannincompoundsintheir crudeextracts.
OurdatashowedthattheIC50andAA%valuesindicated
correla-tionswiththepresenceofthesugarunit,hydroxylorhydrogenof theaglyconelinkedatC-3,whichwereduetothesterichindrance betweentheC-3substituentandtheBringoftheflavonol,whose decreasesignificantlyenhancedfreeradical-scavengingability,as evidencedbycompound5.Thecatechol,␣,-unsaturatedcarbonyl moietyand-hydroxyketonegroupsconferredhigherstabilityto theradicalformandparticipatedinelectrondelocalization(Pietta, 2000).Compound3,whichbelongstotheflavonesubclass,didnot presentcatecholgroupsinthestructure,whichlikelyexplainsthe inactivityofthesample.
Lipinski’s “rule of five” [molecular weight (MW)≤500Da,
logP≤5, H-bond donors (HBD)≤5 and H-bond acceptors
(HBA)≤10], topologicalpolarsurface area (TPSA)and per cent
absorption (%ABS) were calculated and presented in Table 2.
MoleculesviolatingmorethanoneofLipinski’sparametersmay
haveproblemswithbioavailabilityandahighprobabilityoffailure todisplaydrug-likecharacter.Fromthedataobtained,2and4were
foundtoobeyLipinski’srule,showinggoodpermeabilityinthe
cellularplasmatic membrane(78.79% and 50.33%,respectively).
ThecomputationalTPSAfor3(86.98 ˚A2)showedgoodintestinal
absorption,whereas1,3and6violatedthreeparameters. Freeradicalspeciesarealsoresponsibleforactivatingseveral pro-inflammatorytranscriptionfactorsinvolvedinthepromotion
ofinflammatory diseases.Theanti-inflammatory activity(MEST
and 2)was evaluated using carrageenan-induced paw oedema
andassessmentofmyeloperoxidase(MPO).Asshowninthe
lit-eratureresults,carrageenaninjection-inducedpawoedemawas
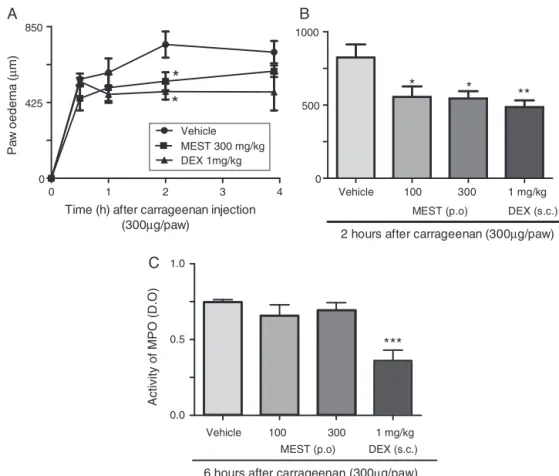
observedatalltime pointsinthepresentwork(Fig.1A).MEST
treatmentsinhibitedoedemaformationwith33±9(100mg/kg)
0.0 0.5 1.0
***
C
Vehicle 100 300 1 mg/kg MEST (p.o) DEX (s.c.)
6 hours after carrageenan (300µg/paw)
Activity of MPO (D.O)
0 1 2 3 4
0 425 850
Vehicle MEST 300 mg/kg DEX 1mg/kg
*
*
Time (h) after carrageenan injection (300µg/paw)
A
Pa
w
oede
ma
(
µ
m)
0 500 1000
*
*
**
Vehicle 100 300 1 mg/kg
MEST (p.o) DEX (s.c.)
2 hours after carrageenan (300µg/paw)
B
Fig.1.Anti-inflammatoryeffectofMESTadministrationoncarrageenan-inducedpawoedemainmice.AnimalsreceivedMEST(100or300mg/kg,p.o.),dexamethasone
(DEX,1mg/kg,s.c.)oravehiclecontrol.After1h,anintraplantarinjectionofcarrageenan(300g/paw)wasperformed.In(A),thetimecourseofinhibition,inducedby
300mg/kgMESTandDEX,ispresented.In(B),thebarsindicatetheeffectofvariousMESTandDEXdosesinpawoedema(m)2haftercarrageenaninjection.In(C),MEST
didnotaltertheincreaseinmyeloperoxidase(MPO)activityinducedbylocalcarrageenaninjections.Thebarsexpressthemean±SEMoffiveanimals.Comparisonswere
Table2
Lipinski’sparameters,topologicalpolarsurfacearea(TPSA)andpercentageofabsorption(%ABS)ofthecompounds(2–7).
Lipinski’sparameters
Compound %ABS TPSAa( ˚A2) nHBAa(nON) nHBDa(nOHNH) logPa MWa nviolationsa
1 −37.26 423.95 25 14 3.65 938.70 3
2 78.99 86.98 5 3 0.84 184.14 0
3 46.28 181.79 10 6 5.16 538.46 3
4 63.68 131.35 7 5 1.68 302.23 0
5 50.33 170.04 10 6 1.13 432.38 1
6 18.12 263.42 16 10 −1.063 610.52 3
awww.molinspiration.com;%ABS=109− 0.345× TPSA;numberofhydrogenbondacceptors(NO)=nHBA≤ 10;numberofhydrogenbonddonors(OHNH)=nHBD ≤ 5;
MW≤500;octanol–waterpartitioncoefficient=logP<5.
0 200 400 600 800 1000
**
**
**
A
Vehicle 100 300 1 DEX 2
2 hours after carrageenan (300µg/paw)
mg/kg Vehicle 100 300 1 mg/kg
Paw oedema (
µ
m)
0.0 0.5 1.0 1.5
B
DEX 2
6 hours after carrageenan (300µg/paw)
*
A
c
ti
v
ity
o
f M
P
O
(
D
.O
)
0.0 0.5 1.0 1.5
6 hours after carrageenan (300µg/paw)
D
Vehicle 10 100 1 2 DEX
**
mg/kg
Activity of MPO (D.O)
0 200 400 600 800 1000
*
*
**
Vehicle 10 100 1 2 DEX
2 hours after carrageenan (300µg/paw)
C
mg/kg
Paw oedema (
µ
m)
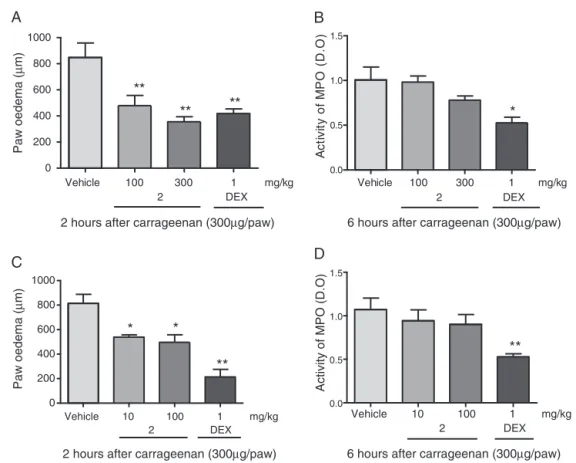
Fig.2.Anti-inflammatoryeffectofmethylgallate(2)oncarrageenan-inducedpawoedemainmice.Animalsreceived2orally(100and300mg/kg,p.o.),2intraplantarly
(10and100mg/kg,p.o.),dexamethasone(DEX,1mg/kg,s.c.)oravehiclecontrol.After1h,themicereceivedanintraplantarinjectionofcarrageenan(300g/paw).In(A),
thebarsindicatetheeffectsofvariousdosesof2andDEXinpawoedema(m)2haftercarrageenaninjection.In(B),2didnotinhibitacarrageenan-inducedincreasein
myeloperoxidase(MPO)activity.In(C),barsdemonstratetheeffectsofvariousdosesofintraplantarinjectionsof2(10and100g/paw)andDEXonpawoedema(mm)2h
aftercarrageenaninjection.In(D),2didnotinhibitcarrageenan-inducedincreaseofMPOactivity.Thebarsexpressthemean±SEMoffiveanimals.Comparisonsweremade
betweenthevehicleandthetreatedgroups.*p<0.05,**p<0.01,***p<0.001,one-wayANOVAfollowedbytheStudent–Newman–Keulsmethod.
and34±6%(300mg/kg)reductions(Fig.1AandB).Moreover,our resultsindicatedthat300mg/kgoftheMESTdisplayedsignificant inhibition2hafteradministration,asindicatedbythetimecourse analysis(Fig.1A).Inaddition,41±5%inhibitionwasobservedin
thedexamethasone-treatedgroup2haftercarrageenaninjection
(Fig.1A).OralMESTtreatment(100and300mg/kg)didnotalter theincreaseinMPOactivityinducedbycarrageenan.Thepositive
control(dexamethasone)inducedinhibitoryactivityintheMPO
analysiswhencomparedwiththecontrolgroup(Fig.1C). Scien-tificworkswithS.terebinthifoliusshowedthatextractsfromleaves exhibitedtopical(dosesof0.1,0.3and1mg/ear)(Fedel-Miyasato etal.,2014a)andsystemicanti-inflammatorypropertiesinanother
inflammatory model including croton oil-induced ear oedema,
arthritis and air pouch models in mice (Fedel-Miyasato et al.,
2014a;Rosasetal.,2015).Previousstudiesusingwound-healing modelsshowedthatextractsfromtheleavesofS.terebinthifolius
wereeffectivefor woundhealing (Nunes etal.,2006; Coutinho
etal.,2006;Martorellietal.,2011).Inrats,Fedel-Miyasatoetal. (2014a)showedthatMEST(80mg/ml)topicalapplication signifi-cantlydecreasedthediameterofthewound.Inthepresentwork, theextractwastestedsystemically(oralroute)inadifferentmodel ofinflammationandcompound3wasassayedviabothanoralroute andlocalinjection(intraplantarinjection).
Compound2alsoexhibitedanti-edematogenicactivity,
inhib-itingapproximately43±9%(100mg/kg)and58±5%(300mg/kg)
(Fig.2A).Dexamethasoneinhibitedoedemaformation(51±4%)2h afterinflammatorystimulus(Fig.2A).Animalstreatedwith intra-plantarcompound2injectionsdisplayed34±2%(10g/paw)and 39±8%(100g/paw)inhibition(Fig.2C).Oral(100and300mg/kg)
orintraplantar(10and100g/paw)administrationofcompound
2didnotalterMPOactivitycomparedtothecontrolgroup(Fig.2B andD).Theanti-inflammatoryeffectsofMESTwereassociatedwith increasedlevelsofmethylgallate(2),whichisalsofoundinother
A
100
75
50
25
0
–25
–50
–75
–100
100
75
50
25
0
–25
–50
–75
–100
100
75
50
25
0
–25
–50
–75
–100 100
75
50
25
0
–25
–50
–75
–100
10–3 10–2 10–1
Cellular growth, %
Cellular growth, % Cellular growth, %
Cellular growth, %
Concentration (µg/ml)
Concentration (µg/ml) Concentration (µg/ml)
Concentration (µg/ml)
U251 MCF7 NCI/ADR-RES 786-0 NCI-H460 HT29 K-562 HaCaT PC-3 OVCAR-3
U251 MCF7 NCI/ADR-RES NCI-H460 PC-3 OVCAR-3 HT29 K-562 HaCaT
U251 MCF7 NCI/ADR-RES 786-0 NCI-H460 HT29 K-562 HaCaT PC-3 OVCAR-3
U251 MCF7 NCI/ADR-RES 786-0 NCI-H460 HT29 K-562 HaCaT PC-3 OVCAR-3
0.25 1002.5 10125 102250
10–3 10–2 10–10.25 1002.5 10125 102250 10–3 10–2 10–10.25 1002.5 10125 102250
10–3 10–2 10–10.25 1002.5 10125 102250
C
D
B
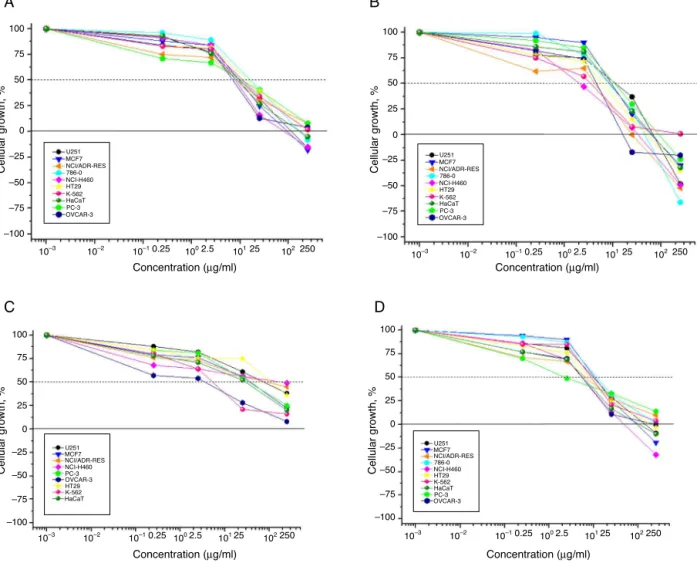
Fig.3.Antiproliferativeactivity.InA:MEST;B:compound1;C:compound5;D:compound6.U251(glioma,CNS),MCF-7(breast),NCI-ADR/RES(ovarianexpressingthe
multipledrugresistancephenotype),786-0(renal),NCI-H460(lung,non-smallcells),PC-3(prostate),OVCAR-3(ovarian),HT-29(colon),K-562(leukaemia)andHaCaT
(humankeratinocytes,immortalizednon-tumouralcells).GI50:concentrationsthatelicit50%inhibitionofcellgrowth(ing/ml).
L,andA.saccharumMarsh(Whangetal.,2005;Kimetal.,2006; Abou-Zaidetal.,2009).
The results demonstrated that sample MEST possesses
in vitro anticancer activity, with GI50 values ranging from 6.3
to9.4g/mlwithselectivityforprostate(PC-3)(GI50=6.3g/ml),
ovarian (OVCAR-3) and resistant ovarian- (NCI-ADR/RES)
(GI50=6.5g/ml), breast (NCI/H460) (GI50=7.6g/ml), glioma
(U251) (GI50=9.1g/ml) and breast (MCF-7) (GI50=9.4g/ml)
cancerandforHaCat(GI50=8.1g/ml)non-tumouralcells(Fig.3A).
Furthermore, 1,2,3,4,6-penta-O-galloyl-O--glucopyranoside
(1) demonstrated high activity with GI50<5.00g/ml against
resistant ovarian (NCI-ADR/RES), breast (NCI/H460)
(GI50=1.9g/ml), leukaemia (K-562) (GI50=2.2g/ml), ovarian
(OVCAR-3)(GI50=2.5g/ml)andcolon(HT-29)(GI50=4.9g/ml)
cancercells (Fig.3B). Quercetrin (5)showed inhibitoryactivity
against prostate (PC-3) (GI50=2.5g/ml), ovarian (OVCAR-3)
(GI50=4.1g/ml), (HaCaT) (GI50=4.3g/ml), ovarian
(NCI-ADR/RES) and breast (NCI/H460) (GI50=4.4g/ml) cancer
cell lines (Fig. 3CD). Luteolin (6) showed activity against
OVCAR-3 (GI50=1.3g/ml), K562 (GI50=4.5g/ml) and
HaCaT (GI50=17.4g/ml) cell lines (Fig. 3D). Compound 3,
robustaflavone,didnotshowinhibitoryactivityagainstanycell lines(GI50>100g/ml)(datanotshown).Ingeneral,S.
terebinthi-foliusand itscompoundsshowedthehighestgrowthinhibitory
activities towards ovarian cancer cells (NCI-ADR/RES;
OVCAR-3) which showed GI50 values in the 1.3–6.5g/ml range. The
Anacardiaceae plant species has already been recorded in the
literature, withvaried growth inhibition potential for different cancercelllines(Kimetal.,2013).Studiesreportthat␣-pinene
isolated from S. terebinthifolius leaves induces apoptosis and
confersantimetastaticprotectioninamelanomamodel(Matsuo
etal.,2011).
In another study by our group, the in vitro
antiprolifera-tive activityagainst ten human cancercell lines of a series of galloylderivativesbearingsubstituted-1,3,4-oxadiazoleand
carbo-hydrazidemoietieswasevaluated.Theresultsdemonstratedthat
methylgallateandintermediarygalloylhydrazideshowedgreat
antiproliferative activity,with GI50 values<5.54M,against all
humantumourcelllinestested(DaSilvaetal.,2015).Therefore, since2exhibitedseveralactivities,thisantioxidantagentisagood naturalproductleadforstructuralmodificationstudiesyielding newagents(Asnaasharietal.,2014).Thisresultisinaccordance withLipinski’sRuleofFive,TPSAand%ABS,whichareimportant forfurtherdevelopmentofdrugsbaseduponthesemoieties.
Conclusions
ThisstudydemonstratedthatMEST,obtainedfromleaves
col-lectedinDourados-MS,haspotentialanti-inflammatoryactivity,
whichsupportspreviousclaimsregardingthetraditionaluseof
S.terebinthifoliusleaves.Themethanolicextractandcompounds
showed the highest growth inhibitory activity, with particular
effectivenessagainstovariancancer(NCI-ADR/RES;OVCAR-3)cell lines.
Ethicaldisclosures
Protectionofhumanandanimalsubjects. Theauthorsdeclare
thattheproceduresfollowedwereinaccordancewiththe regula-tionsoftherelevantclinicalresearchethicscommitteeandwith thoseoftheCodeofEthicsoftheWorldMedicalAssociation (Dec-larationofHelsinki).
Confidentialityofdata. Theauthorsdeclarethattheyhave
fol-lowed theprotocolsof theirworkcenter onthe publicationof
patientdata.
Righttoprivacyandinformedconsent. Theauthorsdeclarethat
nopatientdataappearinthisarticle.
Authors’contributions
MCVidentifiedandcollectedplantmaterial.ASNF,MMSand
KPSpreparedthemethanolicextractandphytochemistrystudy,
assessed antioxidant activity and helped to write and edit the
manuscript.CALKandEKKIdesignedtheanti-inflammatoryassays. MAF,JECandALTGRcontributedtotheantiproliferativeassay.All
authorshavereadandapprovedthefinalmanuscriptfor
submis-sion.
Conflictsofinterest
Theauthorsdeclarenoconflictsofinterest.
Acknowledgements
ThisworkwassupportedbytheConselhoNacionalde
Desen-volvimento Científico e Tecnológico (CNPq, Brazil). We thank
CAPES,CNPqandFundectforfellowships(A.S.N.F,M.A.F,J.E.C.and M.M.S).
References
Abou-Zaid,M.M.,Lombardo,D.A.,Nozzolillo,C.,2009.Methylgallateisanatural
constituentofmaple(Genusacer)leaves.Nat.Prod.Res.23,1373–1377.
Aggarwal,B.,Bharti,A.C.,2003.Anticancerpotentialofcurcumin:preclinicaland
clinicalstudies.AnticancerRes.23,363–398.
Agrawal,P.K.,1989.StudiesinOrganicChemistryCarbon13NMRofFlavonoids.
Elsevier,Amsterdam,564p.
Ajileye,O.O.,Obuotor,E.M.,Akinkunmi,E.O.,Aderogba,M.A.,2015.Isolationand
characterizationofantioxidantandantimicrobialcompoundsfromAnacardium occidentaleL.(Anacardiaceae)leafextract.J.KingSaudUniv.-Sci.27,244–252.
Asnaashari,M.,Farhoosh,R.,Sharif,A.,2014.Antioxidantactivityofgallicacidand
methylgallateintriacylglycerolsofKilkafishoilanditsoil-in-wateremulsion. FoodChem.159,439–444.
Barbieri,D.S.V.,Tonial,F.,Lopez,P.V.A.,SalesMaia,B.H.L.N.,Santos,G.D.,Ribas,M.O.,
Glienke,C.,Vicente,V.A.,2014.AntiadherentactivityofSchinusterebinthifolius
andCrotonurucuranaextractsoninvitrobiofilmformationofCandidaalbicans
andStreptococcusmutans.Arch.OralBiol.59,887–896.
Brand-Williams,W.,Cuvelier,M.E.,Benset,C.,1995.Useoffreeradicalmethodto
evaluateantioxidantactivity.Leb.Wis.Technol.28,25–30.
Carvalher-Machado,S.C.,Rosas,E.C.,Brito,F.A.,Heringe,A.P.,deOliveira,R.R.,
Ka-Plan,M.A.,Figueiredo,M.R.,Henriques,M.,2008.Theanti-allergicactivityofthe
acetatefractionofSchinusterebinthifoliusleavesinIgEinducedmicepawedema andpleurisy.Int.Immunopharmacol.8,1552–1560.
Carvalho,M.G.,Mel,A.G.N.,Aragão,C.F.S.,Raffin,F.N.,Moura,T.F.A.L.,2013.Schinus
terebinthifoliusRaddi:chemicalcompositionbiologicalpropertiesandtoxicity. Rev.Bras.PlantasMed.15,158–169.
CasteloBrancoNetode,M.L.,RibasFilho,J.M.,Malafaia,O.,OliveiraFilho,M.A.,
Czeczko,N.G.,Aoki,S.,Cunha,R.,Fonseca,V.R.,Teixeira,H.M.,Aguiar,L.R.F.,2006.
Avaliac¸ãodoextratohidroalcoólicodeAroeira(SchinusterebinthifoliusRaddi)no processodecicatrizac¸ãodeferidasempelederatos.ActaCir.Bras.21,17–22.
Ceruks,M.,Romoff,P.,Favero,A.O.,Lago,J.H.G.,2007.Constituintesfenólicospolares
deSchinusterebinthifoliusRaddi(Anacardiaceae).Quim.Nova30,597–599.
Coutinho,I.H.I.L.S.,Torres,O.J.M.,Matias,J.E.F.,Coelho,J.C.U.,Stahlke,J.H.J.,Agulham,
M.A.,Bachle,E.,Camargo,P.A.M.,Pimentel,S.K.,Freitas,A.C.T.,2006.Efeitodo
extratohidroalcoólicodearoeira(SchinusterebinthifoliusRaddi)nacicatrizac¸ão deanastomosescolônicas.Estudoexperimentalemratos.ActaCir.Bras.21, 49–54.
Dancey,J.,Sausville,E.A.,2003.Issuesandprogresswithproteinkinaseinhibitors
forcancertreatment.Nat.Rev.DrugDiscov.2,296–313.
DaSilva,M.M.,Comin,M.,Duarte,T.S.,Foglio,M.A.,Carvalho,J.E.,Vieira,M.C.,
Forma-gio,A.S.N.,2015.Synthesis,antiproliferativeactivityandmolecularproperties
predictionsofgalloylderivatives.Molecules20,5360–5373.
DeYoung,L.M.,Kheifets,J.B.,Ballaron,S.J.,Young,J.M.,1989.Edemaandcell
infil-trationinthephorbolester-treatedmouseeararetemporallyseparateandcan bedifferentiallymodulatedbypharmacologicagents.AgentsAct.26,335–341.
Djeridane,A.,Yousfi,M.,Nadjemi,B.,Boutassouna,D.,Stocker,P.,Vidal,N.,2006.
AntioxidantactivityofsomeAlgerianmedicinalplantsextractscontaining phe-noliccompounds.FoodChem.97,654–660.
El-Massry,K.F.,El-Ghorab,A.H.,Shaaban,H.A.,Shibamoto,T.J.,2009.Chemical
com-positionsandantioxidant/antimicrobialactivitiesofvarioussamplesprepared fromSchinusterebinthifoliusleavescultivatedinEgypt.J.Agric.FoodChem.57, 5265–5270.
Ertl,P.,2014.CalculationofMolecularPropertiesandBioactivityScore,Availableat:
http://www.molinspiration.com(accessedonAugust2016).
Farag,S.F.,2008.PolyphenoliccompoundsfromtheleavesofSchinusterebinthifolius
Raddi.Bull.Pharm.Sci.31,319–329.
Fedel-Miyasato,L.E.S.,Kassuya,C.A.L.,Auharek,A.S.,Formagio,A.S.N.,Cardoso,C.A.L.,
Mauro,M.O.,Cunha-Laura,A.L.,Monreal,A.C.D.,Vieira,M.C.,Oliveira,R.J.,2014a.
Evaluationofanti-inflammatory,immunimodulatory,chemopreventiveand woundhealingpotentialsfromSchinusterebinthifoliusmethanolicextract.Rev. Bras.Farmacogn.24,565–575.
Fedel-Miyasato,L.E.S.,Formagio,A.S.N.,Auharek,A.S.,Kassuya,C.A.L.,Navarro,S.D.,
Cunha-Laura,A.L.,Monreal,A.C.D.,Vieira,M.C.,Oliveira,R.J.,2014b.
Antigeno-toxicandantimutageniceffectsosSchinusterebinthifoliusRaddiinAlliumcepa
andSwissmiceacomparativestudy.Gen.Mol.Res.13,3411–3425.
Formagio,A.S.N.,Iriguchi,E.K.K.,Roveda,L.M.,Vieira,M.C.,Cardoso,C.A.L.,Zárate,
N.A.H.,Tabaldi,L.A.,Kassuya,C.A.L.,2011.Chemicalcompositionand
anti-inflammatory activityof the essential oilof Schinus terebinthifoliusRaddi (Anacardiaceae)fruits.ActaFarm.Bonaer.30,1555–1559.
Gazzaneo,L.R.S.,Lucena,R.F.P.,Albuquerque,U.P.,2005.Knowledgeanduseof
medicinalplantsbylocalspecialistsinaregionofAtlanticForestinthestate ofPernambuco(NortheasternBrazil).J.Ethnobiol.Ethnomed.1,1–9.
Gomes,F.S.,Procópio,T.F.,Lima,T.A.,Napoleão,T.H.,Coelho,L.C.B.B.,Paiva,P.M.G.,
2010.IsolationandantimicrobialactivityoflectinfromSchinusterebinthifolius
leaves.J.Biotechnol.150,453.
Jayaprakasha,G.K.,Singh,R.P.,Sakariah,K.K.,2001.Antioxidantactivityofgrape
seed(Vitisvinifera)extractsonperoxidationmodelsinvitro.FoodChem.73, 285–290.
Johann,S.,Sá,N.P.,Lima,L.A.,Cisalpino,P.S.,Cota,B.B.,Alves,T.M.,Siqueira,E.P.,
Zani,C.L.,2010.Antifungalactivityofschinolandanewbiphenylcompound
iso-latedfromSchinusterebinthifoliusagainstthepathogenicfungusParacoccidioides brasiliensis.Ann.Clin.Microbiol.Antimicrob.9,25–30.
Kassuya, C.A.,Cremoneze,A., Barros,L.F.,Simas,A.S.,Lapa,R.,Mello-Silva, R.,
Stefanello,M.E.,Zampronio,A.R.,2009.Antipyreticandanti-inflammatory
prop-ertiesoftheethanolicextract,dichloromethanefractionandcostunolidefrom
Magnoliaovata(Magnoliaceae).J.Ethnopharmacol.124,369–376.
Kim,S.J., Jin,M.,Lee,E.,Moon,T.C.,Quan,Z., Yang,J.H.,Son,K.H.,Kim,K.U.,
Son,J.K.,Chang,H.W.,2006.Effects ofmethylgallateonarachidonic acid
metabolizingenzymes:cyclooxygenase-2and5-lipoxygenaseinmousebone marrow-derivedmastcells.Arch.Pharm.Res.29,874–878.
Kim,K.H.,EunjungMoon,E.,Choi,S.U.,Kim,S.Y.,Lee,K.R.,2013.Polyphenolsfrom
thebarkofRhusvernicifluaandtheirbiologicalevaluationonantitumorand anti-inflammatoryactivities.Phytochemistry92,113–121.
Leite,S.R.R.F.,Amorim,M.M.R.,Sereno,P.F.B.,Leite,T.N.F.,Ferreira,J.A.C.,Ximenes,
R.A.A.,2011.Randomizedclinicaltrialcomparingtheefficacyofthevaginaluse
ofmetronidazolewithaBrazilianpeppertree(Schinus)extractforthetreatment ofbacterialvaginosis.Braz.J.Med.Biol.Res.44,245–252.
Lipinski,C.A.,Lombardo,F.,Dominy,B.W.,Feeney,P.J.,1997.Experimentaland
com-putationalapproachestoestimatesolubilityandpermeabilityindrugdiscovery anddevelopmentsettings.Adv.Drug.DeliveryRev.23,3–25.
Martorelli,S.B.F.,Pinheiro,A.L.B.,Souza,I.A.,Higino,J.S.,Bravo,F.,2011.Extrato
hidroalcoólicodeSchinusterebinthifoliusRaddi(aroeira)30%emorabase.Int. J.Dent.10,80–90.
Matsuo,A.L.,Figueiredo,C.R.,Arruda,D.C., Pereira,F.V.,Scutti,J.A.,Massaoka,
M.H.,Travassos,L.R.,Sartorelli, P.,Lago,J.H.,2011.␣-Pineneisolatedfrom
SchinusterebinthifoliusRaddi(Anacardiaceae)inducesapoptosisandconfers antimetastaticprotectioninamelanomamodel.Biochem.Biophys.Res. Com-mun.411,449–454.
Moyo, M., Ndhlala, A.R., Finnie,J.F., Van Staden, J.,2010. Phenolic
composi-tion,antioxidantandacetylcholinesteraseinhibitoryactivitiesofSclerocarya birreaandHarpephyllumcaffrum(Anacardiaceae)extracts.FoodChem.123, 69–76.
Monks,A.,Scudiero,D.,Skehan,P.,Shoemaker,R.,Paull,K.,Vistica,D.,Hose,C.,
Langlet,J.,Cronise,P.,Vaigro-Wolff,A.,Ray,G.M.,Campbell,H.,Mayo,J.,Boyd,
M.,1991.Feasibilityofahigh-fluxanticancerdrugscreenusingdiversepanelof
Morton,J.F.,1978.Brazilianpepper:itsimpactonpeopleanimalsandthe environ-ment.Econ.Bot.32,353–359.
Newman,D.J.,Cragg,G.M.,Holbeck,S.,Sausville,E.A.,2002.Naturalproductsand
derivativesasleadstocellcyclepathwaytargetsincancerchemotherapy.Curr. CancerDrugTarg.2,279–308.
Newman,D.J.,Cragg,G.M.,O’Keefe,B.R.,2005.In:Knablein,J.(Ed.),Modern
Bio-pharmaceuticals,Design,DevelopmentandOptimization,vol.2.Wiley-VCH, Weinheim,pp.451–496.
Newman,D.J.,Gragg,G.M.,2012.Naturalproductsassourcesofnewdrugsoverthe
30yearsfrom1981to2010.J.Nat.Prod.75,311–335.
NunesJr.,J.A.T.,Ribas-Filho,J.M.,Malafaia,O.,Czeczko,N.G.,Inácio,C.M.,Negrão,
A.W.,Lucena,P.L.H.,Moreira,H.,WagenfuhrJr.,J.,Cruz,J.J.,2006.Avaliac¸ão
doefeitodoextratohidroalcoólicodeSchinusterebinthifoliusRaddi(aroeira) no processo de cicatrizac¸ão da línea alba de ratos. Acta Cir. Bras. 21, 8–15.
Okoth,D.A.,Hafizah,Y.,Chenia,H.Y.,Koorbanally,N.A.,2013.Antibacterialand
antioxidantactivitiesofflavonoidsfromLanneaalata(Engl.)Engl. (Anacar-diaceae).Phytochem.Lett.6,476–481.
Pietta,P.G.,2000.Flavonoidsasantioxidants.J.Nat.Prod.63,1035–1043.
Rosas,E.C.,Correa,L.B.,Pádua,T.deA.,Costa,T.E.,Mazzei,J.L.,Heringer,A.P.,Bizarro,
C.A.,Kaplan,M.A.,Figueiredo,M.R.,Henriques,M.G.,2015.Anti-inflammatory
effectofSchinusterebinthifoliusRaddihydroalcoholicextractonneutrophil migrationinzymosan-inducedarthritis.J.Ethnopharmacol.175,490–498.
Santana,J.S.,Sartorelli,P.,Lago,J.H.G.,Matsuo,A.L.,2012.Isolamentoeavaliac¸ão
dopotencialcitotóxicodederivadosfenólicosdeSchinusterebinthifoliusRaddi (Anacardiaceae).Quim.Nova35,2245–2248.
Santos,L.C.,Amorim,M.M.R.,2002.Usodaaroeira(SchinusterebinthifoliusRaddi)
paratratamentodeinfecc¸õesvaginais.Femin30,339–342.
Uliana,M.P.,Fronza,M.,Dilva,A.G.,Vargas,T.S.,DeAndrade,T.U.,Scherer,R.,2016.
CompositionandbiologicalactivityofBrazilianrosepepper(Schinus terebinthi-foliusRaddi)leaves.Ind.CropsProd.83,235–240.
Whang,W.K.,Park,H.S.,Ham,I.H.,Oh,M.,Namkoong,H.,Kim,H.K.,Hwang,D.W.,
Hur,S.Y.,Kim,T.E.,Park,Y.G.,Kim,J.R.,Kim,J.W.,2005.Methylgallateand
chemicalsstructurallyrelatedtomethylgallateprotecthumanumbilicalvein endothelialcellsfromoxidativestress.Exp.Mol.Med.37,343–352.
Zhao,M.,Bi,L.,Wang,W.,Wang,C.,Baudy-Floc’h,M.,Ju,J.,Peng,S.,2006.Synthesis