IN ST IT U TE O F B IO M ED IC A L S CI EN CE S A BE L S A LA ZA R
D
iana
S
an
tia
go
d
os
S
an
to
s. C
ha
ra
cte
riz
ati
on o
f b
ro
w
n t
ro
ut
fitn
es
s a
nd
g
on
ad
al m
atu
ra
tio
n a
lo
ng
th
e r
ep
ro
du
cti
ve
c
yc
le
Ch
ara
cte
riz
ati
on o
f b
ro
w
n t
ro
ut fi
tn
es
s a
nd
go
nad
al m
atu
ra
tio
n a
lo
ng
the
re
pro
du
cti
ve
c
yc
le
D
iana
S
an
tia
go
d
os
S
an
to
s
Characterization of brown trout fitness
and gonadal maturation along the
reproductive cycle
Diana Santiago dos Santos
M
2018
M
.ICBAS
2018
MASTER’S DEGREE IN MARINE SCIENCES - MARINE RESOURCES SPECIALIZATION IN MARINE BIOLOGY AND ECOLOGY
MASTER’S DEGREE IN MARINE SCIENCES – MARINE RESOURCES SPECIALIZATION IN MARINE BIOLOGY AND ECOLOGY
Characterization of brown trout fitness and
gonadal maturation along the reproductive
cycle.
Diana Santiago dos Santos
M
2018
Diana Santiago dos Santos
Characterization of brown trout fitness and gonadal maturation
along the reproductive cycle
Dissertation application to the
master’s degree in
Marine Sciences - Marine Resources, submitted to the
Institute of Biomedical Sciences
Abel Salazar,
University of Porto
Supervisor – Tânia Vieira Madureira
Category
– Auxiliary Researcher and Invited Auxiliary
Professor
Affiliation
– Interdisciplinary Centre of Marine and
Environmental Research and Institute of Biomedical
Sciences Abel Salazar, University of Porto
Co-supervisor – Eduardo Jorge Sousa da Rocha
Category – Full Professor
Affiliation
– Institute of Biomedical Sciences Abel
Salazar, University of Porto
iii
Acknowledgements
I would like to thank everyone who, with words or gestures, helped me through this incredible experience. The empathy and friendship I felt were overwhelming and kept me going through the toughest times.
To Professor Doctor Tânia Madureira, my supervisor, I cannot thank enough for all the encouragement and dedication. I feel eternally grateful for your guidance and for continuously trying to help me improve myself.
To Professor Doctor Eduardo Rocha, my co-supervisor, I’m very thankful for your outstanding leadership and integrity, you are an inspiration to me. Thank you so much for the enthusiasm and for the time dedicated to my dissertation.
Thank you so much, Célia Lopes and Fernanda Malhão, you have no idea how much your endless help meant to me. I am very thankful for all the kindness and generosity you have shown me over the past year, it has been an invaluable gift.
To all the staff and students of the laboratory of Histology and Embryology at ICBAS, I thank you for kindly receiving me and providing me with a supportive environment.
To all my friends, for entertaining me in the most stressful moments. I thank you for all the care and affection you’ve given me, you have made the good times better and the hard times a lot easier.
To Telmo Silva, I want to thank you for all the wonderful ways you make me smile, for all the love and for always trying to understand me. It has been a very challenging year and knowing that I have you by my side made all the difference.
To my family, I thank you for the unconditional love and affection. I also thank you for trusting me in my decisions and always supporting me.
To my sister, I know I can always count on you in the most difficult times and for that, I’ll always be grateful. Thanks for looking out for me, sis.
To my mother, I will never stop being grateful to you for how amazing you have been and still are as a parent to me. You are the best doctor, the best teacher and the best listener I could ever ask for. You have helped me in every away during this transition.
To my parents, my eternal gratitude, for all the sacrifices, the difficulties and the efforts to get me where I am today. I will forever hold you close to my heart.
iv This investigation was supported by ICBAS, the FCT – Foundation for Science and Technology, via the Strategic Funding project UID/Multi/04423/2013, the European Regional Development Fund (ERDF) via COMPETE (INNOVMAR, ECOSERVICES NORTE-01-0145-FEDER-000035) and ICNF. A special thanks for the opportunity given to me through this funding responsible entities.
v
Table of Contents
Acknowledgements ... iii
Resumo ... vii
Abstract ... ix
List of Abbreviations ... xi
List of Figures ... xv
List of Tables ... xix
I.
INTRODUCTION ... 1
1.1. Reproduction and Gonad Types in Teleost Fish ...3
1.1.1. Reproductive strategies ...3
1.1.2. Description of gonad structure ...4
1.1.3. Spermatogenesis ...7
1.1.4. Oogenesis... 10
1.2. Endocrinology of Teleost Reproduction ...12
1.2.1. Primary mediators and secondary hormones ... 12
1.3. Reproductive Seasonal Patterns in Salmonids ...14
1.4. The Brown Trout ...15
1.5. Background and Aims of the Study ...17
II.
MATERIALS AND METHODS ... 19
2.1. Study Animals ...21
2.2. Physico-Chemical Parameters of Water ...21
2.3. Sampling Procedures ...21
2.4. Biochemical Analyses of Blood, Plasma and Serum ...22
2.5. Hormone Plasmatic Levels ...23
2.6. Histological Processes of the Gonads ...23
2.6.1. Paraffin procedures ... 23
2.6.2. Methacrylate procedures ... 24
2.7. Grading of Gonadal Maturation ...24
vi
2.9. Data Analysis and Statistics ...25
III.
RESULTS ... 27
3.1. Physico-Chemical Parameters of Water ...29
3.2. Biometric Measurements ...29
3.3. Biochemical Analyses ...32
3.4. Quantification of Plasma Steroid Hormones ...34
3.5. Histological Criteria Established for the Classification of Adult Brown Trout Gonadal Maturation Stages ...35
3.6. Grading Stages of Gonadal Maturation in Adult Brown Trout ...39
3.7. Immunolocalization of CYP17-I, CYP19 and 17β-HSD in Adult Brown Trout Gonads ...40 3.7.1. Testis ... 40 3.7.2. Ovaries ... 44
IV.
DISCUSSION ... 49
V.
CONCLUSION ... 59
VI.
REFERENCES ... 63
VII.
APPENDIXES... 85
Appendix I – Histological processing protocols ...87
Appendix II – Hematoxylin-eosin staining (H&E) protocol for paraffin sections ...88
Appendix III – Technovit 7100 adapted protocol ...89
Appendix IV – Sirius red staining protocol for methacrylate sections (Kiernan)...90
Appendix V – Reticulin staining protocol for methacrylate sections (Bancroft et al., 2013) ...91
Appendix VI – Adapted immunohistochemistry protocol from Novolink™ Polymer Detection Systems (Leica Biosystems RE7280-K, United Kingdom) ...92
Appendix VII – ELISA protocol for 17-OHP ...94
Appendix VIII – ELISA protocol for FSH and LH ...97
vii
Resumo
Existe um extenso interesse ambiental e económico na truta fário (Salmo trutta
fario) para a pesca desportiva, a pesca comercial e a aquacultura. A falta de uma
caracterização sistemática do ciclo reprodutivo da truta fário reduz a oportunidade de utilizar esta espécie num maior número de estudos. O objetivo desta Dissertação é caracterizar a condição física e reprodutiva de machos e fêmeas adultas de truta fário nas fases de maturação/desova (dezembro), regressão (março) e regeneração (julho).
Para isso, uma ampla seleção de parâmetros, desde indicadores bioquímicos de sangue, plasma e soro a parâmetros biométricos foram analisados. Além disso, estabeleceram-se classificações histomorfológicas quer quantitativas, quer qualitativas das fases de desenvolvimento da gónada em microscopia ótica. Adicionalmente, os níveis plasmáticos de FSH – hormona folículo estimulante, LH – hormona luteinizante e 17-OHP – 17-hidroxiprogesterona foram medidos através de ensaios imunoenzimáticos, e a localização imunofenótipica de três enzimas esteroidogénicas (CYP17-I – citocromo P450c17-I, CYP19 – aromatase e 17-HSD – 17β-hidroxiesteroide desidrogenase) foi avaliada em ovários e testículos de truta fário nas diferentes estações.
Tanto para fêmeas como para machos, o índice gonadossomático foi significativamente maior na época de desova comparando com as demais estações, enquanto o peso e o comprimento total atingiram os valores mais baixos em dezembro. Foi obtido um diâmetro dos ovos maior e um número total de ovos menor também em dezembro. O colesterol e as lipoproteínas de baixa densidade (LDL) apresentaram os níveis mais baixos em dezembro, possivelmente devido a um maior investimento de energia na reprodução, particularmente na época de desova. Para além disso, os valores de proteína total, glicose e potássio foram menores na época de desova, tanto para machos como para fêmeas. Não ocorreram variações significativas dos níveis de hemoglobina, hematócrito, triglicerídeos, lipoproteína de densidade muito baixa (VLDL), cálcio total, cálcio ionizado e sódio entre estações. Em relação aos níveis de cloro e fósforo verificaram-se valores significativamente menores nas fêmeas em março, em comparação com dezembro, mas os valores dos machos permaneceram aproximadamente constantes. O magnésio foi significativamente menor em dezembro para as fêmeas, enquanto que nos machos os valores mais baixos foram obtidos em março, e foram diferentes dos obtidos em dezembro e julho. Relativamente às hormonas esteroides plasmáticas quantificadas, a concentração de 17-OHP foi superior em dezembro em relação a março e julho, pois está intimamente relacionada com a maturação do ovário e dos testículos. Nenhuma alteração significativa foi observada para
viii as concentrações de FSH e LH. Na classificação histomorfológica das gónadas foi possível diferenciar quatro fases de maturação nos testículos e cinco nos ovários. A maturação das gónadas em ambos os sexos foram superiores em dezembro, em comparação a março e julho. Foram utilizadas duas técnicas de coloração para avaliar a variabilidade na distribuição das fibras de colagénio nas gónadas ao longo das três estações do ano. A coloração de Sirius red mostrou uma distribuição descontínua e com fraca intensidade nas fibras de colagénio totais dos cortes de testículo de dezembro, que se tornaram contínuas e mais intensas em março e julho. Os vasos sanguíneos e a túnica albugínea apresentaram intensidade moderada em todas as estações do ano. A coloração das fibras reticulares não mostrou grandes diferenças nos testículos. Nos ovários, não houve diferenças aparentes entre estações, tanto com a coloração Sirius red como com a coloração da reticulina. No entanto, ambas as técnicas coraram camadas foliculares de ovócitos primários a ovócitos maduros, e vasos sanguíneos, com uma intensidade moderada.
Em relação à imunolocalização do CYP17-I, um forte sinal foi detetado nas células de Leydig, e também nas espermatogónias e células de Sertoli. Em março e julho, os grupos de células intersticiais foram menores, resultando numa imunomarcação menos intensa/abundante. Nos ovários, a reatividade nas camadas da granulosa e da teca aumentou de dezembro para março e julho. Um forte sinal positivo também foi detetado no ooplasma dos ovócitos primários. A imunoexpressão do CYP19 foi leve/moderada no interstício e nas espermatogónias em dezembro, diminuindo nas outras estações. Uma coloração mais forte foi também observada no endotélio dos vasos sanguíneos. Em dezembro, foi observada uma imunorreação intensa na granulosa e na teca que diminuiu ao longo do ciclo. A marcação da 17β-HSD foi específica e intensa em espermatogónias, em todas as estações do ano. Nos ovários, foi encontrado um sinal positivo na granulosa, que aumentou de dezembro para março e julho. Verificou-se igualmente uma imunorreação forte no epitélio dos canais eferentes (células colunares e cúbicas) com todos os anticorpos.
De um modo geral, os resultados apontam para variações específicas da biometria da truta fário, bioquímica do sangue, plasma e soro, fases de maturação das gónadas e imunolocalização das três enzimas que metabolizam esteroides nas gónadas, ao longo do ciclo reprodutivo. Estes novos dados podem servir como valores fisiológicos de referência para futuros ensaios experimentais com esta espécie.
ix
Abstract
There is an extensive environmental and economic interest in brown trout (Salmo
trutta fario) for sports, commercial fisheries and aquaculture. The lack of a systematic
characterization of brown trout reproductive cycle reduces the opportunity of a wider range of studies using this species. The aim of this Dissertation is to characterize the fitness and reproductive stages of female and male adult brown trout at spawning capable (December), regressing (March) and regenerating (July) seasonal stages.
For that purpose, a large portfolio of distinct parameters was selected including blood, plasma and serum biochemical indicators, along with fish biometry. A quantitative and qualitative histomorphological grading of the gonadal stages was established under light microscopy. Also, the plasma levels of FSH – follicle stimulating hormone, LH – luteinizing hormone and 17-OHP – 17-hydroxyprogesterone were measured by enzyme-linked immunosorbent assays (ELISA), and the immunophenotype location of three key steroidogenic enzymes (cytochrome P450c17-I – CYP17-I, aromatase – CYP19 and 17β-hydroxysteroid dehydrogenase – 17β-HSD) was assessed in brown trout ovaries and testis during the distinct seasonal stages.
For both females and males, the gonadosomatic index was significantly higher in the spawning capable season comparing with the other seasons, while weight and length reached the lowest levels in December. Females showed the larger egg diameter and the lowest total number of eggs also in December. Cholesterol and low-density lipoproteins (LDL) showed the lowest values in December, possibly due to a higher energy investment in reproduction, particularly in spawning capable season. Also, values of total protein, glucose and potassium were lower in the spawning capable season, for both males and females. No significant variations occurred for hemoglobin, hematocrit, triglycerides, very-low density lipoprotein (VLDL), total calcium, ionized calcium and sodium during the sampled seasons. Regarding chlorine and phosphorus levels, there were significantly lower levels in females from March, comparing to the ones from December, but male values were roughly constant. Magnesium was significantly lower in December for females, while for males the lower values from March differed from the ones obtained in December and July. Among the plasma steroid hormones quantified, the 17-OHP was higher in December than in March and July, because it is closely related to the maturation of the ovary and the testis. No significant alterations were observed for FSH and LH. Four maturation stages were identified in the testes and five in the ovaries. The maturation stage of both gonads was higher in December comparing with March and July. Two staining techniques were used to evaluate the variability in the distribution of collagen
x fibers in gonads along the three seasons. The Sirius red staining showed a discontinuous distribution and weak intensity of total collagen fibers in testis sections from December, which became continuous and more intense in March and July. Blood vessels and the tunica albuginea showed moderate intensity in all seasons. The reticulin staining in the testis did not changed too much. In the ovaries, there was not apparent differences between seasons by using both Sirius red and reticulin staining. Despite that, both techniques stain the follicular layers of primary to mature oocytes, and blood vessels with a moderate intensity.
Regarding the immunolocalization of CYP17-I, a strong signal was detected in the Leydig cells, in spermatogonia and Sertoli cells. In March and July, the interstitial cell groups were smaller, resulting in a less intense/abundant immunoreaction. In the ovaries, the reactivity in the granulosa and theca layers increased from December to March and July. A strong positive signal was also detected in the ooplasm of primary oocytes. CYP19 immunostaining was mild/moderate in the interstitium and spermatogonia in December, which declined in the other seasons. A stronger staining was also noted in the endothelium of blood vessels. A strong immunoreaction in the granulosa and theca layers was observed in December, which decreased along the cycle. The 17β-HSD immunostaining was specific and intense in spermatogonia in all seasons. In the ovary, a positive signal was found in the granulosa, which increased from December to March and July. A strong immunoreaction in the epithelium of efferent ducts (columnar and cuboidal cells) was noted with all antibodies.
Overall, data point to specific variations of brown trout biometry, blood, plasma and serum biochemistry, maturation stages of gonads and immunolocalization of three steroid-metabolizing enzymes in the gonads, along the reproductive cycle. These new outputs may serve has baseline physiological reference levels for future experimental trials with the species.
xi
List of Abbreviations
11-KT – 11-Ketotestosterone 11β-HSD – 11β-Hydroxysteroid dehydrogenase 17-OHP – 17-Hydroxyprogesterone 17α,20β-DP – 17α,20β-Dihydroxy-4-pregnen-3-one 17β-HSD – 17β-Hydroxysteroid dehydrogenase 20β-HSD – 20β-Hydroxysteroid dehydrogenase 3β-HSD – 3β-Hydroxysteroid dehydrogenase BM – Basement membraneBSA – Bovine serum albumin BV – Blood vessel
Cao – Cortical alveolar oocytes
CYP11 – P450c11/Cytochrome P450 family 11 CYP17 – P450c17/Cytochrome P450 family 17
CYP17a1 – P450c17a1/Cytochrome P450 family 17 subfamily A member 1 CYP17-I – Cytochrome P450c17-I
CYP19 – Aromatase/Cytochrome P450 family 19 DAB – 3,3’-Diaminobenzidine
E2 – 17β-Estradiol ED – Efferent ducts
EDTA – Ethylenediamine tetraacetic acid ELISA – Enzyme-linked immunosorbent assay EU – European Union
Evo – early vitellogenic oocytes FSH – Follicle stimulating hormone
xii GC – Germinative compartment GE – Germ cells GH – Hardness GnRH – Gonadotropin-releasing hormone Gr - Granulosa
GSI – Gonado-somatic index GTH – Pituitary gonadotropins H&E – Hematoxylin and eosin HDL – High-density lipoprotein HRP – Horseradish peroxidase HSI – Hepato-somatic index IC – Interstitial compartment In – Interstitium K – Condition factor LC – Leydig cells LDL – Low-density lipoprotein LH – Luteinizing hormone Lvo – Late vitellogenic oocytes MIS – Maturation-inducing steroid MPF – Maturation-promoting factor Mso – Mature/spawning oocytes Mvo – Mid-vitellogenic oocytes
N02- – Nitrite
N03- – Nitrate
NH3 – Ammonia
xiii OD450 – Optical density measured with a 450nm filter
OECD – Organisation for Economic Co-operation and Development Oo – Ooplasm
P450scc – P450 side-chain-cleavage PBS – Phosphate buffered saline PGC – Primordial germ cells PM – Peritubular myoid cells Po – Perinucleolar oocytes RSI – Reno-somatic index SC – Sertoli cells
SSI – Spleno-somatic index
StAR – Steroidogenic acute regulatory SZ – Spermatozoa T – Testosterone TBS – Tris-buffered saline Temp – Temperature Th – Theca TMB – 3,3′,5,5′-Tetramethylbenzidine VLDL – Very-low-density lipoprotein Vtg – Vitellogenin Zr – Zona radiata
xv
List of Figures
Fig. 1 – Schematic illustration of teleost fish testis (A), including both interstitial and germinative compartments (B), based on Schulz et al., 2011. IC – interstitial compartment; GC – germinative compartment; BM – basement membrane; LC – Leydig cells; BV – blood vessel; PM – peritubular myoid cells; SC – Sertoli cells; GE – germ cells; SZ – spermatozoa. ...6 Fig. 2 – Illustration of the fundamental structural compartments of fish oocyte and of an intact ovarian follicle, based on Tyler et al., (1996)...7 Fig. 3 – Representation of steroid synthesis in teleost thecal and granulosa layers of the ovarian follicle and the testis, Leydig cells and sperm. Steroid hormones are in boxes, enzymes are in bold and next to arrows that connect the boxes, based on (Lubzens et al., 2010), Bain et al., (2015) and Nagahama, (1994). P450scc – P450 side-chain-cleavage; 3β-HSD - 3β-hydroxysteroid dehydrogenase; CYP17 – cytochrome P450c17; 17β-HSD – 17β-hydroxysteroid dehydrogenase; 17-OHP – 17-hydroxyprogesterone; HSD – 20β-hydroxysteroid dehydrogenase; CYP19 – aromatase; CYP11 – cytochrome P450c11; 11β-HSD – 11β-hydroxysteroid dehydrogenase...14 Fig. 4 – Egg diameter (mm) (A) and total number of eggs (B) in female adult brown trout gonads in distinct sampling seasons (December, March and July). Graphical data correspond to median (line in the box) and maximum and minimum values (in the whiskers). Different low case letters mean significant differences (p ≤ 0.05) between seasons, by the Tukey test. N = 6 animals/season, except in December (N = 7). ...30 Fig. 5 – Plasma concentrations (ng/mL) of 17-OHP (A), FSH (B) and LH (C) in male and female adult brown trout along distinct sampling seasons (December, March and July). Graphical data correspond to median (line in the box) and maximum and minimum values (in the whiskers). N = 6 animals/gender/season. ...34 Fig. 6 – Representation of paraffin sections (H&E staining) of adult brown trout testes at distinct developmental stages: (A) undeveloped; (B) early spermatogenic; (C) mid-spermatogenic; (D) late mid-spermatogenic; Arrows ( ) point to cists with spermatozoa; Arrow heads (►) point to interstitial compartment. ...35 Fig. 7 – Representation of methacrylate sections of adult brown trout testes stained with Sirius red (December – A; March – B and July – C) and reticulin (December – D; March – E and July – F). Arrows (➝) point to collagen. Arrow heads (►) correspond to reticulin. .36
xvi
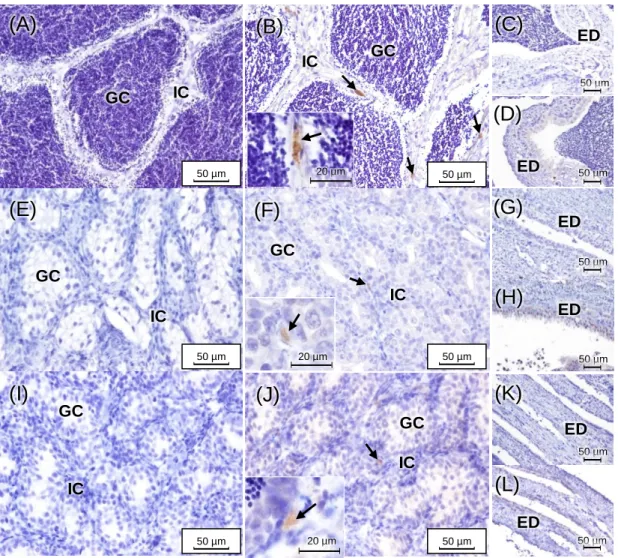
Fig. 8 – Representation of paraffin sections (H&E staining) of adult brown trout ovaries at distinct developmental stages: (A) early development; (B) mid-development; (C) late development; (D and E) late development/hydrated; (F) post-ovulatory. Oo – ooplasm; Zr – zona radiata; Gr – granulosa; Th – theca; In – interstitium; Po – perinucleolar oocytes; Cao – cortical alveolar oocytes; Evo – early vitellogenic oocytes; Mvo – mid-vitellogenic oocytes; Lvo – late vitellogenic oocytes; Mso – mature/spawning oocytes. ...38 Fig. 9 – Representation of methacrylate sections of adult brown trout ovaries stained with Sirius red (A – March and B – July) and reticulin (C – March and D – July). Arrows (➝) point to collagen. Arrow heads (►) correspond to reticulin. ...39 Fig. 10 – Adult brown trout gonadal maturation stages for both genders along the distinct sampling seasons (December, March and July). Ovary maturation stages: 1 – early development; 2 – mid-development; 3 – late development; 4 – late development/hydrated; 5 – post-ovulatory. Testes maturation stages: 0 – undeveloped; 1 – early spermatogenic; 2 – mid-spermatogenic; 3 – late spermatogenic. Values correspond to the medians with their respective maximum and minimum. Different low case letters mean significant differences (p ≤ 0.05) among sampling seasons within a gender by the Tukey test. N = 6 animals/gender/season, except in females from December (N = 7) and males from July (N = 8). ...40 Fig. 11 – CYP17-I immunoreactivity in adult brown trout testes in December (A–D), March (E–H) and July (I–L). Negative controls for each maturing phase are also shown (December – A and C; March – E and G; and July – I and K). GC – germinal compartment; IC – interstitial compartment; ED – efferent ducts; Arrows (➝) point to Leydig cells. ...41 Fig. 12 – 17β-HSD immunoreactivity in adult brown trout testes in December (A–D), March (E–H) and July (I–L). Negative controls for each maturing phase are also shown (December – A and C; March – E and G; and July – I and K). GC – germinal compartment; IC – interstitial compartment; ED – efferent ducts; Arrow heads (►) correspond to spermatogonia. ...42 Fig. 13 – CYP19 immunoreactivity in adult brown trout testes in December (A and B), March (C and D) and July (E and F). Negative controls for each maturing phase are also shown (December – A; March – C; and July – E). GC – germinal compartment; IC – interstitial compartment; ED – efferent ducts; Arrow heads (►) correspond to spermatogonia. ...43
xvii
Fig. 14 – CYP19 immunoreactivity in adult brown trout testes in December (A, B, E and F), March (C, G, H and K) and July (D, I, J and L). Negative controls are also shown for blood vessels – A and efferent ducts – E (December), G (March) and I (July). GC – germinal compartment; IC – interstitial compartment; ED – efferent ducts; BV – blood vessel. Arrows (➝) correspond to peritubular myoid cells. ...44 Fig. 15 – CYP17-I (B and F), CYP19 (C and G) and 17β-HSD (D and H) immunolocalization in adult brown trout ovaries in December. Negative controls are shown in (A) and (E). Arrows (➝) and arrow heads (►) point to granulosa and theca cells, respectively. ...46 Fig. 16 – CYP17-I (B and F), CYP19 (C and G) and 17β-HSD (D and H) immunolocalization in adult brown trout ovaries in March. Negative controls are shown in (A) and (E). Arrows (➝) and arrow heads (►) point to granulosa and theca cells, respectively. ...47 Fig. 17 – CYP17-I (B and F), CYP19 (C and G) and 17β-HSD (D and H) immunolocalization in adult brown trout ovaries in July. Negative controls are shown in (A) and (E). BV – blood vessel; Arrows (➝) and arrow heads (►) point to granulosa and theca cells, respectively. ...48
xix
List of Tables
Table 1 – Biometric data of adult brown trout along distinct sampling seasons (December, March and July). ...31 Table 2 – Biochemical data obtained from total blood, plasma or serum of adult brown trout from distinct sampling seasons (December, March and July). ...33 Table 3 – Detailed steps of the routine and long histological processing protocols...87 Table 4 – Stages of methacrylate manual processing using fragments stored in ethanol 70%. All fragments were processed in the short processing except gonads with eggs from December, which were handled using the long protocol. ...89
1
3
1.1. Reproduction and Gonad Types in Teleost Fish
1.1.1. Reproductive strategies
Reproduction in teleost fish occurs by distinct reproductive strategies, due to the substantial number of species and diversity of aquatic environments (Jalabert, 2005). The majority of teleost fish are gonochoristic, which means that individuals retain distinct sexes, being either male or female (Patzner, 2008). In primary gonochorism, an indifferent gonad directly turns into an ovary or a testis, and in secondary gonochorism, the gonad begins by being intersexual, and then differentiates into ovary or testis (Rocha et al., 2008). In contrast to gonochorism, the hermaphroditism is less frequent in teleosts, and the most usual variants are protandry and protogyny, two forms of sequential hermaphroditism (Wootton et al., 2015). Protandrous individuals are mature males throughout a part of their life cycles, up until sex inversion into breeding females (Jalabert, 2005), as barramundi (Lates calcarifer) (Guiguen et al., 1994). The protogynous individuals, such as the grouper (Epinephelus
tauvina) (Tan et al., 1974), undergo the opposite inversion from mature females to males,
producing spermatozoa. Sex inversion may occur more than once in a lifetime (Baroiller et al., 1999). Simultaneous hermaphroditism is characterized by the presence of both active gonads (ovary and testis), at the same time. This synchronized occurrence of both male and female haploid gametes may enable the occurrence of self-fertilization, as in mangrove killifish (Kryptolebias marmoratus) (Tatarenkov et al., 2012), or allow individuals to outcross and mate with each other (Wootton et al., 2015).
Another reproductive strategy that is rare, but can also be present in teleosts is parthenogenesis, which happens when only the female genome is transmitted through generations. Parthenogenesis is divided in two forms: gynogenesis and hybridogenesis, that have been previously reviewed in detail (Chevassus, 1998). In gynogenic species, such as in amazon molly (Poecillia formosa) (Hubbs et al., 1932), spermatozoa are only needed for the activation of the oocytes. In hybridogenesis, as in the genus Poeciliopsis (Schultz, 1969), both maternal and paternal genomes are expressed at a somatic level, but after a non-recombinant meiosis, one of them is discarded (Jalabert, 2005).
The fertilization in teleosts is usually external, right after the synchronized release of the eggs, so-called spawning (Jalabert, 2005). Teleosts that form yolk comprising eggs are named oviparous (Schultz, 1969), such as the common carp, (Cyprinus carpio) (Linhart et al., 1995), cod (Gadus morhua) (Marteinsdottir et al., 2000), Atlantic salmon (Salmo salar) (Saltveit et al., 2013). Internal fertilization using an intromittent organ is also possible, but
4 occurs only occasionally in teleost species, where the fertilized egg may develop inside the ovary or be deposited after fertilization (Jalabert, 2005, Wootton et al., 2015). When embryos grow inside the female reproductive system, they are classified as viviparous (Lubzens et al., 2010), as in mosquito fish (Gambusia affinis), guppy (Poecilia reticulata) (Jalabert, 2005) or bulldog goodeid (Alloophorus robustus) (Mendoza, 1962).
An important feature of fish reproduction is the ovarian development, which can be classified as synchronous, group-synchronous and asynchronous (Wallace et al., 1981). In synchronous development, all the oocytes are in the same stage of growth and ovulate at the same time. Group-synchronous occurs when there are at least two noticeable groups of developing oocytes in the ovary, throughout the cycle. An example species is the yellowtail flounder (Pleuronects ferrugineus), which is a batch spawner (Manning et al., 1998). In salmonids, egg spawning is an annual occurrence, as in brown trout (Salmo trutta) (Bagenal, 1969), or once in a lifetime event, as in pink salmon (Oncorhynchus gorbuscha) (Dye et al., 1986). Lastly, asynchronous development involves the presence of a heterogeneous group of oocytes, without any predominant stage, but all stages co-exist (Lubzens et al., 2010). There are several marine species known to be asynchronous, like the sea bass (Dicentrarchus labrax) (Carrillo et al., 1989) and the Atlantic halibut (Hippoglossus
hippoglossus) (Norberg et al., 1991).
1.1.2. Description of gonad structure
Ovaries and testes are endocrine organs that generate gametes and produce several steroid hormones (Grier et al., 2009). Teleost gonads develop in the dorsolateral covering of the peritoneal cavity, and it is suggested that the primordium tissue is responsible for the ontogeny of both organs (Baroiller et al., 1999). Major morphologic variations are present in differentiated gonads, which allow the identification of both sexes. The vertebrate gonads include somatic cells and germ cells, which latter differentiate into gametes (Wootton et al., 2015). In both males and females, somatic tissues have the same embryological origin (Nakamura et al., 1998). In some teleosts, the male gonads start developing as proto-ovary, before differentiating into testis (Wootton et al., 2015). Germ cells are first denominated as primordial germ cells (PGCs) and are originated out of the gonad, then migrating to the primordial gonad, where the somatic cells remain (Yamaha et al., 2010). At this point, both PGCs and somatic cells have the potential to develop male or female features. The conversion occurs in the differentiation process, much depending on the chemical communication between the somatic cells and PGCs. Oocytes grow by maintaining the same
5 spherical shape of the PGC, while sperm goes through remarkable transformations, becoming flagellated and motile spermatozoa, which are produced in large quantities by the testis.
▪ Testis
The majority of teleosts have an elongated pair of testes with a single sperm duct ascending from the posterior mesodorsal area of each testis (Nagahama, 1983). There is a great diversification of testicular structures in teleost species, but the basic forms two tubular types, recognized according to the distribution of spermatogonia (Grier et al., 1980). Primitive bony fish, such as the ones belonging to the orders of Salmoniformes and Cypriniformes have an “anastomosing tubular testis” structure (Grier, 1993, Schulz et al., 2010), in which tubules anastomose around the efferent ducts and do not terminate at the periphery of the testis, as described by (Uribe et al., 2014). In this unrestricted spermatogonial testis, spermatogonia occur along the full length of the tubule. In contrast, usually Atheriniformes present a restricted spermatogonial testis type with spermatogonia located on the distal terminus of tubules (Grier, 1981). Bony fish testis are constituted of two compartments, the interstitial and the germinative compartment separated by a basement membrane (Fig. 1) (Uribe et al., 2014). The interstitial tissue consist of steroidogenic Leydig cells, blood/lymphatic vessels and connective tissue components, as collagen fibers and peritubular myoid cells (Schulz et al., 2010), also previously called tubule boundary cells (Grier et al., 1980, Grier, 1981). The germinative compartment is composed by the germinal epithelium, which contains somatic Sertoli cells and germ cells in distinct stages of development from spermatogonia to differentiated spermatozoa (Schulz et al., 2010, Schulz et al., 2011). Among the vertebrates, Sertoli cells rest on a supporting layer, the basement membrane (Uribe et al., 2014). The number of germ cells and their stage of development depends on the age of the male fish and the maturation stage along the natural reproductive cycle (Schulz et al., 2011). Fish spermatogenesis occurs in a cystic structure, and during its course there is an increase in both germ and Sertoli cells (details in section 1.1.3) (Schulz et al., 2005).
6 Fig. 1 – Schematic illustration of teleost fish testis (A), including both interstitial and germinative compartments (B), based on Schulz et al., 2011. IC – interstitial compartment; GC – germinative compartment; BM – basement membrane; LC – Leydig cells; BV – blood vessel; PM – peritubular myoid cells; SC – Sertoli cells; GE – germ cells; SZ – spermatozoa.
▪ Ovary
The ovary of most teleosts is a paired organ, although some species fuse the organ in one, during early development as in the medaka (Oryzias latipes) and in some viviparous clades, such as the livebearers (Poeciliidae) and goodeids (Goodeidae) (Grier et al., 2009). Synchronous ovary is present in species that spawn once and then die, like the anadromous
Oncorhynchus species. Group-synchronous ovary relates to teleosts that spawn once a year,
and have a small breeding season, as the rainbow trout (Oncorhynchus mykiss). Lastly, an asynchronous ovary spawns many times a year, and has a long breeding season, as the goldfish (Carassius auratus) (Nagahama, 1983). Viviparous teleosts, such as the poeciliid guppy, have a single ovary, where the mature eggs go through an internal fertilization and are kept inside until the embryonic development is achieved (Nagahama, 1983).
(B)
GC
IC
(A)
Testis
IC
BV
LC
PM
BM
GC
SZ
SC
GE
50 µm7 Oocytes, the female germ cells, grow within the ovarian follicles, which are supported by a basement membrane (basal lamina) (Reading et al., 2017). The granulosa cell layer surrounds the oocyte and is composed of follicle cells, which multiply forming a continuous follicular layer. Externally, there is a peripheral layer composed by thecal cells, which results from the organization of the surrounding stromal connective tissue elements (Fig. 2) (Nagahama, 1983). The development and growth of these structures during maturation, leads to the oocyte transformation into an egg during the ovulation (Reading et al., 2017). The complex egg formation features enormous structural and functional variations (Cerdà et al., 2008), during a process called oogenesis, which will be detailed above (section 1.1.4).
Fig. 2 – Illustration of the fundamental structural compartments of fish oocyte and of an intact ovarian follicle, based on Tyler et al., (1996).
1.1.3. Spermatogenesis
Cysts formation starts with a meiotic division of spermatogonia that differentiate into primary spermatocytes. By the end of the first meiotic division, the secondary spermatocytes are formed. The latter become spermatids through the second meiotic division, but still aren’t capable of functioning as gametes. Differentiation must occur into spermatozoa, a procedure denominated spermiogenesis (Schulz et al., 2011). In late spermatogenesis, a reduction of the proliferative spermatogonia and Sertoli cells is observed (Uribe et al., 2014). When spermatogenesis is completed, spermiation is induced by the disruption of the connection between germ and Sertoli cells, opening the cysts and becoming continuous with the lumen
8 of the tubules (Schulz et al., 2011). This process comprises a reorganization of the nucleus and the cytoplasm, to form the flagellum (Nagahama, 1983). To better understand the spermatogenesis process, it is essential to recognize the main characteristics of the distinct cellular components, as follows.
▪ Sertoli cells
Sertoli cells play a crucial role by supporting and enveloping a group of spermatogenic cells, forming the spermatocyst. These cells have a clearly defined elongated or triangular nucleus, variable nucleoli, and a frequently indistinct cytoplasm. Sertoli cells are generally present as single cells, and in some cases, they may be similar to spermatogonia (OECD, 2009). Cytoplasmic lipids in the cytoplasm of Sertoli cells vary among the species, which may reflect the minor differences in the role of these cells (Grier et al., 1980). Their ultrastructure reflects characteristics of steroid-producing cells, suggesting a role in steroid synthesis or storage locations of steroid hormones (Grier et al., 1977). In the beginning of the reproductive cycle, most of the tubules are empty and are lined only by Sertoli cells. The greatest development of Sertoli cells correlates with the maximum spermiogenic activity in the testis, thus having an important role in germ cell maturation. They involve the developing cysts and occupy the unfilled areas among them in this stage (Cruz-Landim et al., 2005). When the testis are fully developed, Sertoli cells assume a reabsorption function, particularly during regression stages, phagocytizing residual sperm and bodies, and apoptotic germ cells (Cruz-Landim et al., 2005, Schulz et al., 2010).
• Leydig cells
Leydig cells are interstitial components of the testes, in close association with blood vessels (Grier, 1981), and it is suggested that Leydig cells have the characteristics of steroidogenic cells and are involved in male sex steroidogenesis (Chung et al., 2010). These cells have round or oval dark and dense nuclei with varying amounts of vacuolated cytoplasm. In comparison to germ cells, interstitial cells are usually present in low numbers, as single cells or small aggregates, within the interlobular interstitium (OECD, 2009). Leydig cells remain undifferentiated through the germ cell maturation, and its development occurs with the degeneration of somatic cells, in spermiation, which is accompanied by the increasing levels of steroid hormones (Pudney, 1987).
9
▪ Peritubular myoid cells
Teleost peritubular cells are located outside of the basement membrane forming a peritubular discontinuous layer bordered by Leydig cells or blood vessels. They comprehend several microfilaments parallel to the axis of the cell, elongated nucleus and collagen fibrils that may be detected between peritubular cells and the basement membrane (Grier et al., 1980, Grier, 1981). The long and flat cells with dense bodies have the same ultrastructural features as myoid cells characterized in trout and carp, and may be related to the sperm transportation, identical to the peritubular cells in mammals (Timmermans et al., 1992, Cauty et al., 1995).
▪ Spermatogonia
Spermatogonia are located in the germinal epithelium and are present during almost the entire reproductive cycle. Primary spermatogonia are the largest germ cells, usually having centered spherical nuclei, one or two prominent nucleoli and light cytoplasmic granules. They form clusters of secondary spermatogonia by dividing mitotically. The secondary ones are smaller and spherical, with a sphere-shaped nucleus and one or two nucleoli. They are limited by cytoplasmatic extensions of Sertoli cells, forming clusters (Grier et al., 1980, OECD, 2009, Uribe et al., 2014).
▪ Spermatocytes
When spermatogonia replicate their chromosomes and enters meiosis, they become primary spermatocytes. Meiosis comprises two cell divisions, one involving the primary spermatocytes and the other involving the secondary ones. Primary cells are round and with a comparable size to secondary spermatogonia. Secondary spermatocytes are also spherical, in addition to the smaller size and filamentous chromosomes, prepared for the second division of meiosis (Uribe et al., 2014). Spermatocytes are one of the most usual spermatogenic cells, and often lead to a larger spermatocyst (OECD, 2009).
▪ Spermatids
Spermatids have round shape and nucleus and they don’t divide. Instead, its morphology changes to become spermatozoa, through spermiogenesis. The process includes the establishment of the sperm head with the condensed nucleus, the intermediate portion and the flagellum (Uribe et al., 2014). Spermatids have dense nuclei and narrow borders of
10 eosinophilic cytoplasm. These are the smallest in the germinal epithelium, and they lose their cytoplasmic attachments in spermiogenesis (OECD, 2009).
▪ Spermatozoa
Spermatozoa have the smallest size among spermatogenic cells and they are present as individual cells in the tubular lumen. The nuclei is dark, round with a minimal or indistinct cytoplasm, and tails are usually not visible in histological sections (OECD, 2009). As spermiogenesis progresses, spermatozoa become motile (Uribe et al., 2014). These cells reach their maximum total when the testes have developed completely (Cruz-Landim et al., 2005). During spermiation, the cysts are exposed and the spermatozoa is release (Uribe et al., 2014).
1.1.4. Oogenesis
Oogenesis, the development of oogonia into mature oocytes (prepared to be ovulated) (Wallace et al., 1981), is relatively consistent across species. Even though the duration of the its stages changes between species (Tyler et al., 1996). This process can last a few days, which happens in the zebrafish (Danio rerio), as well as a few months, as in salmonids (Knoll-Gellida et al., 2007). The time spent in each stage is predominantly regulated by pituitary-produced gonadotropins, follicle-stimulating hormone (FSH), in the earlier development, and luteinizing hormone (LH), in later stages (Wootton et al., 2015) (detailed in section 1.2). The most usual, morphological criteria for staging ovaries include size, amount and distribution of various cell inclusions, particularly yolk granules (Nagahama, 1983). A brief description of the main aspects of ovarian germ cells can be reviewed below.
▪ Oogonia
Oogonia proliferate through mitosis until they develop to be primary oocytes (chromatin nucleolar oocytes and perinucleolar oocytes) (McMillan, 2007). They are round and the smallest oocytic cells with an prominent nucleolus in the large nucleus. Oogonia have a confined area of clear cytoplasm and are solitary cells or appear in small nests in the ovarian germinal epithelium, conventionally in small numbers (Janssen et al., 1995, OECD, 2009).
11
▪ Chromatin nucleolar oocytes
These primary oocytes are slightly larger than oogonia. Pre-granulosa cells surround the oogonia forming a primordial follicle enclosed by a basement lamina. The central nucleus grows, giving the chromatin nucleolar oocyte a relatively large nucleus that has a single, large and basophilic nucleolus. Scarce cytoplasm becomes lightly denser and granular (Khoo, 1979, Janssen et al., 1995, OECD, 2009).
▪ Perinucleolar oocytes
The oocyte develops by size expansion, the enlargement of the cytoplasm and nucleoli multiplication, usually at the periphery of the nucleus (Janssen et al., 1995, OECD, 2009). The nucleoli proportions and morphology vary among teleosts, but its presence is universal (Wallace et al., 1981). Early perinucleolar oocytes have homogeneous and basophilic cytoplasm, unlike late ones, which undergo modifications in the basophilic nature, originating a less dense staining with hematoxylin. These cells are also present in adult ovaries (Janssen et al., 1995, OECD, 2009).
▪ Cortical alveolar oocytes
Cortical alveolar oocytes are usually larger than perinucleolar oocytes and are differentiated by yolk-like vesicles resembling cortical alveoli located in various depths of the ooplasm. It is not yolk, because it doesn’t nourish the embryo. It increases in size and number as they allocate in the periphery of the ooplasm (Wallace et al., 1981). The chorion, which is an acellular multilayered cover of the oocyte (Cotelli et al., 1988), becomes visible as well as the perifollicular cells (OECD, 2009). The cortical alveoli multiplies and becomes heterogenous in the ooplasm. At the end of this phase, almost all cytoplasm is occupied by cortical alveoli (McMillan, 2007).
▪ Early to late vitellogenic oocytes
As cortical alveolar oocytes increase in size, they become early vitellogenic. Other indication of the development of this process is the arise of the centralized, spherical and eosinophilic vitellogenic yolk granules (OECD, 2009). Early maturation is distinguished by the fast volume increase of the follicles. Also, endocytic activity is maintained at their surface, which does not happen in late maturation (McMillan, 2007).
12 Late maturation is characterized by an increasing number of vitellogenic granules. Cortical alveolar matter is shifted and the nucleus starts migrating to the periphery of the cell (OECD, 2009). The follicle keeps growing due to the hydration process (McMillan, 2007).
▪ Mature/Spawning oocytes
At this stage, oocytes reach their critical proportion and hydration just before spawning. The nucleus as already reached the periphery and is being dissolved, although it is frequently not detectable in larger oocytes because of the plane sectioning. The oocyte is surrounded by a thick zona radiata layer and by noticeable granulosa and theca layers (Janssen et al., 1995, OECD, 2009).
1.2. Endocrinology of Teleost Reproduction
1.2.1. Primary mediators and secondary hormones
Environmental factors, such as biorhythms, nutrition and seasonal changes mediate the production of gonadotropin-releasing hormone (GnRH) by the hypothalamus, which induces the production of the pituitary gonadotropins (GTHs). Fish produce two GTHs, with homology to other vetebrates, the FSH and LH (Kawauchi et al., 1989). The release of these hormones may be inhibited by hypothalamus secreted dopamine (Yaron et al., 2011). GTHs act indirectly through the biosynthesis of steroid hormones, mediating gametogenesis stages and generating responses by the gonads (Nagahama, 1994, Yaron et al., 2011).
In the early 80’s an in vitro approach with theca and granulosa layers of amago salmon (Oncorhynchus rhodurus) oocytes evidenced that both types of cells are needed for 17β-estradiol (E2) synthesis after GTH stimulation. The process has been described as occurring biphasically; first the theca produces testosterone (T), that is then aromatized to E2 in the granulosa (Fig. 3) (Kagawa et al., 1982). The E2 secreted into the bloodstream induces hepatic vitellogenin (Vtg) synthesis, which is subsequently incorporated into the oocytes promoting its enlargement during secondary growth (Tyler et al., 1988). In rainbow trout, FSH seems to have a leading role in regulating the vitellogenic growth stage (Tyler et al., 1997), but also in the previtellogenic oocyte growth in salmon, showing positive correlations with E2 and other factors (Campbell et al., 2006). In addition, there are in vitro studies pointing to the interference of the 11-ketotestosterone (11-KT) androgen in the early oocyte growth in Atlantic cod (Gadus morhua) (Kortner et al., 2009) and coho salmon (Oncorhynchus kisutch) (Forsgren et al., 2012). In salmon, the oocyte maturation is greatly induced by LH in
13 granulosa cells, through the conversion of 17α-hydroxyprogesterone (17-OHP), produced in theca layer, into the the maturation-inducing steroid (MIS), namely 17α,20β-Dihydroxy-4-pregnen-3-one (17α,20β-DP) by the 20β-hydroxysteroid dehydrogenase (20β-HSD) (Suzuki et al., 1988). MIS stimulates folicullar maturation and ovulation by inducing the production of maturation-promoting factor (MPF) (Nagahama, 1994, Yaron et al., 2011). In salmonids, the 17α,20β-DP is the progestin acepted as the main MIS (Jalabert et al., 1986). During oocyte maturation, the granulosa layer decreases the E2 synthesis and increases the 17α,20β-DP levels (Kanamori et al., 1988).
In males, low concentrations of E2, which is considered a female hormone, are also detected (Schulz et al., 2011). E2 controls mitotic spermatogonia divisions, in germ cell renewal, and is produced by interstitial Leydig cells, which are controled by FSH (Yaron et al., 2011). Estrogen receptors are present in Sertoli cells and spermatids, these estrogens are important to spermatogenesis, being involved in metabolism and cellular comunication or proliferation (Schulz et al., 2011). The major hormones supporting spermatogenesis are androgens, T and 11-KT, also produced by Leydig cells, which are regulated mainly by FSH (Yaron et al., 2011). Androgens participate in many steps of the spermatogenesis, namely, spermatogonial multiplication and spermatocyte formation or maturation, as well as spermiation (Ueda et al., 1985, Billard, 1986, Nagahama, 1994). In comparison to progestins, androgens such as T and 11-KT, are less efective in inducing spermiation (Ueda et al., 1985). One of the most common progestins is 17α,20β-DP, which in addiction to spermiation, increases milt production and stimulates spermatozoa motility (Baynes et al., 1985, Tubbs et al., 2008).
Briefly, the process of steroidogenesis, involves the turning of cholesterol into sexual steroids, and initiates with the transport of cholesterol from cytoplasm to the internal membrane of a mitochondrion, which requires the presence of steroidogenic acute regulatory (StAR) protein (Fig. 3). Then, cholesterol is converted to pregnenolone by P450 side-chain-cleavage enzyme (P450scc). Pregnenolone can be converted to progesterone by 3β-hydroxysteroid dehydrogenase (3β-HSD), or to 17-hydroxypregnenolone by 17α-hydroxylase activity of cytochrome P450c17 (CYP17). Both progesterone and 17-hydroxypregnenolone are tranformed to 17-OHP, that can form 17α,20β-DP by 20β-HSD activity (Nagahama et al., 2008). The conversion of 17-OHP or dehydroepiandrosterone in androstenedione by CYP17 (17,20-lyase activity) or 3β-HSD, respectively, preceeeds the formation of T by 17β-HSD. From this process, T can result in the androgen 11-KT by 11β-hydroxilase action or be converted to E2 by aromatase (CYP19) (Nagahama, 1994, Bain et al., 2015).
14 Fig. 3 – Representation of steroid synthesis in teleost thecal and granulosa layers of the ovarian follicle and the testis, Leydig cells and sperm. Steroid hormones are in boxes, enzymes are in bold and next to arrows that connect the boxes, based on (Lubzens et al., 2010), Bain et al., (2015) and Nagahama, (1994). P450scc – P450 side-chain-cleavage; 3β-HSD - 3β-hydroxysteroid dehydrogenase; CYP17 – cytochrome P450c17; 17β-HSD – 17β-hydroxysteroid dehydrogenase; 17-OHP – 17-hydroxyprogesterone; 20β-HSD – 20β-hydroxysteroid dehydrogenase; CYP19 – aromatase; CYP11 – cytochrome P450c11; 11β-HSD – 11β-hydroxysteroid dehydrogenase.
1.3. Reproductive Seasonal Patterns in Salmonids
The rotation of the Earth enforces temporal cycles, which deeply control, not only the reproduction of salmonids, but also the life cycle of all teleosts (Ricklefs et al., 2000). Environmental changes determined by these cycles, may have serious consequences in fish reproductive success and survival (Wootton et al., 2015).
Salmonids display a series of reproductive strategies, varying from iteroparous, with more than one reproduction in a life cycle, to semelparous, with only one reproductive cycle in a lifetime (Willson, 1997). The degree of iteroparity and lifespan diverges in several species (Behnke, 1992), as in brown trout, where additional food supply extends their life and rises their growth rate nearing the end of the expected lifespan (Willson, 1997).
3β-HSD 3β-HSD P450scc 3β-HSD Cholesterol Pregnenolone Progesterone Dehydroepiandrosterone Androstenedione Androstenediol Testosterone CYP17 CYP17 CYP17 CYP17 17β-HSD 17β-HSD 3β-HSD 17-hydroxypregnenolone 17-OHP 20β-HSD CYP19 17β-Estradiol Vitellogenesis Oocyte maturation
Theca/Testis
17,20β-dihydroxy-4-pregnen-3-one Sperm maturation Spermatogenesis 11-ketotestosterone CYP11 11β-HSDLe
yd
ig
ce
lls
Granulosa/Sperm
11β-hydroxytestosterone15 Fish require a high adaptation ability to the changes in the seasonal cycle, in particular, those which are migratory species (Bernard, 2005). This seasonality and processes of development and maturation are highly correlated with the fluctuations in climate, photoperiod and food supplies (Bromage et al., 2001). Fish reproduction is under the control of not only the peripheral endocrine target organs, through endogenous feedback, but also, natural impacts of the environment (Rocha et al., 2008). Internal processes, such as reproduction, rely on the external changes in environment during the distinct seasons (Bromage et al., 2001). The most crucial factor controlling the timing of reproduction in salmonids appears to be day length, among other factors such as photoperiod, temperature, rainfalls, food supplies and pheromones (Bromage et al., 2001).
1.4. The Brown Trout
Brown trout belongs to the kingdom Animalia, phylum Chordata, class Actinopterygii, order Salmoniformes and family Salmonidae (Freyhof, 2011). This species is endemic from Europe, Northern Africa and Western Asia (MacCrimmon et al., 1970), although it is globally distributed (Elliott, 1994). The geographical dissemination of this trout is apparently due to its ecological adaptation capability and ability to colonize new water streams (Klemetsen et al., 2003). Brown trout requires high standard levels of water quality, making it a valuable bioindicator to detect effects of potential pollutants and endocrine disruptors (Körner et al., 2007, a Marca Pereira et al., 2011). In several countries, it has high economic value, for sport and commercial fisheries and aquaculture (Şahin et al., 2010). According to FAO, it was the first fish to be artificially produced in Germany around 1739 (Vandeputte et al., 2012) Brown trout has a facultative migratory character, meaning resident trout are able to migrate to feed in the sea, alike the migratory trout (Kallio-Nyberg et al., 2010). It is also able to remain in freshwater throughout its entire life-cycle as a resident fish, or it can migrate into lakes or into the sea, returning to the initial environment to spawn (de Leeuw et al., 2007, Kallio-Nyberg et al., 2010).
The several documented morpho types of brown trout are still controversial. These are distinguished by phenotypic characteristics and life history variations, as a result of its wide distribution and presence in many ecologically diverse habitats (Caputo et al., 2010). There are three major subspecies usually mentioned, including the anadromous, the lake dwelling and the stream resident trout. Although the subspecies have very different characteristics, this doesn’t mean that they represent monophyletic groups (Hindar et al., 1991). Individuals are born in rivers or streams and posteriorly, assume behavioral variances. The anadromous
16 or sea trout (so-called S. trutta morpha trutta), migrates into the sea, up until its maturation, then returning to their inborn location to spawn (Antunes et al., 1999). The lacustrine ecotype (S. trutta m. lacustris) migrates into lakes and returns to spawn, while the resident form (S.
trutta m. fario) stays in the same river or stream, spawning in small areas (Elliott, 1994).
Resident individuals are widely disseminated, unlike the anadromous trout, which is only present in North Atlantic, from Scandinavia to the North Iberian Peninsula (Elliott, 1994). Kallio-Nyberg et al. (2010), analyzed if resident trouts were capable of migration, and some of them migrated to the sea and had potential to evolve to migratory trouts. Therefore, migration might be dependent on genetic factors, but it may also rely on environmental factors (Jonsson, 1982, Olsson et al., 2006).
The Iberian Peninsula represents the south western limit of brown trout European distribution (Gasith et al., 1999, Tierno de Figueroa et al., 2013). Populations which inhabit peripheral limits differ from central populations, by experiencing different environmental conditions and natural selection (Lesica et al., 1995). For example, temperature variances correlated to geographical latitude influence brown trout growth rates (Jensen et al., 2000, Ojanguren et al., 2001). Spawning is also affected by latitude, taking place earlier in higher latitudes because of the lower water temperatures, resulting in longer egg incubation periods (Klemetsen et al., 2003). Furthermore, these reproductive traits may be fluctuate as a consequence of specific genetic or environmental factors (Larios-López et al., 2015). In Portugal, the occurrence of the anadromous migratory variant it’s only known in populations of the rivers Minho and Lima (Antunes et al., 2001), and it is considered “critically endangered” due to the not-reversible reduction of the population in the last 10 to 15 years (Cabral et al., 2006, Freyhof, 2011). The non-migratory form is considered “least concerned”, and it’s known in the rivers Minho, Lima, Cávado, Ave, Douro, Vouga and Mondego and in the northeast of river Tejo (Cabral et al., 2006).
Brown trout usually claims the highest predator position as an opportunistic carnivore, and depending on age, habitat and geographic area, it can feed from aquatic invertebrates to fishes (Antunes et al., 1999). In the Iberian Peninsula, specimens can reach no more than 50 centimeters of length and 2 kilograms of weight (Lobon-Cervia et al., 1986). Resident trout reaches sexual maturity between 1 and 10 years and age/size usually vary between sexes, due to differences at age of maturation (Klemetsen et al., 2003). Reproductive female potential is determined by the number and quality of their eggs, meaning larger eggs will produce larger offspring, which will grow and compete harder for food resources (Elliott, 1994).
17
1.5. Background and Aims of the Study
Given the high economic and environmental interest of brown trout, there is very little information about the seasonal characterization of its reproductive cycle. Brown trout gametogenesis and gonad development have already been described for both sexes, at the first reproductive cycle, on individuals originally from the United Kingdom (Billard, 1987). Spawning capable season, maturation age, fecundity and egg size were evaluated in a brown trout population from the Yadong River, Tibet (Hao et al., 2008), while oogenesis features as the oocyte growth, histological characteristics and plasma levels of key steroid hormones were studied in females of Chile (Estay et al., 2003). There are also very few investigations on the reproductive endocrinology of this species and even of other salmonids, over the natural reproductive cycle. The hormones most frequently quantified in plasma/blood of salmonids are T, E2 and 11-KT. The latter is found in males, however, exceptionally, it is analyzed in females and has revealed seasonal fluctuations (Schultz et al., 2005, Akhtar et al., 2017). Other hormones which have been showing specific variations according to certain phases of the reproductive cycle are: FSH and LH, which induce the production of steroid hormones in the gonads (Santos et al., 2001, Chauvigné et al., 2014) and 17-OHP, a steroid involved in the production of T (Yeung et al., 1985, Wootton et al., 2015).
The accurate identification of fish reproductive phases and its relationship with either the histomorphology of the gonads or/and the biochemical and hormonal profiles is not commonly investigated, although it is a tool with enormous potential. The identification of the follicular phase by monitoring calcium and E2 levels was studied in Salmo trutta m. caspius (Jamalzadeh et al., 2012). Like this, many other studies focus on keeping the track of plasma and/or blood parameters, such as Vtg, glucose, hemoglobin and calcium (Denton et al., 1975, Norberg et al., 1989, Vuorinen et al., 2003). However, it is not usual to establish the correlation between the plasma biochemistry and the histology of the gonads.
To hinder the existing background, the available brown trout studies used very distinct fish populations from different environmental habitats, which difficult cross comparisons between the reported data. Many studies refer to brown trout that inhabit distinct environments, precluding the direct comparisons across data (Billard, 1987, Şahin et al., 2010, Rawat et al., 2011).
This study aims to characterize the fitness and reproductive stages of adult male and female brown trout (with about 3 years old), obtained from a national government-owned
18 aquaculture (Torno, Amarante, Portugal), along three distinct stages of the reproductive cycle (December, spawning capable; March, regressing and July, regenerating). A set of plasma, blood or serum parameters, including hematologic and biochemical ones (hemoglobin, cholesterol, triglycerides, glucose, and potassium, among others) and three hormones, namely, FSH, LH and 17-OHP will be monitored along the distinct stages. Complementary, a qualitative and quantitative histomorphological study in male and female gonads will be addressed, in order to evaluate the gametogenic stages in each phase of the reproductive cycle. Immunohistochemistry will also be used to identify the distribution of three main steroidogenic enzymes (CYP17-I, CYP19 and 17β-HSD) in brown trout gonads and check the possible immunophenotype changes during the reproductive phases.
19