h ttp : / / w w w . b j m i c r o b i o l . c o m . b r /
Industrial
Microbiology
High
levels
of

-xylosidase
in
Thermomyces
lanuginosus
:
potential
use
for
saccharification
Juliana
Moc¸o
Corrêa
a,∗,
Divair
Christi
a,
Carla
Lieko
Della
Torre
b,
Caroline
Henn
c,
José
Luis
da
Conceic¸ão-Silva
b,
Marina
Kimiko
Kadowaki
b,
Rita
de
Cássia
Garcia
Simão
baCentrodeCiênciasExataseTecnológicas
bCentrodeCiênciasMédicaseFarmacêuticas,UNIOESTE,Cascavel,PR,Brazil cCentralHidrelétricadeItaipu,ItaipuBinacional,FozdoIguac¸u,PR,Brazil
a
r
t
i
c
l
e
i
n
f
o
Articlehistory:
Received5November2015 Accepted20February2016 Availableonline27April2016 AssociateEditor:SolangeInes Mussatto
Keywords:
Experimentaldesign Saccharification -Xylosidase
Thermomyceslanuginosus AtlanticForest
a
b
s
t
r
a
c
t
AnewstrainofThermomyceslanuginosuswasisolatedfromtheAtlanticForestbiome,and its-xylosidasesoptimizationinresponsetoagro-industrialresidueswasperformed.Using statisticalapproachasastrategyforoptimization,theinductionof-xylosidasesactivity wasevaluatedinresidualcornstraw,andimprovedsothattheoptimumconditionachieved high-xylosidasesactivities1003U/mL.Accordingourknown,thisstudyisthefirsttoshow sohighlevelsof-xylosidasesactivitiesinduction.Inaddition,theapplicationofan experi-mentaldesignwiththismicroorganismtoinduce-xylosidaseshasnotbeenreporteduntil thepresentwork.TheoptimalconditionsforthecrudeenzymeextractwerepH5.5and60◦
C showingbetterthermostabilityat55◦C.Thesaccharificationabilityof-xylosidaseinthe
presenceofhemicelluloseobtainedfromcornstrawrawandxylanfrombeechwood sub-stratesshowedaxylo-oligosaccharidetoxyloseconversionyieldof80and50%,respectively, at50◦C.Ourdatastronglyindicatedthatthe
-xylosidasesactivitieswasnotsubjectedtothe effectsofpotentialenzymeinhibitorsoftenproducedduringfermentationprocess.These datasuggesttheapplicationofthisenzymestudiedforsaccharificationofhemicellulose, anabundantresidueintheAmericancontinents,thusprovidinganinterestingalternative forfuturetestsforenergyproduction.
©2016SociedadeBrasileiradeMicrobiologia.PublishedbyElsevierEditoraLtda.Thisis anopenaccessarticleundertheCCBY-NC-NDlicense(http://creativecommons.org/ licenses/by-nc-nd/4.0/).
Introduction
Thermomyceslanuginosus,previouslyknownasHumicola lanug-inosa, is a thermophilic fungus widely distributed and
∗ Correspondingauthor.
E-mail:julianacb6974@hotmail.com(J.M.Corrêa).
frequently isolated from organic waste.1 Several strains of
Themomyces lanuginosus have been shown to be potential producersofenzymeswithdifferentapplications.2–4 In gen-eral,microbialenzymeshavereceivedattentionduetobeing
http://dx.doi.org/10.1016/j.bjm.2016.04.028
precursorstoclean technologyfortheproductionof indus-trialandcommerciallyimportantcompoundsusingresidual biomassassubstrates.
Thebiomasspresentinagro-industrial residuesis com-posedofhemicellulose,andtheenzymaticdepolymerization ofits structure is commercially interesting due to its soft conditionandnoformationoftoxiccompoundsduringthe degradationoftheproducts.Thecompletedeconstructionof the hemicellulose structure requires the synergistic action of several enzymes, including xylanases (EC 3.2.1.8) and -xylosidases (EC 3.2.1.37). -Xylosidase cleaves  -1,4-xylo-oligosaccharides releasedby xylanasesproducing xylose, a monosaccharidethatcanbeusedbydifferentmicroorganisms intheprocessofsaccharificationandproductioncompounds ofthebiotechnologicalapplication.1,5
Currently,marketanalysesindicatethatglobalmaize pro-ductionisabundant,andtheUSisthelargestproducerofthe cropfollowedbyArgentinaandBraziltotalingapproximately 53.2milliontonsperharvest.Cornismainlyusedforanimal feedproductionbutisalsousedtoalesserextentforthe pro-ductionofoils,floursandbreakfastcereals.Thus,theresidual biomassofthecrop,suchasstrawandcorncobs,shouldbe consideredfortheproductionofenergyandotherproductsof biotechnologicalinterest.1,2
Brazilianindicesrelatedtocorncropsshowthatonly5%of productionisintendedforhumanconsumption.Theresidues fromtheprocessingofthiscropmakeup58%oftheproduced biomass,andthisportion isstillneglected andcould alter-nativelycontributeasasourceofrenewableenergysourcesto generatewealthandtominimizetheaccumulationofresidues thatcanbeharmfultotheenvironment.1,2
Inthepresentreport,anisolatefromtheBrazilianAtlantic Forestbiome was optimized forthe production total of -xylosidases.Theoptimizedenzymecrudeextractwasused for testing the saccharification of xylan from beechwood andhemicellulosederivedfromcornstrawemphasizingthe potentialbiotechnologicalrolefortheenzyme.
Materials
and
methods
Isolation,identificationandmaintenanceofculture
ThefunguscatalogedundertheIDofPC-7S-1-Twasisolated fromasoilsampleobtainedfromtheAtlanticForestbiome ofthe BeautifulViewRefuge (latitude 24◦55′16′′ S and lon-gitude53◦54′35′′W)FozdoIguac¸u,Paraná,Brazil(collection authorizedbyItaipuBi-national).Theisolatewassubjectedto morphologicalidentificationfollowedbymolecular identifica-tionbynucleotidesequenceanalysisoftheITSregionofDNA correspondingtotherRNAofthemicroorganism.This analy-siswasperformedattheLaboratoryofMolecularBiochemistry attheStateUniversityofWestParaná(Brazil)byextractingthe totalDNAofthefungusfollowedbyreplicationofthetarget ampliconusingspecificprimers.Theampliconsobtainedwere sequencedbythesequencing serviceprovidedbyHELIXXA (Sequencing Service, Brazil), and the sequences obtained were analyzed using sequence alignment tools (Blast-n from the National Center for Biotechnology Information; NCBI).
ThegeneratedsequencewasdepositedinGenBankunder theaccessnumberKJ934703.1.Inbothanalysesusedto iden-tifythefungus,theisolatewasclassifiedasbelongingtotheT. lanuginosusspecies.Themicroorganismwasgrownat42◦Cfor 7daysintubescontainingsolidPDAmedium(20%potato infu-sion(w/v),1.5%dextrose(w/v)and1.5%agar(w/v))andwas subsequentlystoredunderrefrigerationat4◦Cwithperiodic maintenanceevery30days.
Preparationofagro-industrialresiduesandplantbiomass
Thefollowingcarbonsourcestestedforuseintheexperiments offungalgrowthandinductionof-xylosidasesenzyme activ-itieswereselectedaccordingtoavailability:cornstraw,orange peels,bananapeelsandpassionfruitpeels.Theplantbiomass residues weredriedat70◦Cfor24hfollowedbytrituration (SL30slicerWilley)usinga20meshsieve, andtheresidues werethenstoredinglassvialsatroomtemperature.
CultivationconditionsofT.lanuginosus
Newlygrownfungalconidiawereusedtopreparea suspen-sion (1×105conidiamL−1)insterilewater, whichwas then inoculatedinto25mLofmineralmedium(distilledwater,0.3g ofNaNO3,0.1gofK2HPO4,0.05gofMgSO4·7H2O,0.05gofKCl,
0.001gofFeSO4·7H2O,and0.1gofyeastextract;pH6.0)
supple-mentedwith1%(w/v)differentselectedcarbonsources.The submergedfermentation(SMF)cultivationswereperformed twodifferentwaysasfollows:stationaryliquidandagitated liquid(150rpm)at42◦Cfor7daysinbiologicalduplicates.The carbonsourcemosteffectiveininducingthehighestenzyme activitywasusedforfurtheroptimizationexperiments.
Theenzymeproductionwasmonitored,bycollectingthe mycelialcellsofT.lanuginosusbyvacuumfiltrationofthe cul-turetosterileWhatmanpaper.Theobtainedmycelialcells werefrozen,andtheywerethenmaceratedwith1gofglass beads,resuspendedin5mLofice-colddistilledwaterand cen-trifugedat8000×gand4◦Cfor5min.Thesupernatantwas collectedandusedintheanalyticaltests.
Determinationoffungalbiomass
Thesamplesusedforthedeterminationofbiomasswere fil-tered through5mfilterpaper (Whatman®), driedfor48h at105◦C and weighed onan analytical balance.Theassay formeasurethefungalbiomasswasconductedusinga con-trol fordetermination ofbiomass.In thecontrol flaskwas addedtheresidueintheabsenceofinoculum.Thebiomass wasquantifiedbythedifferenceofweightobtainedinthetest experimentscontainingresidueandfungus,and,thecontrol containingonlytheresidue.
Table1–LevelsoffactorsusedinCCRD.
Factorsa −1.68 −1 0 1 +1.68
F1 0.04g
(0.2%)
0.125g (0.5%)
0.25g (1%)
0.375g (1.5%)
0.46g (2%)
F2 23◦C 28◦C 35◦C 42◦C 46◦C
F3 0.005g
(0.01%)
0.025g (0.05%)
0.0525g (0.2%)
0.0807g (0.3%)
0.1g (0.4%)
a F1,cornstraw;F2,temperature;F3,yeastextract.
ofcornstrawwith0.0525gofyeastextractasacentralpoint asthiswasdefinedastheoptimalconditionfor-xylosidases activitiesinpreliminarytests.
Thevalueswere then addedtothe central pointofthe design,and the influenceoftemperatures between23 and 46◦Cwasalsoincludedinthetests(Table1).Statistical analy-siswasperformedtoobtainthelineareffects,andthemodel wasadjustedaccordingtoEq.(1)todescribetheresponse sur-face.
ˆ y=b0+
3
i=1 biFi+
3
i,j=1
bijFiFj (1)
Itwasused Derringer’s6 desirability functionacriterion decision-makingmethodproposedbyDerringerandSuichin 1980.Thisisthemostcurrentandfrequentlyusedtechnology forresponseoptimization.Theprocedureinvolvesthe trans-formationofindividualresponsetoadesirabilityfunction(di), definedasadimensionlesspartialdesirabilityfunctionthat variesfrom0(consideredacompletelyundesirableresponse) to1(consideredafullydesiredresponse).
ˇ-Xylosidasesactivitiesandproteindetermination
The-xylosidaseactivitywasdeterminedusing0.5mLofp -nitrophenyl--d-xylo-pyranoside (pNPX) at a concentration
of10mM in50mM sodiumphosphate buffer (pH6.0) with 0.05mLofthecrudeenzymeextract(withorwithout dilu-tion),whichwasincubatedforaperiodof10–60minat50◦C inawaterbath.Thereactionwasstoppedwithasaturated solutionofsodiumtetraborate.Thesampleswereanalyzedby spectrophotometerreadingat410nmusingp-nitrophenol (0–0.6mol/mL)forthestandardcurve.Oneunitofenzyme activity was defined as the amount ofenzyme capable of releasingonemolofp-nitrophenolperminuteofreaction. Theproteinconcentration wasdetermined bythe Bradford methodusingbovineserumalbuminasthestandard.
The enzymatic activity was expressed in U/mL of the homogenateorinspecificenzymaticactivity(U/mg)that cor-respondtotheenzymaticactivityobtainedbymgofthetotal proteinproducedinthecell.
Influenceofthetestconditionsontheproductionof
ˇ-xylosidase
AftergrowthofT.lanuginosuscellsindifferenttestconditions, itwaspossibletodefineparameterstobeconsideredinthe
experimentaldesignaswellasthecarbonsource,typeofcrop andtheoptimalproductiontimefor-xylosidasesactivities.
InfluenceofpHandtemperatureonintracellular
ˇ-xylosidasesactivities
Theintracellular-xylosidaseactivitywasanalyzedfor vari-ationsindifferentpHconditions(McIlvainebufferpH5–7.5). Toanalyze theeffectoftemperature,theoptimumpHwas maintained,andthecrudeextractcontainingtheenzymewas incubatedattemperaturesrangingfrom50to70◦Cin incre-mentsof5◦.TheconditionsforoptimumpHandtemperature wereusedforanalysisofthermostabilityinthe3optimal tem-peratureconditionsdisplayed.
Pretreatmentforhemicelluloseextractionfromcornstraw
Samplesofcornstrawwereplacedinsealedtubescontaining 0.35goffreshcornstrawwith10mLofdeionizedwater,and thesampleswereplacedinablockdigesterfor1hat200◦C. Thesampleswerethen immediatelyplacedinanicebath. Afterseparationofthesolidfromtheliquidphase,theliquid portion containingthehemicellulosewasprecipitatedwith absoluteethanol(three-foldthevolume)for48h.Theobtained precipitatewasrecoveredbyfiltrationandthendriedat37◦C foraperiodof3days.
Enzymatichydrolysisofxyloseandeffectonˇ-xylosidases
activities
withoutenzyme,and2–incubationofDNScontainingthe crude extract of enzymes without addition of hemicellu-lose from corn straw or xylan. Reducing sugars present in boththexylanfrombeechwoodandhemicellulosefromcorn strawweremeasuredbeforestartingthehydrolysis,andthe values obtained were used to present normalized results. Samplesobtainedaftertheenzymaticsaccharificationwere subjectedtothinlayerchromatographytoevaluatethe con-versionofthexylo-oligosaccharidestoxylosebyT.lanuginosus
-xylosidase.Inparallel,theresidualxylosewasdetermined inthehemicellulosesamplesat12husingtheMegazyme®kit. Theinfluenceofxyloseon-xylosidasesactivitieswasalso investigatedbyincubatingthecrudeintracellularextract con-taining-xylosidaseswithxyloseatthelevelsdetectedinthe saccharificationprocess.
TLCsilicaproductofthehydrolysis
Analysisofhydrolysisproductsformedfrom thecombined actionoftheenzymes,namelyxylanase (extracellular)and -xylosidases (intracellularcrude extract), ofT. lanuginosus wasperformedbyascendingthin layerchromatographyon 10cm×10cm silica (Alufolien DC-Kieselgel60 without flu-orescent indicator, Merck®). The hydrolysis products were collected atbothtemperatures and atdifferent times. The productswerethenboiledfor10minandfrozen.Thexylose standard (Sigma®) (2mg/mL; 5L) and xylobiose/xylotriose standard (Megazime®) (3mg/mL; 4L) (Sigma®) and each
sample(4L)wereaddedtothesilica.Theplatewasresolved twicewithamixtureofbutanol,pyridineanddistilledwater (7:3:1,v/v/v).Disclosureofthehydrolysisproductswas per-formedusingasolutionof0.3%orcinol(w/v)sulfuricacidin methanol(1:9).Subsequently,theplatewasplacedinanoven at100◦Cuntilthexylo-oligosaccharidedegradationproducts weregenerated.
Results
Determinationofoptimalconditionsforˇ-xylosidases
production
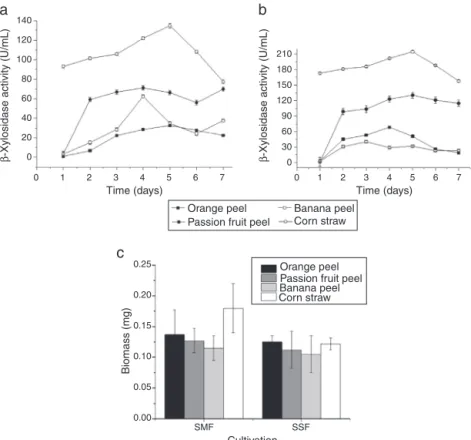
The T. lanuginosus fungus was grown under different con-ditions(typeofresidue,cultivationandincubationtime)to determine the optimal conditionsfor the productionof  -xylosidases with high activity. The plant biomass residue thatledtohigherenzymaticactivityofT.lanuginosuswas1% cornstraw(w/v)ascomparedtotheotherresidues,namely 1% orange peel (w/v), 1% passion fruit peel (w/v) and 1% bananapeel(w/v).Withbothsteadycultivationandagitated cultivation, corn straw was the highest inducer of intra-cellular-xylosidasesleadingtoamaximum productionof 214U/mL after5daysofcultivationunder shaking(Fig.1A and B) The agitatedcultivation using corn straw was also the most effective for the production ofmycelial biomass (Fig.1C).
140
210 180 150 120 90 60 30 0
a
c
0.25
0.20
0.15
0.10
Biomass (mg)
0.05
0.00
SMF SSF
Cultivation
b
120 100 80 60
β
-Xylosidase activity (U/mL) β-Xylosidase activity (U/mL)
40 20 0
0 1 2 3 4
Time (days) Time (days)
5 6 7 0 1 2 3 4 5 6 7
Banana peel Corn straw
Banana peel Corn straw Orange peel
Passion fruit peel
Orange peel Passion fruit peel
Fig.1–(A)-xylosidasesactivitiesinthecrudeextractofThermomyceslanuginosusafter7daysofcultivationinmineral mediumsupplementedwith1%ofvariousagro-industrialresiduesasacarbonsourceat42◦Candatstationaryconditions.
(B)Productionoftheintracellular-xylosidasesenzymeofT.lanuginosusduring7daysinculturecontainingmineral mediumsupplementedwith1%ofagro-industrialresiduesasacarbonsourceat42◦Candagitationat150rpm.(C)
Experimentaldesign
Theparametersforenzymeproductionwiththeoptimal con-ditionswereappliedtothe CCRDexperimentaldesignasa waytofurtherimprovetheyieldsofenzymaticactivity.The experimentaldesignwasbasedonthreedifferentfactorsas follows:concentration of residue as a carbon source(corn straw),temperatureandconcentrationofyeastextract (nitro-gensource)during5daysinSMFcultureunderagitationat 150rpm.The23experimentalmatrixconsistedofthree cen-tralpointsandsixaxialpointswherethetwofactorscombined withthree levels totaling 17 tests. Weselected the combi-nationof0.25gofcornstraw with0.0525gofyeastextract asacentralpointasthiswastheoptimumconditionfound forthe activityofintracellular-xylosidases inpreliminary tests(Fig.1).Therefore,thesevalueswereaddedtothecentral pointofthedesign.Theeffectoftemperaturewasalso com-binedinthetestsusingtemperaturesbetween23and46◦C duetothethermophilicconditionshownbytheisolatedstrain (Table1).
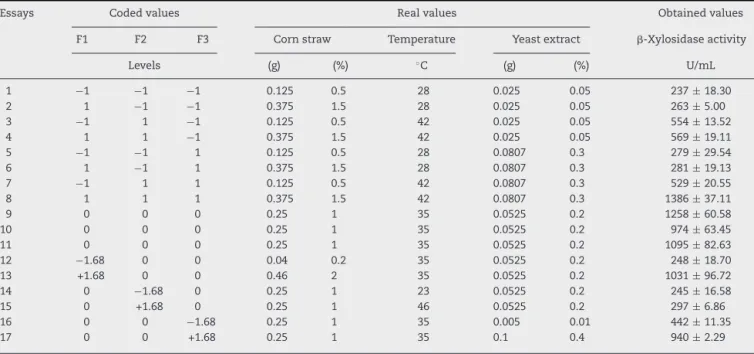
Table2showsthecodedandrealvaluesofthe experimen-taldesignperformedwiththreecenterpointsandsixaxial pointstotaling17tests.Thevaluesof-xylosidasesactivities arepresentedindividuallyaccordingtoeachassayincluding thecorrespondingstandarderror.
The estimated effects of the variables evaluated were positiveinthisanalysisastheydemonstratedthatthe tem-perature,cornstrawandyeastextractledtoanincreasein enzymeactivityof302,324and247U/mL,respectively. Analy-sesofvariablesgeneratedamathematicalmodelofthesecond order(Eq.(2))wherepmiscornstraw,tistemperatureandel isyeastextract.
ˆ
ˇ=(1107,73+(162,435pm−163,005pm2)
+(151,434t−293,537t2)+(123,753el−144,798t2)) (2)
Thesummaryoftheanalysisofvariance(ANOVA)ofthe experimentaldesignispresentedintermssignificantto10% ofprobability(p<0.10)inTable3.Thecoefficientof determi-nation (R2)was0.86,andtheF-testshowedthatthemodel isappropriatetopredicttheresultsviaresponsesurface,thus confirmingthemathematicalmodelpresentedinEq.(2).Fig.2
showsthebehaviorofthedesigndatawiththethreevariables tested,thusindicatingthepreferredoptimization.
Thesurfaceresponsesrepresentthemathematicalmodel thatenablestheverificationofthecombinationofthree fac-torsanalyzedintheexperiment, theinfluenceofeachone and the maximum enzymeactivity of-xylosidase. Condi-tionsofincreasedproductionof-xylosidasewereobtainedat concentrationsthatwereclosetothecentralpointcondition forthethreefactorsstudied.However,contourcurves were presentindicatingslightlylowervaluesofthecenterpointfor allthreevariables,thusleadingtothesameresponses.
Optimizationoftheconditionsthatmaximizeenzymatic production of -xylosidase requires analysis of individual behavioral responsesinacceptableregions.Thedesirability conditions (Fig. 3) showed that the combination of factors that achieved the highestproductionof-xylosidase must contain0.287gofcornstrawwith0.06gofyeastextractata temperatureof36◦C.Theprofileofthepredictedvaluesand desirabilityofthemodelshowedthatitispossibletoobtain a maximum of1194U/mL of-xylosidase enzymewithan expectedyieldof84%.
Validationofoptimizedexperimentalconditions
The conditions determined for desirability of the experi-ment were validated after performing 6 repetitions with duplicatebiologicalandenzymatic dosagesfortheextracts obtainedfrom mycelia.Thecultivationswereperformedin
Table2–Matrixofdesign(CCRD)withthecoded,realgivenvaluesandvaluesobtainedintheexperiments(residue concentration,temperatureandconcentrationoftheyeastextract).
Essays Codedvalues Realvalues Obtainedvalues
F1 F2 F3 Cornstraw Temperature Yeastextract -Xylosidaseactivity
Levels (g) (%) ◦C (g) (%) U/mL
1 −1 −1 −1 0.125 0.5 28 0.025 0.05 237±18.30
2 1 −1 −1 0.375 1.5 28 0.025 0.05 263±5.00
3 −1 1 −1 0.125 0.5 42 0.025 0.05 554±13.52
4 1 1 −1 0.375 1.5 42 0.025 0.05 569±19.11
5 −1 −1 1 0.125 0.5 28 0.0807 0.3 279±29.54
6 1 −1 1 0.375 1.5 28 0.0807 0.3 281±19.13
7 −1 1 1 0.125 0.5 42 0.0807 0.3 529±20.55
8 1 1 1 0.375 1.5 42 0.0807 0.3 1386±37.11
9 0 0 0 0.25 1 35 0.0525 0.2 1258±60.58
10 0 0 0 0.25 1 35 0.0525 0.2 974±63.45
11 0 0 0 0.25 1 35 0.0525 0.2 1095±82.63
12 −1.68 0 0 0.04 0.2 35 0.0525 0.2 248±18.70
13 +1.68 0 0 0.46 2 35 0.0525 0.2 1031±96.72
14 0 −1.68 0 0.25 1 23 0.0525 0.2 245±16.58
15 0 +1.68 0 0.25 1 46 0.0525 0.2 297±6.86
16 0 0 −1.68 0.25 1 35 0.005 0.01 442±11.35
Table3–SummaryofANOVAofthe2ndordermathematicalmodelfortheproductionof-xylosidase.
Variationsource SS GF MS Fvalue Ftab
Regression 194,460.20 6 324,100.33 5.34 2.46
Residues 60,731.30 10 60,731.30
Total 255,191.50 16
SS,sumofthesquares;GF,degreesoffreedom;MS,meansquares;Fvalue,calculatedFvalue;Ftab,tabulatedFvalue;R2=0.86;p-value<0.10.
liquidmediumwithshaking(150rpm)for5daysat36◦Cas
indicated by the desirability settings (Fig. 3). The
intracel-lular crude enzymatic extract and dosages of -xylosidase activityfollowedtheprotocolin“Materialsandmethods” sec-tion.Theaverageenzymaticactivityobtainedinthetestswas 1003U/mL,andthespecificactivitywas1683U/mg.Initially, the experiments without application of the experimental designhad a maximum -xylosidase activity of 214U/mL. However,optimizationachievedalmost500%morethanthe initialvalueswitha4.7-foldincreasein-xylosidaseactivity.
Theˇ-xylosidaseactivityinthecrudeextractwas
subjectedtoapartialcharacterization
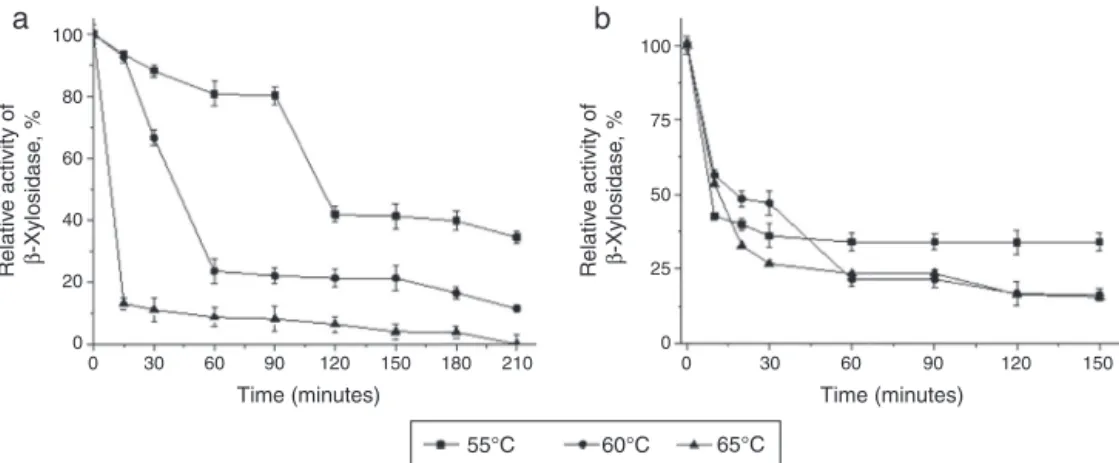
The-xylosidaseactivityinthecrudeextractwassubjected toapartialcharacterization.Thetestconditionsatdifferent pHvalues(McIlvainebuffer) indicatedthat theoptimal pH
valuewas5.5(Fig.4A)at60◦C(Fig.4B).Inthisstudy,the intra-cellular-xylosidaseofT.lanuginosuswasmorethermostable at55◦CandpH7(Fig.5A)retaining80%activityfor90min. However,thethermostabilityof-xylosidasewaslowatthe optimumpHastherewasasignificantdecreaseinenzyme activityafter30minofincubation,buttheactivityremained stablethereafteratthethreetestedtemperatures(Fig.5B).In the sameextractwerequantified the-glucosidaseactivity at4.9U/mL,␣-l-arabinofuranosidase2.6U/mLand Xylanase
35.59U/mL.
Enzymaticsaccharificationusingxylanaseand
ˇ-xylosidase
The intracellular T. lanuginosus-xylosidase was subjected toatestforenzymaticsaccharificationattwotemperatures of 37 and 50◦C with and without pre-hydrolysis with the
1600 1600 1400 1200 1000 800 600 400 200 –200 –400 –600 0.1 0.0525 0.0807 0.025 0.0050.005 0.025 0.0525 0.807 0.1 0 1600 1400 1200 1000 800 600 400 200 –200 –400 –600 0.1 0.0807 0.0525
Corn str
aw (g) Cor n str aw (g) 0.025 0.005 0.125 0.25 0.3750.46 0.04 0 1400 1200 1000 800 600 β -xylosidase U/mL β -xylosidase U/mL β -xylosidase U/mL 400 200 –200 –400 –600 –800 –1000 42 Temper ature ( °C) 35 28 23 0.04
Corn str
aw (g) 0.12 5 0.25 0.37 5 0.46
Yeast e xtract(g)
Yeast e xtract(g) 0 >1000 <1000 <600 <200 <–200 <–600 >1000 <1000 <800 <600 <400 <200 <–200 <0 >1000 <1000 <800 <600 <400 <200 <–200 <0
a
c
b
Corn straw
1800
1194
84%
0.125 g 0.375 g
0.287 g
28°C 46°C
36°C
0.025 g
0.06 g 0.0807 g
Temperature Yeast extract
0 5
1 1386
811.69
β
-Xylosidase (U/mL)
236.89
Desirability
Fig.3–DesirabilityfortheproductionofT.lanuginosus-xylosidase.
100
a
b
Relativ
e activity of
β
-Xylosidase
, %
Relativ
e activity of
β
-Xylosidase
, %
75
50
25
0
100
75
50
25
0 5.0 5.5 6.0 6.5
pH
7.0 7.5 50 55 60 65 70
Temperature °C
Fig.4–(A)EffectofpHontheactivityofintracellular-xylosidaseofT.lanuginosusincubatedinMcIlvainebuffer(pH 5.0–7.5)followedbydeterminationof-xylosidaseusingNPXasasubstrate.(B)Effectoftemperatureontheactivityof intracellular-xylosidaseofT.lanuginosusincubatedinMcIlvainebuffer(pH5.5)during10minattemperaturesof50–70◦C
withsubsequentstandarddeterminationof-xylosidase.
Table4–Productionofreducingsugarsintheenzymatichydrolysisbyactionofxylanase(X)or-xylosidase(BX)tested individuallyorincombination(X+BX).
Essay 37◦C 50◦C
Reducingsugarsmol/mL Reducingsugarsmol/mL
X X+BX BX X X+BX BX
Xylanfrombeechwood(1%,w/v) 6.77 3.17 0.64 7.04 8.15 0.30
100
a
b
80
60
40
20
0
100
75
50
25
0 0 30 60 90 120 150 180
Time (minutes) Time (minutes)
55°C 60°C 65°C
210 0 30 60 90 120 150
Relativ
e activity of
β
-Xylosidase
, %
Relativ
e activity of
β
-Xylosidase
, %
Fig.5–ThermostabilityprofileoftheintracellularT.lanuginosus-xylosidaseactivity.(A)Thermostabilitydisplayedinthe topthreetemperaturesof55◦C,60◦Cand65◦Cduring210minofincubationtime.(B)TheenzymestabilityatpH5.5at
temperaturesof55◦C,60◦Cand65◦Cduring150min.
filtrate containing high extracellular xylanase activity and totalabsenceof-xylosidaseactivityobtainedfromT. lanug-inosus. Each crude enzyme(2U/mL), including intracellular -xylosidaseandextracellularxylanase,wasincubatedwith thefollowingtwodifferentsubstrates:1%xylanfrom beech-wood(w/v)and1%hemicellulosefromcornstraw (w/v).In thehydrolysisperformedattwotemperaturesinthepresence ofxylanfrombeechwood,theenzymeshadsimilarbehavior whentheyweretestedindividuallyorincombination(Table4). However,inthe presenceofhemicellulosefromcorn straw andinassayscontainingxylanasepreviouslyadded,the -xylosidasesofT.lanuginosuswasmoreefficientinpromoting saccharification.Theseresultssuggestacombinedactionof bothenzymes,andindicateanimportantroleforthe decon-structionstructureofhemicellulosefromvegetablebiomasses derivedfromresidues(Table4).
Theyieldofthereducingsugarsgeneratedfromthe indi-vidualactionof-xylosidaseintheassaydemonstratedthe highconversioncapacityoftheenzymebecausethe hydroly-sisat50◦Cwas80%comparedtosaccharificationperformed onlybyxylanaseinhemicellulosederivedfrom corn straw (Table4).Aslightlylowerconversionwasfoundusingxylan
asasubstrateatthesametemperaturewheretheefficiency of-xylosidasewasonly50%higherthanthatofthe individ-ualactionofthexylanaseenzyme.Theenzymaticactivityof -xylosidasewasmonitoredduringtheentiresaccharification processandshowedaconstancymaintainingapproximately 80%ofitsactivityovertimeinthepresenceofthetest hemi-cellulosesubstratefromcornstraw(Fig.6A).
The yield of -xylosidase conversion into xylo-oligosaccharides was represented by the amount of total reducing sugars which indicated the ability to generate 0.64 and 0.30mol/mL from xylan and between 2.12 and 2.75mol/mL from hemicellulose from corn straw in the twotestconditionsof37and50◦C,respectively.Thexylose content was also measured using the d-xylose Assay Kit
(Megazyme®)whichindicatedaproductionof207mol/mL ofxyloseat37◦Cand205mol/mLofxyloseat50◦Cin24h oftesting.TheamountofxyloseproducedisshowninFig.7.
Asaparameterdeterminingthelevelsofxylosedetected duringsaccharificationofhemicellulosefromcornstrawwith -xylosidaseofT.lanuginosus,anexperimentwasconducted toinvestigatethebehaviorofenzymeactivityintheabsence andpresenceof200mMofxyloseevenwithoutthepresenceof
100
a
b
80
60
40
20
0
100
75
50
25
0 0 2
Time (hours) Treatments
Xylose–(37°C) Xylose+(37°C) Xylose–(50°C) Xylose+(50°C)
4 6 8 10 12
Relativ
e activity of
Beta-Xylosidase
, %
Relativ
e activity of
β
-xylosidase
, %
Fig.6–PerformanceT.lanuginosus-xylosidasesactivitiesduringsaccharification.Relativeactivityafterhydrolysisby intracellular-xylosidaseofT.lanuginosusfor12hinxylanfrombeechwood(square)(1%,w/v)andhemicellulosefromcorn straw(circle)(1%,w/v)at37◦C(closedcircleandclosedsquare)and50◦C(opencircleandopensquare).Relativeactivity
a
P XL H XLC H 37°C
C P XL H 50°C
b
XLC HC
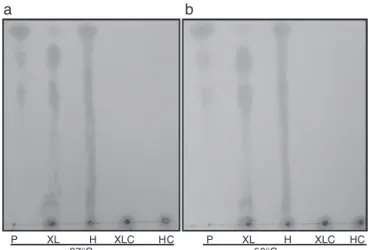
Fig.7–Analysisofthinlayerchromatography.TLCofthe hydrolysisofT.lanuginosus-xylosidaseproductsobtained after12hofincubationwithxylanfrombeechwood(1%, w/v)andhemicellulosefromcornstraw(1%,w/v)at37◦C
(A)and50◦C(B).P,xylose,xylobioseandxylotriose;XL,
xylanfrombeechwood(1%,w/v);H,hemicellulosefrom cornstraw(1%,w/v);XLC,xylanfrombeechwood(1%,w/v) control;HC,hemicellulosefromcornstraw(1%,w/v) control.
anothersubstrateatbothtemperaturesusedinthehydrolysis (37and50◦C)for12h.Thisassayshowedthatthe
-xylosidase activityat37◦Cremainedatapproximately85%ofthe origi-nalactivity at50◦Candthattheenzymeactivityremained slightlymorethan56%oftheenzymeactivitywhencompared withthetotalabsenceofxylose(Fig.6B).Importantly,inthe actualtestconditionsofsaccharification,thehydrolysis prod-uctsof-xylosidasearegraduallyreleasedintothereaction mediumfromthenaturalsubstrate,eitherxylanor hemicel-lulosefromcornstraw.However,whentryingtoevaluatethe inhibitionbythefinalhydrolysisproductof-xylosidase in thecaseofxylosealone,thecompoundisaddedinthe pres-enceofenzyme,whichjustifiesagreaterinhibitionbyproduct, thusoverestimating whatactuallyoccurs intheprocess of saccharification(Fig.6A).
Discussion
Despite of the fact the -xylosidase activity in the crude extractwashightoptimized(1003U/mL),otherenzymes intra-cellularxylanase,-glucosidaseand␣-L-arabinofuranosidase wereobtainedinlowlevels.Thisresultsuggeststhatthehigh levelof-xylosidaseintracellularobtainedinthecrudeextract isaconsequenceoftheextracellulardeconstructionofcorn strawresiduebyotherxylanoliticenzymes.
Ingeneral,the growthoffungicanbeinfluencedbythe rateofaerationincultivationduetothehigheravailabilityof oxygenduringgrowth,whichcanpositivelyinfluenceenzyme productivity.7 However,variousmicroorganismsbehave dif-ferentlytogrowthconditions,soitisessentialtoevaluatethe combinationofdifferenteffectsexertedontheenzymes of interest.Severalcarbonsourceshavebeenusedtoevaluate
theinductionofhemicellulaseactivityinotherisolatesofT. lanuginosus.Amongthesestudies,thereisanemphasisonthe useofmaterialsderivedfromcornprocessingaspromising enzymeinducers.2,7,8
Thecorncobhasbeenreportedasacomplexresiduein the inductionofxylanase inT. lanuginosusDSM 5826 com-paredtoxylan8toinducelowenzymeproductivity.Theeffects ofaerationoncultivatingwerealsoinvestigatedthe produc-tionofhemicelluloseinT.lanuginosusSSBPcellsgrownina bioreactor.3Thesetestsshowedanoverallinductionof hemi-cellulaseproportionaltotheincreasedagitation.2
Although some studies have focused on T. lanuginosus, theuseofagro-industrialresiduesinfermentationprocesses withthisfilamentous fungusforenzymeproductionisstill incipient.However,thecorncob,7,9 citruspectin/beetpulp,4 wheatstraw10 andwheatbran11 havebeen showntohave potentialapplicabilityforenzymeinductionofthexylanolytic andhemicellulolyticcomplex.However,theapplicationofan experimental designwiththis microorganism toinduce  -xylosidaseshasnotbeenreporteduntilthepresentwork.
DatageneratedusingStatistica7®softwaretoanalyzethe combinedfactorsindicatedamaximumpredictedactivityof 1194U/mL,andtheexperimentalconditionsindicateda pro-ductionof1003U/mL.Thus,84%ofthepredictedvaluewas achieved with the application of the experimental model. Accordingourknown,thisstudyisthefirsttoshowsohigh levelsof-xylosidaseactivityinduction.Inaddition,the appli-cationofanexperimentaldesignwiththismicroorganismto induce-xylosidaseshasnotbeenreporteduntilthepresent work.
Inthisstudy,theneedforexperimentaldesignto deter-mine the optimal conditions for enzyme induction was demonstrated.Oneofthemajoradvantagesusingthis statis-ticalapproachtoobtainhighenzymaticactivityislaboratory economics; inarecentwork hasreported5 the presenceof beta-xylosidasesinthermophilicfungiinsmallerquantities than this study,the abilityto obtainhigh enzymelevelsis desirable.
Thenumberoftestsperformedtoachievetheoptimalstate islesswithhigherefficiencyduetoless timespentforthe determinationoffurtherparametersandthereduceduseof reagentsneededintheculturesandenzymedosages.Thedata indicatedthatstrawcornresidue(abundantintheAmerican continents,largelyunexploredandconsideredpoorin nutri-ents)isanexcellentinducerofintracellular-xylosidasefrom T.lanuginosusinoptimizedconditions.
TheAspergillusfumigatusgrownoncorncobsat tempera-turesof30and42◦CshowedanoptimumpHof5.4andan optimumtemperature of70◦C.9 Lactobacillusbrevis NCDC01 grown in 1% wheat bran has an optimum pH and tem-perature of 6.0 and 40◦C, respectively.12 In the bacterium
Selenomonas ruminantiumGA192,showedanoptimumpHof 5.3andtemperatureof25◦Cfor-xylosidase.12,13
Alicyclobacil-lusacidocaldariusinoptimizedconditionsisassayedatpH5.5 andat50◦C.14Allofthesedatasuggesttheversatilityand het-erogeneityofbiochemicalcharacteristicsof-xylosidasesin pro-andeukaryoticmicroorganisms.
contributionof-xylosidase inthe process of deconstruct-ing hemicellulose, and they showed increased production ofreducing sugars withthepurifiedenzymes ascompared totheamountproduced withpre-hydrolysisbyxylanase.15 Inotherstudy withrecombinantproteins-xylosidase and xylanasepurifiedfromHumicolainsolensexpressedinE.coli tohydrolyzexylanfrombirchwoodandbeechwoodat37◦C, which resulted in saccharification increases of 1 and 1.29 times,respectively,indicatingthesynergisticactionofthese enzymes.16
Xylanaseand-xylosidaseisolatedfromNeurosporacrassa havealsobeenusedforthesaccharificationofxylan result-ingin18%hydrolysiswiththeindividualactionofxylanase, 68% with the combination of both and 48% with the individual action of -xylosidase, thereby indicating that -xylosidaseisanimportanttoolfordepolymerizationof xylo-oligosaccharides.17
In this study, the -xylosidase activity was monitored throughoutthetestshowinghighperformancewith hemicel-lulosefromcornstraw,anditretainedapproximately80%of theactivityovertime(Fig.6A).Mostofthetestsshowed sta-bilitythatlastedfor1hafterthestartofthesaccharification, whichstronglysuggestedthattheenzymewasnotsubjected totheeffectsofpotentialenzymeinhibitorsoftenproduced duringthistypeofprocess.
Ingeneral,thedatapresentedherestronglysuggestthat -xylosidasefromT.lanuginosuscanbeusedforthe sacchari-ficationofhemicellulosederivedfromcornstraw,anabundant residueintheAmericancontinents,thusprovidingan alter-native forfuture trials toproduce energythat dependson the conversion of plant biomass. Successful strategies for theproductionofhemicellulaseswithbiotechtraitsnotonly dependontheselectionofnewmicroorganismsbutalsoon theimprovementofexperimentalstrategiesthattake maxi-mumadvantageofitspotential.18,19
Conclusions
Thisstudydemonstratesthepotentialofusingcornstrawasa substrateforachievinghighlevelsofintracellular-xylosidase (1003U/mL/1683U/mg)forT.lanuginosus.Thisabundant agro-industrialwastebiomassiseasilyaccessibleandcanleadto significantcostreductionsforproductionofenzymeswhen usedasacarbonsource.Althoughitiswidelyusedforfeed, corn straw is underutilized in biotechnological processes, thusleadingtoitsaccumulationintheenvironment.Inthis study, we tested the optimal conditions for producing  -xylosidaseusinganoptimizedexperimentaldesignresulting inanincreaseofapproximately500%or4.7timesinterms ofenzymeyield.Ourresultssuggestanapplicationforthe useofcorn strawnotonlyforenzymeproductionbut also forprocessesinvolvingthesaccharificationofhemicellulose. Additionalstudiesarestillneededandarebeingconducted fortheapplicationofcornstrawintheproductionofbiofuels.
Conflict
of
interest
Theauthorsdeclarethattheyhavenoconflictofinterest.
Acknowledgements
J.M. Corrêa was fellow of the Coordination of Improve-ment of Higher Education Personnel(CAPES, Brazil). R.C.G. Simão (process 630/2014) was partially supported by the Araucária Foundation. M.K. Kadowaki were supported by the Araucária Foundation (process 395/2013) and National CouncilforScientificandTechnologicalDevelopment(CNPq 563260/2010-6/Brazil).
r
e
f
e
r
e
n
c
e
s
1.JuturuV,WuJC.Insightintomicrobialhemicellulasesother thanxylanases:areview.JChemTechnolBiotechnol.
2013;88:353–363.
2.SuY,ZhangX,HouZ,ZhuX,GuoX,LingP.Improvementof xylanaseproductionbythermophilicfungusThermomyces lanuginosusSDYKY-1usingresponsesurfacemethodology.
NewBiotechnol.2011;28:40–46.
3.ReddyV,ReddyP,PillayB,SinghS.Effectofaerationonthe productionofhemicellulasesbyT.lanuginosusSSBPina30l bioreactor.ProcessBiochem.2002;37:1221–1228.
4.PuchartV,KatapodisP,BielyP,etal.Productionofxylanases, mannanases,andpectinasesbythethermophilicfungus
Thermomyceslanuginosus.EnzymeMicrobTechnol. 1999;24:355–361.
5.BenassiVM,LucasRC,JordeJA,PolizeliMLT.Screeningof thermotolerantandthermophilicfungiaiming-xylosidase andarabinanaseproduction.BrazJMicrobiol.
2014;45:1459–1467.
6.DerringerG,SuichR.Simultaneousoptimizationofseveral responsevariables.JQualTechnol.1980;12:214–219.
7.SinghS,MadlalaAM,PriorBA.Thermomyceslanuginosus: propertiesofstrainsandtheirhemicellulases.FEMSMicrobiol Rev.2003;27:3–16.
8.BennetNA,RyanJ,BielyP,VranskaM.Biochemicaland catalyticpropertiesofanendoxylanasepurifiedfromthe culturefiltrateofThermomyceslanuginosusATCC46882.
CarbohydrRes.1998;306:445–455.
9.LenartoviczV,SouzaCGM,MoreiraFG,PeraltaRM. Temperatureandcarbonsourceaffecttheproductionand secretionofathermostable-xylosidasebyAspergillus fumigatus.ProcessBiochem.2003;38:1775–1780.
10.BokhariSAI,RajokaMI,JavaidA,Shafiq-ur-Rehman, Ishtiaq-ur-Rehman,LatifF.Novelthermodynamicsof xylanaseformationbya2-deoxy-d-glucose
resistantmutantofThermomyceslanuginosus
anditsxylanasepotentialforbiobleachability.Bioresour Technol.2010;101:2800–2808.
11.HoqMM,HempelC,DeckwerWD.Cellulase-freexylanaseby
ThermomyceslanuginosusRT9:effectofagitation,aeration, andmediumcomponentsonproduction.JBiotechnol. 1994;37:49–58.
12.LasradoLD,GudipatiM.Purificationandcharacterizationof
-d-xylosidasefromLactobacillusbrevisgrownon xylo-oligosaccharides.CarbohydrPolym.2013;92: 1978–1983.
13.JordanDB,LiXL,DunlapCA,WhiteheadTR,CottaMA. -Xylosidaseforconversionofplantcellwallcarbohydrates tosimplesugars.USPatent20,090,280,541;2009.
15.CorrêaJM,GracianoL,AbrahãoJ,etal.Expressionand characterizationofaGH39-xylosidaseIIfromCaulobacter crescentus.ApplBiochemBiotechnol.2012;168:2218–2229.
16.YangX,ShiP,HuangH,etal.Twoxylose-tolerantGH43 bifunctional-xylosidase/␣-arabinosidasesandoneGH11 xylanasefromHumicolainsolensandtheirsynergyinthe degradationofxylan.FoodChem.2014;148:381–387.
17.DeshpandeV,LachkeA,MishraC,KeskarS,RaoM.Modeof actionandpropertiesofxylanaseand-xylosidasefrom
Neurosporacrassa.BiotechnolBioeng.1986;28:1832–1837.
18.MallerA,ViciAC,FacchiniFDA,etal.Increaseofthephytase productionbyAspergillusjaponicusanditsbiocatalyst potentialonchickenfeedtreatment.JBasicMicrobiol. 2014;54:S152–S160.