w ww.e l s e v i e r . c o m / l o c a t e / b j p
Original
Article
Turbo-extraction
of
glycosides
from
Stevia
rebaudiana
using
a
fractional
factorial
design
Paula
M.
Martins
a,
Aurea
D.
Lanchote
c,
Bhaskar
N.
Thorat
b,
Luis
A.P.
Freitas
c,∗aFaculdadedeCeilândia,UniversidadedeBrasília,Brasília,DF,Brazil
bInstituteofChemicalTechnology,NathalalParekhMarg,Matunga,Mumbai,India
cFaculdadedeCiênciasFarmacêuticasdeRibeirãoPreto,UniversidadedeSãoPaulo,RibeirãoPreto,SP,Brazil
a
r
t
i
c
l
e
i
n
f
o
Articlehistory:
Received29November2016 Accepted24February2017 Availableonline1April2017
Keywords:
Designofexperiments Stevioside
RebaudosideA Hydroethanolicextract Desirability
a
b
s
t
r
a
c
t
Steviarebaudiana(Bertoni)Bertoni,Asteraceae,leafextracthasrecentlycalledtheattentionoffood industryasaproposalfornaturalsweetener.Thesweetflavorisattributedtotheglycosides,inespecial steviosideandrebaudiosideA,whicharetheplantmainchemicalmarkers.Theaimoftheworkreported herewastooptimizetheturbo-extractionofstevialeavesusingwater,ethanol70%and90%(w/w)as greensolvents.A25-2factorialdesignwasappliedtostudythelineareffectsofthedrugsize,solventto
drugratio,temperature,timeandalsotheturbolysisspeedontheextractionofglycosides.Theglycosides exhaustiveextractionshowedthatethanol70%gavebetterresultsandwasusedforturbo-extraction. ThesteviosideandrebaudiosideAcontentswerequantifiedbyavalidatedmethodbyhighperformance liquidchromatographicwithphotodiodearraydetector.ThecontentsofsteviosideandrebaudiosideAin fluidextractincreasedwiththedrugsize,butdecreasedathighshearingspeedsandsolventtodrugratio, whiletheiryieldsdecreasedathighertemperatureandwerenotaffectedbyturbospeed.Anincreasein solventtodrugratioreducedsignificantlytheglycosidespercentindriedextract.Optimalsolutionfor
S.rebaudianaleavesturbo-extractionwasdeterminedbydesirabilityfunctions.Theoptimalextraction conditioncorrespondedtodrugsizeof780m,solventtodrugratioof10,extractiontimeof18min;
temperatureof23◦Candturbospeedof20,000rpm,resultinginyieldsof4.98%and2.70%,forstevioside
andrebaudiosideA,respectively.Theseyieldsarecomparabletotheonesrecentlypublishedfordynamic maceration,butwiththeadvantageofshorterextractiontimes.Thisworkdemonstratesthatturbolysis ispromisingforS.rebaudianaglycosidesextractionandstimulatenewresearchonthepurificationof theseextracts,whichmaybecomeaninterestingsourceofincomefordevelopingcountriessuchasIndia andBrazil.
©2017SociedadeBrasileiradeFarmacognosia.PublishedbyElsevierEditoraLtda.Thisisanopen accessarticleundertheCCBY-NC-NDlicense(http://creativecommons.org/licenses/by-nc-nd/4.0/).
Introduction
The “estevia” is a shrub popularly known worldwide and belongstothe261membersofgenusinthefamilyAsteraceaeand hasthebotanicalnameofSteviarebaudiana (Bertoni)Bertoni.It originatesfromtheValleyofAmambay,whichextendsfrom north-eastParaguaytosouthBrazilandsouthernArgentina.Theplantis aperennialshrubheightupto30cm.Itsleavesaresessile,3–4cm long,shapedasspatulaorlongbladewithsawnedges.Theadaxial surfaceisslightlyglandular.Thebranchesarefragileandtheroots arerhizometype, slightlybranched.Theflowersare composite,
∗ Correspondingauthor.
E-mail:lapdfrei@usp.br(L.A.Freitas).
surroundedbyepicalyceshousing,colorwine,lightand pentamer-ous.Thefruitisstriated(Madanetal.,2010).
Stevialeavescontaineightterpeneglycosides,identifiedas ste-vioside,rebaudosideA,B,C,DandE,dulcosideAandC.Themain glycosidesaresteviosideandrebaudosideA,summingupto5–10% ofthedrug(SinghandRao,2005;Abou-Arabetal.,2010;Yadav etal.,2011)andgiveapronouncedsweetflavor.Steviaisdescribed in theBrazilianPharmacopoeia 5th Ed.(FarmacopeiaBrasileira, 2010)indicatingdriedleavesasthepartusedandmustcontain atleast12%oftotalcarbohydratesand4%steviosides.
AccordingtoMadan etal.(2010),thespecies hasbeenused for centuries by the Guarani Indians as a sweetener in some drinks,especiallythematetea,andtheactivecomponentthathas thehighestsweetnessindexisrebaudosideAwhilesteviosideis themaincomponentandgivesapostdigestivebittertaste.The
http://dx.doi.org/10.1016/j.bjp.2017.02.007
concentrationofthesediterpeneglycosidesmayvarydepending oncultivars,soiland climate,andagronomictechniques(Yadav etal.,2011).
The medicinal use of the species is described in the con-trol of obesity, high blood pressure, digestive problems and contraceptivebyindigenous peoplesinmany countriesof Latin America(Sardhara,2015;Madanetal.,2010).Preclinicaland clini-calstudieshavebeenconductedtoevaluatetheantioxidantactivity (Sharmaetal.,2012),thereproductivesystem,kidneyfunction, bloodpressure,theeffectonbloodglucoseandanti-viralactivity, aswellasstudiesonpharmacokinetic(Madanetal.,2010),toxicity, sideeffectsandallergenic(Urbanetal.,2015).
Inmanycountriessteviahasitsuseregulatedasadietary sup-plementandthusisnotusedasasweetener,suchasinEurope. Butin2008,theFDAhascategorizedthespeciesasGenerally Rec-ognizedasSafe(GRAS)afterpublicationbytheJointCommitteeof ExpertsontheFAOFoodAdditives/WHO–JECFA,whichestablished themonographofglycosidesofsteviol(WHO,2004).Theproduct approvedforindustrialandcommercialuseisapowderwith min-imumof95%ofsteviolglycosidesandthesumofsteviosideand rebaudosideoAnotlessthan70%.
Steviaproductsareconsideredhealthyandnaturalfoodsand havegained attentionininternationalmarkets(Gasmallaetal., 2014) with China, India, Brazil, Korea, Mexico, United States, Indonesia,TanzaniaandCanadaasproducers(Ahmadetal.,2014). Recently,thecommercialgiantCocaColaCompanyhaslaunched the“green”product“CokeLife”andisexpectedtohighlyincrease thesteviaworldsales(Ausfood,2015).
The separation of the glycosides from the plant extract is hamperedbyseveralfactorsincludingimpuritiessuchasresins, proteins, organic acids and especially pigments (chlorophyll, caroteneandxanthophyll). Theaqueousextractionofthe pow-dered leaveswas themost indicated choice until recently and presentsthehighestyieldsof steviosideand rebaudosídeo Ain crude extract (Abou-Arab et al., 2010; Chhaya et al., 2012a,b; MondalandChhaya,2012;Raoetal.,2012;Pericheetal.,2014; Sardhara,2015).
Theuseofmethanolwasalsoproposedtogiveselective extrac-tionandeasypurification(Rajabetal.,2009),buttheendproduct isnolongerclassifiedasGRAS.Becauseofthebloominginterest in steviaproducts, there is a largenumber of publications and patentsontheextractionandpurificationofsteviosideusing dif-ferent methods (Periche et al.,2015; Jentzer etal., 2015), such asenzymatic extraction(Purietal.,2012),bleachingagentsand selectiveprecipitationprocessesusingmetalions(Kienle,2010; Abou-Arabetal.,2010),selectiveextractionbysupercriticalfluid extractionandseparationprocessesforadsorptivemethodsusing chromatographiccolumns,chemicalsolventsandactivatedcarbon (Rajabetal.,2009),ionexchangeresins,micro,ultraand nanofil-tration(Zhangetal.,2000;Reisetal.,2009;Raoetal.,2012;Chhaya etal.,2013).
Recently,theglycosidesextractionfromsteviausingethanol 70 and 90% wasstudied by dynamic maceration coupled with a multivariateapproachand resultedin stimulatingadvantages over previous methods (Martins et al., 2016). Meanwhile, the turbo-extractionorturbolysiscanbeadvantageousoverdynamic macerationandisbasedonextractionwithstirringand simulta-neousreductionofparticlesizeasaresultofapplicationofhigh shearingforce,sothatwiththedisruptionofthecellsthereisa rapiddissolutionoftheactivesubstances,resultinginextraction timesoftheorderofminutesandalmostcompletelyexhausted theplantdrug(Sonaglioetal.,2003).
Nevertheless, turbolysisof stevia hasnever beenattempted (Martinsetal.,2016)andtogetherwiththeuseofdifferentgrade ethanolasgreenandGRASsolventandtheapplicationof experi-mentaldesigns(Costa-Machadoetal.,2013;Pauluccietal.,2013;
Martinsetal.,2013)thisprocesscouldgiveapromisingand opti-mizedmethodforsteviaextraction.
Thus,theaimofthisworkwastostudytheturbo-extractionof steviosideandrebaudosídeoAfromS.rebaudianadriedand pow-deredleavesbyapplyingafractionalfactorialdesignthatallowthe evaluationofthemaineffectsofdrugpowdersize,weightratio sol-venttodrug,temperature,agitationandtimeontheyieldofthese glycosides.
Materialandmethods
Steviadrugcharacterization
TheleavesofSteviarebaudiana(Bertoni) Bertoni,Asteraceae, camefromaplantationlocatedatlatitude22◦79′81.059′′Sand lon-gitude47◦11′56.138′′W,grownbytheCPQBA–Multidisciplinary CenterforChemical,BiologicalandAgriculturalSciencesfromState University ofCampinas(Campinas– SP,Brazil), witha voucher depositedattheCPQBAherbariumwithfilenumber273.Theplant wassuppliedtoCPQBAbyCenargen(Brasília,DF,Brazil)in1998, accordingtoDrIllioMontanariJr.
Thepharmacognosticcharacterizationofdrugwasperformed accordingtotheBrazilianPharmacopoeia5thEdition(Farmacopeia Brasileira,2010)afterdryingat45±2◦Cinacirculatingairoven andmilling.Thephysicochemicalparametersdeterminedwerethe moisturecontent,totalash,swellingindexandsolventuptake.
Turbolysisextractionstudy
InordertodeterminethesteviosideandrebaudiosideAcontent inthedrug,a preliminaryexhaustiveextractionstudywas per-formedbyusingwater,alcohol70%and90%assolvents.Finedrug powder(5g)wasaddedto50mlofsolventandstirredatroom tem-perature(25◦C)for2h.Thedrugpowderasfilteredandthenthe procedurewasrepeatedthree-foldwith50mloffreshsolvent.The fourthandfinalextractionstepwasrunfor12hatsameconditions (50mlfreshsolventunderstirringand25◦C).Theextractionruns werecarriedoutintriplicateandthestevioside content(CEST), rebaudosideAcontent(CREB)andthetotalsolidscontent(TSOL) weredetermined.
Theturbo-extractionorturbolysiswasperformedusingahigh shearstirrerUltraturraxT-25(IkaLtda,SaoPauloBrazil).Grounded stevialeavestogetherwith50mlofethanol70%asextracting sol-ventwerepouredintoa50mlbeakerandsubmittedtothehigh shear stirring according to temperature, velocity and time pre determinedbytheexperimentaldesign.Theextractswerefiltered onWhatman® qualitativefilterpapergrade1(poresize11m) andstoredat−8◦Cforfurtherchromatographicdeterminationof TSOLandquantificationofCESTandCREBbyHPLC-PAD.
Theeffectoffiveturbolysisextractionfactorswereinvestigated byapplyinga25-2fractionalfactorialdesign,asshowninTable1 (Moura-Costaetal.,2012;Araújoetal.,2014;Martinsetal.,2016). Theextractionfactorsselected,accordingtoMartinsetal.(2016), werethesolventtodrugmassratio(S/D),averagedrugpowdersize (D50),highshearspeed(SS),extractiontime(t),andtemperature (T).TheireffectontheextractglycosidescontentCESTandCREB werequantifiedbyliquidchromatography.Toallowtheelucidation oftheeffectofdrugdivisionlevelontheextraction,thedrugwas dividedintwosizefractions,calledfinepowder(150–212m)and coarsepowder(710–850m),withaveragesizesof181mand 780m,respectively.
Table1
Steviarebaudianaturbo-extractionfractionalfactorialdesign25-2presentingfactors,theirlevelsaandextractionresults.
Run D50(m) S/D(g/g) t(min) T(◦C) SS(rpm) CEST(mg/ml) CREB(mg/ml) YST(%) YREB(%) DST(%) DREB(%)
1 −1 −1 −1 −1 +1 2.37 1.25 4.65 2.45 6.19 3.27
2 +1 −1 −1 +1 −1 2.83 1.52 4.26 2.28 6.24 3.35
3 −1 +1 −1 +1 −1 0.12 0.07 0.11 0.06 0.76 0.45
4 +1 +1 −1 −1 +1 0.17 0.10 0.15 0.09 1.19 0.70
5 −1 −1 +1 +1 +1 2.22 1.21 4.50 2.46 5.28 2.88
6 +1 −1 +1 −1 −1 2.75 1.49 5.22 2.83 6.63 3.59
7 −1 +1 +1 −1 −1 0.14 0.08 0.12 0.07 1.24 0.71
8 +1 +1 +1 +1 +1 0.21 0.12 0.15 0.09 1.52 0.87
aActualvaluesforeachfactor:drugmeansizeD50(−1)181m,D50(+1)780m;solventtodrugratioS/D(−1)10g/g,S/D(+1)30g/g;extractiontimet(−1)3min,t
(+1)18min;extractiontemperatureT(−1)23◦C,T(+1)80◦C;turbospeedSS(−1)5000rpm,SS(+1)20,000rpm.SteviosideandrebaudiosidecontentsinfluidextractCEST
andCREB(mg/ml);SteviosideandrebaudiosideyieldsYSTandYREB(%).Steviosideandrebaudiosidecontentsindriedextract,DSTandDREB(%).
calculatetheglycosidescontentindriedextracts,DSTandDREB.
TheliquidextractsweredriedinErlenmeyer’sundernitrogenflux
of10ml/minatroomtemperature.Thedefinitionsusedtocalculate
DSTandDREBaregiveninEqs.(3)and(4).
YST (%)= CEST×V
Wd ×100 (1)
YREB (%)=CREB×V
Wd ×100 (2)
DST (%)= (CEST×V)
(TSOL×V)×100 (3)
DREB (%)= (DREB×V)
(TSOL×V)×100 (4)
whereV,volumeofliquidextractcollected;Wd,weightofdrug usedinextractionrun.
GlycosidesquantificationbyHPLC-PDA
Theglycosidesstevioside andrebaudosideAwerequantified byhighperformanceliquidchromatography,HPLC-PDA.TheHPLC equipmentusedwasanUltimate3000(ThermoFischerScientific, Sunnyvale,CA,USA)withachromatographiccolumnPhenomenex C18(5m, 250×4.6mm) and a photodiode arraydetector set to210nm(Liuetal.,2010;Woelwer-Riecketal.,2010; Aranda-Gonzalezetal.,2014;Jentzeretal.,2015).ThesoftwareChromeleon 7.1.2ChromatographicDataSystem(ThermoFischerSci, Sunny-vale,CA,USA)wasusedfordataacquisitionandanalysis.Theoven wassetto50◦Candtheacetonitrile/watermobilephasewas oper-atedingradientmodevaryingfrom10:90to90:10,v/vin45min atflowof0.7ml/min.Thepureanalyticalstandardsofthe glyco-sides(Sigma–AldrichCo,SãoPaulo,Brazil)wereusedasreference standardstopreparethecalibrationcurves.Solutionscontaining concentrationsof50,100,200, 400and600g/ml for rebaudo-sideAand28,84,140,224,560and840g/mlforsteviosidein methanolwerepreparedandinjectedinquintuplicateforthe cal-ibrationcurvesconstruction.Dependingonglycosidescontentof theextracts,thesampleinjectionvolumevariedfrom5to25l. Bothextractsamplesandanalyticalstandardsweresolubilizedin methanolandfilteredthrough0.45mfilter(MilliporeCo., Biller-ica,MA,USA)priortoinjection.Allextractssampleswereinjected intriplicate.Methodresolutionwascalculatedbasedonselectivity (separationfactor),efficiencyandretention(capacityfactor)bythe softwareChromeleon7.1.2.
Multivariateanalysis
The25-2fractionalfactorialdesign,asshowninTable1( Moura-Costaetal.,2012;Araújoetal.,2014;Martinsetal.,2016)was appliedtoinvestigatethelineareffectsof S/D,D50, SS,t,and T ontheTSOL, CEST,CREB,YST,YREB,DSTandDREBbyresponse
surfacemethodology,RSM,usingMinitab14.0(Minitab,State Col-lege,USA),adoptingthesignificancelevelof5%(p<0.05).Amodel consideringlineartermsofthefactorswasfittedtothedata, accord-ingtoEq.(5).
Yi=A1+A2X1+A3X2+A4X3+A5X4+A6X5 (5)
TheresponsesurfacesfittedtoEq.(5)wereusedtofind opti-malextractionconditionbyapplyingthe“DesirabilityFunctions Method”,using“ResponseOptimizer”(Minitabv14,StateCollege, USA).
Resultsanddiscussion
TheS.rebaudiana leavespresented totalmoisturecontentof 8.62±0.20%,ashcontentof8.16±1.08%,swellingindexof67%and solventuptakeof149%inethanol90%,33%and177%inethanol70% and167%and245%inwater.Thedrugmoistureandashcontents areunderthemaximumlimitsestablishedbytheBrazilian Pharma-copoeia(FarmacopéiaBrasileira,2010)13%and9.5%,respectively, and can beattributedto adequatepost-harvest processing, e.g. dryingandstorage,andalsothatthedrugisacceptableforthe forth-comingextractionstudy.Totalashrepresentsapuritytestforthe presenceofnon-volatileorganicsubstancesbyincineration,giving thesumofinorganicorintrinsicashinS.rebaudianaandthe con-taminantsfromearthyorigin.Ontheotherhand,swellingindex andsolventuptakebydrugareusedtoestimatethevolumeof solventthatwillberetainedorabsorbedbyvegetalmatterafter extractionandthefinalextractvolumerecovered.Thistraditional pharmacognosticcharacterizationisessentialtoguaranteeagood phytopharmaceuticaltechnologystudyoftheplant.TheS. rebaudi-anaswellingindexandsolventuptakewere,indescendingorder, water,ethanol90%andethanol70%.Inordertochoosethebest solventforglycosidesextractionfromS.rebaudianaapreliminary exhaustiveextractionbymacerationwascarriedout.
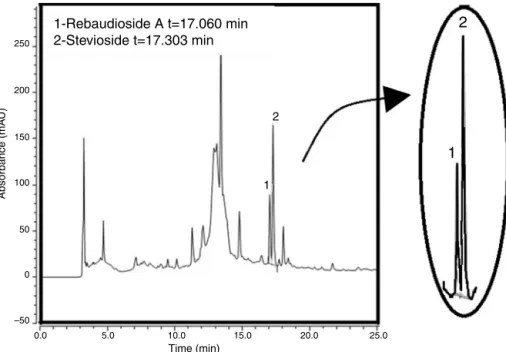
TheHPLC-PDAchromatogramshowingsteviosideand rebau-dioside A peaks in extract injection can be seen in Fig. 1. Stevioside and rebaudiosideA had elutiontimes of17.303 and 17.060min,respectively.Thecalibrationcurvesobtainedandtheir correlationcoefficientswereC=0.0557A+0.2918(R2=0.9996)and C=0.0491A+0.1154(R2=0.9999),forsteviosideandfor rebaudio-sideArespectively.Furthermore,theresolutionscalculatedbythe softwareChromeleon7.1.2(ThermoFischerScientific,Sunnyvale, CA,USA)variedfrom1.98to1.99and1.48to1.64forstevioside andrebaudiosideA,respectively.
2
1
1 2
1-Rebaudioside A t=17.060 min
2-Stevioside t=17.303 min
250
200
150
100
50
0
–50
0.0 5.0 10.0 15.0
Time (min)
Absorbance (mA
U)
20.0 25.0
Fig.1.HPLC-PDAchromatogramofSteviarebaudianaleafextractobtainedbyturbolysisusingethanol70%,solventtodrugweightratio1:10,drugpowdersize780m, temperature50◦C,turbolysisspeed20,000rpmand1hextraction.Peak1rebaudosídeA(17.060min)andpeak2estevioside(17.303min).
Table2
Cumulativestevioside,rebaudiosideAandtotalsolidscontentsinSteviarebaudianaextractsobtainedinafour-stepexhaustiveextractionbydynamicmaceration.
Extractiontime(h) Solvent
Water Ethanol70% Ethanol90%
CEST(mg/ml) CREB(mg/ml) TSOL% CEST(mg/ml) CREB(mg/ml) TSOL(%) CEST(mg/ml) CREB(mg/ml) TSOL(%)
2 1.83 1.12 3.08 2.52 1.48 3.84 1.77 0.95 2.72
4 2.15 1.33 3.38 2.69 1.59 4.76 1.98 1.07 3.73
6 2.17 1.39 3.50 2.78a 1.65a 4.94a 2.25 1.25 4.01
18 2.17 1.39 3.67 2.80a 1.66a 5.20a 2.41 1.37 4.27
Final 2.17 1.39 3.67 2.80 1.66 5.20 2.41 1.37 4.27
aSteviosideandrebaudiosidecontentsinfluidextractCESTandCREB(mg/ml);totalsolidscontentinfluidextractTSOL(%,w/w).
obtainedwas100ml.RebaudosideAextractionfromS.rebaudiana withdifferentgradesofethanolupto60%(Gasmallaetal.,2014) resultedinhigheryieldswithethanol30%,justifiedbytheswelling index,accordingtotheauthors.However,inotherwork(Martins etal.,2016)highswellingindexandextractionyieldsfor stevio-sideandrebaudiosideAwereobservedforethanol70%,whichis inagreementwiththis workand canbeexplained bythe ade-quatepolarityofthissolventforsteviacomponentsextraction,like organicacids,flavonoids,alkaloids,xanthophyll,oligosaccharides andglycosides(Muandaetal.,2011).Ethanol70%hasalsogiving excellentresultsinothernaturalproductsextraction,suchasfor curcuminoidsfromCurcumalonga(Martinset al.,2013), actives fromofSchinusterebenthifoliusshells(Mouraetal.,2005)andin theturbolysisofpopularplantsfromancienttribes,usingethanol 38–56%(Moura-Costaetal.,2012).BasedonliteratureforS. rebau-dianaglycosidesextraction(Liuetal.,2010;Gasmallaetal.,2014; Sardhara,2015;Pericheetal.,2015;Jentzeretal.,2015;Koubaa etal.,2015;Martinsetal.,2016)and theresultsinTable2the solventchosenfortheturbolysisstudywasethanol70%.Besides, ethanolisclassifiedasGRAS(Rajabetal.,2009)andalsoasagreen solvent(Capelloetal.,2007;Chematetal.,2015)andsohighly rec-ommendedforphytotherapicsandnutraceuticals(Chematetal., 2012;Torrietal.,2016).
Theresultsofextractionbythefractionalfactorialdesignare showninTable1,whichdisplaysthevalues ofCEST,CREB,YST, YREB, DST and DREB for all conditions studied. This data was analyzed by response surface methodology using Minitab 14.0
(Minitab,StateCollege,USA)andresultedinlinearmodels, accord-ingtoEq.(1),foreachextractparameterasshowninTable3,where thesignificanttermsandrespectivecoefficientsforthemodelare indicated.Thesteviosidecontentinfluidextract,CEST(mg/ml) var-iedfrom0.12mg/mlto2.83mg/mlandwasaffectedbythesolvent todrugmassratio–S/D,averagedrugpowdersize–D50andhigh shearorturbolysisspeed–SS,atthesignificancelevelof5%,which wasadoptedforallanalysisintheworkherein.Ontheotherhand, rebaudiosideAcontentinfluidextract,CREB(mg/ml),rangedfrom 0.07mg/mlto1.52mg/mlandwassimilarlyaffectedbythesame factorssignificantforstevioside,S/D,D50andSS,whichisexpected bychemicalsimilaritybetweenthesetwoglycosidesfromS. rebau-diana.Allfivefactorsstudiedinthefractionalfactorialdesignwere chosenbasedonthe“spaceofknowledge”assuggestedbythe up-to-dateapproachoftheQualitybyDesign(Yanetal.,2014),since previousknowledgeindicatethesearethemostimportantin turbo-extraction.However,theresultsshowthatonlyS/D,D50andSS affectedtheextraction,whileextractiontime(t)andtemperature (T),didnot.Theextractiontimeandtemperatureareusuallyvery importantfactorsinherbaldrugextractiveprocessesbecausethey mayalterthekineticsofactiveremovalfromplantmatrixby equi-libriumdislocation.However,turbolysisisahighenergyextraction requiringreducedtimeandtemperature.Probablythesetwo fac-torswerenotsignificantduetotheshortextractiontimesof3and 18min,aswellasmoderatetemperaturesof23and80◦C.
Table3
SummaryoffactorialANOVAfortheprincipaltermsinSteviarebaudianaturbo-extractionfractionalfactorialdesign25-2.
Factor Responses
CEST(mg/ml) CREB(mg/ml) YST(%) YREB(%) DST(%) DREB(%)
D50(m) p=0.000a
ki=1.345
p=0.000a
ki=0.080
p=0.302 p=0.283 p=0.106 p=0.016a
ki=0.059
S/D(g/g) p=0.000a
ki=−1.185
p=0.000a
ki=−0.633
p=0.000a
ki=−2.24
p=0.000a
ki=−1.205
p=0.000a
ki=−2.668
p=0.000a
ki=−1.415
t(min) p=0.092 p=0.467 p=0.09 p=0.047a
ki=0.071
p=0.860 p=0.223
T(◦C) p=0.473 p=0.724 p=0.022a
ki=−0.15
p=0.046a
ki=−0.071
p=0.767 p=0.280
SS(rpm) p=0.000a
ki=−0.114
p=0.000a
ki=−0.061
p=−0.04 p=0.442 p=0.513 p=0.493
R2
adj 99.8% 99.9% 99.4% 99.3% 99.6% 99.8%
D50,drugmeansize;S/D,solventtodrugratio;t,extractiontime;T,extractiontemperature;SS,turbospeed.SteviosideandrebaudiosidecontentsinfluidextractCEST (mg/ml)andCREB(mg/ml);SteviosideandrebaudiosideyieldsYST(%)andYREB(%).Steviosideandrebaudiosidecontentsindriedextract,DST(%)andDREB(%).
aSignificantat5%level(p<0.05);ki,coefficientsforthemodel,R2
adj,adjustedRsquared.
A
CEST mg/ml < 0.5 0.5 - 1.0 1.0 - 1.5 1.5 - 2.0 2.0 - 2.5 > 2.5
CEST mg/ml < 0.5 0.5 - 1.0 1.0 - 1.5 1.5 - 2.0 2.0 - 2.5 > 2.5
1.0 1.0
0.5 0.5
0.0 0.0
–0.5 –0.5
–1.0 –1.0
–1.0 –0.5 0.0
SS S/D
D50 D50
0.5 1.0 –1.0 –0.5 0.0 0.5 1.0
B
Fig.2.Contourplotsforsteviosidecontent(CEST,mg/ml)influidextractasfunctionsof:(A)druggranulometry(D50)andhighshearspeed(SS);and(B)druggranulometry (D50)andsolventtodrugratio(S/D).
1.0
A
B
0.5
CREG mg/ml <0.2 0.2 - 0.4 0.4 - 0.6 0.6 - 0.8 0.8 - 1.0 1.0 - 1.2
> 1.4 1.2 - 1.4
CREG mg/ml <0.2 0.2 - 0.4 0.4 - 0.6 0.6 - 0.8 0.8 - 1.0 1.0 - 1.2
> 1.4 1.2 - 1.4
D50 D50
SS S/D
0.0
–0.5
–1.0
1.0
0.5
0.0
–0.5
–1.0
–1.0 –0.5 0.0 0.5 1.0 –1.0 –0.5 0.0 0.5 1.0
Fig.3. ContourplotsforrebaudiosideAcontent(CREB,mg/ml)influidextractasfunctionsof:(A)druggranulometry(D50)andhighshearspeed(SS);and(B)drug granulometry(D50)andsolventtodrugratio(S/D).
seeninFigs.2and3,respectively.Thecontourplotofstevioside content,CEST(mg/ml),asafunctionofD50andSSisshown in Fig.2A,whileCESTvaluesasafunctionofD50andS/Disshown inFig.2B.Fig.2AshowsthatCESTvaluesarehigherinthecorners ofthecontourplot,withcombinationsoflargeD50andlowSSor theopposite.Thisresultisinterestingbecauseitshowsthatcoarse powdersneedmoresolventfortheextraction,butatthesametime finepowdercangivegoodextractiveresultwithlesssolvent.The
A
1.0YST % < 1 1 - 2 2 - 3 3 - 4 4 - 5 > 5
YST % < 1 1 - 2 2 - 3 3 - 4 4 - 5 > 5 0.5
0.0
–0.5
–1.0
1.0
0.5
0.0
–0.5
–1.0 –1.0 –0.5 0.0
T T
S/D S/D
0.5 1.0 –1.0 –0.5 0.0 0.5 1.0
B
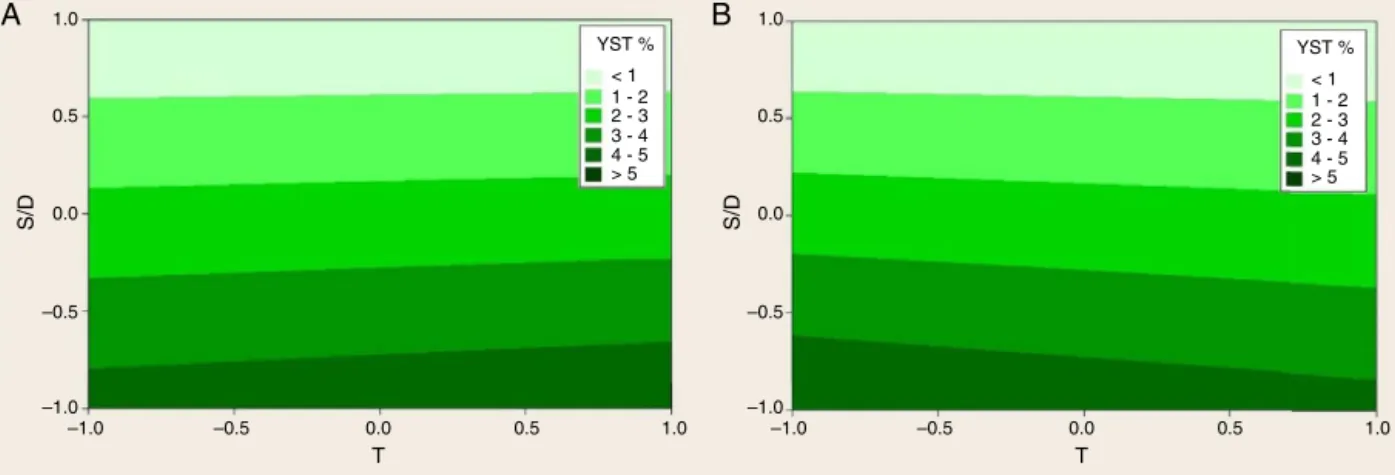
Fig.4. Contourplotsforsteviosideextractionpercentyield(YST,%)fromSteviarebaudianaleavesasfunctionsof:(A)solvent/drugratio(S/D)andtemperature(t);and(B) solventtodrugratio(S/D)andextractiontemperature(T).
1.0
YREB % YREB %
< 0.5 0.5 - 1.0 1.0 - 1.5 1.5 - 2.0 2.0 - 2.5 > 2.5
< 0.5 0.5 - 1.0 1.0 - 1.5 1.5 - 2.0 2.0 - 2.5 > 2.5 0.5
0.0
–0.5
–1.0
1.0
0.5
0.0
–0.5
–1.0 –1.0 –0.5 0.0
T T
S/D S/D
A
B
0.5 1.0 –1.0 –0.5 0.0 0.5 1.0
Fig.5.ContourplotsforrebaudiosideAextractionpercentyield(YREB,%)fromSteviarebaudianaleavesasfunctionsof:(A)solvent/drugratio(S/D)andtemperature(t);and (B)solventtodrugratio(S/D)andextractiontemperature(T).
inFigs.2A-3Aand2B-3B.Asamatteroffact,thesignificant fac-torswerethesameforsteviosideandrebaudiosideAcontentsin fluidextract,whichcouldbeexplainedbythechemicalsimilarity betweenthesetwoglycosides.Duetothisonemayalsoconclude thatfinerpowderneedlesssolventbutlargeramountsofsolvent givesbetterextraction.AgainforrebaudiosideAthefactorstand Tdidnotaffectextractionsignificantly,duetocharacteristicsof theturbolysistechnique.Itisimportanttoreinforcethatthe deter-minationcoefficients,ortheadjustedRsquared,giveninTable3 showverygoodfitsofthemodelsbeing99.8%and99.9%forCEST andCREB,respectively.Thefittedequationsforthemultiplelinear CESTandCREBdependencyonthethreesignificantfactors,D50,SS andS/D,areshowinEqs.(6)and(7):
CEST (mg/ml)=1.345+0.145
D50−480.5 299.5−1.185
SD−20 10−0.114
SS−12,500 7500(6)
CREB (mg/ml)=0.725+0.080
D50−480.5 299.5−0.633
SD−20 10−0.061
SD−20 7500(7)
ThesteviosideandrebaudiosideAyieldsarerepresentedas per-centagesofthedrugweightusedintheexperimentalruns.The valuesareshowninTable1andcorrespondto0.11%to5.22%for steviosideandfrom0.06%to2.83%forrebaudiosideA.Thecontour plotshowingtheresponseofsteviosideyield,YST,asfunctionof S/DandtisshowninFig.4A,whileitsresponseasfunctionofS/D andTisshowninFig.4B.Fig.4AshowshigherYSTdecreaseswith S/Dincrease,e.g.decreasesforhigheramountsofsolvent.Although theeffect oft resulted significantin RSManalysis,the contour linesshowthatS/Dinfluenceismuchstronger.Thecontourplotin Fig.4BshowsthatS/DstronglyaffectsYSTaswell,andlower sol-ventamountsgavehighersteviosideyields.TheeffectofT,shown incontourlinesofFig.4BisalsolessimportantthantheS/D.These resultsareconfirmedbythecoefficientsinEq.(8),sincecoefficient forS/Dismorethanten-foldhigher.TherebaudiosideAyieldsare showninFig.5AandB.TheYREBvaluesarehigherforcoarse parti-clesandlowtemperatures,asshowninthecontourplotinFig.5A. ThetopographicviewofYREBresponsesasfunctionofS/Dandt canbeseeninFig.5B,whichshowsastrongeffectofS/D.Thedata ofYSTandYREBwerefittedtothemodelfromEq.(5),andresulted inEqs.(8)and(9),withadjustedR squaredof99.1%and99.0%, respectively(Fig.6).
YST (%)=2.382−2.24
SD−20 10−0.15
T−51.5 28.5Optimal D 0.93881
Hi Cur
Lo
ST % Pla
Maximum
y = 4.9795
d = 0.89978
REV % PI
Maximum
y = 2.6907
d = 0.86333
ST % PO
Maximum
y = 6.5606
d = 1.0000
REB % PO
Maximum
y = 3.5423
d = 1.0000
D50 1.0 [1.0] –1.0
S/D 1.0 [–1.0]
–1.0
t 1.0 [1.0] –1.0
TEMP 1.0 [–1.0]
–1.0
RPM 1.0 [–1.0]
–1.0
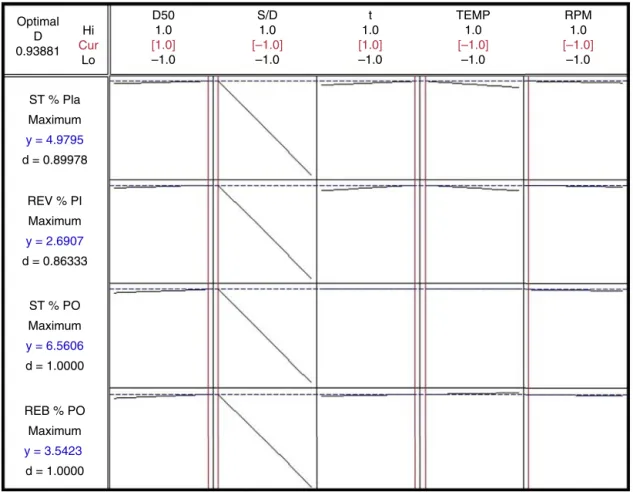
Fig.6.Plotshowingthedesirabilityresponsesforsteviosideandrebaudiosideyields,YSTandYREB,andtheircontentsindriedextract,DSTandDREB,foreachofthe followingstudiedfactors.Drugpowdersize:D50;temperature:T;time:t;turbolysisspeed:SS,andsolventtodrugratio:S/D.
YREB (%)=1.281−1.2057
SD−20 10+0.071
t−10.5 7.5−0.071
T−51.5 28.5(9)
Thevalues ofsteviosideand rebaudiosideAcontentindried extract, DST and DREB, respectively are given in Table 1. The steviosidecontentvariedfrom0.76to6.19%dependingon turbo-extractioncondition,but wasaffected significantly only bythe solventtodrugratio,S/D.Ontheotherhand,DREBwasaffected significantlybyS/DandalsoD50.ThemodelswerefittedtoDST andDREBwithadjustedRsquaredof99.6and99.8%,respectively, andaregivenbyEqs.(10)and(11).
DST (%)=3.787−2.669
SD−20 10(10)
DREB (%)=2.049+0.059
D50−480.5 299.5−1.415
SD−20 10(11)
Recently,theapplicationofmultivariateanalysisfor pharma-ceuticaldevelopmenthasbeenrecommendedbytheICH-Q8-R2 (ICH,2009)becausethissortofapproachturnspossiblethe opti-mizationoftheprocess.Somemethodsofoptimization,suchas thedesirabilityfunctions,allowthedeterminationofthemost suit-ableconditionwithintherangeoffactorsstudied(Huetal.,2008). Consideringthis,themodelsfittedinEqs.(6)–(11)wereusedto carryoutadesirabilityfunctionsanalysis(Huetal.,2008)using Minitab14(Minitab,StateCollege,USA).Thesettingstosolvethe
Table4
Summaryofdesirabilityfunctionsoptimization.
Settingsforoptimization Desirability Maximizedvalue
Responses GOAL LO TGT WT IMP
YST(%) Maximize 3.00 5.20 1 1 0.8998 4.980
YREB(%) Maximize 2.00 2.80 1 1 0.8633 2.691
DST(%) Maximize 5.00 6.50 1 1 1.000 6.488
DREB(%) Maximize 3.00 3.50 1 1 1.0000 3.513
Globaldesirability:0.9388.
LO,lowervalue;TGT,targetvalue;WT,weightofresponse;IMP,importanceofresponse.
OptimumsetofconditionsforturboextractionofSTEVIA:D50=780m,S/D=10(g/g);t=18min;T=23◦C;SS=5000rpm. SteviosideandrebaudiosideyieldsYST(%)andYREB(%).Steviosideandrebaudiosidecontentsindriedextract,DST(%)andDREB(%).
Conclusions
The GRASand green solventethanol 70% showed tobethe
bestsolventforglycosidesextractionfromS.rebaudiana leaves,
accordingtotheturbolysisatroomtemperature.Highyieldsof
glycosidesextractioncanbeattainedbylowsolventtodrugratios
inturbo-extraction,coarsedrugpowder,lowtemperatures, low
shearratesandshortertimes.ThesteviosideandrebaudiosideA
yieldsobtainedinthisworkaresuperiortotheonesfound
previ-ouslyusingothersolventsandothertechniques.Theresultsforthe
twoglycosidesturbolysisextractionarecomparable totheones
recentlypublishedfordynamicmaceration,butwiththe
advan-tageofshorterextractiontimes.Theresultshereinstimulatenew
researchonS.rebaudianaglycosidesextractionbyturbolysisand
theevaluationofthoseextractsinfurtherpurificationsteps.
Authors’contributions
PMMcontributedinrunningthepharmacognostic
characteri-zation,extractionsandmanuscriptpreparation.ADLcontributed
to the chromatographic analysis and quantification of the
gly-cosides.BNTcontributedtomanuscript preparationand critical
reading.LAPFcontributed toexperimentaldesign,dataanalysis
andmanuscript preparation.Alltheauthorshaveread thefinal
manuscriptandapprovedthesubmission.
Conflictsofinterest
Theauthorsdeclarenoconflictsofinterest.
Acknowledgements
FinancialsupportfromCNPqforgrant401044/2013-0underthe
“CooperationAgreementMCTI-CNPq(Brazll)&DST(India)”andthe
scholarship(CNPq202564/2014-1)toProfP.M.Martins
(Universi-dadedeBrasília)aregratefullyacknowledged.
References
Abou-Arab,A.E.,Abou-Arab,A.A.,Abu-Salem,M.F.,2010.Physico-chemical assess-mentofnaturalsweetenerssteviosidesproducedfromSteviarebaudianaBertoni plant.Afr.J.FoodSci.4,269–281.
Ahmad,S.,Farooq,A.K.,Abdul,H.,Muhammad,S.N.,2014.Areviewonpotential toxicityofartificialsweetnersvssafetyofStevia:anaturalbio-sweetner.J.Biol. Agric.Healthc.4,137–147.
Aranda-Gonzalez,I.,Moguel-Ordonez,Y.,Betancur-Ancona,D.,2014.RapidHPLC methodfordeterminationofrebaudiosideDinleaves ofSteviarebaudiana
BertonigrowninthesoutheastofMéxico.Am.J.Anal.Chem.5,813–819. Araújo,A.A.,Soares,L.A.L.,Ferreira,M.R.A.,Neto,M.A.S.,Silva,G.R.,AraújoJr.,R.F.,
Guerra,G.C.B.,Melo,M.C.N.,2014.Quantificationofpolyphenolsand evalu-ationofantimicrobial,analgesicandanti-inflammatoryactivitiesofaqueous andacetone–waterextractsofLibidibiaferrea,ParapiptadeniarigidaandPsidium guajava.J.Ethnopharmacol.156,88–96.
Ausfood, 2015. Australia Food News, Stevia to Take Off Globally Through CokeLife Expansion http://www.ausfoodnews.com.au/2015/03/30/stevia-to-take-off-globally-through-coke-life-expansion.html(accessed11.08.16). Capello,C.,Fischer,U.,Hungerbuhler,K.,2007.Whatisagreensolvent?A
compre-hensiveframeworkfortheenvironmentalassessmentofsolvents.GreenChem. 9,927–934.
Chemat,F.,Rombaut,N.,Fabiano-Tixier,A.S.,Pierson,J.T.,Bily,A.,2015.Green extrac-tion:fromconceptstoresearch,educationandeconomicalopportunities.In: Chemat,F.,Struhe,S.(Eds.),GreenExtractionofNaturalProducts:Theoryand Practice.,1sted.Wiley-VCHVerlagGmbH&Co,Berlin,pp.1–36.
Chemat,F.,Vian,M.A.,Cravotto,G.,2012.Greenextractionofnaturalproducts: conceptandprinciples.Int.J.Mol.Sci.13,8615–8627.
Chhaya,C.S.,Majumdar,G.C.,Sirshendu,D.,2013.PrimaryclarificationofStevia
extract:acomparisonbetweencentrifugationandmicrofiltration.Sep.Sci. Tech-nol.48,113–121.
Chhaya,C.S.,Sourav,M.,Majumdar,G.C.,Sirshendu,D.,2012a.Clarificationof ste-viaextractbyultrafiltration:selectioncriteriaofthemembraneandeffectsof operatingconditions.FoodBioprod.Proc.90,525–532.
Chhaya,C.S.,Sourav,M.,Majumdar,G.C.,Sirshendu,D.,2012b.Clarificationofstevia extractusingcrossflowultrafiltrationandconcentrationbynanofiltration.Sep. Pur.Technol.89,125–134.
Costa-Machado,A.R.M.,Bastos,J.K.,Freitas,L.A.P.,2013.Dynamicmacerationof
Copaiferalangsdorfiileaves:a technologicalstudy usingfractionalfactorial design.Rev.Bras.Farmacogn.23,79–85.
FarmacopeiaBrasileira,2010.MétodosGerais,volI.,5thed.AgênciaNacionalde VigilânciaSanitária,Brasília,DF.
Gasmalla,M.A.A.,Yang,R.,Musa,A.,Hua,X.,Ye,F.,2014.Influence of sonica-tion processparameters to thestate of liquidconcentration ofextracted rebaudosideAfromstevia(SteviarebaudianaBertoni)leaves.Arab.J.Chem., http://dx.doi.org/10.1016/j.arabjc.2014.06.012.
Hu,Z.,Cai,M.,Liang,H.,2008.Desirabilityfunctionapproachfortheoptimizationof microwave-assistedextractionofsaikosaponinsfromRadixbupleuri.Sep.Purif. Technol.61,266–275.
Jamal,P.,Azmi,S.M.N.,Amid,A.,Salleh,H.M.,Hashim,Y.Z.H-Y.,2014. Develop-mentandimprovementofanti-goutpropertyfromaqueous-methanolextractof
Morindaellipticausingcentralcompositedesign.Adv.Environ.Biol.8,734–742. Jentzer,J.B.,Alignan,M.,Vaca-Garcia,C.,Rigal,L.,Vilarem,G.,2015.Responsesurface methodologytooptimiseacceleratedsolventextractionofsteviolglycosides fromSteviarebaudianaBertonileaves.FoodChem.166,561–567.
Kienle,U.,2010.WelchesSteviahaettenSiedenngerne?AnbauundHerstellung– Perspektivenweltweit.J.FoodProt.FoodSaf.5,241–250.
Koubaa,M.,Rosello-Soto,E.,Zlabur,J.S.,Jambrack,A.R.,Brncic,M.,Grimi,N., Bous-setta,N.,Barba,F.J.,2015.Currentandnewinsightsinthesustainableandgreen recoveryofnutritionallyvaluablecompoundsfromSteviarebaudianaBertoni.J. Agric.FoodChem.63,6835–6846.
ICH-Q8,2009.ICHTripartiteGuidelines:PharmaceuticalDevelopment,Q8(R2). ICH Expert Working Group, http://www.ich.org/fileadmin/PublicWebSite/ ICH Products/Guidelines/Quality/Q8R1/Step4/Q8R2Guideline.pdf (accessed 29.12.16).
Liu,J.,Li,J.W.,Tang,L.,2010.Ultrasonicallyassistedextractionoftotalcarbohydrates fromSteviarebaudianaandidentificationofextracts.FoodBioprod.Proc.88, 215–221.
Madan,S.,Ahmad,S.,Singh,G.N.,Kohli,K.,Kumar,Y.,Singh,R.,Garg,M.,2010.Stevia rebaudiana(Bert.)Bertoni–areview.IndianJ.Nat.Prod.Res.1,267–286. Martins,R.M.,Pereira,S.V.,Siqueira,S.,Salomão,W.F.,Freitas,L.A.P.,2013.
Curcum-inoidcontentandantioxidantactivityinspraydriedmicroparticlescontaining turmericextract.FoodResInt.50,657–663.
Martins,P.M.,Lanchote,A.D.,Thorat,B.N.,Freitas,L.A.P.,2016.Greenextraction ofglycosidesfromSteviarebaudiana(Bert.)withlowsolventconsumption:a desirabilityapproach.Resour.Effic.Technol.2,247–253.
Mondal,S.,Chhaya,S.D.,2012.Predictionofultrafiltrationperformanceduring clar-ificationofsteviaextract.J.Membr.Sci.396,138–148.
Muanda,F.N.,Soulimani,R.,Diop,B.,Dicko,A.,2011.Studyonchemical composi-tionandbiologicalactivitiesofessentialoilandextractsfromSteviarebaudiana
Bertonileaves.LWTFoodSci.Technol.44,1865–1872.
Paulucci,V.P.,Couto,R.O.,Teixeira,C.C.C.,Freitas,L.A.P.,2013.Optimizationofthe extractionofcurcuminfromCurcumalongarhizhomes.Rev.Bras.Farmacogn. 23,94–100.
Periche,A.,Koutsidis,G.,Escriche,I.,2014.Compositionofantioxidantsandamino acidsinStevialeafinfusions.PlantFoodsHum.Nutr.69,1–7.
Periche,A.,Castelló,M.L.,Heredia,A.,Escriche,I.,2015.Influenceofextraction meth-odsontheyieldofsteviolglycosidesandantioxidantsinSteviarebaudiana
extracts.PlantFoodsHum.Nutr.70,119–127.
Puri,M.,Sharma,D.,Barrow,C.J.,Tiwary,A.K.,2012.Optimisationofnovelmethod fortheextractionofsteviosidesfromSteviarebaudianaleaves.FoodChem.132, 1113–1120.
Rajab,R.,Mohankumar,C.,Murungan,K.,Harish,M.,Mohanan,P.V.,2009. Purifica-tionandtoxicitystudiesofsteviosidefromSteviarebaudianaBertoni.Toxicol. Int.16,49–54.
Rao,A.B.,Prasad,E.,Roopa,G.,Sridhar,S.,Ravikumar,Y.V.L.,2012.Simpleextraction andmembranepurificationprocessinisolationofsteviosideswithimproved organolepticactivity.Adv.Biosci.Biotechnol.3,327–335.
Reis,M.H.M.,daSilva,F.V.,Andrade,C.M.G.,Rezende,S.L.,Wolf-Maciel,M.R., Berga-maso,R.,2009.Clarificationandpurificationofaqueoussteviaextractusing membraneseparationprocess.J.FoodProc.Eng.32,338–354.
Sardhara,A.M.,(MasterThesis)2015.Extraction,PurificationandFormulationof ExtractFromNaturalProduct.InstituteofChemicalTechnology,Mumbai,India, pp.90.
Sharma,R.,Yadav,R.,Manivannan,E.,2012.StudyofeffectofSteviarebaudiana
Bertonionoxidativestressintype-2diabeticratmodels.Biomed.AgingPathol. 2,126–131.
Singh,S.D.,Rao,G.P.,2005.Stevia:theherbalsugarof21stcentury.SugarTechnol. 7,17–24.
Sonaglio,D.,Ortega,G.G.,Petrovick, P.R.,Bassani,V.L.,2003.Desenvolvimento tecnológicodeproduc¸ãodefitoterápicos.In:Simões,C.M.O.,etal.(Eds.), Farma-cognosia:Daplantaaomedicamento.UFRGS/UFSC,PortoAlegre/Florianópolis, pp.289–326.
Torri,L.,Frati,A.,Ninfali,P.,Mategna,Cravotto,G.,Morini,G.,2016.Comparison ofreducedsugarhighqualitychocolatessweetenedwithsteviosideandcrude stevia‘green’extract.J.Sci.FoodAgric.,http://dx.doi.org/10.1002/jfsa.8045. Urban,J.D.,Carakostas,M.C.,Taylor,S.L.,2015.Steviolglycosidesafety:arehighly
purifiedsteviolglycosidesweetenersfoodallergens?FoodChem.Toxicol.75, 71–78.
WHO,2004.JointFAO/WHOExpertCommitteeonFoodAdditives.Sixty-Third Meet-ing.WorldHealthOrganization,Geneva,pp.1–18.
Yadav,A.K.,Singh,S.C¸.,Dhyani,D.,Ahuja,P.S.,2011.Areviewontheimprovement ofsteviaSteviarebaudiana(Bertoni).Can.J.PlantSci.91,1–27.
Yan,B.,Yao,L.,Guo,Z.,Qu,H.,2014.Qualitybydesignforherbaldrugs:a feedfor-wardcontrolstrategyandanapproachtodefinetheacceptablerangesofcritical qualityattributes.Phytochem.Anal.25,59–65.
Zhang,S.Q.,Kumar,A.,Kutowy,O.,2000.Membrane-basedseparationschemefor processingsweetenersfromstevialeaves.FoodRes.Int.33,617–620. Woelwer-Rieck,U.,Lankes,C.,Wawrzun,A., Wuest,M.,2010. ImprovedHPLC