R e v is ta d a S o c ie d a d e B r a s ile ir a d e M e d ic in a T r o p ic a l 2 2 (1 ): 1 9 -23 , j a n - m a r , 1 9 8 9
HEM OCULTURES FO R THE PARASITO LO GICAL D IA G N O SIS OF H U M A N
CHRONIC C H A G A S’ D ISE A SE
Egler Chi a r il, João Carlos Pinto Dias2 , Marta L a n a 3 and Clea Andrade Chiari"*
W ith the p u rp o se o f sta n d a r d iza tio n o f an hem ocu ltu re techn ique p re sen tin g a h igh er p o s itiv e ra te in th e p a r a sito lo g ic a l d ia g n o sis o f ch ronic C h a g a s ’ d isea se in p a tie n ts w ith re a ctive sero lo g y (IF T, H A, C F T ) th e fo llo w in g sch edu le w as used. T h irty m l o f ven ou s b lo o d w a s co llected w ith h ep arin a n d th e p la s m a w as se p a ra te d by cen trifug ation ( 2 .0 0 0 rp m /30'). The p a c k e d cells w ere w a sh ed w ith L I T m ediu m o r P B S w hich w a s then rem o ved b y cen trifug ation ( 2 .0 0 0 r p m /1 5 ’). Th is m a te ria l w as sa m p le d in 6 screw -tu bes 1 8 x 2 0 0 w ith 6 m l o f L I T m ed iu m a n d in cu b a ted a t 28°C . These in cu b a ted cu ltu res a t 2 8 ° C w ere e x a m in e d a fter 15, 30, 4 5 a n d 6 0 days. W hen the h em ocu ltu re w a s n o t im m ed ia tely p ro c e sse d a fter b lo o d collection , th e p la s m a w as rem o ved a n d th e se d im e n t en rich ed w ith L I T m ed iu m a n d p re se rv e d a t 4°C . The X en o-d ia g n o sis w a s p e rfo rm e o-d acco ro-d in g to S c h e n o n e s m eth o o-d u seo-d here a s a reference tech nique.
A m o n g the va rio u s g ro u p s o f p a tie n ts ex a m in ed b y both tech n iq u es th e b est resu lts o b ta in e d were: 5 5 .0 8 % o fp o s itiv ity f o r hem ocultures a g a in st 2 7 .5 % fo r x e n o d ia g n o s is (X ^ = 4.54 , p = 0 .0 5 ), w ith a tu b e p o s itiv ity o f 2 6 .6 % .
R eco m m e n d a tio n f o r screen in g tria ls o f dru g a ssa y s is the rep etition o f m eth o d on a sa m e p a tie n t 2 o r m ore tim e s in d ifferen t occasion s, a s u se d in x en od ia gn o sis.
Key-words: Chagas’ disease. Parasitological diagnosis. Hemoculture and xeno diagnosis. T ryp a n o so m a cru zi.
For several years the hemocultures were not currently performed for the diagnosis o f chronic Chagas' disease because authors such as Pedreira de Freitas7 and Pifano16 obtained negative results and a low level o f positivity (6.3% ).
Since Chiari & Brener4 obtained 31.8% o f positive blood culture in LIT medium, the opinion about the method changed and new possibilities o f research appeared. It was possible, by using mainly liquid media with direct seeding (or after centrifugation o f the material in different schedules) to use experi mental and human blood culture for diagnostic pur poses.
Several attempts have been made to improve the parasitological diagnosis o f human Chagas’ disease by using hemocultures1 4. Mourào & M ello12 were enga ged in the development o f their idea to remove the plasma, to wash the cells with the purpose o f taking off antibodies or other inhibition factors for the growth
1. Universidade Federal de Minas Gerais.
2. Fundação Oswaldo Cruz, Universidade Federal de Minas Gerais e SUCAM (Divisão de Doença de Chagas). 3. Universidade Federal de Ouro Preto.
Financiado em parte pelo CNPq, FINEP.
Endereço para correspondência: Prof. Egler Chiari. Depar tamento de Parasitologia/ICB. CP: 2486 - 30161 Belo Horizonte, M G - Brasil.
Recebido para publicação em 14/06/88.
o f T ryp a n o so m a cru zi. A t the end o f same year Chiari & Dias®, reproducing the technique o f above men tioned authors started a pilot project in which they attempted to change the blood volume from 10 to 30 ml to obtain better positivity earlier and also to try new media like W arren^2^ and others. Experimental approaches made by N eal and N e a l13 & M iles14 15 in further investigations with Warren’s medium showed that between 10 and 20 trypomastigotes can reliably give positive cultures. A ll these above mentioned papers suggest the use o f hemocultures associated or not with xenodiagnosis in patients with chronic Cha gas’ disease. The importance o f hemoculture techni que for parasitological diagnosis is the assessment of drug activity in clinical trials with human chronic Chagas’ disease. The ideal method should be simple and practical, giving a quick growth o f flagellates. Furthermore, hemoculture methods are also desirable since xenodiagnosis presents some practical limita tions such as allergic reactions to the insect bite, low sensitivity in human chronic disease and several problems to maintain a large insectary.
M A T E R IA L A N D M E T H O D S
C h i a n E , D i a s J C P , L a n a M , C h ia r i C A . H e m o c u ltu r e s f o r th e p a r a s ito lo g ic a l d ia g n o s is o f h u m a n c h r o n ic C h a g a s ’ d is ea s e. R e v is ta d a S o c ie d a d e B r a s ile ir a d e M e d ic in a T r o p ic a l 2 2 : 1 9 -2 3 , j a n - m a r , 1 989.
this material immediately blood can be kept in the refrigerator at 4°C , after the substitution o f the plasma by equal volume o f LIT medium. Plasm a is removed by centrifugation at 2,000 rpm for 30 minutes. This material should be kept in the refrigerator or ice-bath untill the complete processing o f the whole technique.
H em ocu ltu re p ro cessin g
After the removal o f the plasma, the packed cells must be washed with LIT medium or physiological buffer saline (PBS) that is then removed by centrifu gation (2 ,0 0 0 rpm/15 minutes). This material is sampled in 6 screw-tubes 18x200 with 6 ml o f LIT medium and incubated at 28°C . Every 2 days the hemocultures tubes must to be agitated in order to homogenize the material.
H em ocu ltu re ex a m in a tio n
Fresh preparations are examined after 15, 30, 45 and 60 days after seeding, between slide and cover-glass 22x22, with lOOx and 4 0 0 x magnifications. The sample volume must be always 0 .1 0 ml to give a good slide preparation, collected on the surface o f sedi-mented cells, and the LIT medium liquid phase. Such process permits to obtain an uniform suspension that facilitates the search o f flagellates and amastigotes clumps, almost always without movement. After 60 days (or 75 days) the negative tubes are centrifugated at 2 ,0 0 0 rpm/15 minutes and the sedimented material is examined.
Xenodiagnosis were carried according to Schenone et alii18 19 method with 4 0 3 nd instar o f
T ria to m a in festan s examined after 30 and 60 days. The chi-square ( X 2) test was used. The signifi cance level o f 5% was accepted for all tests.
R E SU L T S
T able 1 shows results o f xenodiagnosis done in a group o f 4 0 pacients, from which 30ml o f venous blood was collected for hemoculture. The packed cells obtained from the 30m l of venous blood were washed in PBS and distributed in 6 tubes containing LIT medium.
Table 2 shows the results o f the comparison of the material in which the plasma is immediately removed after collection or when the plasma is not removed.
Table 3 shows the results o f xenodiagnosis and hemoculture when blood-plasma is removed and equal volume o f LIT medium is additioned.
W hen the heparinized blood of 2 0 patients was kept at room temperature for 24-48 hours, the percen tage of positive tubes was reduced to half. The methodology was the same as in the previous experi ments.
Table 1 - Positivity of xenodiagnosis (Schenone method) against the hemoculture in LIT medium (Mourao and M ello technique modified) performed simultaneously in human chronic phase o f Chagas’ disease (Bambui, M G ).
Method Positive Negative Total % o f positive
Xenodiagnosis 12 28 40 30.0
Hemoculture 22 18 40 55.0
Total 34 46 80
Positivity/tube 11% X 2 o f 5.11 p = 0.0238
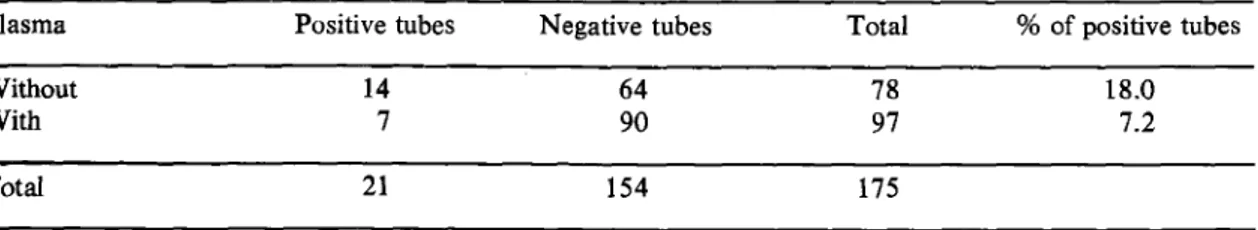
Table 2 - Positivity o f hemoculture tubes which the plasma was or was not removed after to have blood collected and before to process 24-48 hours in refrigerator or ice-bath in transport to laboratory using same technique.
Plasma Positive tubes Negative tubes Total % o f positive tubes
Without 14 64 78 18.0
With 7 90 97 7.2
Total 21 154 175
C h ia r i E , D i a s J C P , L a n a M , C h ia r i C A . H e m o c u ltu r e s f o r th e p a r a s ito lo g ic a l d ia g n o s is o f h u m a n c h r o n ic C h a g a s ’ d is ea s e. R e v i s t a d a S o c ie d a d e B r a s ile ir a d e M e d ic in a T r o p ic a l 2 2 : 1 9 -2 3 , j a n - m a r , 1 9 8 9 .
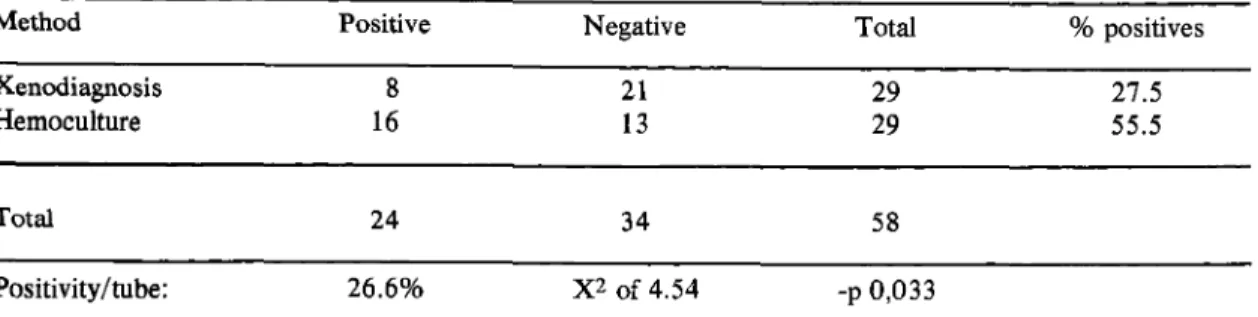
Table 3 - Positivity o f xenodiagnosis (Schenone) and hemoculture with 30 ml of blood-heparinised collected. Plasma is removed by centrifugation and addition o f equal volume o f LIT medium in a group o f 29 patients o f chronic Chagas’ disease (Bambui, M G).
Method Positive Negative Total % positives
Xenodiagnosis 8 21 29 27.5
Hemoculture 16 13 29 55.5
Total 24 34 58
Positivity/tube: 26.6% X2 o f 4.54 -p 0,033
D IS C U S S IO N
Our hemoculture studies supported the findings o f Mourao & M elo 12. After the first confirmation of the results by Chiari & Dias®, some technical changes, were introduced. Instead o f using 10ml o f blood (as used in their original method), or 2 0 ml as they previously tested, 30m l o f blood was used in order to increase positivity. Another change was the use o f LIT medium to wash the packed cells in place o f PBS. In Table 2 the reduction from 18.0 to 7.2% positivity/ tubes is probably due to the lytic action o f immunoglo bulins present in the plasma of chronic patients or, according to Pifano1®, due to macrophage potential of existing lymphocytes in the material. In order to study the inference o f “ chagasic” plasma on the parasite development we added different amounts of plasma collected from normal and chronic infected individuals in T. c ru zi cultures. Such experiments show an important inhibition o f “ chagasic” plasma on the cultures growth in comparision to the normal deve lopment o f those in which not infected plasma was added (Chiari et ali5). The addition o f LIT medium after the removal o f the plasma aims at adapting the present flagellates to a new metabolic pathway in the LIT medium, as accepted by Pifano1®. Such addition does not increase the positivity o f the hemoculture, but increases the number o f positive tubes per patient, thus facilitating the detection o f flagellates in microscopic examination (26.6% ) against 11.0 to 18.0% o f other technical procedures (Tables 1, 2 and 3).
After many changes, some attempts to standar dize the technique are being made. W hen the hemocul ture is not processed immediately, 30m l o f heparinized blood is used, with the removing o f the plasma and its substitution for LIT medium. W hen the method is immediately processed the cells are washed in LIT medium, the medium is then removed by centrifuga tion and the cells are seed in new LIT medium. In the first instances the material must be kept at 4°C. The
LIT-wash is also incubated at 28°C and none o f the 50 seeded plasma showed positive result of T. cruzi.
According to the literature3 1 both LIT and Warren media support growth o f T. c ru zi at least for 3-4 months. Laboratories performing hemoculture can use both media, depending on their facilities.
The association of hemoculture with xenodiag nosis for Chagas was suggested by Chiari & B rener4 .
W e have now evidence that this association should be used specially when blood is collected in field condi tions or in areas o f Chagas disease where patients show low levels o f parasites in the blood.
Repetition o f the reported method is recom mended, in the same patient two or more times in different occasions, as used in xenodiagnosis in clini cal trials (Cançado et al2). According to Galvão et ali8 in 51 untreated patients with 45% positive in a total o f 69 hemocultures with only 12% o f patients being examined more than twice, and in a group o f 32 treated patients considered as a therapeutic failure with 31% positive in 69 cultures, with 49% being examined more than twice (2-5 times).
The positivity o f hemoculture depends upon the group of chronic chagasic patients and the level o f low rates o f persisting ongoing T. cru zi infections.
Hemocultures were important tools for the isolation o f strains for biochemical studies (isoenzy mes and restricted endonucleases) according to Ro-manha et ali17 and Morel et ali10 to characterize T. c ru zi in human, wild and domestic animals.
Other changes must be tested with the purpose o f obtaining higher percentage o f positivity to hemo culture technique.
R E S U M O
C h ia r i E , D i a s J C P , L a n a M, C h ia r i C A . H e m o c u ltu r e s f o r th e p a r a s ito lo g ic a l d ia g n o s is o f h u m a n c h r o n ic C h a g a s ’ d is e a s e . R e v is ta d a S o c ie d a d e B r a s ile ir a d e M e d ic in a T r o p ic a l 2 2 : 1 9 -2 3 , ja n - m a r , 1 98 9.
H A , R F C ) u tiliz a m o s o seg u in te esq uem a: o volu m e d e 3 0 m l d e sa n g u e f o i co lh id o h ep a rin iza d o e o p la s m a sep a ra d o p o r cen trifu ga çao (2 0 0 r p m /3 0 ’); o sed i m en to f o i la v a d o co m m eio L I T ou sa lin a fisio ló g ic a ta m p o n a d a e rem o vid o p o r n o va centrifug ação (2 0 0 0 r p m /1 5 ) ; a s célu la s se d im e n ta d a s fo r a m d istrib u í d a s em 6 tu b o s tipo “sc re w ” 18 x 2 0 0 con ten do 6 ,0 m l d e m eio L IT ; a s cu ltu ra s in cu b a d a s a 28°C , fo r a m e x a m in a d a s a p ó s 15, 30, 4 5 e 6 0 dias; q u a n d o a he-m o cu ltu ra nã o f o i p ro c e ssa d a ihe-m ed ia ta he-m en te a p ó s a co lh eita d e sangue, o p la s m a f o i rem o vid o e o se d i m ento, a d ic io n a d o d e m eio L IT , con servad o a 4°C; o x en o d ia g n ó stico (S ch en on e) r e a liza d o no m esm o d ia d a co lh eita d o sa n gu e f o i u tiliza d o co m o técn ica d e referência.
E n tre os vá rio s gru p o s de p a c ie n te s ex a m in a dos, os m elh o res resu lta d o s fo rn ecera m u m a p o s iti-vid a d e de 5 5 ,0 % p a r a h em ocu ltu ra co n tra 2 7 ,5 % d e x e n o d ia g n ó stico s p o s itiv o s (X 2 = 4 ,5 4 ; p = 0 ,0 5 ), com p o s itiv id a d e /tu b o d e 2 6 ,6 % . A con servaçã o do m a te ria l a 4 ° C d u ra n te 4 8 -7 2 h o ra s não a ltero u o p e rc e n tu a l d e p o s itiv id a d e . A p o s itiv id a d e d o s tu b o s d e h em ocu ltu ras m ostro u u m a red u ção d e 1 8 ,0 p a r a 7 ,2 % q u a n d o o p la s m a nã o f o i rem o vid o logo a p ó s a co lh eita d o sangue, e m a n tid a s p o r 2 4 -4 8 h oras a 4 °C an tes d a técn ica s e r p ro c e ssa d a . É p r o v á v e l q u e isto se d eva à a çã o lític a d e im u n o g lo b u lin a sp resen tes no p la sm a , ou a o p o te n c ia l m a crofágico d o s linfócitos. R eco m en d a m o s: (a ) a rea liza çã o d e d u a s ou m a is h em o cu ltu ra s do m esm o p a c ie n te co m a fin a li d a d e de a u m en ta r a p o s itiv id a d e , p rin c ip a lm e n te em con dições d e c a m p o e em á re a s on de o s p a cien tes crô n icos a p resen ta m b a ix o s n íveis d e p a ra site m ia ; (b ) o em prego d esta técn ica na tria g em d e p a c ie n te s e no co n tro le p a ra sito ló g ic o d e cura, em en sa io s clí-n ico-terapéu tico s; (c ) a execu çã o d este m éto d o em ou tro s la b o ra tó rio s com a fin a lid a d e d e c o m p ro v a ra via b ilid a d e d e seu em prego no d ia g n ó stico d e rotin a u tiliza n d o o m eio L I T ou W arren.
Palavras-chaves: D oença de Chagas. Diagnós tico parasitológico. Hemocultura e xenodiagnóstico.
T ryp a n o so m a cruzi.
ACKNOWLEDGEMENTS
W e wish to thank Mr. A fonso da Costa Viana and Mr. Orlando Carlos Magno for their technical assistance.
R E FE R E N C E S
1. Albuquerque RDR, Fernandes LAR, Funayama GH, Ferriolli Filho F, Siqueira AF. Hemoculturas seriadas com o meio de Warren em pacientes com reação de Guerreiro-Machado positiva. Revista do Instituto de Medicina Tropical de São Paulo 14: 1-5, 1972,
2. Cançado J R Brener Z. Terapêutica. In: T r y p a n o s o m a c r u z i e Doença de Chagas (Brener, Z. & Andrade, Z.), pp. 362-422. Guanabara Koogan, Rio de Janeiro, 1979. 3. Cerisola JA. El xenodiagnostico. International Sympo
sium on New Approaches in American Trypanosomiasis Research Pan American Health Organization. Belo Horizonte, Brazil, 18-21 March, Scientific Publication n.° 318, 1975.
4. Chiari E, Brener Z. Contribuição ao diagnóstico parasi tológico da doença de Chagas na sua fase crônica. Revista do Instituto de Medicina Tropical de São Paulo 8: 134-148, 1966.
5. Chiari E, Lana M, Dias JCP. Crescimento e inibição do T r y p a n o s o m a c r u z i em hemoculturas de pacientes na fase crônica da doença de Chagas. X IV Congresso da Sociedade Brasileira de Medicina Tropical de João Pessoa, Paraíba, 1978.
6. Chiari E, Dias JCP. N ota sobre uma nova técnica de hemocultura para diagnóstico parasitológico na doença de Chagas na sua fase crônica. Revista da Sociedade Brasileira de Medicina Tropical 9: 133-136, 1975. 7. Freitas JLP. O diagnóstico de laboratório da moléstia de
Chagas. Revista Clinica de São Paulo 28: 1-20, 1952. 8. Galvão LMC, Cançado JR , Brener Z, KretÜi AU.
Hemoculture repeatedly negative in humans with Cha gas’ disease displaying negative complement-mediated lysis tests after specific treatment suggest cure in infection. Memórias do Instituto Oswaldo Cruz, Rio de Janeiro (suppl.) 81:111, 1981. Proceeding of the X III Annual Meeting on Basic Research in Chagas’ Disease. Caxambu, Brazil, nov. 1986.
9. Minter-Goedbloed E. Hemoculture compared with xe- nodiagnosis for the detection of T. c r u z i infection in man and in animals. In: New Approaches in American Trypanosomiasis Research. Proceedings of an Interna tional Symposium (Belo Horizonte, 1975). PAHO, Scientific Publication n,° 318 (Washington), p. 245-252, 1976.
10. Morel CM, Chiari E, Camargo EP, M attei DM, Roma- nha AJ, Simpson L. Strains and clones of T r y p a n o s o m a c r u z i can be characterized by pattern of restriction endonuclease products of kinetoplast D N A minicircles. Proceeding N ational Academy Sciences U SA 77:6810- 6814, 1980.
11. Mourão OG, Chiari E. Comprovação parasitológica na fase crônica da doença de Chagas por hemoculturas seriadas em meio LIT. Revista da Sociedade Brasileira de Medicina Tropical 5: 215-219, 1975.
12. Mourão OG, Mello OC. Hemocultura para o diagnós tico parasitológico na fase crônica da doença de Chagas. ; Revista da Sociedade Brasileira de Medicina Tropical 9:
183-188, 1975.
13. Neal RA. Superiority of the culture technique over xenodiagnosis for detection of trypanosomes in Chagas’ disease. In: International Congress ofTropical Medicine
and Malarial, Athens, Annals, p. 56, 1973.
C h ia r i E , D i a s J C P , L a n a M , C h ia r i C A . H e m o c u ltu r e s f o r th e p a r a s ito lo g ic a l d ia g n o s is o f h u m a n c h r o n ic C h a g a s ’ d is ea s e. R e v is ta d a S o c ie d a d e B r a s ile ir a d e M e d ic in a T r o p ic a l 2 2 : 1 9 -2 3 , j a n - m a r , 1 9 8 9 .
15. Neal RA, Miles RA. The number of tripomastigotes of T r y p a n o s o m a c r u z i required to infect R h o d n i u s p r o li-x u s . Revista do Instituto de Medicina Tropical de São
Paulo 19: 177-181, 1977.
16. Pifano FC. El diagnóstico parasitológico de la enferme- dad de Chagas en fase crónica. Estúdio comparativo entre la gota gruesa, el xenodiagnóstico, el hemocultivo y las inoculaciones experimentales en animales sensibles. Archivos Venezolanos de Patologia Tropical 2: 89-120, 1954.
17. Romanha AJ, Silva Pereira AA, Chiari E, Kilgour R. Isoenzymes patterns in T r y p a n o s o m a c r u z i
comparati-ve. Biochemistry and Phisiology 62B: 139-142, 1979. 18. Schenone H, Alfaro E, Reyes H, Taucher E. Valor del
xenodiagnóstico en la infección chagásica crónica. Bole- tín Chileno de Parasitologia 23: 149-154, 1968.
19. Schenone H, Alfaro E, Rojas A. Bases y rendimiento dei xenodiagnóstico en la infección chagásica humana. Bo- letín Chileno de Parasitologia 29: 24-26, 1974.