ABSTRACT
http://dx.doi.org/10.1590/1678-775720160185
Silv er n an op ar t icle in cor p or at ion ef f ect on
m echanical and t herm al propert ies of dent ure
base acrylic resins
$\úHJO.g52ö/812QXUù$+ø11,úÕQ.h5.dh2ö/82'R÷XgPU'('(37RQJXog='(0ø54, Baki HAZER5
%OHQW(FHYLW8QLYHUVLW\)DFXOW\RI'HQWLVWU\'HSDUWPHQWRI3URVWKRGRQWLFV=RQJXOGDN7XUNH\ 6OH\PDQ'HPLUHO8QLYHUVLW\)DFXOW\RI'HQWLVWU\'HSDUWPHQWRI3URVWKRGRQWLFV,VSDUWD7XUNH\
3- Ordu University, Faculty of Dentistry, Department of Prosthodontics, Ordu, Turkey.
4- Mersin University, Faculty of Engineering, Department of Chemical Engineering, Mersin, Turkey.
%OHQW(FHYLW8QLYHUVLW\)DFXOW\RI$UWVDQG6FLHQFHV'HSDUWPHQWRI&KHPLVWU\=RQJXOGDN7XUNH\
Corresponding address:2QXUùDKLQ%OHQW(FHYLW8QLYHUVLW\)DFXOW\RI'HQWLVWU\'HSDUWPHQWRI3URVWKRGRQWLFV=RQJXOGDN7XUNH\3RVWFRGH
- Phone: +903722613536 - Fax: +903722613403 - e-mail: sonurs60@hotmail.com
6XEPLWWHG$SULO0RGL¿FDWLRQ-XO\$FFHSWHG$XJXVW
O
bj ect ive: The aim of t he present st udy was t o evaluat e t he m echanical and t herm al charact erist ics of t wo dent ure base acrylic resins cont aining silver nanopart icles ( AgNPs) . Material and Methods: Two different acrylic denture base resins ( heat-polym erized and m icrowave polym erized) cont aining 0.3, 0.8 and 1.6 wt % AgNPs were evaluat ed for ÀH[XUDOVWUHQJWKHODVWLFPRGXOXVDQGLPSDFWVWUHQJWK7KHJODVVWUDQVLWLRQWHPSHUDWXUH ( Tg) and relat ive heat capacity ( Cp) of t he sam ples were det erm ined from t he Different ial Scanning Calorim et ry ( DSC) result s. For st at ist ical analysis, t wo-way ANOVA and Tukey-HSD t est s were perform ed. Result s: Addit ion of 0.8% and 1.6% AgNPs in m icrowave-SRO\PHUL]HGUHVLQVLJQL¿FDQWO\GHFUHDVHGWKHWUDQVYHUVHVWUHQJWKDQGHODVWLFPRGXOXV,Q t erm s of im pact st rengt h, t he addit ion of AgNPs has no effect on bot h resin groups. Glass t ransit ion t em perat ure ( Tg) was decreased wit h t he addit ion of AgNPs for bot h dent ure base resins. Conclusions: The incorporat ion of AgNPs, generally used for ant im icrobial HI¿FLHQF\DIIHFWHGWKHWUDQVYHUVHVWUHQJWKRIWKHGHQWXUHEDVHDFU\OLFUHVLQVGHSHQGLQJ on t he concent rat ion of nanopart icles. Tg was decreased wit h t he addit ion of AgNPs for bot h dent ure base resins.Ke yw or ds: Nanopart icles. Acrylic resin. Flexural st rengt h. I m pact st rengt h. Different ial scanning calorim et ry.
I N TROD UCTI ON
Poly( m et hyl m et hacrylat e) ( PMMA) is widely used in t he preparat ion of part ial and t ot al dent ure bases. The proliferat ion of cert ain pat hogens such as Candida albicans and St rept ococcus m ut ans is induced by t he surface roughness of acrylic resins and local or system ic factors1,6,25. The im provem ent
of oral hygiene is generally achieved by t he use of ant im icr obial m out hwashes and appr opr iat e t oot h- br ushing m et hods along w it h t he use of denture cleansing tablets and prophylactic system ic an t ibiot ics. How ev er, all t h ese m et h ods h av e lim it ed success in reducing t he effect iveness of these pathogens7,19. For these reasons, research on
broad-spectrum antim icrobial acrylic resin m aterials has at t ract ed m uch int erest in recent t im es13.
Silver ( Ag) salt s have been used for t housands RI\HDUVEHFDXVHRIWKHLUDQWLPLFURELDOHI¿FLHQF\ against Gram - positive and Gram - negative bacteria, prot ozoa and fungi, as well as viruses18. Nowadays,
elem ental Ag and associated com pounds are used to reduce the risk of infection in the treatm ent of burns, prevent bacterial colonization on m edical devices, in VXUJLFDOWH[WLOHIDEULFVIRUZDWHUSXUL¿FDWLRQERQH cem ent s, and dent al m at erials11- 14,18,24.
I n dental applications, different form s of Ag such as Ag ions ( Ag+) , Ag nanopart icles ( AgNPs) , and
however, rest rict s it s pract ical im plem ent at ion. The problem can be resolved by prot ect ing t he Ag+ wit h
a polym eric m at rix sheat h. The m aj or advant age of using AgNPs arises from t heir large rat io of surface area t o volum e. AgNPs exhibit m ore effect ive ion release and enhanced antim icrobial activity5. AgNPs
are preferred for t his reason, alongside addit ional funct ional asset s such as t heir duct ilit y, elect rical and t herm al conduct ivity7,17,22,25.
Al t h o u g h t h e a n t i m i cr o b i a l ch a r a ct er i st i c of AgNPs in acr ylic r esins has been pr eviously illust rat ed1,13,19,23,27, t here are few st udies report ing
WKHLQÀXHQFHRI13VRQWKHPHFKDQLFDOSURSHUWLHV of dent ure base resins10,13,25. Alt hough t he addit ion
of AgNPs has ant im icrobial advant ages on acrylic resins, it s effect on t he m echanical propert ies of t he resin should be exam ined25. Therefore, t he
aim of t his in v it r o st udy was t o evaluat e t he HIIHFW RI $J13V RQ WKH ÀH[XUDO VWUHQJWK HODVWLF m odulus, im pact strength, and differential scanning calorim et ry ( DSC) propert ies of t wo dist inct dent al acrylic resins.
M ATERI AL AN D M ETH OD S
Tw o acr y lic r esins used in t his st udy w er e; ( 1) convent ional heat - poly m er ized PMMA r esin ( Meliodent , Bayer Dent al, Berkshire, UK) ; and ( 2) m icrowave- polym erized PMMA resin ( Acron MC, GC Dent al, Tokyo, Japan) .
Syn t h e sis a n d ch a r a ct e r iz a t ion of AgN Ps
Th e Ag NPs w e r e p r e p a r e d b a se d o n t h e Turkevich m et hod26. Silver nit rat e ( AgNO
3) was
dissolved in wat er ( 208 m g AgNO3/ 100 m L H2O) and t he solut ion was brought t o a boil ( at 100° C) . After 2 m in of boiling, an aqueous solution of sodium cit rat e ( Na3C6H5O7) ( 49 m g sodium cit rat e/ 1.25 g H2O) was added. The form at ion of AgNPs was perceived from t he em ergence of a yellow colour in t he previously colourless solut ion. The solut ion was boiled furt her for anot her 6 m in and t hen allowed t o cool. Ult raviolet ( UV) visible absorpt ion spect roscopy ( T80+ , PG I nst rum ent s, Leicest er, UK) and Transm ission elect ron m icroscopy ( TEM) ( Technai G2 Spirit BioTWI N, FEI , OR, USA) were
used t o charact erize t he form at ion of AgNPs. Also, a Zet a Sizer I nst rum ent ( Malvern I nst rum ent s Lt d., Malvern, UK) was used t o det erm ine t he part icle size. Distilled water was used as a dispersion m edia during t he part icle size- det erm inat ion process.
Spe cim e n pr e pa r a t ion
I n t ot al, 56 specim ens ( n= 7) were prepared for ÀH[XUDODQGLPSDFWVWUHQJWKWHVWVZLWKGLPHQVLRQV of 65× 10× 2.5 m m and 50× 6× 4 m m , according t o t h e ADA Specif icat ion No. 1 2 an d I SO/ DI S 1567: 1998 st andards, respect ively. The powder/
liquid rat ios for heat- polym erized and m icrowave-polym erized resin were 35 g/ 14 m L and 100 g/ 43 m L, respectively. For the study groups to determ ine t he effect of AgNPs, t he suspension of AgNPs was m ixed wit h each resin m onom er in concent rat ions of 0.3, 0.8, and 1.6 wt % and sonicat ed for 15 m in. Aft erwards, t he liquid com ponent was m ixed w it h t he pow der par t , in accor dance w it h t he m anufact urer’s inst ruct ions. The heat- polym erized specim ens were cured in a wat er bat h at 100° C for 30 m in while the m icrowave-polym erized specim ens w er e ir r ad iat ed f or 3 m in at 6 0 0 W. Bef or e GHÀDVNLQJDOOWKHVSHFLPHQVZHUHEHQFKFRROHG Test specim ens w er e w et gr ound w it h silicone carbide grinding papers of 200, 400, and 600- grit sizes using an aut om at ic polishing m achine ( Grin 329JULQGHUSROLVKHU0HWNRQ$ù%XUVD7XUNH\ %HIRUHWHVWLQJIRUIXOOVDWXUDWLRQWKHÀH[XUDOWHVW specim ens were kept in dist illed wat er at 37° C for 50± 2 h, and im pact t est specim ens were st ored at 37° C for 2 weeks8,15.
Fle x u r a l st r e n gt h t e st in g
7KH ÀH[XUDO VWUHQJWK WHVW ZDV SHUIRUPHG E\ using a Lloyd universal t est ing m achine ( Lloyd I nst rum ent s, LRX, Fareham , UK) wit h a crosshead speed of 5 m m / m in. Flexural st rengt h ( FS) was det erm ined using t he following form ula;
FS= 3Fl/ 2bh2
where F is t he m axim um load applied ( N) , l is t he dist ance bet ween support s ( span lengt h= 50 m m ) , b is t he widt h of t he specim en ( 10 m m ) and h is t he t hickness of t he specim en ( 2.5 m m ) .
Elast ic m odulus ( E) was calculat ed from t he form ula;
EM= Fl3/ 4bh3d
ZKHUHGPPLVWKHGHÀHFWLRQ
I m pa ct st r e n gt h t e st in g
I m pact st rengt h t est was perform ed using a Charpy- t ype im pact t est er ( Coesfeld, Pendulum I m p act Test er, Dor t m u n d , Ger m an y ) . I m p act st rengt h ( I S) was calculat ed using t he following form ula;
I S= E/ wt
where E is t he energy required t o break t he specim en ( J) , w is t he widt h ( 6 m m ) and t is t he t hickness of t he specim en ( 4 m m ) .
Th e r m a l a n a lysis
(Tg) was taken as the peak tem perature of the glass transition region. Tg and relative heat capacity ( Cp) of the sam ples (control sam ples and 1.6 wt% AgNPs incorporat ed int o m icrowave and heat polym erized sam ples) were det erm ined from t he DSC t est s.
St a t ist ica l a n a lysis
I n each group, the m ean and standard deviation values were calculated. For statistical analysis, two-way ANOVA and Tukey- HSD t est s were perform ed. 6WDWLVWLFDOVLJQL¿FDQFHZDVVHWDWp< 0.05.
RESULTS
Syn t h e sis a n d ch a r a ct e r iz a t ion of AgN Ps
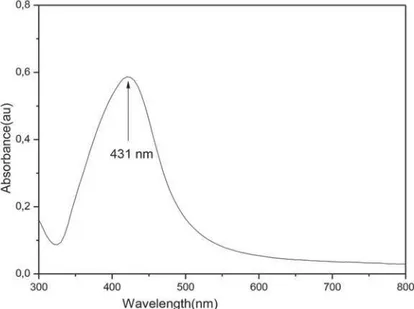
Ac c o r d i n g t o t h e UV v i s i b l e a b s o r p t i o n spect roscopy AgNPs have a UV absorpt ion band wit h a peak cent ered around 431 nm ( Figure 1) . Shape and size dist r ibut ion of t he sy nt hesized AgNPs were charact erized by TEM st udy. The TEM im age shown in Figure 2 was obt ained by high cont rast TEM [ FEI Technai G2 Spirit Bio ( TWI N) ] .
The TEM im age showed that the nano particles have rat her sim ilar and m ainly spherical- like shapes.
Figure 1- UV absorption spectra of AgNP solution
The result s of t he nanopart icle size dist ribut ion according t o t he Zet asizer I nst rum ent showed t hat t he m ean nanopart icle size was about 68 nm and was illust rat ed in Figure 3.
Fle x u r a l st r e n gt h t e st r e su lt s
For each t est group, t he calculat ed m ean and st andar d dev iat ion values of flex ural st r engt h in Table 1 and t he elast ic m odulus are given in Table 2. Am ong t he eight t est groups, t he highest ÀH[XUDOVWUHQJWKDQGHODVWLFPRGXOXVYDOXHVZHUH
found in t he m icrowave- polym erized resin group wit h 0.3 wt % of added AgNPs, while t he lowest values were observed for the 1.6 wt% AgNPs-added conventional heat- polym erized resin group. For the convent ional heat- polym erized resin group, t he DGGLWLRQRI$J13VKDGQRHIIHFWVRQÀH[XUDOVWUHQJWK and elast ic m odulus. However, t he addit ion of 0.8 and 1.6 wt % AgNPs in t he m icrowave- polym erized UHVLQVLJQL¿FDQWO\GHFUHDVHGWKHÀH[XUDOVWUHQJWK and elast ic m odulus ( p< 0.05) .
Denture Base Material
Control 0.3 % AgNPs 0.8 % AgNPs 1.6 % AgNPs
Meliodent 104.30 (5.82)a 102.71 (3.45)a 99.37 (7.94)a 97.34 (9.21)a
Acron 192.43 (3.05)c 197.60 (6.07)c 155.01 (5.81)b 146.56 (5.29)b
Results of Tukey post-hoc comparisons were shown as superscripts and values having the same letters do not differ VLJQL¿FDQWO\S!
Table 1- Means and standard deviations of transverse strength for the groups tested (in MPa)
Denture Base Material
Control 0.3 % AgNPs 0.8 % AgNPs 1.6 % AgNPs
Meliodent 1.90 (0.21)a 1.87 (0.25)a 1.92 (0.24)a 1.81 (0.17)a
Acron 3.96 (0.58)c 4.04 (0.22)c 3.02 (0.47)b 2.79 (0.67)b
Results of Tukey post-hoc comparisons were shown as superscripts and values having the same letters do not differ VLJQL¿FDQWO\S!
Table 2- Means and standard deviations of elastic modulus for the groups tested (in GPa)
Denture Base Material
Control 0.3 % AgNPs 0.8 % AgNPs 1.6 % AgNPs
Meliodent 12.32 (0.81)b 10.78 (0.72)ab 11.64 (1.12)ab 11.14 (1.39)ab
Acron 10.93 (0.97)ab 10.80 (0.97)ab 10.35 (0.45)a 10.37 (1.23)a
Results of Tukey post-hoc comparisons were shown as superscripts and values having the same letters do not differ VLJQL¿FDQWO\
Table 3- Means and standard deviations of impact strength for the groups tested (in kJ/m2)*
I m pa ct st r e n gt h t e st r e su lt s
For each t est group, t he calculat ed m ean and st andard deviat ion values of im pact st rengt h are given in Table 3. The highest im pact st rengt h was observed for t he convent ional heat- polym erized resin wit hout AgNPs and t he lowest was for t he m icr ow av e- r esin g r ou p w it h 0 . 8 w t % Ag NPs cont ent . The addit ion of AgNPs had no effect on t he im pact st rengt h of bot h resin groups ( p> 0.05) .
Th e r m a l a n a lysis r e su lt s
The result s of t he DSC analysis, nam ely DSC t herm ogram s, onset t em perat ures of t he glass
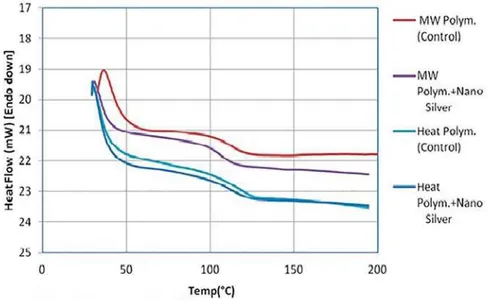
t ransit ion and glass t ransit ion t em perat ures ( Tg) are given in Figure 4 and Figure 5. I t was observed that Tg was higher for the heat-polym erized denture base resin. Tg was found t o decrease wit h t he addit ion of AgNPs for bot h t ypes of dent ure base resins. The relat ive change in heat capacity was FDOFXODWHGIURPWKHKHDWÀRZGDWDDQGLVLOOXVWUDWHG in Figure 6. This was probably due t o a decrease in int er and int ra- m olecular forces wit hin t he polym er m at r ix upon incor porat ion of t he nanopar t icles t herein.
Meliodent Control Meliodent & AgNPs Acron Control Acron & AgNPs
2QVHW7HPSHUDWXUH& 108.63 106.51 101.19 102.46
3HDN7HPSHUDWXUH& 126.04 124.71 114.7 113.73
Figure 4- DSC test results
Figure 5-'6&WHVWUHVXOWV+HDWÀRZ
D I SCUSSI ON
Th er e ar e m an y ar t icles in t h e lit er at u r e regarding AgNPs incorporat ion in dent ure base acrylic resins1,9,10,13,19,25. I n t his st udy, t he AgNPs
w er e sy n t h esized by t h e Tu r k ev ich m et h od2 6,
t hrough t he reduct ion of silver nit rat e wit h sodium cit rat e. The nanopart icles are insoluble and t heir size is t ypically less t han 100 nm28. I n t he present
st udy, t he m ean part icle size det erm ined via Zet a Sizer was about 68 nm . The result s dem onst rat e t hat nanost ruct ures were achieved and t he part icle size dist r ibut ion has a Max w ell- Bolt zm ann like dist ribut ion. Panacek, et al.21 ( 2006)and Baker, et
al.2 ( 2005) report ed t hat t he sm aller part icles are
m ore effect ive against bact eria due t o t he large surface area. Also, t he shape of t he nanopart icle DIIHFWVWKHDQWLPLFURELDOHI¿FLHQF\,WZDVUHSRUWHG t hat t riangular AgNPs expressed great er biocidal act ivit y against E. coli t han rod- or
spherically-shaped nanopart icles20. I n t he current st udy, as
seen in t he TEM im age ( Figure 2) , AgNPs are in spherical- like shapes.
For t he preparat ion of nanocom posit es, t hree appr oach es h av e been dev eloped: ( a) m ix in g nanopar t icles w it h t he polym er, ( b) generat ing nanopart icles during polym erizat ion, ( c) adding nanopar t icles t o t he m onom er3. I n t he cur r ent
st udy, t o reduce t he agglom erat ion and t o readily ach iev e p oly m er / silv er n an ocom p osit es, t h e prepared aqueous solut ion of AgNPs was dispersed in acryl liquid in t he desired rat io and t hen m ixed wit h t he powder part of t he acrylic m at erial.
Th e n a n o p a r t i cl es a d d ed t o t h e p o l y m er PDWHULDOVZHUHDSSURSULDWHO\TXDQWL¿HGVXFKWKDW t he quant it ies used do not cause adverse effect s18.
Yen HJ, Hsu SH and Tsai CL29 ( 2009) report ed t hat
depending on t he part icle size and concent rat ion, AgNPs showed various degrees of adverse effect s on m acrophages. I n a st udy t hat invest igat ed t he effect of nanosilver on t he m echanical and t herm al properties of the acrylic base of com plete dentures, 5 wt% of nanosilver was added, in order to m inim ize probable unfavourable changes in t he m echanical and chem ical propert ies of t he acrylic base of t he dent ure10. I n anot her st udy exam ining t he effect
of AgNPs on t he m echanical propert ies of acrylic resins, 0.2 and 0.05 % of AgNPs were used. This st udy revealed t hat t he effect of AgNPs on t he ÀH[XUDO VWUHQJWK RI 300$ GHSHQGHG RQ VHYHUDO fact ors such as t he t ype of acrylic resin and t he concent rat ion of nanopart icles25. I n t he current
st udy, 0.3, 0.8, and 1.6 wt % of AgNPs were used. By ut ilizing low concent rat ions of nanopart icles, m at erial cost s and t he am ount of m onom er used can be reduced, t hus rendering our process cost effect ive. As a result , t he m echanical propert ies and aest het ic appearance of t he cured polym er
pose less risk9.
I n t he present st udy, t he int eract ion bet ween acrylic resin t ypes and t he addit ion of AgNPs at GLIIHUHQW UDWLRV ZDV QRW VLJQL¿FDQW IRU ÀH[XUDO st r engt h, elast ic m odulus and im pact st r engt h t est s ( p> 0.05) . Result s of t his st udy indicat e t hat t he addit ion of 0.8 and 1.6 wt % AgNPs alt ers t he ÀH[XUDOVWUHQJWKDQGHODVWLFPRGXOXVRIPLFURZDYH cured PMMA. This m ay have result ed from t he aforem ent ioned AgNPs rat ios act ing as im purit ies wit hin t he resin, which led t o a decrease in t he m echanical st rengt h of t he polym er. Also, upon adding nanopar t icles, t he unr eact ed m onom er qu an t it y m ay be in cr eased depen din g on t h e decrease of t he m onom er react ion and it act ed like a plast icizer9. For im pact st rengt h values, t he
presence of AgNPs had no effect on the test groups. The st udy of Sodagar, et al.25 ( 2012)dem onst rat ed
that the addition of 0.05% AgNPs caused a decrease LQWKHÀH[XUDOVWUHQJWKRIRQHEUDQGRIVHOIFXULQJ resin but led t o an increase in t he ot her brand’s st rengt h. I t is hence report ed t hat t he t ype of acrylic resin and t he am ount of NPs incorporat ed t herein are t he im port ant fact ors which affect t he ÀH[XUDOVWUHQJWKRI300$,QFRQWUDVWWRRXUVWXG\ t he st udy by Kassaee, et al.13 ( 2008)indicat ed t hat
adding 0.5% AgNPs int o t he self- curing acrylic UHVLQ V\VWHP LQFUHDVHV WKH ÀH[XUDO VWUHQJWK DQG ant ibact erial effect of t he m at erial. The differences in acrylic resin t ype, t he am ount of NPs, and polar int eract ions which form ed bet ween C= O groups of t he PMMA chains and AgNPs, m ay be responsible for t his sit uat ion25. Chladek, et al.4 ( 2013)report ed
t hat t he m echanical and physical propert ies of t he ¿QDOSRO\PHUDUHDGYHUVHO\DIIHFWHGZLWKLQFUHDVLQJ AgNPs concent rat ion. I n a st udy t hat evaluat ed t he effect of adding AgNPs t o PMMA at t wo different weight percent ages ( 0.2 and 2 wt % ) , t he following was observed: t he effect of AgNPs depended on it s rat ios, and an increm ent in AgNPs increased com pressive st rengt h but led t o a decrease in t he WHQVLOHVWUHQJWKRIWKHUHVLQV$VDUHVXOWWKH¿QDO m at erial becam e m ore brit t le t han t he pure resin itself9. According to the results of the present study,
in low concent rat ions AgNPs, clinically, have no negat ive effect s on t he m echanical propert ies of acrylic resins.
increased wit hin t he glass t ransit ion region ( Figure 6) , due t o an increm ent in free volum e wit hin t he polym eric m atrix and second order phase transition in t he glass t ransit ion region. I ncrease of t he free volum e would decrease t he heat t o be t ransferred via t he conduct ion m ode due t o t he increase of t he int erm olecular dist ances of t he polym eric chains.
This in vit ro study had som e lim itations. Different n an op ar t icle con cen t r at ion s, t h eir r esp ect iv e m icrobiological aspect s and t heir effect s on colour changes should be taken into consideration in future invest igat ions.
CON CLUSI ON S
Wit hin t he lim it at ions of t his in vit ro st udy, t he following conclusions were drawn:
I n cor p or at ion of 0 . 8 an d 1 . 6 w t % Ag NPs GHFUHDVHGWKHÀH[XUDOVWUHQJWKDQGHODVWLFPRGXOXV of m icrowave- polym erized acrylic resin but had no effect on t he ot her t est groups;
For t he t wo resin groups and t est specim ens, t he addit ion of AgNPs had no effect s in t erm s of im pact st rengt h;
The glass transition tem perature ( Tg) decreased wit h t he addit ion of AgNPs for bot h of t he dent ure base resins.
REFEREN CES
1 - Acost a-Tor r es LS, Men diet a I , Nu ñ ez- An it a RE, Caj er o-Juárez M, Cast año VM. Cyt ocom pat ible ant ifungal acrylic resin cont aining silver nanopart icles for dent ures. I nt J Nanom edicine. 2012; 7: 4777- 86.
2- Baker C, Pradhan A, Pakst is L, Pochan DJ, Shah SI . Synt hesis and ant ibact erial propert ies of silver nanopart icles. J Nanosci Nanot echnol. 2005; 5( 2) : 244- 9.
3- Balan L, Schneider R, Lougnot DJ. A new and convenient route to polyacrylat e/ silver nanocom posit es by light- induced cross- linking polym erizat ion. Prog Org Coat . 2008; 62( 3) : 351- 7.
4- Chladek G, Kasperski J, Barszczewska- Rybarek I , Zm udzki J. Sorpt ion, solubilit y, bond st rengt h and hardness of dent ure soft lining incorporat ed wit h silver nanopart icles. I nt J Mol Sci. 2013; 14( 1) : 563- 74.
5- Dam m C, Münst edt H. Kinet ic aspect s of t he silver ion release from ant im icrobial polyam ide/ silver nanocom posit es. Appl Phys A Mat er Sci Process. 2008; 91( 3) : 479- 86
6- Dar- Odeh NS, Shehabi AA. Oral candidosis in pat ient s wit h rem ovable dent ures. Mycoses. 2003; 46( 5- 6) : 187- 91.
7- Fan C, Chu L, Rawls HR, Norling BK, Cardenas HL, Whang K. Developm ent of an ant im icrobial resin - a pilot st udy. Dent Mat er. 2011; 27( 4) : 322- 8.
8- Ganesh S, Guj j ori AK, S SK, B RM, S S, S M. Com parat ive st udy t o assess t he effect iveness of various disinfect ant s on t wo
PLFURRUJDQLVPVDQGWKHHIIHFWRIVDPHRQÀH[XUDOVWUHQJWKRI
acrylic dent ure base resin - an in vit ro st udy. J I nt Oral Healt h. 2013; 5: 55- 62.
9- Ghaffari T, Ham edirad F, Ezzat i B. I n vit ro com parison of com pressive and t ensile st rengt hs of acrylic resins reinforced by silver nanopart icles at 2% and 0.2% concent rat ions. J Dent Res Dent Clin Dent Prospect s. 2014; 8( 4) : 204- 9.
10- Ham edi- Rad F, Ghaffari T, Rezaii F, Ram azani A. Effect of nanosilver on t herm al and m echanical propert ies of acrylic base com plet e dent ures. J Dent ( Tehran) . 2014; 11( 5) : 495- 505.
11- Hazer DB, Mut M, Dinçer N, Saribas Z, Hazer B, Özgen T. The
HI¿FDF\RIVLOYHUHPEHGGHGSRO\SURS\OHQHJUDIWHGSRO\HWK\OHQH
glycol-coated ventricular catheters on prevention of shunt catheter infect ion in rat s. Childs Nerv Syst . 2012; 28( 6) : 839- 46.
.DOD\FÕ g$ &|PHUW )% +D]HU % $WDOD\ 7 &DYLFFKL .$
Cakm ak M. Synt hesis, charact erizat ion, and ant ibact erial act ivit y of m etal nanoparticles em bedded into am phiphilic com b-type graft copolym ers. Polym Bull. 2010; 65( 3) : 215- 26.
13- Kassaee MZ, Akhavan A, Sheikh N, Sodagar A. Ant ibact erial effects of a new dental acrylic resin containing silver nanoparticles. J App Polym er Sci. 2008; 110( 3) : 1699- 703.
14- Kim JS, Kuk E, Yu KN, Kim JH, Par k SJ, Lee HJ, et al. Ant im icr obial effect s of silver nanopar t icles. Nanom edicine. 2007; 3( 1) : 95- 101.
15- Kim SH, Wat t s DC. The effect of reinforcem ent wit h woven
(JODVV ¿EHUV RQ WKH LPSDFW VWUHQJWK RI FRPSOHWH GHQWXUHV
fabr icat ed w it h h igh - im pact acr y lic r esin . J Pr ost h et Den t . 2004; 91: 274- 80.
16- Lyut akov O, Goncharova I , Rim pelova S, Kolarova K, Svanda J, Svorcik V. Silver release and ant im icrobial propert ies of PMMA
¿OPVGRSHGZLWKVLOYHULRQVQDQRSDUWLFOHVDQGFRPSOH[HV0DWHU
Sci Eng C Mat er Biol Appl. 2015; 49: 534- 40.
17- Mat sum ura Y, Yoshikat a K, Kunisaki SI , Tsuchido T. Mode of bact ericidal act ion of silver zeolit e and it s com parision wit h t hat of silver nit rat e. Appl Environ Microbiol. 2003; 69( 7) : 4278- 81. 18- Mont eir o DR, Gor up LF, Takam iya AS, Ruvollo- Filho AC, Cam argo ER, Barbosa DB. The growing im port ance of m at erials t hat prevent m icrobial adhesion: ant im icrobial effect of m edical devices cont aining silver. I nt J Ant im icrob Ag. 2009; 34( 2) : 103- 10 1 9 - Na m KY, Le e C- H, Le e CJ. An t i f u n g a l a n d p h y si ca l
FKDUDFWHULVWLFVRIPRGL¿HGGHQWXUHEDVHDFU\OLFLQFRUSRUDWHGZLWK
silver nanopart icles. Gerodont ology 2012; 29( 2) : 413- 9.
20- Pal S, Tak YK, Song JM. Does t he ant ibact erial act ivit y of silver nanopart icles depend on the shape of nanoparticle? A st udy of the Gram - negative bacterium Escherichia coli. Appl Environ Microbiol. 2007; 73( 6) : 1712- 20.
3DQiþHN$.YtWHN/3UXFHN5.RODU09HFHURYD53L]~URYD1
et al. Silver colloid nanopart icles: synt hesis, charact erizat ion, and t heir ant ibact erial act ivit y. J Phys Chem B. 2006; 110( 33) : 16248-53.
22- Rai M, Yadav A, Gade A. Silver nanopar t icles as a new generat ion of ant im icrobials. Biot echnol Adv. 2009; 27( 1) : 76- 83. 23- Shinonaga Y, Arit a K. Ant ibact erial effect of acrylic dent al
GHYLFHVDIWHUVXUIDFHPRGL¿FDWLRQE\ÀXRULQHDQGVLOYHUGXDOLRQ
im plant at ion. Act a Biom at er. 2012; 8( 3) : 1388- 93.
24- Silver S, Phung LT, Silver G. Silver as biocides in burn and wound dressings and bact erial resist ance t o silver com pounds. J I nd Microbiol Biot echnol. 2006; 33( 7) : 627- 34.
25- Sodagar A, Kassaee MZ, Ak havan A, Javadi N, Arab S,
.KDUD]LIDUG0-(IIHFWRIVLOYHUQDQRSDUWLFOHVRQÀH[XUDOVWUHQJWK
of acrylic resins. J Prost hodont Res. 2012; 56( 2) : 120- 4.
26- Turkevich J, St evenson PC, Hillier J. A st udy of t he nucleat ion and growt h processes in t he synt hesis of colloidal gold. Discuss Faraday Soc. 1951; 11: 55- 75.
27- Wady AF, Machado AL, Zucolot t o V, Zam perini CA, Berni E,
9HUJDQL&((YDOXDWLRQRI&DQGLGDDOELFDQVDGKHVLRQDQGELR¿OP
for m at ion on a dent ur e base acr y lic r esin cont aining silver nanopart icles. J Appl Mirobiol. 2012; 112( 6) : 1163- 72.
28- Weir E, Lawlor A, Whelan A, Regan F. The use of nanopart icles in ant i- m icrobial m at erials and t heir charact erizat ion. Analyst . 2008; 133: 835- 45.