ABSTRACT
http://dx.doi.org/10.1590/1678-775720160058
Evaluation of phenyl-propanedione on yellowing and
chem ical- m echanical pr oper t ies of exper im ent al
dent al r esin- based m at er ials
Dayane Carvalho Ramos Salles de OLIVEIRA1, Eduardo José SOUZA-JUNIOR1, Adam DOBSON2, Ana Rosa Costa
CORRER1, William Cunha BRANDT3, Mário Alexandre Coelho SINHORETI1
1- Universidade Estadual de Campinas, Faculdade de Odontologia de Piracicaba, Departamento de Odontologia Restauradora, Piracicaba, SP, Brasil. 2- Oregon Health & Science University, Department of Biomaterials, Portland, Oregon, United States of America.
3- Universidade de Santo Amaro, Departamento de Implantodontia, São Paulo, SP, Brasil.
Corresponding address: Dayane Carvalho Ramos Salles de Oliveira - Faculdade de Odontologia de Piracicaba - UNICAMP - Departamento de Odontologia
Restauradora - Av. Limeira, 901 - Areião - Piracicaba - SP - Brazil - 13414903 - Phone: +55(21)981666214 - e-mail: oliveira.day@icloud.com
6XEPLWWHG)HEUXDU\0RGL¿FDWLRQ0D\$FFHSWHG-XQH
O
EMHFWLYH7RHYDOXDWHWKHLQÀXHQFHRISKHQ\OSURSDQHGLRQHRQ\HOORZLQJDQGFKHPLFDOm echanical pr oper t ies of ex per im ent al r esin- based m at er ials phot oact ivat ed using different light curing unit s ( LCUs) . Mat erial and Met hods: Experim ent al resin- based m at erials w it h t he sam e or ganic m at r ix ( 60: 40 w t % BisGMA: TEGDMA) w er e m echanically blended using a cent r ifugal m ixing device. To t his blend, differ ent phot oinit iat or syst em s w er e added in equim olar concent rat ions w it h aliphat ic am ine doubled by w t % : 0.4 w t % CQ;ZW33'RUZW&4DQGZW33'7KHGHJUHHRIFRQYHUVLRQ'&ÀH[XUDO
st r engt h ( FS) , Young’s m odulus ( YM) , Knoop har dness ( KNH) , cr osslinking densit y ( CLD) , and yellow ing ( Y) w er e evaluat ed ( n= 10) . All sam ples w er e light cur ed w it h t he follow ing LCUs: a halogen lam p ( XL 2500) , a m onowave LED ( Radii) , or a polywave LED ( Valo)
w it h 16 J/ cm27KHUHVXOWVZHUHDQDO\VHGE\WZRZD\$129$DQG7XNH\¶VWHVWĮ
Result s: No st at ist ical differ ences w er e found bet w een t he differ ent phot oinit iat or syst em s
WR.1+&/6)6DQG<0SURSHUWLHVS33'&4DVVRFLDWLRQVKRZHGWKHKLJKHU'&
values com par ed w it h CQ and PPD isolat ed syst em s w hen phot oact ivat ed by a polywave
/('S<YDOXHVZHUHKLJKHVWIRUWKH&4FRPSDUHGZLWKWKH33'V\VWHPVS
Conclusion: PPD isolat ed syst em prom ot ed sim ilar chem ical and m echanical propert ies and less yellow ing com par ed w it h t he CQ isolat ed syst em , r egar dless of t he LCU used.
Ke y w or ds: Dent al adhesive. Dent al cur ing light s. Dent al phot oinit iat or s. Physical and
chem ical pr oper t ies.
I N TROD UCTI ON
Cam phor quinone ( CQ) is t he m ost w idely and su ccessf u lly u sed ph ot oin it iat or in den t al r esin m at er ials6. Despit e t heir high clinical accept ance, phot oinit iat or syst em s based on CQ are responsible for a yellow ish colour in r esin- based m at er ials2,6,13.
Alt er nat ive phot oinit iat or sy st em s have been suggest ed t o subst it ut e CQ in dent al m at er ials in or der t o r educe t his yellow ing effect , especially in r esin- based m at er ials t o bleached t eet h2,6,13,16. On t he ot her hand, alt er nat ive phot oinit iat or s syst em s for r esin m at er ials m ust not only have accept able init ial and long- t erm est het ical appearance, but also
appr opr iat ed m echanical pr oper t ies.
Phenyl- pr opanedione ( PPD) is suggest ed as an effect ive alt ernat ive phot oinit iat or in order t o reduce t his yellow ing5,6. As a Nor r ish t ype I phot oinit iat or, PPD r eact s by phot oly sis, in w hich t he cleavage of t he C- C bond bet w een t he car bonyls funct ional gr oups of it s m olecule leads t o t he for m at ion of fr ee radicals st ar t ing t he polym er izat ion. How ever, PPD can also r eact via a co- init iat or, since it bear s t h e sam e d ik et on e g r ou p as cam p h or q u in on e. Then, radicals der ived fr om t he am ine- based co-init iat or H- t ransfer ar e r esponsible for st ar t ing t he polym er izat ion12.
m ech an ical pr oper t ies of r esin - based m at er ials associat ed w it h alt er nat ive phot oinit iat or s, such as PPD, dipheny l( 2 , 4 , 6 - t r im et hy lbenzoy l) phosphine oxide (TPO) , and phenylbis( 2,4,6- t rim et hylbenzoyl) phosphine oxide ( BAPO) , showing sim ilar or superior per for m ance com par ed w it h t he CQ syst em s1,4,17. PPD is an alt er n at iv e ph ot oin it iat or t h at sh ow s r educed yellow ing com par ed w it h t he CQ syst em s, but it s chem ical and m echanical propert ies st ill need t o be fur t her evaluat ed1,17.
Unlike CQ, t he absor pt ion peak of PPD is in t he near UV r egion ( UVA) and ext ends slight ly int o t he v isible wavelengt h. Thus, it could be consider ed
DEHWWHU89LQLWLDWRUWKDQDQHI¿FLHQWYLVLEOHOLJKW
phot oinit iat or6. How ever, som e st udies have show n t hat PPD pr oduces sim ilar degr ee of conver sion com par ed w it h t he CQ syst em s w hen a halogen lig h t is u sed f or p h ot oact iv at ion3 , 8 , 1 4 , 1 5. Bu t it s
HI¿FLHQF\ ZLWK GLIIHUHQW OLJKWFXULQJ XQLWV /&8V
for phot oact ivat ion of t hese r esins st ill needs t o be evaluat ed5.
Th e aim of t h e t h is st u d y w as t o ev alu at e t h e y ellow in g ( Y) an d t h e ch em ical- m ech an ical p r o p e r t i e s, su ch a s Kn o o p h a r d n e ss ( KNH) , cr osslinking densit y ( CLD) , degr ee of conver sion
'&ÀH[XUDOUHVLVWDQFH)5DQG<RXQJ¶VPRGXOXV
( EM) of r esi n m at er i al s con t ai n i n g PPD i n i t s com posit ion com par ed w it h t hose cont aining CQ, phot oact ivat ed by differ ent LCUs. The alt er nat ive hypot heses t est ed w er e as follow s:
( i) PPD- based r esin s w ill pr om ot e sim ilar or super ior chem ical- m echanical pr oper t ies, but less yellow ing com par ed w it h t he CQ- based r esins;
( ii) Br oadban d spect r u m u n it s, su ch as t h e halogen light or t he polywave LED, w ill pr om ot e super ior chem ical pr oper t ies for t he PPD- based resins com pared wit h t he narrowed m onowave LED; ( iii) Broadband spect rum unit s, such as t he halogen light or t he polywave LED, w ill pr om ot e super ior m echanical pr oper t ies for t he PPD- based r esins com par ed w it h t he nar r ow ed m onowave LED.
M ATERI AL AN D M ETH OD S
Re sin ble n ds
Exper im ent al r esins w er e m echanically blended using a cent r ifugal m ixing device SpeedMixer DAC 1 5 0 . 1 FVZ- K ( Hau sch ild En g in eer in g ; Ham m , Nor t h Rhine- West phalia, Ger m any) w it h t he sam e or g an ic m at r ix b ased on 6 0 w t % BisGMA an d 40 w t % TEGDMA. To t his r esin, blend equim olar p h o t o i n i t i at o r co n cen t r at i o n w er e ad d ed w i t h t w ice t he concent rat ion by w t % aliphat ic am ine, DMAEMA ( Sigm a- Aldr ich I nc; St . Louis, MO, USA) , an d t h e follow in g ph ot oin it iat or w t % : 0 . 4 w t % cam phor quinone ( Sigm a- Aldr ich I nc; St Louis, MO, USA) ; 0 . 3 6 w t % ph eny l- pr opan edion e ( Sigm a-Aldr ich I nc; St Louis, MO, USA) , or bot h in m olar
concent rat ion, 0.2 w t % CQ and 0.18 w t % PPD.
Ligh t - cu r in g u n it e v a lu a t ion
Th e m ean an d m ax im u m r ad ian t em it t an ce ( m W/ cm2) and radiant exposur e ( J/ cm2) accor ding
t o t he differ ent wavelengt h ranges of each light curing unit , XL 2500 ( 3M/ ESPE; St . Paul, MN, USA) , Radii ( SDI , Bay sw at er ; Vict or ia, Aust ralia) , and Valo Cor dless ( Ult radent ; Sout h Jor dan, UT, USA) w er e evaluat ed using a MARC Resin Calibrat or sp ect r op h ot om et er ( Blu eLig h t An aly t ics; Nov a Scot ia, Canada) .
Absor pt ion spe ct r oph ot om e t r ic a n a ly sis A solut ion of each phot oinit iat or was pr epar ed u sin g 1 m L of > 9 9 . 5 % et h an ol ( Sigm a- Aldr ich , St . Lou is, MO, USA) . Th e solu t ion ab sor p t ion spect r oph ot om et r ic an aly sis w as det er m in ed in t he 200–600 nm range using a U- 2425 UV–Vis sp ect r op h ot om et er ( Hit ach i Hig h -Tech n olog ies; Chiyoda, Tokyo, Japan) . The spect ra w ere collect ed using a quar t z cell w it h a pat h lengt h of 1 cm .
D e gr e e of Con v e r sion ( D C)
Th e DC f or each r esin w as m easu r ed u sin g Fou r ier t r an sf or m in f r ar ed spect r oscopy ( FTI R)
FRXSOHGWRDQDWWHQXDWHGWRWDOUHÀHFWDQFH$75
Spect r um 100 ( Per kinElm er ; Walt ham , MA, USA) . Rect angular bar- shaped specim ens ( 10 m m x 2 m m , 1 m m t hick ) w er e phot oact ivat ed t hr ough Mylar st r ip using one of t he light - cur ing unit s t est ed w it h 16 J/ cm2 ener gy dose ( n= 10) . The t ransm ission
spect ra were recorded using 16 scans at a resolut ion of 1 cm- 1 for each uncur ed and post - cur ed sam ple
r espect ively. The num ber of r em aining uncover ed carbon double bonds w ere calculat ed by com paring t he per cent age of aliphat ic C= C ( vinyl) absor pt ion ( 1638 cm- 1) w it h ar om at ic C= C absor pt ion ( 1608
cm- 1) bet w een post - cur ed and uncur ed sam ples, in
w hich t he ar om at ic double bond st r et ching bands r em ain const ant dur ing poly m er izat ion r eact ion and ser ve as an int er nal st andar d. The DC for each r esin was calculat ed by subt ract ing t he per cent age of aliph at ic dou ble bon ds pr esen t f r om 1 0 0 % , accor ding t o t he follow ing equat ion:
DC ( % ) = { 1- ( Xa/ Ya) / ( Xb/ Yb) } × 100, w her e Xa ( post - cur ed) and Xb ( uncur ed) r epr esent t he ar eas u n der t h e ban ds of t h e poly m er izable aliph at ic double bonds, and Ya ( post- cured) and Yb ( uncured) r epr esent t he ar eas under t he bands of ar om at ic double bonds.
Fle x ur a l st r e ngt h ( FS) a nd Young’s m odulus ( YM )
suppor t s of 6.0 m m ; cr osshead speed of 0.5 m m / m in unt il failur e) .
Kn oop h a r dn e ss ( KN H )
Cylindr ical specim ens ( 2 m m diam et er, 2 m m t hick , n= 10) w er e used t o m easur e KNH t aken on t op and bot t om sur faces using a Knoop har dness m et er, HMV- 2 ( Sh im ad zu ; Ch iy od a- k u , Tok y o, Japan ) , u n der a 0 . 4 9 N load ( equ ivalen t t o 5 0 Kgf ) for 15 s. Five r eadings w er e per for m ed for each sur face, and t he m ean was r ecor ded as t he KNH values ( Kgf/ m m2) . The sam e specim ens w er e
im m ediat ely used t o m easur e Y.
Ye llow in g e v a lu a t ion
Yellow in g m easu r em en t s of each sp ecim en per for m ed for KNH an aly sis w er e t ak en w it h a D65 illum inant over w hit e ( CI E L* = 91.1, a* = 1.2 and b* = - 3.4, Y= 78.8) back gr ound using a pr e-calibrat ed spect r ophot om et er, CM- 7 0 0 d ( Konica Minolt a; Chiyoda- ku, Tokyo, Japan) wit h a diam et er t ip of 4 m m . The t ip of t he spect r ophot om et er was placed in t he m iddle of each specim en using a
7HÀRQMLJDQGWKUHHPHDVXUHPHQWVZHUHWDNHQRI
each specim en. The yellow ing was det er m ined by b* axis coor dinat e param et er value, in w hich, + b*
\HOORZDQGíE EOXH
Cr oss- lin k in g de n sit y ( CLD )
Aft er colour m easur em ent s, t he init ial r eadings of KNH w er e r ecor ded as t he init ial KNH num ber ( KNH1) for each specim en. Then, t he specim ens w er e st or ed in 100% et hanol for 24 h at r oom t em perat ure, and a second hardness m easurem ent w as r ecor ded as KNH2. The CLD w as est im at ed by t he soft ening effect pr om ot ed by t he et hanol w it h har dness decr ease calculat ing t he per cent age decr ease of KNH5.
St a t ist ica l a n a ly sis
Dat a w er e subm it t ed t o t he Shapir o- Wilk t est
IRU QRUPDOLW\ GLVWULEXWLRQ YHUL¿FDWLRQ DQG WR WKH
Levene’s t est for hom ogeneit y of var iances. A t w o-way analysis of var iance was used for st at ist ical
DQDO\VHVRI'&)6<0.1+&/'DQGƩEYDOXHV
Tukey’s t est was applied for m ult iple com par isons ( p= 0.05) bet w een t he differ ent phot oinit iat or s and light - cur ing unit s t est ed. All st at ist ical analy ses w er e per for m ed using St at a/ MP 13.0 ( St at aCor p; College st at ion, TX, USA) .
RESULTS
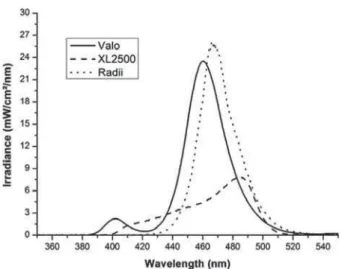
The LCUs wavelengt hs and t he spect ra of t he phot oinit iat or s used in t his st udy ar e show n in Figur es 1 and 2 r espect iv ely. Radii show ed t he highest spect ral ir radiance values ( 25.5 m W/ cm2/
nm ) at t he 465 nm em ission peak, w her eas Valo pr esent ed 23 m W/ cm2/ nm at t he 460 nm em ission
peak . The halogen lam p XL 2500 pr esent ed t he lowest spect ral irradiance values ( 7.6 m W/ cm2/ nm )
at t he 485 nm em ission peak. The light absor pt ion analysis of dent al phot oinit iat or s show ed t hat CQ exhibit ed absor pt ion cent r ed in t he blue r egion of t he light spect r um , w it h Absm ax at 470 nm and
İNJPD[/PROFPZKLOH33'LQLWLDWHVWKHFXUYHLQ WKH89UHJLRQZLWK$EVPD[DWQPDQGİNJPD[
101 L/ m ol.cm , and ext ends t he absor pt ion cur ve int o t he visible r egion.
Tables 1- 6 show t he m ean values and st andar d deviat ions for DC ( % ) , FS ( MPa) , YM ( GPa) , KNH ( Kgf/ m m 2 ) , Y, an d CLD ( % ) r espect iv ely. As it can be obser ved, no st at ist ical differ ences w er e found bet w een t he differ ent phot oinit iat or syst em s
WR .1+ &/6 )6 DQG <0 SURSHUWLHV S
However, PPD/ CQ associat ion showed t he higher DC values com pared wit h CQ and PPD isolat ed syst em s
ZKHQSKRWRDFWLYDWHGE\DSRO\ZDYH/('S
Figure 1- Absolute irradiance (mW/cm2/nm) x wavelength
(nm) for each light-curing unit tested
Figure 2- Absolute absorbance x wavelength (nm) for
Photoinitiator Light Curing Unit
Radii-Cal Valo XL 2500
CQ 76.4 (4.1)Aa 79.3 (4.3)Ab 78.1 (2.1)Aa
CQ/PPD 75.4 (2.6)Ba 87.5 (3.2)Aa 79.3 (5.0)Ba
PPD 75.7 (4.1)Ba 82.8 (3.6)Aab 77.7 (3.9)Ba
0HDQVIROORZHGE\GLIIHUHQWFDSLWDOOHWWHUVLQWKHVDPHOLQHDQGVPDOOOHWWHUVLQWKHVDPHFROXPQZHUHVLJQL¿FDQWO\GLIIHUHQW
(p<0.05)
Table 1- Mean DC value (%) and standard error (±) provided for each of the photoinitiator systems
Photoinitiator Light Curing Unit
Radii-Cal Valo XL 2500
CQ 165.2 (30.6)Aa 158.4 (13.4)Aa 126.7 (13.4)Ba
CQ/PPD 174.6 (24.6)Aa 174.8 (20.0)Aa 134.1 (15.2)Ba
PPD 168.7 (25.1)Aa 166.7 (32.1)Aa 103.8 (20.9)Ba
0HDQVIROORZHGE\GLIIHUHQWFDSLWDOOHWWHUVLQWKHVDPHOLQHDQGVPDOOOHWWHUVLQWKHVDPHFROXPQZHUHVLJQL¿FDQWO\GLIIHUHQW
(p<0.05)
Table 2- Mean FS value (MPa) and standard error (±) provided for each of the photoinitiator systems
Photoinitiator Light Curing Unit
Radii Valo XL 2500
CQ 19.9 (4.1)Aa 23.5 (7.7)Aa 23.6 (6.9)Aa
CQ/PPD 20.9 (3.3)ABa 28.1 (6.7)Aa 16.4 (3.7)Ba
PPD 14.8 (4.7)Aa 23.0 (4.2)Aa 16.2 (4.5)Aa
0HDQVIROORZHGE\GLIIHUHQWFDSLWDOOHWWHUVLQWKHVDPHOLQHDQGVPDOOOHWWHUVLQWKHVDPHFROXPQZHUHVLJQL¿FDQWO\GLIIHUHQW
(p<0.05)
Table 4- Mean KNH value (Kgf/mm2) and standard error (±) provided for each of the photoinitiator systems
Photoinitiator Light Curing Unit
Radii-Cal Valo XL 2500
CQ 3.60 (0.77)Aa 3.74 (0.50)Aa 1.93 (0.22)Ba
CQ/PPD 3.74 (0.93)Aa 3.43 (0.53)Aa 1.83 (0.35)Ba
PPD 4.14 (0.43)Aa 3.73 (0.70)Aa 1.45 (0.39)Ba
0HDQVIROORZHGE\GLIIHUHQWFDSLWDOOHWWHUVLQWKHVDPHOLQHDQGVPDOOOHWWHUVLQWKHVDPHFROXPQZHUHVLJQL¿FDQWO\GLIIHUHQW
(p<0.05)
Table 3- Mean YM value (GPa) and standard error (±) provided for each of the photoinitiator systems
Photoinitiator Light Curing Unit
Radii Valo XL 2500
CQ +4.17 (0.20)Ba +5.3 (0.58)Aa +4.34 (0.14)Ba
CQ/PPD +3.94 (0.09)Cb +5.09 (0.19)Aa +4.23 (0.11)Ba
PPD +3.48 (0.17)Bc +4.78 (0.11)Ab +4.08 (0.12)Bb
0HDQVIROORZHGE\GLIIHUHQWFDSLWDOOHWWHUVLQWKHVDPHOLQHDQGVPDOOOHWWHUVLQWKHVDPHFROXPQZHUHVLJQL¿FDQWO\GLIIHUHQW
(p<0.05)
and Y values w er e highest for t he CQ com par ed
ZLWKWKH33'V\VWHPVS
D I SCUSSI ON
7KH¿UVWDOWHUQDWLYHK\SRWKHVLVWKDW33'EDVHG
r esins w ill pr om ot e sim ilar or super ior chem ical-m echanical pr oper t ies but less yellow ing coical-m par ed w it h t he CQ- based r esins, could not be r ej ect ed.
7KHUHZHUHQRVLJQL¿FDQWGLIIHUHQFHVLQ.1+&/'
FS, and YM bet w een t he phot oinit iat or sy st em s
SDQG33'DOVRSURPRWHGVLPLODURUKLJKHU
DC an d low er y ellow in g af t er cu r in g com par ed w it h t he CQ syst em , as show n in Tables 1 and 5 r espect ively.
PPD h a s b e e n s t u d i e d a s a n a l t e r n a t i v e p h o t o i n i t i at o r i n o r d er t o d ecr ease y el l o w i n g caused by CQ because of it s less yellow ish colour in com par ison w it h CQ2,5,6,14,15. Also, as a Nor r ish t ype
I phot oinit iat or, PPD does not r equir e a yellow ed colour ed co- init iat or, such as t er t iar y am ines, t o generat e free radicals t o st art t he polym erizat ion12.
Man y st u d ies sh ow ed t h at PPD r ed u ced t h e Y com par ed w it h CQ and pr om ot ed sim ilar har dness t o CQ2,6,14. As obser ved in t his st udy, PPD was able
t o r educe Y and also pr om ot e sim ilar or super ior ch em ical an d m ech an ical p r op er t ies com p ar ed w it h CQ.
Th e secon d alt er n at iv e h y p ot h esis t h at t h e br oadband unit s, such as t he halogen light or t he p oly w av e LED, w ill p r om ot e su p er ior ch em ical p r op er t ies f or t h e PPD- b ased r esin s com p ar ed w it h t he nar r ow ed m onowave LED could not be r ej ect ed, sin ce su per ior DC w as fou n d in PPD-based r esins com par ed w it h CQ- PPD-based sy st em s only w hen phot oact ivat ed by t he polywave LED. Th e h i g h e st D C f o r PPD - b a se d sy st e m s w a s achiev ed using t he poly w av e LED, as obser v ed in Tab le 1 . Un lik e CQ, t h e ab sor p t ion p eak of PPD i s n ear t h e UV r eg i on ( UVA) ( Fi g u r e 1 ) , t hus t he violet spect r um ir radiat ion by polywave LED p r o m o t ed m o r e ef f i ci en t p h o t o act i v at i o n o f t h i s a l t e r n a t i v e p h o t o i n i t i a t o r3 , 6 , 8 , 1 5 - 1 7,
explaining t he higher DC w hen pr oper spect r um em ission w as u sed f or p h ot oact iv at ion9 , 1 0 , 1 1 , 1 3.
The t hird alt ernat ive hypot hesis t hat t he broadband unit s, such as t he halogen light or t he polywave
LED, w ill pr om ot e super ior m echanical pr oper t ies f o r t h e PPD - b ased r esi n s co m p a r ed w i t h t h e nar r ow ed m onowave LED, how ever, was r ej ect ed. Sim ilar it ies in chem ical and m echanical pr oper t ies bet w een CQ and PPD for m ulat ions ar e explained by sim ilar it ies in DC and CLD achieved in t hese p h o t o i n i t i at o r sy st em s3 , 6. As o b ser v ed i n t h i s
st udy, even w hen higher DC was achieved by PPD isolat ed syst em phot oact ivat ed w it h t he polywave LED, no differ ences in t he CLD w er e found. The CLD in cr eases as t h e p oly m er izat ion r eact ion s incr ease t he polym er chains. Ther efor e, t he CLD p l ay essen t i al r o l es i n m ech an i cal p r o p er t i es developm ent in com parison w it h t he DC7. How ever,
despit e no im pr ovem ent s in m echanical pr oper t ies were found for PPD- based resins in com parison wit h CQ, CLD sim ilarit y regarding t he higher DC m ight be
SURPLVLQJIRU¿OOHGUHVLQPDWHULDOV6LQFHYROXPHWULF
shr inkage occur s sim ult aneously w it h t he incr ease in t h e CLD of t h e poly m er7, PPD m igh t r edu ce
< LQ ¿OOHG UHVLQ PDWHULDOV ZLWKRXW FRPSURPLVLQJ
m a r g i n a l a d a p t i o n o f d i r e c t r e s t o r a t i o n s .
7KXV DFFRUGLQJ WR WKH ¿QGLQJV RI WKLV VWXG\ LW
was possible t o conclude t hat PPD is a pr om ising alt er nat ive phot oinit iat or com par ed w it h CQ, since it reduced yellowing wit hout com prom ising chem ical or m echanical pr oper t ies of t he r esins, r egar dless of t h e LCU u sed. PPD in cr eased t h e degr ee of conver sion w hen t he polywave LED was used as
WKH/&8EXWQRVLJQL¿FDQWGLIIHUHQFHZDVIRXQGIRU
CLD. Then, furt her st udies are necessary t o evaluat e
33' DGGLWLRQ WR UHVLQ EOHQGV ZLWK ¿OOHU DGGLWLRQ
and polym er izat ion shr inkage should be evaluat ed t o det er m ine if t he incr ease in DC could pr om ot e less Y w it hout affect ing t he m ar ginal adapt at ion of dir ect r est orat ions.
CON CLUSI ON
$FFRUGLQJWRWKH¿QGLQJVRIWKLVVWXG\LWZDV
possible t o conclude t hat PPD pr om ot ed sim ilar ch em i cal an d m ech an i cal p r o p er t i es an d l ess yellow ing on r esins com par ed w it h t he CQ- syst em , r egar dless of t he LCU used.
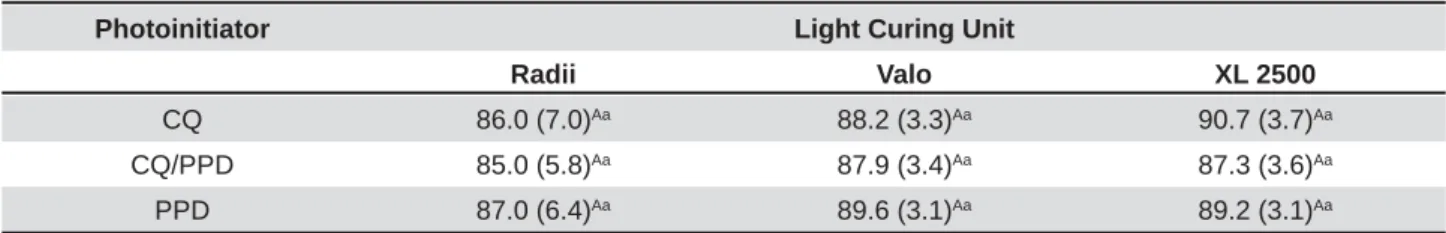
Photoinitiator Light Curing Unit
Radii Valo XL 2500
CQ 86.0 (7.0)Aa 88.2 (3.3)Aa 90.7 (3.7)Aa
CQ/PPD 85.0 (5.8)Aa 87.9 (3.4)Aa 87.3 (3.6)Aa
PPD 87.0 (6.4)Aa 89.6 (3.1)Aa 89.2 (3.1)Aa
0HDQVIROORZHGE\GLIIHUHQWFDSLWDOOHWWHUVLQWKHVDPHOLQHDQGVPDOOOHWWHUVLQWKHVDPHFROXPQZHUHVLJQL¿FDQWO\GLIIHUHQW
(p<0.05)
ACKN OW LED GM EN TS
This st udy was support ed by FAPESP - São Paulo
5HVHDUFK)RXQGDWLRQJUDQWV
REFEREN CES
1 - Albu qu er qu e PP, Mor eir a AL, Mor aes RR, Cav alcan t e LM, Sch n eid er LFJ. Colou r st ab ilit y, con v er sion , w at er sor p t ion and solubilit y of dent al com posit es for m ulat ed w it h differ ent phot oinit iat or syst em s. J Dent . 2012; 41( Suppl 3) : e67- 72. 2- Alvim HH, Alecio AC, Vasconcellos WA, Fur lan M, Oliveira JE, Saad JR. Analysis of cam phor quinone in com posit e r esins as a funct ion of shade. Dent Mat . 2007; 23: 1245- 9.
$VPXVVHQ(3HXW]IHOGW$,QÀXHQFHRIFRPSRVLWLRQRQUDWHRI
polym er izat ion cont ract ion of light - cur ing r esin com posit es. Act a Odont ol Scand. 2002; 60: 146- 50.
4- Brandt WC, Schneider LF, Frollini E, Correr- Sobrinho L, Sinhoret i MA. Effect of differ ent phot o- init iat or s and light cur ing unit s on degr ee of conver sion of com posit es. Braz Oral Res. 2010; 24: 263-70.
5- Brandt WC, Silva CG, Fr ollin E, Souza-Júnior EJ, Sinhor et i MA. Dynam ic m echanical t her m al analysis of com posit e r esins w it h CQ and PPD as phot o- init iat or s phot oact ivat ed by QTH and LED unit s. J Mech Behav Biom ed Mat er. 2013; 24: 21- 9.
6- Brandt WC, Tom aselli LO, Cor r er- Sobr inho L, Sinhor et i MA.
&DQ SKHQ\OSURSDQHGLRQH LQÀXHQFH .QRRS KDUGQHVV UDWH RI
polym erizat ion and bond st rengt h of resin com posit e rest orat ions? J Dent . 2011; 39( 6) : 438- 47.
7- Dauvillier BS, Feilzer AJ, De Gee AJ, Davidson CL. Visco- elast ic param et er s of dent al r est orat ive m at er ials dur ing set t ing. J Dent Res. 2000; 79( 3) : 818- 23.
(PDPL16|GHUKROP.-,QÀXHQFHRIOLJKWFXULQJSURFHGXUHV
and phot o- init iat or / co- init iat or com posit ion on t he degr ee of conversion of light - curing resins. J Mat er Sci Mat er Med. 2005; 16: 47- 52.
9- I lie N, Hickel R. Can CQ be com plet ely r eplaced by alt er nat ive init iat or s in dent al adhesives? Dent Mat J. 2008; 27: 221- 8. 10- Milet ic V, Sant ini A. Micr o- Ram an spect r oscopic analysis of t he degr ee of conver sion of com posit e r esins cont aining differ ent init iat or s cur ed by poly wave or m onowave LED unit s. J Dent . 2012; 40( 2) : 106- 13.
1 1 - Mi l e t i c V, Sa n t i n i A. Op t i m i zi n g t h e co n ce n t r a t i o n o f 2 , 4 , 6 - t r i m et h y l b en zoy l d i p h en y l p h o sp h i n e ox i d e i n i t i at o r i n com posit e r esins in r elat ion t o m onom er conver sion. Dent Mat er J. 2012; 31( 5) : 717- 23.
1 2 - Neu m an n MG, Sch m it t CC, Fer r eir a GC, Cor r êa I C. Th e
LQLWLDWLQJ UDGLFDO \LHOGV DQG WKH HI¿FLHQF\ RI SRO\PHUL]DWLRQ IRU
var ious dent al phot oinit iat or s excit ed by differ ent light cur ing unit s. Dent Mat er. 2006; 22( 6) : 576- 84.
13- Oliveira DC, Rocha MG, Gat t i A, Cor r er AB, Fer racane JL, Sinhoret MA. Effect of different phot oinit iat ors and reducing agent s
RQFXUHHI¿FLHQF\DQGFRORUVWDELOLW\RIUHVLQEDVHGFRPSRVLWHV
using differ ent LED wavelengt hs. J Dent . 2015; 43( 12) : 1565- 72. 1 4 - Sch n eid er LF, Con san i S, Sak ag u ch i RL, Fer r acan e JL. Alt er n at iv e ph ot oin it iat or sy st em r edu ces t h e r at e of st r ess
GHYHORSPHQW ZLWKRXW FRPSURPLVLQJ WKH ¿QDO SURSHUWLHV RI WKH
dent al com posit e. Dent Mat . 2009; 25: 566- 72.
15- Schneider LF, Pfeifer CS, Consani S, Prahl SA, Fer racane JL.
,QÀXHQFH RI SKRWRLQLWLDWRU W\SH RQ WKH UDWH RI SRO\PHUL]DWLRQ
degr ee of conver sion, har dness and yellow ing of dent al r esin com posit es. Dent Mat . 2008; 24: 1169- 77.
16- Schr oeder W, Ar enas G, Vallo C. Monom er conver sion in a light - cur ed dent al r esin cont aining 1- phenyl- 1,2- pr opanedione phot osensit izer. Polym I nt . 2007; 56: 1099- 105.