ContentslistsavailableatScienceDirect
Journal
of
Hazardous
Materials
jou rn a l h om ep ag e :w w w . e l s e v i e r . c o m / l o c a t e / j h a z m a t
Batch
and
fixed-bed
assessment
of
sulphate
removal
by
the
weak
base
ion
exchange
resin
Amberlyst
A21
Damaris
Guimarães
∗,
Versiane
A.
Leão
Bio&HydrometallurgyLaboratory,DepartmentofMetallurgicalandMaterialsEngineering,UniversidadeFederaldeOuroPreto,CampusMorrodoCruzeiro, s.n.,Bauxita,OuroPreto,MG,35400-000,Brazil
h
i
g
h
l
i
g
h
t
s
•AmberlystA21(weakbaseresin)wasappliedforthefirsttimeinsulphateremoval. •Itisacomprehensivestudyofthemainaspectsofsulphateadsorption,neverdonebefore. •SulphateadsorptionprocessbyAmberlystA21isfastandfeasibleinacidmedium. •Efficientsulphateelutionwasobtainedusingsimpleandaccessibleconditions.
a
r
t
i
c
l
e
i
n
f
o
Articlehistory:
Received20February2014
Receivedinrevisedform4July2014
Accepted26July2014
Availableonline13August2014
Keywords:
Sulphateremoval
Sorption
Ionexchange
AmberlystA21
Weakbaseresins
a
b
s
t
r
a
c
t
ThispaperinvestigatedsulphateremovalfromaqueoussolutionsbyAmberlystA21,apolystyreneweak baseionexchangeresin.BoththepHandinitialsulphate concentrationwereobservedtostrongly affectsorptionyields,whichwerelargestin acidicenvironments.Working underoptimum opera-tionalconditions,sulphatesorptionbyAmberlystA21wasrelativelyfastandreachedequilibriumafter 45minofcontactbetweenthesolidandliquidphases.Sorptionkineticscouldbedescribedbyeither thepseudo-firstorder(k1=3.05×10−5s−1)orpseudo-secondordermodel(k2=1.67×10−4s−1),and boththeFreundlichandLangmuirmodelssuccessfullyfittedtheequilibriumdata.Sulphateuptakeby AmberlystA21wasaphysisorptionprocess(H=−25.06kJmol−1)thatoccurredwithentropyreduction (S=−0.042kJmol−1K−1).Elutionexperimentsshowedthatsulphateiseasilydesorbed(∼100%)from theresinbysodiumhydroxidesolutionsatpH10orpH12.Fixed-bedexperimentsassessedtheeffectsof theinitialsulphateconcentration,bedheightandflowrateonthebreakthroughcurvesandtheefficiency oftheAmberlystA21inthetreatmentofarealeffluent.Inallstudiedconditions,themaximumsulphate loadingresinvariedbetween8and40mg(SO42−)mL(resin)−1.
©2014ElsevierB.V.Allrightsreserved.
1. Introduction
Whenoxidizing conditions are present,sulphate is the pre-dominantsulphurspeciesinliquideffluentsassociatedwiththe processingsulphur-ladenrawmaterials[1,2].Highsulphatelevels inwaterandwastewatersarerelatedtotheoccurrenceofpiping andequipmentcorrosion,and,whensucheffluentsaredischarged intotheenvironment,theymayincreasetheacidityofbothsoils andbodiesofwater[1,3].
Amongwastewaterscontaininghighsulphateconcentrations,
acidminedrainage(AMD)isoneofthemostimportant.AMDis
generatedwhensulphidemineralsareoxidizedinthepresenceof
∗Correspondingauthor.Tel.:+553135591102;fax:+553135591561.
E-mailaddress:guimaraes.damaris@yahoo.com.br(D.Guimarães).
waterandoxygen,eitherwithorwithouttheaidofbacteria.This acidicsolutionactsasaleachingagentfordifferentminerals, pro-ducingadrainagerichindissolvedmetals,whichcanrendernatural waters unsafefor use,even after miningactivitieshave ceased
[4]. Highsulphate concentrations indrinkingwater cause taste alteration and, when the sulphate content exceeds 600mgL−1, diarrhoea[2].Becauseoftheseadverseeffectstohumanhealthand theenvironment,miningcountriesusuallysetlimitsforsulphate concentrationsinwatersandwastewatersrangingfrom250mgL−1 to500mgL−1[2,5].
Sulphate emissions can be controlled by combinations of
differenttechniquessuchaslimeneutralizationandgypsum pre-cipitation, reverse osmosis, electrodialysis and adsorption [1]. Althoughtheapplicationofadsorptionisoftenlimitedtothe pol-ishingstepofwastewatertreatment,itisapromisingmethoddue toitsabilitytoreducesulphateionconcentrationstoverylowlevels
http://dx.doi.org/10.1016/j.jhazmat.2014.07.071
[6].Moreover,itsrelativecostcanbereducedbyselectinga suit-ableresinandworkingunderconditionsthatmaximizeadsorption andalsofacilitateregeneration.Sulphateremovalbyshrimp peel-ings[7],modifiedcellulosecontainedinbothsugarcanebagasse
[8]andricestraw[9],coconutpith[10],limestone[11]and mod-ifiedzeolites[12]havebeenproposed.Fengetal.[13]deviseda processforAMDtreatmentcomprisedofmetalprecipitationand sulphatesorptiononionexchangeresins.Nevertheless,a funda-mentalassessmentofsulphatesorptionmechanismsisyettobe carriedout.Withinsucha context,thepresentstudysoughtto investigatetheutilizationofAmberlystA21,aweakbaseresin,for sulphatesorptionbecausethismaterialconferstheadvantageof easyelutionviapHshift.
2. Experimental
2.1. AmberlystA21resin
AmberlystA21isa weakbase(tertiaryamine)macroporous
polystyreneresin withanexchangecapacity of1.25eqL−1 [14]. Priortotheexperiments,theresinwassievedusingTylersieves,
and a particle size range between 0.71mm and 0.84mm was
selectedforallexperiments.Sampleswerekeptunderwaterfor atleast40hbeforeuseintheexperiments.
2.2. Batchsorptionandelution
TheinfluenceofbothpH(2,4,6,8and10)andinitialanion concentrationonsulphateuptakewasstudiedbyplacing1mLof hydratedresin incontactwith100mLofsodiumsulphate solu-tionswithSO42−concentrationsrangingfrom100to1200mgL−1. Theexperimentswereperformedover5h,duringwhichthe sys-temwasstirredat180min−1 whiletemperature(34◦C,50◦Cor
70◦C)andsolutionpHwereconstant.Attheendofthe
experi-ments,thesampleswerefiltered,andthesulphateconcentration intheaqueousphasewasdeterminedbyICP–OES(Inductively Cou-pledPlasma–OpticalEmissionSpectroscopy).Sulphateloadingsin theresinsweredeterminedbymassbalance.
Thekineticofthesorptionwasalsostudied.Inthisstep,5mL
of hydrated resin were mixed with 1L of solution containing
160mg(SO42−)L−1 at28±1◦CandpH4,whichwerekept
con-stantthroughouttheexperiment.Thesystemwassampledevery
5mininthefirsthour,every10mininthesecondhour,andat140, 160,180,210,240and360min.Sorptiondatawerethenfittedto pseudo-firstorder,pseudo-secondorderandintraparticlediffusion models[15].
Thepseudo-firstordermodelassumesthatthesorptionprocess isreversibleandreachesequilibrium.Thismodelisrepresentedby Eq.(1),wherek1 istheoverallrateconstantforthepseudo-first ordermodel,tistimeandUtthefractionalattainmentof equilib-rium.UtisobtainedbyEq.(2).Suchamodelcanonlybeapplied todatafromtothelowerpartoftheresinloadingcurve;i.e.,the modeldoesnotapplytoequilibriumdata[15].
ln (1−Ut)=−k1t (1)
Ut= q
qeq
= Co−C
Co−Ceq (2)
Thepseudo-secondordermodel(Eq.(3))wasproposedbyHo andMcKay[18]todescribesorptionprocessesinwhichthe rate-determiningstepisthesorptionreaction.In thisequation,k2 is therateconstantofthepseudo-secondordermodel,andqt and
q∞arethesulphateloadingachievedattimetandatequilibrium,
respectively.
qt= tk2(q∞) 2
1+tk2q∞
(3)
TheintraparticlediffusionmodelproposedbyWeberand Mor-ris[19]isvalidifadsorbatediffusionintheliquidfilmistheonly controllingstepoftheprocess.ThismodelisdescribedbyEq.(4), inwhichkipistherateconstantofintraparticlediffusion,andCis aconstantrelatedtothethicknessoftheboundarylayer.
q=kip(t)1/2+C (4)
Sorption equilibrium was investigated using adsorption
isothermsproduced atdifferent temperatures,which enabled a
thermodynamicanalysisofsulphatesorption.Theisothermswere producedbymixing1mLofhydratedresinwith100mLofsodium sulphate solutions whose initialsulphate concentrations varied between30mgL−1 and1800mgL−1.Eachsystemwasstirredat 180min−1,pH4andatemperatureof34◦C,50◦Cor70◦Cuntil
equilibrium wasreached.The aqueousphase wassubsequently
separated from the solid phase, and the residual aqueous
sul-phateconcentrationwasdeterminedbyICP-OES.Resinloadingwas determinedbymassbalance.Equilibriumdatawerethenfittedto theFreundlichandLangmuirmodels[14]priortothermodynamic analysis.
TheLangmuirmodel(Eq.(5))wasoriginallydevelopedto rep-resentmonolayeradsorptiononidealsurfaces.Itassumesthatthe heatofadsorptionisindependentofsurfacecoverageandissimilar tothatobservedinchemicalreactions.Furtherassumptionsare:(i) thereisalimitedandmeasurableareaforadsorption;(ii)the mono-layerisonemoleculethick;and(iii)adsorptionisreversible,and equilibriumconditionsareattained[6].Conversely,theFreundlich isotherm(Eq.(6))isanempiricalequationthatdescribesnon-ideal adsorptionprocesses.Itassumesheterogeneoussurfacesand mul-tilayeradsorption[20],resultinginanexponentialdistributionof theheatsofadsorption[6].
qeq=qmaxbCeq
1+bCeq (5)
qeq=Kf
Ceq 1/n(6)
InEqs.(5)and(6),qeqistheresinloadingatequilibrium,Ceqis theequilibriumconcentrationoftheadsorbateinsolution,qmaxis themaximumloading,bisaconstantrelatedtotheaffinitybetween theresinandtheadsorbate,Kfisthecapacityfactor,andnisthe intensityparameter[6].
Athermodynamicanalysisuseddataproducedfortheinitial sulphateconcentrationof300mgL−1wascarriedouttoassessthe influenceoftemperatureonthesorptionprocess.Equilibrium con-stant(Keq)valuescorrespondingtothesorptionprocessdeveloped atdifferenttemperaturesweredeterminedutilizingEq.(7):
Keq= qeq
Ceq (7)
Theequilibriumconstant, aswrittenin Eq.(7),is alsocalled thedistributioncoefficient(KD)[24–26].AfterobtainingKeq,the enthalpy(H)andentropy(S)ofsulphatesorptionwere deter-minedfromthevan’tHoffequation(Eq.(8)),andtheGibbsfree energywascalculatedbyEq.(9).
lnKeq=−H RT +
S
R (8)
G=−RTlnKeq (9)
In Eqs. (8) and (9), R is the universal gas constant
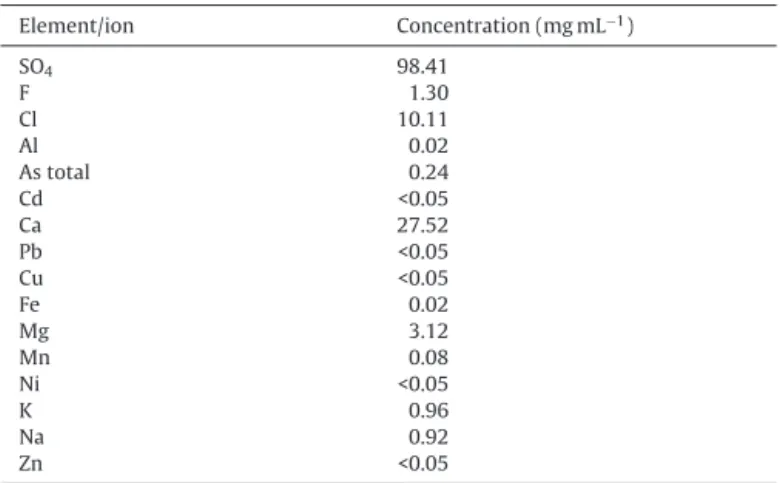
Table1
Compositionoftheindustrialeffluentusedexperimentally.
Element/ion Concentration(mgmL−1)
SO4 98.41
F 1.30
Cl 10.11
Al 0.02
Astotal 0.24
Cd <0.05
Ca 27.52
Pb <0.05
Cu <0.05
Fe 0.02
Mg 3.12
Mn 0.08
Ni <0.05
K 0.96
Na 0.92
Zn <0.05
SulphateelutionfromAmberlystA21wasassessedbymixing 100mLofsodiumhydroxidesolutions(atpH10andpH12)with 1mLofpre-loadedresin(10.4mg(SO42−)mL(resin)−1).Thesystem waskeptunderagitationfor24hat30◦C,andthefinalsulphate
concentrationintheaqueousphasewasdeterminedbyICP–OES priortocalculatingresinelutionefficiencies
2.3. Fixed-bedexperiments
Sulphateadsorptioninfixed-bedcolumnswereperformedin ordertoinvestigatetheeffectsofconcentration,bedheightand flowrateonbedloading.Differentvolumesofresin(0.71–0.84mm particlesizes)weretransferredtoaglasscolumn(13mm diam-eter×142mmheight)toproducedifferentbedlengths,Z(6cm, 9cmand12cm).Afterloading,distilledwaterwaspassedthrough thecolumn(60min)toremovefineparticlesthatcouldhavebeen loadedinthecolumn.Thecolumnwasfedupwardsbyperistaltic pumpssothatanypreferentialpathwayforthesolutionwouldbe avoided.Theflowrate(Q)wasvariedbetween10and20mLmin−1. Sampleswerecollectedregularlyfromthecolumneffluent.The sul-phateconcentrationsinthesesampleswereanalysedbyICP–OES. Theinlet sulphate concentration(C0)rangedfrom55mgL−1 to 160mgL−1,andtheanionloadingontheresinwasdeterminedby massbalance.TheexperimentswerecarriedoutatapHof4.0and atemperatureof28±1◦C.
ToassesstheefficiencyofAmberlystA21inthetreatmentofa realeffluent(fromtheminingindustry),adsorptionexperiments wereperformedina fixed-bedcolumn.The compositionofthe acidiceffluent(pH3.2)ispresentedinTable1andtheexperiments werecarriedoutat25±1◦C,usingabedheightof9cm,whichwas
fedataflowrateof10mLmin−1.Immediatelyafterloadingthebed, itwaselutedfor3hwithapH12sodiumhydroxidesolutionata flowrateof10mLmin−1.Thesampleswerecollectedinintervals of10minandthechlorideandfluoridecontentweredetermined
byionchromatography(Metrohm).
3. Resultsanddiscussion
3.1. Batchexperiments
AscanbeseeninFig.1,sulphateloadingsinAmberlyst A21 weresignificantlyreducedasthesolutionaciditydecreased.This outcomewasexplainedbythepresenceoftertiaryaminegroups, whichmustbepositivelychargeinordertobindsulphate(Eq.(10)). Therefore,theconcentrationofprotonatedaminegroupsincreased with increasing acidity, resulting in greater sulphate loading (Eq.(11)).Fig.1 also indicatesthat increasingthe initialanion
2 4 6 8 10
5 10 15 20 25 30 35
(a)
Su
lpha
te
c
on
c.
in
th
e
re
si
n
(m
g
m
L
-1
)
pH
Initial Sulpha
te con
c.
100 mg L
-1300 mg L
-1700 mg L
-11200 mg L
-12 4 6 8 10
0 5 10 15 20 25 30 35
Su
lpha
te
c
on
c.
in
th
e
re
si
n
(m
g
m
L
-1
)
(b)
pH
Initial Sulpha
te con
c.
100 mg L
-1300
mg L
-1700
mg L
-12 4 6 8 10
0 5 10 15 20 25 40 42 44
Su
lpha
te
c
on
c.
in
th
e
re
si
n
(m
g
m
L
-1
)
(c)
pH
Initial Sulpha
te con
c.
100
mg L
-1300
mg L
-1700
mg L
-1Fig.1.EffectofpHonsulphatesorptionbyAmberlystA21resinat34◦C(a),50◦C(b)
and70◦C(c).Experimentalconditions:1mLofhydratedresin,100mLofsolution
andstirringrateof180min−1.
concentrationresultedinlargersulphate loadingsonAmberlyst A21.Conversely,sulphateloadingswereslightlyreducedathigher temperatures;thiswillbefurtherdiscussedduringtheanalysisof theeffectoftemperatureonsorptionequilibrium.
R–NH3(resin)+H2O⇆RNH4+(resin)+OH−(aq) (10)
0 50 100 150 200 250 300 0
3 6 9 12 15
k = 1.67
x 10
-4min
-1q
eq= 13
.9 mg mL(res
in)
-1R
2= 0.96
Time (min)
q
t(m
g (S
O
4 2-) m
L(r
es
in
)
-1
)
Fig.2. Loadingsulphate(qt)infunctionoftimeandfitofpseudo-secondorder
modelfromadsorptionessaysbyAmberlystA21.Experimentalconditions:5mLof
hydratedresin,1Lofsodiumsulphatesolution(150mg(SO42−)L−1),at28±1◦C,pH
4,stirringrateof200min−1.
ThepH4 wasselected for furthersulphatesorption studies
becausethis pHis encounteredinmanyAMDsites.Workingat
thispH,Fig.2 depictstheprofileof thesulphateconcentration intheresinasafunctionoftimeinabatchstudy.Thesorption process,whichwascomprisedoftwodifferentstages,resin pro-tonation(Eq.(10))andsulphatesorption(Eq.(11)),wasrelatively fast,attaininganequilibriumconcentrationof11.6mgmL(resin)−1 innearly45min.
The kinetic data were fitted to pseudo-first order,
pseudo-second order and intraparticle diffusion models. Among these
models, only the pseudo-second order model showed a good
fit to the experimental data and described all thedata gener-atedinthekineticexperiments.Thus,itcanbeinferredthatthis
model describes the adsorption of sulphate by Amberlyst A21
resin.AsshowninFig.2,thepseudo-secondorderrateconstant was1.67×10−4s−1andthetheoreticalequilibriumsulphate load-ing(q∞)was13.9mgmL(resin)−1,which isconsistentwiththe
experimentalvalue(11.6mgmL(resin)−1).Instudiesofsulphate adsorptionbyanothertypeofadsorbent,Oliveira[12]investigated theadsorptionof sulphatein zeolites functionalizedbybarium salts.Inthiscase,theprocesswasdescribedbythepseudo first-ordermodelwithanoverallrateconstantof0.4s−1.
Subsequently, sorption equilibrium was investigated utiliz-ingisotherms produced at differenttemperatures and fittedto both Langmuir(Eq.(5))and Freundlich (Eq.(6))models.Fig. 3
presentsthe fittingsof the experimentaldata to theLangmuir
0 200 400 600 800 1000 1200 1400
0 5 10 15 20
Aqueou
s sulpha
te con
c.
(mg L
-1)
Su
lpha
te
c
on
cen
tr
a
tion
(m
g
m
L
(r
es
in)
-1
)
34o
C
50o
C
70o C
Fig.3.AdsorptionisothermsfittedtoLangmuirmodel,constructed withdata
obtainedfromessayscarriedoutindifferentconditionsoftemperature,pH4and
stirringrateof180min−1,withtheAmberlystA21resin.
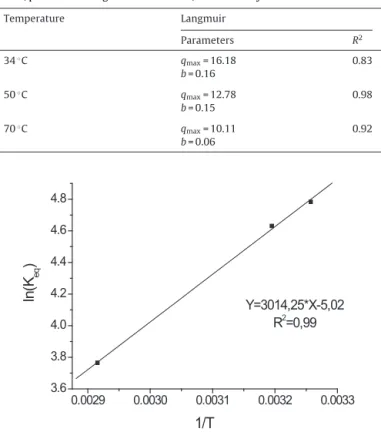
Table2
AdsorptionisothermsparametersofLangmuirmodelobtainedatdifferent
temper-atures,pH4andstirringrateof180min−1,withAmberlystA21resin.
Temperature Langmuir
Parameters R2
34◦C q
max=16.18 0.83
b=0.16
50◦C
qmax=12.78 0.98
b=0.15
70◦
C qmax=10.11 0.92
b=0.06
0.0029 0.0030 0.0031 0.0032 0.0033
3.6 3.8 4.0 4.2 4.4 4.6 4.8
Y=3014
,25
*X-5,02
R
2=0,99
1/T
ln
(K
eq
)
Fig.4.van’tHoffplotforsulphatesorptiononAmberlystA21.
equationat differenttemperatures(34◦C, 50◦C and 70◦C); the
Langmuirisothermcanbeutilized todescribesulphate adsorp-tionbyAmberlystA21,withqmaxvaluescloseto15mgmL(resin)−1 (Table2).Theobservedbconstantvalues,alsolistedinTable2, gen-erallydecreasedwithincreasingtemperature.Asthisconstantis relatedtotheaffinitybetweensulphateandresin,itcanbeinferred thattheaffinitydecreasesastemperatureincreases[6].Sorption datawerealsofittedtotheFreundlichmodel,however,agood fit-tingwasnotobservedinthiscase. Basedontheanalysisofthe
R2 values,itcanbestatedthatsulphateadsorptioninAmberlyst A21isdescribedbytheLangmuirmodel.Thisway,thesurfaceof theloadedresiniscomposedofamonolayerofsulphateanions. SimilarresultswerealsoobservedbyHaghshenoetal.[21]forthe
ionexchangeresinLewaitK6362,byNamasivayamandSangeetha
[10]usingZnCl2activatedcarbonproducedfromcoconutshell,and byOliveira[12]instudieswithzeolites.Intheseworks,the maxi-mumsulphateloadingsobservedwere55.6mgg−1,4.9mgg−1and 53.8mgg−1,respectively.
Aspredictedbyconstant bin theLangmuirmodel,thevan’t Hoffequation(Eq.(8))[27]confirmedthatsulphatesorptionby AmberlystA21is anexothermicprocess (H=−25.06kJmol−1) related to a decrease in entropy (S=−0.042kJmol−1K−1) of
the system (Fig. 4, Table 3). A study of sulphate sorption
on activated carbon revealed it to be an endothermic process
Table3
Changesofenthalpy,H,Gibbsfreeenergy,Gandentropy,S,relatedtosulphate
adsorptionprocessbyAmberlystA21resin,atpH4andstirringrateof180min−1.
Temperatura(◦C) H(kJmol−1) S(kJmol−1K−1) G(kJmol−1)
34 −12.21
50 −25.06 −0.042 −10.25
0 50 100 150 200 250 0.0
0.2 0.4 0.6 0.8 1.0
Time (min)
C
t/C
oC
o= 55
mg(SO
42-) L
-1C
o=80
mg(SO
42-) L
-1C
o=160
mg(SO
42-) L
-1(a)
0 50 100 150 200 250
0.0 0.2 0.4 0.6 0.8 1.0
C
t/C
oTime (min)
H = 6 cm
H = 9 cm
H = 12
cm
(b)
0 50 100 150 200 250
0.0 0.2 0.4 0.6 0.8 1.0
C
t/C
oQ = 10
mL min
-1Q = 15
mL min
-1Q = 20
mL min
-1Time (min)
(c)
Fig.5.BreakthroughcurvesforsulphatesorptionbyAmberlystA21resin,at
differ-entinletconcentrations(C0)(a),bedheight(H)(b)andflowrate(Q)(c).Experimental
conditions:pH4,28±1◦Cand0.35bedporosity.Fixedconditions:inletsulphate
concentrationof80mgL−1,bedheightof9cmandflowrateof15mLmin−1.
(H=15.4kJmol−1) that occurred with an increase in entropy (S=0.133kJK−1mol−1)[10].
TheHvaluelowerthan40kJmol−1(inabsoluteterms)implied
that physisorption was the main sorption mechanism [6,28],
whereas thenegativeSvalueswere interpretedtoindicate a
reductioninsulphateaccessibilitytotheresincorewithincreasing temperature[27].ItisalsoworthnotingthattheGibbsfreeenergy relatedtosulphatesorptionbyAmberlystA21wasalsomeasured (Eq.(9)),andtheresultsarepresentedinTable3.Such thermody-namicparameters,however,refertotheoverallsorptionprocess, i.e.,theyincludeboththeprotonationofthefunctionalgroupsand sulphateadsorptionsteps.
AnimportantfeaturetobeaddressedinapplyingAmberlystA21 inthetreatmentofsulphate-ladeneffluentsisresinelution[29].As observedinthestudyoftheinfluenceofpHonsulphateadsorption byAmberlystA21,aminefunctionalgroupsarenotprotonatedin
Table4
SulphateelutionofAmberlystA21resin,at30◦
Candstirringrateof180min−1.
Initialsulphateloading
(mg(SO42−)mL(resin)−1)
Dessorption
efficiency(%)
Eluant
10.4 ∼100 SolutionofNaOH
(pH10)
∼100 SolutionofNaOH
(pH12)
alkalineconditions,resultinginlowsulphateloadings.Therefore, elutionwascarriedoutbysettingthepHoftheeluentsolutionto 10and12,whichgaveyieldsapproaching100%(Table4).Similar tothecurrentstudy,Fengetal.[13]observedelutionyieldsfor theionexchangeresinsDuoliteA161,DuoliteA375andAmberlite IRA67between90%and95%whenapplyingCa(OH)2solutions con-taining2%NaOH.Moreover,MoretandRubio[7]observednearly 96%sulphateelutionfromshrimppeelingsfunctionalizedwith ter-tiaryaminegroupsafterincreasingthepHto12.Efficientsulphate desorptionfromcarbonproducedfromcoconutshellandactivated withZnCl2wasalsopromotedinanalkalinemedium(pH11)with 90%efficiency[10].
3.2. Fixed-bedexperiments
Fig.5(a)and (b)presentsthebreakthrough curvesproduced fromsolutionscontainingdifferentsulphate concentrationsand beddepths, respectively,ata flow rateof15mLmin−1.Table5 depictsthemeasuredsorptionparameters.Thecurvesdemonstrate that either increasingthe solution concentration or decreasing thebeddepthresultedinreachingbreakthroughandsaturation pointsmorerapidly;thus,asmallervolumeofsolutionistreated at breakthrough.Theseoutcomes werea resultof thefactthat theadsorption capacity isfinite and wasreachedmore rapidly atlargerinletconcentrationsorlowerbedheights.Irrespectiveof theparameterinvestigated,thesamecapacitiesofapproximately 10mg(SO42−)mL(resin)−1weredetermined.Fig.5(c)andTable5 demonstratethatincreasingtheflowratereducedthetimeneeded toreachthebreakthroughandsaturationpoints.Becauseincreased flowratesimplylesscontacttimebetweentheresinandthe solu-tion,therewasaslightreductioninsorptioncapacitytoavalue around8mgmL(resin)−1,whichisconsistentwiththestudyby Haghshenoetal.[21]ontheresinLewaitK6362.
0 20 40 60 80 100 120 140 160 180 200 0.0
0.5 1.0 1.5 2.0 2.5 3.0 3.5 4.0
C
t/C
oTime (min)
SO
4Cl
F
-Fig.6.Breakthroughcurvesfortheadsorptionofthemajoranionspresentinthe
metallurgicalindustryeffluenttreatedinafixed-bedofAmberlystA21.
Experimen-talconditions:pH3.2,25±1◦C,bedheightof9cm,0.35bedporosityandflowrate
Table5
Experimentaldataobtainedwithfixedbedexperimentscarriedoutat28±1◦C,pH4,porosityof0.35,varyingthesulphateinletconcentration,bedheightandflowrate.
Varied experimental condition
Breakthrough
volume(mL)
Breakthrough
time(min)
Saturation
volume(mL)
Saturationtime
(min)
Bedcapacityof
adsorption
(mg(SO42−))
Maximumloadingofresin
(qmax)
(mg(SO42−)mL(resin)−1)
Sulphateinletconcentration
(mg(SO42−)L−1)
55 600 40 1500 100 93.8 8.52
80 450 30 1200 80 105.7 9.61
160 150 10 750 50 126.7 11.5
Bedheight
(cm)
6 150 10 1200 80 101.6 12.7
9 300 20 1350 90 111.6 10.1
12 450 30 1650 110 138.7 9.6
Flowrate
(mLmin−1)
10 400 40 1100 110 88.5 8.1
15 450 30 1200 80 95.6 8.7
20 400 20 1200 60 96.8 8.8
0 20 40 60 80 100 120 140 160 180
0 100 200 300 400 500 600
Su
lpha
te
c
on
cen
tr
a
tio
n
mg
mL
(r
es
in)
-1
Time (min)
Fig.7.Desorptioncurveofthebedloadedbythemetallurgicaleffluentbyusingof
sodiumhydroxide(pH12).Experimentalconditions:25±1◦C,bedheightof9cm,
0.35bedporosityandflowrateof10mLmin−1.
Fig.6 showsthebreakthroughcurveobtainedinthe experi-mentsperformedwithanindustrialeffluent(Table1).Atlowresin loadings,therewerefreetertiaryaminefunctionalgroupsandthus bothchlorideandsulphateionswereadsorbed.Asthesegroups wereoccupied,acompetitionbetweenchlorideandsulphatewas thenobserved.Becausesulphatehasahigheraffinityfortheresin, chlorideovershootingwasobservedduringtheloadingasindicated byCt/Co ratioslargerthan1,forchloride.Therefore,nochloride loadingontheresinwasobservedattheendoftheexperiment. Thatwasconfirmedbythedesorptionexperimentswhoseresults arepresentedinFig.7andrevealednochlorideintheeluate,i.e. onlysulphateanionsweredetected.Furthermore,theindustrial effluentalsocontainedfluorideionsbutthelatterwasnotadsorbed
atanymoment.
Approximately 150min were necessary to saturate the
resinwhosemaximumloadingwas14.27mg(SO42−)mL(resin)−1 whereasapproximately80minwererequiredduringelution.Resin loadingintheexperimentwiththeindustrialeffluentwashigher
thanthoseobtainedinbatchtests(carriedoutatpH4).Itislikely thatthehigherloadingobservedintheexperimentwithareal efflu-entisduetothepHoftheeffluentwhichwas3.2.AccordingtoFig.1, thelowerthepH,thehighertheloadingcapacityoftheresindue toitsfunctionalgroupsprotonation(Eqs.(1)and(2)).
4. Conclusions
Thepresentstudyshowedtheapplicationoftheionexchange resin Amberlyst A21in sulphate sorption for thefirst time. By
a comprehensive investigation of different parameters related
to its sulphate sorption performance, it was possible to
con-cludethattheprocesshaspositivefeatures thatmakeitagood
candidate for use in sulphate removal applications. In acidic
conditions, the process is fast (k=1.67×10−4s−1), exothermic (H=−25.06kJmol−1)andcanbecarriedoutinbothbatchand fixed-bedcolumnswithnodifferenceinthemaximumadsorption capacity(11.6mg(SO42−)mL(resin)−1).Althoughthisadsorption capacityis lowcompared tostrongbaseresins,100%resin elu-tioniseasilyaccomplishedbyincreasingthepHto10and12with sodiumhydroxidesolutions.Theexperimentswithanindustrial effluentindicatedthatfluorideionsdonotinterfereonthesulphate loading.Conversely,althoughchloridewasalsoloaded,itwaslater desorbedasthebedbecamesaturatedwithsulphate,whichhada higheraffinityfortheresin.
Acknowledgements
FinancialsupportfromthefundingagenciesFINEP,FAPEMIG, CNPq,CAPESandValeisgratefullyappreciated.
References
[1]INAP,Treatmentofsulphateinmineeffluentes,in:InternationalNetworkfor
AcidPrevention,2003,p.129.
[2]WHO,in:GuidelinesforDrinking-WaterQuality,Genebra,2008,p.668.
[3]R.J.Bowell,Sulphateandsaltminerals:theproblemoftreatingminewaste,in: MineEnvironmentalMangazine,2000,pp.11–13.
[5]USEPA,in:SulfateinDrinkingWater,U.S.EnvironmentalProtectionAgency,
Washington,DC,1999.
[6]T.D.Reynolds,P.Richards,UnitOperationsandProcessesinEnvironmental Engineering,2nded.,PWSPublishingCompany,Boston,1995.
[7]A.Moret,J.Rubio,Sulphateandmolybdateionsuptakebychitin-basedshrimp shells,Miner.Eng.16(2003)715–722.
[8]D.R.Mulinari,M.L.C.P.daSilva,Adsorptionofsulphateionsbymodificationof sugarcanebagassecellulose,Carbohydr.Polym.74(2008)617–620. [9]W.Cao,Z.Dang,X.-Q.Zhou,X.-Y.Yi,P.-X.Wu,N.-W.Zhu,G.-N.Lu,Removalof
sulphatefromaqueoussolutionusingmodifiedricestraw:preparation, charac-terizationandadsorptionperformance,Carbohydr.Polym.85(2011)571–577. [10]C.Namasivayam,D.Sangeetha,Applicationofcoconutcoirpithfortheremoval
ofsulfateandotheranionsfromwater,Desalination219(2008)1–13. [11]A.M.Silva,R.M.F.Lima,V.A.Leão,Minewatertreatmentwithlimestonefor
sulfateremoval,J.Hazard.Mater.221–222(2012)45–55.
[12]C.R.Oliveira,Adsorc¸ão–remoc¸ãodesulfatoeisopropilxantatoemzeólita
nat-uralfuncionalizada,MSc,UniversidadeFederaldoRioGrandedoSul,Porto
Alegre,2006,107pp.
[13]D.Feng,C.Aldrich,H.Tan,Treatmentofacidminewaterbyuseofheavymetal precipitationandionexchange,Miner.Eng.13(2000)623–642.
[14]D.Guimarães,TratamentodeEfluentesRicosemSulfatoporAdsorc¸ãoem
ResinadeTroca-Iônica,MasterThesis,UniversidadeFederaldeOuroPreto,
OuroPreto,MG,Brasil,2010,170pp.
[15]H.Qiu,L.Lv,B.C.Pan,Q.J.Zhang,W.M.Zhang,Q.X.Zhang,Criticalreviewin adsorptionkineticmodels,J.ZhejiangUniv.Sci.A10(2009)716–724.
[18]Y.S.Ho,G.McKay,Pseudo-secondordermodelforsorptionprocesses,Process Biochem.34(1999)451–465.
[19]J.Weber,J.C.Morris,Kineticsofadsorptiononcarbonsolution,J.Sanit.Eng.89 (1963)31–59.
[20]M.Chabani,A.Amrane,A.Bensmaili,Kineticmodellingoftheadsorptionof nitratesbyionexchangeresin,Chem.Eng.J.125(2006)111–117.
[21]R.Haghsheno,A.Mohebbi,H.Hashemipour,A.Sarrafi,Studyofkineticand fixedbedoperationofremovalofsulfateanionsfromanindustrialwastewater byananionexchangeresin,J.Hazard.Mater.116(2009)961–966.
[24]S.S.Tahir,N.Rauf,ThermodynamicstudiesofNi(II)adsorptionontobentonite fromaqueoussolution,J.Chem.Thermodyn.35(2003)2003–2009. [25]H.Zheng,L.Han,H.Ma,Y.Zheng,H.Zhang,D.Liu,S.Liang,Adsorption
char-acteristicsofammoniumionbyzeolite13X,J.Hazard.Mater.158(2008) 577–584.
[26]B.Kemer,D.Ozdes,A.Gundogdu,V.N.Bulut,C.Duran,M.Soylak,Removalof fluorideionsfromaqueoussolutionbywastemud,J.Hazard.Mater.168(2009) 888–894.
[27]E.L.Schineider,Adsorc¸ãodecompostosfenólicossobrecarvãoativado,93pp., UniversidadeEstadualdoOestedoParaná,Toledo,2008.
[28]G.Bayramoglu,B.Altintas,M.Y.Arica,Adsorptionkineticsandthermodynamic parametersofcationicdyesfromaqueoussolutionsbyusinganewstrong cation-exchangeresin,Chem.Eng.J.152(2009)339–346.