w w w . r e u m a t o l o g i a . c o m . b r
REVISTA
BRASILEIRA
DE
REUMATOLOGIA
Review
article
Recommendations
for
the
treatment
of
Sjögren’s
syndrome
夽
Valéria
Valim
a,∗,
Virgínia
Fernandes
Moc¸a
Trevisani
b,c,
Sandra
Gofinet
Pasoto
d,
Erica
Vieira
Serrano
e,f,
Sandra
Lúcia
Euzébio
Ribeiro
g,
Tania
Sales
de
Alencar
Fidelix
b,
Verônica
Silva
Vilela
h,
Leandro
Lara
do
Prado
d,
Leandro
Augusto
Tanure
i,
Tatiana
Nayara
Libório-Kimura
j,
Odvaldo
Honor
de
Brito
Filho
k,
Liliana
Aparecida
Pimenta
de
Barros
l,
Samira
Tatiyama
Miyamoto
m,
Silvia
Vanessa
Lourenc¸o
n,
Maria
Carmen
Lopes
Ferreira
Silva
Santos
o,
Luis
Antonio
Vieira
p,
Consuelo
Bueno
Diniz
Adán
p,
Wanderley
Marques
Bernardo
qaHospitalUniversitárioCassianoAntôniodeMoraes(HUCAM),EmpresaBrasileiradeServic¸osHospitalares(EBSERH),
DepartmentofInternalMedicine,HealthSciencesCenter,UniversidadeFederaldoEspíritoSanto(UFES),Vitória,ES,Brazil bDepartmentofInternalMedicine,UniversidadeFederaldeSãoPaulo(Unifesp),SãoPaulo,SP,Brazil
cDisciplineofRheumatology,UniversidadedeSantoAmaro(Unisa),SãoPaulo,SP,Brazil
dHospitaldasClínicas,MedicineSchool,DepartmentofInternalMedicine,DivisionofRheumatology,UniversidadedeSãoPaulo(USP),
SãoPaulo,SP,Brazil
eHospitalUniversitárioCassianoAntôniodeMoraes(HUCAM),EmpresaBrasileiradeServic¸osHospitalares(EBSERH),Universidade
FederaldoEspíritoSanto(UFES),Vitória,ES,Brazil
fEscolaSuperiordeCiênciasdaSantaCasadeMisericórdiadeVitória(EMESCAM),Vitória,ES,Brazil
gDepartmentofInternalMedicine/Rheumatology,UniversidadeFederaldoAmazonas(UFAM),Manaus,AM,Brazil hDisciplineofRheumatology,UniversidadedoEstadodoRiodeJaneiro(UERJ),RiodeJaneiro,RJ,Brazil
iDepartmentofLocomotorSystem,HospitaldasClínicas,UniversidadeFederaldeMinasGerais(UFMG),BeloHorizonte,MG,Brazil jDepartmentofPathologyandForensicMedicine,MedicineSchool,UniversidadeFederaldoAmazonas(UFAM),Manaus,AM,Brazil kDentistandPeriodontist,Brazil
lDepartmentofDentalClinic,HealthSciencesCenter,UniversidadeFederaldoEspíritoSanto(UFES),Vitória,ES,Brazil mDepartmentofHealthCareEducation,UniversidadeFederaldoEspíritoSanto(UFES),Vitória,ES,Brazil
nDisciplineofGeneralPathology,DentalSchool,UniversidadedeSãoPaulo(USP),SãoPaulo,SP,Brazil
oDepartmentofPathology,HealthSciencesCenter,UniversidadeFederaldoEspíritoSanto(UFES),Vitória,ES,Brazil pDepartmentofOphthalmology,UniversidadeFederaldeSãoPaulo(Unifesp),SãoPaulo,SP,Brazil
qProjectGuidelines,Associac¸ãoMédicaBrasileira(AMB),SãoPaulo,SP,Brazil
夽
StudyconductedatSjögren’sSyndromeCommitteeoftheBrazilianSocietyofRheumatology.
∗ Correspondingauthor.
E-mail:val.valim@gmail.com(V.Valim).
http://dx.doi.org/10.1016/j.rbre.2015.08.002
a
r
t
i
c
l
e
i
n
f
o
Articlehistory:
Received29March2015
Accepted23July2015
Availableonline8September2015
Keywords:
Sjögren’ssyndrome
Treatment Recommendations Guidelines
Systematicreview
a
b
s
t
r
a
c
t
TherecommendationsproposedbytheSjögren’sSyndromeCommitteeoftheBrazilian
Soci-etyofRheumatologyforthetreatmentofSjögren’ssyndromewerebasedonasystematic
reviewofliteratureinMedline(PubMed)andtheCochranedatabasesuntilOctober2014and
onexpertopinionintheabsenceofstudiesonthesubject.131articlesclassifiedaccording
toOxford&Gradewereincluded.Theserecommendationsweredevelopedinordertoguide
themanagementandfacilitatetheaccesstotreatmentforthosepatientswithan
appropri-ateindication,consideringtheBraziliansocioeconomiccontextandpharmacologicalagents
availableinthiscountry.
©2015ElsevierEditoraLtda.Allrightsreserved.
Recomendac¸ões
para
o
tratamento
da
síndrome
de
Sjögren
Palavras-chave:
SíndromedeSjögren
Tratamento Recomendac¸ões Diretrizes
Revisãosistemática
r
e
s
u
m
Asrecomendac¸õespropostaspelaComissãodeSíndromedeSjögrendaSociedadeBrasileira
deReumatologiaparatratamentodasíndromedeSjögrenforambaseadasemumarevisão
sistemáticadaliteraturanasbasesdedadosMedline(PubMed)eCochraneatéoutubrode
2014eopiniãodeespecialistasnaausênciadeartigossobreoassunto.Foramincluídos
131artigosclassificadosdeacordocomOxford&Grade.Essasrecomendac¸õesforam
elab-oradascomoobjetivodeorientaromanejoadequadoefacilitaroacessoaostratamentos
paraaquelespacientescomadequadaindicac¸ãoderecebê-los,considerandoocontexto
socioeconômicobrasileiroeosmedicamentosdisponíveisnopaís.
©2015ElsevierEditoraLtda.Todososdireitosreservados.
Introduction
Sjögren’ssyndrome(SS)isarelativelycommonautoimmune
rheumaticdisease,whichismostcommoninwomeninthe
fifthdecadeoflife.1AccordingtoaBrazilianpopulationstudy,
theprevalenceofprimarySSis0.17%.2SScanoccurin
associ-ationwithotherautoimmunediseasessuchassystemiclupus
erythematosus(SLE)andrheumatoidarthritis(RA)invariable
rates,upto22.2%inpatientswithRA.3,4
SSisaslowlyprogressivechronicdisease,characterizedby
alymphocyticinfiltratethataffectstheepitheliumofexocrine
(mainlysalivaryandtear)glands,leadingtoadecreased
pro-ductionoftearsandsaliva.Thisisasystemicdisease with
highriskoftransformationtolymphomathatprimarilyaffects
the joints,lungs, central nervous system (CNS), peripheral
nervoussystem(PNS)andkidneysinapproximately50%of
patients.5
Morerecentstudieshaveshownthattherearesubgroups
ofpatientswithdifferentclinicalmanifestations,histological
patterns (presenceofgerminative centers),cytokine profile
and prognosis.6,7 In the nearfuture, bettergenetic8–10 and
phenotypic characterization will be able to determine
dif-ferent treatment patterns. But nowadays it is possible to
definetreatmentstrategiesbasedonsymptoms(symptomatic
treatment),andonthetypeandseverityofsystemic
mani-festations.TheseguidelinesrecommendtheuseofEULAR
Sjö-gren’sSyndromeDiseaseActivity (ESSDAI),atoolvalidated
bothinternationally11andinBrazil,12ascriteriafordisease
activityandresponsetotreatment.
Theserecommendationsweredevelopedinordertoguide
the managementand facilitatethe accesstotreatmentfor
those patients with an appropriateindication, considering
the Brazilian socioeconomic context and pharmacological
agents available in the country. Due to the length of this
review,specifictopicssuchasmanagementduringpregnancy
and treatment of lymphoma associated with SS were not
addressed.Therecommendationswerebasedonstudieson
primarySjögren’ssyndrome.Consideringthelackofstudies
inpatientswithassociationwithotherautoimmunediseases,
theserecommendationscanbeextrapolatedtosecondary
Sjö-gren’ssyndrome.
Materials
and
methods
Articles were reviewed in MEDLINE (PubMed) and the
CochranedatabasesuntilDecember2013.Themanualupdate
was held in October 2014. The search strategy was based
on structured questions according to PICO (i.e. “Patient”,
“Intervention”, “Controls” and “Outcome”) format. These
descriptorswereusedforcross-searchinginaccordancewith
thethemeproposedineachofPICOquestions.ForallPICOs,
thefilter“random”wasused,limitingthesearchtocontrolled
studies.StudiespublishedfromJanuarytoOctober2014and
somecaseseriesonextraglandularmanifestationswere
man-uallyincluded.Afteranalyzingtitlesandabstracts,131articles
relatedtoquestionsthatgeneratedtheevidencethatprovided
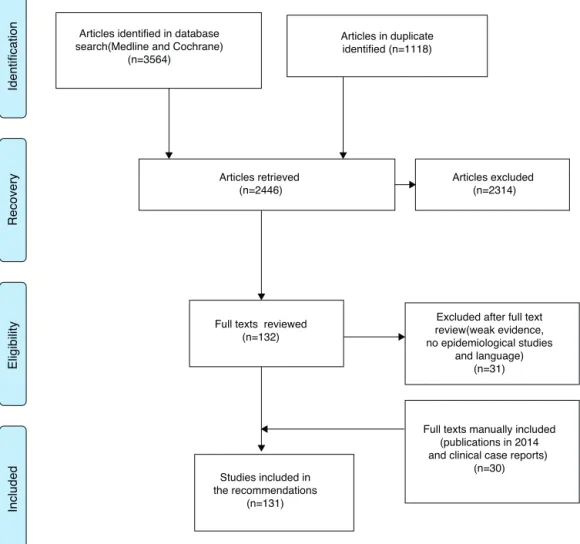
Articles identified in database search(Medline and Cochrane)
(n=3564)
Articles in duplicate identified (n=1118)
Articles retrieved (n=2446)
Articles excluded (n=2314)
Excluded after full text review(weak evidence, no epidemiological studies
and language) (n=31)
Studies included in the recommendations
(n=131)
Full texts manually included (publications in 2014 and clinical case reports)
(n=30) Full texts reviewed
(n=132)
Identification
Recovery
Eligibility
Included
Fig.1–FlowdiagramoftheselectionofevidenceforSjögren’ssyndrometreatment.
were classifiedaccording toOxford& GRADE indegreesof
recommendationandstrengthofevidenceasfollows:
A:Experimentalor observationalstudies ofbetter
consis-tency.
B:Experimental or observational studies oflower
consis-tency.
C:Casereports(non-controlledstudies).
D:Opiniondevoidofacriticalevaluation,basedon
consen-sus,physiologicalstudies,oranimalmodels.
Somerecommendationswerebasedsolelyontheopinion
ofexpertsfrom the Sjögren’sSyndromeScientific
Commit-tee of the Brazilian Society of Rheumatology (BSR), in the
absenceofstudiesonsubjects.Theserecommendationswere
alsogradedasaDlevelandarenotprecededbythereference.
PICOswerestructuredbyamultidisciplinaryteamthatwas
comprisedofninerheumatologists,membersoftheSjögren’s
SyndromeScientificCommitteeofBSR,witheight
profession-alsfromdifferentareas(dentists,ophthalmologists,
patholo-gistsandphysiotherapists);allweremembersoftheExpanded
StudyGrouponSjögren’sSyndrome(GrupoAmpliadodeEstudos
emSíndromedeSjögren–GAESS–Brazil).Therecommendations
wereformulatedmainlybasedonevidenceandreviewedby
allparticipantsattwomeetingsandseveralroundsof
commu-nicationviatheInternet,fromApril2013untilOctober2014.
Recommendations
Basedonthose 14PICOs usedinthissurvey,weorganized
didactically16questionswith44recommendations,divided
intothreemajortopicsandsummarizedinFigs.2–5:
Part1.Generalandpatienteducationrecommendations.
Part2.Symptomaticmanagementofdryness.
Part3.Systemictreatmentforglandularandsystemic
man-ifestations.
Part1.Generalandpatienteducationrecommendations
Q1.Whatarethegeneralrecommendationsbasedonexpert
opinion?
1. ThemanagementofSSmustbeperformedbya
multidisci-plinaryteamincludingatleastarheumatologist,adentist
andanophthalmologist.Therheumatologististhe
Eye
Patient education and preventive measures (D)
Lubricant eyedrops (A)
Patient education and preventive measures (D)
Saliva substitutes (C) Gustatory stimulants (D)
Patient education and preventive measures N-acetylcysteine (A)
Muscarinic agonists (A)
Punctal occlusion (A) Muscarinic agonists (A)
Mild
Severe
Moderate
Mouth Other locations
Topical Cyclosporin (A) Omega 3 (A)
Fig.2–FlowchartforthesymptomatictreatmentofdrynessofSS.
2. Patients with early diagnosis and improved glandular
reserveshowabetterresponsetotreatment(D).
3. Itisrecommendedthattreatmentstrategiesofsystemic
manifestationsarestratifiedaccordingtodiseaseseverity,
basednotonlyonclinicalimpressionofthespecialist,but
alsowiththeuseofESSDAI.Systemictreatmentshouldbe
institutedinthefaceofamoderateESSDAI(≥5).Aresponse
totreatmentwasconsideredwhereadecreaseofESSDAI
≥3pointswasdetected(D).
4. PatientswithSSshouldavoidtheuseofcaffeinateddrinks,
alcoholandtobacco(D).
5. It isrecommended that patients receive general
educa-tionforhygieneandmeasurestopreventdehydrationand
irritationofmucousmembranes(Table1)(D).
Q2.ExerciseiseffectiveintreatingpatientswithSS?
6. WomenwithSShave alowerphysicalability,13 and the
practice of aerobic exercise at moderate to high
inten-sityleadstotheimprovementofaerobiccapacity,fatigue,
perceivedexertionanddepression.14(B)
Q3.Whatare theeffectivenessandsafety ofvaccinesin
patientswithSS?
7. Itisrecommendedthatgeneralguidelinesforvaccination
inpatientswithautoimmunediseasesarefollowed15(C),
andthevaccinationstatusofthepatientmustbechecked
atthepatientbaselineassessment.Itisalsorecommended
Fatigue Myositis
GC (D)
Arthritis Noninflammatory
arthralgia and myalgia
Patient education (D) Aerobic exercise (B)
Methotrexate (C)
Rituximab (C) Management as for
chronic pain (D)
Third line First line
Second line
Patient education (D) Physiotherapy (D) Corticosteroids or NSAIDs +
hydroxychloroquine (C)
Intravenous MP + CYC (C)
Intravenous MP + CYC (C) GC (C)
IV Immunoglobulin (C)
IV Immunoglobulin (C) GC +
Immunosuppressant (C) Intravenous MP +
CYC (C)
Multiple mononeuritis
CNS
vasculitis MS-like Meningitis
Third line
First line
Second line
Rituximab (C)
Immunosuppressant (C) Sensory PN
sensory neuropathy
Sensory-motor PN
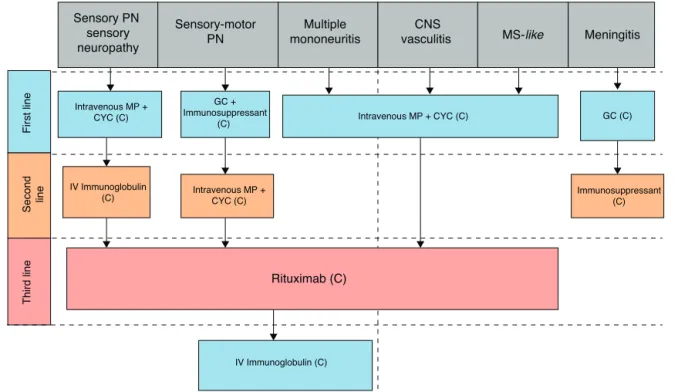
Fig.4–Flowchartforthetreatmentofneurologicalsystemicmanifestations.PN,peripheralpolyneuropathy;CNS,central nervoussystem;MS,multiplesclerosis;GC,glucocorticoids;MP,methylprednisolone;CYC,cyclophosphamide.
toadminister vaccines in stable periodsof the disease;
liveattenuatedvaccinesshouldbeavoided.Immunization
againstinfluenza16,17(A)andPneumococcusareindicated,18
(A) with application of other vaccines according to the
immunizationschedule(D).
Q4.SSpatientsshouldreceivesupplementationwith
vita-minD?
8. VitaminDdeficiencyshouldbeinvestigatedand,if
neces-sary,itssupplementationshouldbeinstituted16–23(C).
Part2.Symptomaticmanagementofdryness
Q5.Whatisthetopicaltreatmentfordrymouth?
9. Saliva substitutes improve comfort24–31 (C) and ideally
shouldcontainfluoride,bactericidesandbuffered
solu-tionsthathelptocombatbiofilm,theformationofcaries
andcandidiasis32(D).
10. Mechanical and/or chemical gustatory stimulating
agents,forinstance,hardcandiesandsugar-freechewing
Interstitial lung disease
Cutaneous
vasculitis vasculitis GN TIN
Bronchiolitis
Oral GC (C)
Immunosuppressant (C)
Intravenous MP + CYC (C)
Intravenous MP + CYC (C) K+ and HCO3-replacement (C)
Oral or intravenous
GC (C) GC + Immunosuppressant (C)
(azathioprine, cyclophosphamide, mycophenolate)
Rituximab (C)
Inhaled corticosteroids
(C)
Third line
First line
Second line
Table1–Patienteducation:measuresforhygieneand
hydrationofmucousmembranes.
SSpatientsshouldbeencouragedtodrinkfluidsfrequently, preferablywater.
SSpatientsshouldbeinformedthattobacco,caffeineandvarious medicationssuchasdiuretics,betablockers,antidepressants andanxiolytics,areknowncausesofdrynessandcanworsen theglandularsymptoms.
Topreventtearevaporation,thepatientmustavoiddry environments,airconditioning,wind,andalsoactivitiesthat decreaseeyeblinking,suchasusingthecomputerorreadingfor alongperiodoftime.
Patientswithsymptomaticdryeyeshoulduseenvironment humidifiers,andglasseswithsideshieldsduringexposureto windorwhenpracticingoutdoorsports.
Preventivemeasuresfororalhealthaimtocontrolbiofilm,caries, lossofteethandoralcandidiasis.Forthispurpose,itis importanttomaintainagoodoralhygiene,keepingintraoralpH (basic)withtheuseofbuffersandrestrictingconsumptionof sugar.
Carefuloralhygieneincludesthesamerecommendationsforthe generalpopulation,withspecialattentiontoprosthesishygiene andchoosinglessabrasiveproducts,avoidingproductsthat increasedrynessofthemucosa,forexample,toothpastes containingsodiumlaurylsulfateandtheuseof
alcohol-containingmouthrinse.
Removabletotaldentalprosthesisusersshoulddailycleanedtheir denturesusingadentalbrushwithstiffbristleswithtoothpaste, switchingtoasoftbristlesbrushfororalmucosacleaning.Once aweek,theprosthesismustbeimmersedinanaqueous solutioncontaining1%sodiumhypochloritesolutionduring 30min;thentheprosthesisshouldberinsedinrunningwater. Onecanobtainthisconcentrationofsodiumhypochloriteby dilutingatablespoonofchlorinebleachinaglasscontaining 300mLoffilteredwater.
gum,32 (D) may behelpful. Solutions or mouthwashes
containingmalicacid,fluorideand xylitolhavesimilar
efficacytotraditionalstimulatingagentscontainingcitric
acid,butwiththe advantageofmaintainingalessacid
pH.33(B)
Q6.Whatisthetopicaltreatmentfordryeye?
11. Thefrequentuse oflubricanteyedropscontaining
glu-cans or carboxymethylcellulose improves comfort and
functional tests34–40 (A). These eyedrops should be
preservative-free,hypotonic,highercolloidalosmolality
products.37,41 (A) Gel formulations are of longer
dura-tionandgenerategreaterrelief,butmaycausetemporary
blurredvision.
12. Topicalcyclosporin0.05%bidfor6–12weeksiseffectivefor
symptomaticandfunctionalimprovementofdryeye42–56
(A).Theoccurrenceofeyeirritationiscommon;thus,this
drugisrecommendedatthelowesteffective
concentra-tion(0.05%)55(A).
13. Topicalglucocorticoidscanbeusedforthemost
symp-tomaticcases57,58(A)foralimitedperiod,becauseofthe
risksubcapsularcataract, glaucomaandinfection34 (D).
Non-steroidal anti-inflammatory drugs (NSAIDs) must
not be routinely used, due to the high risk of corneal
perforation59,60(D).
14. Lacrimalpunctaocclusionimprovessymptomsand
out-comesofocular tear tests.This strategyis superiorto
lubricanteyedrops,andisindicatedinseverecases
refrac-torytotopicaltreatmentwitheyedrops61–65(A).
Q7.What arethe effectivenessand safetyofmuscarinic
agonistsandmucolyticsinsystemicsymptomatictreatment
ofdryness?
15. Muscarinicagonistssuchaspilocarpine(5mgbid,qid)and
cevimeline(30mg,tid),aremorebeneficialinthe
symp-tomatictreatment ofdry mouth66–69 (A), but they may
alsobeuseful in treating moderate tosevere41 (D) dry
eye66,67,69–73(A).
16. Itisrecommendedthatthedoseandtherangeof
pilo-carpinebeadjustedastolerated66,67(A).
17. AlthoughnotavailableinBrazil,cevimelineisthesafest
muscarinicagonist,withlowerratesofsideeffectsand
oftreatmentdiscontinuation,thankstoamoreselective
actiononM3receptors74,75(C).
18. Themostfrequentsideeffectofmuscarinic agonistsis
sweating69,72,74(A).Oneshouldbeawareofthe
contraindi-cations for the use of muscarinic agonists, especially
pilocarpine,inasthmaandcardiacdiseasepatients41(D).
19. ThemucolyticagentN-acetylcysteine,atadoseof200mg
tid,maybeanoptionformuscarinicagonistsinpatients
withintolerance, and also in patients withdryness in
otherplaces,suchastheskin,vaginaandairways76(A).
Q8.Whataretheeffectivenessandsafetyof
supplemen-tationwithfattyacidsinpatientswithSS?
20. Supplementationoffatty acids (omega-3) canbe used,
sincethis is alow-risk intervention and promotes the
improvementofsymptomsandoffunctionaldryeyetests,
althoughtheresultshavebeencontroversialindifferent
studies77–82(A).
Part3.Systemictreatmentforglandularandsystemic
manifestations
Q9.Whataretheeffectivenessandsafetyof
hydroxychloro-quineandimmunosuppressantsinthetreatmentofpatients
withSS?
21. Thereisnoevidenceofsignificantimprovementin
glan-dularsymptomswiththeuseofhydroxychloroquinein
SS. However, there is improvement of laboratory and
inflammatoryparameters83–90(A).
22. Thereisnoevidencefortheuseofsystemic
immunosup-pressantsintreatingsymptomsofdryness.Whilesome
open and controlledstudies have shownimprovement
inlaboratoryand inflammatoryparameters, ahigh
fre-quency of adverse events precluded their use for dry
syndrome91–98(B).
23. Thechoiceofimmunosuppressivedrugtreatmentfor
sys-temicmanifestationswilldependontheaffectedorgan
Q10.Whataretheeffectivenessandsafetyofbiologic
ther-apiesinthetreatmentofpatientswithSS?
24. Anti-TNF therapy isnotindicatedfor thetreatment of
glandularorsystemicmanifestationsofSS99–102(A).
25. Rituximabiseffectiveinimprovingmanymanifestations
inSS,asglandularinvolvement(A),fatigue(A),disease
activity(C),immunologicalparameters(A),glandular
lym-phocyticinfiltration,systemicmanifestations,andquality
oflife103–105
26. Rituximab is not indicated as sole treatment of the
symptomsofdryness.Thisdrugisatherapeuticoption
for systemic manifestations that failed conventional
treatment103–106(C).Byclinicalcriteria,inselectedcases,
rituximabcanbeconsideredforseriousandspecific
glan-dularmanifestations,suchasrefractoryparotiditis62(D).
27. Abatacept106–109 (C)andbelimumab110(C)arepromising
drugstoimprovediseaseactivity,immunologicalprofile
andqualityoflife.Thesedrugscanbeconsideredinthe
treatmentofSSinrefractorycasesandwithhighsystemic
diseaseactivity.
Q11.What isthe treatment of articular manifestations,
myositisandfatigueinpatientswithSS?
28. Initial treatment of arthritis resulting from SS can be
carried out with hydroxychloroquine, with or without
lowdosesofglucocorticoidsorNSAIDsforsymptomatic
relief83 (C). In case oftreatment failure with
hydroxy-chloroquine,thisdrugcanbereplacedorassociatedwith
methotrexate60,96(D).Inthoserarerefractorycasesunder
anoptimalmethotrexatedosage,theuseofrituximabis
recommended106(C).
29. Myositis characterized by weakness, elevated creatine
kinase(CK)andelectroneuromyographicchangesshould
initiallybetreatedwithprednisone.Inthoserare
refrac-torycases,methotrexateisrecommended(D).
30. Noninflammatorypatternarthralgiawithdiffusepainin
the absence ofmyositisshouldbetreated asapainful
amplification syndrome, with analgesia and exercise,
withattentiontothepotentialriskofworseningof
dry-ness,causedbyapharmacologicaladverseeffect(D).
31. The treatment of fatigue includes the prescription
of aerobic exercise of moderate to high intensity
14 (B), and a proper management of the
underly-ing disease. Different classes of drugs have been
tested and have not proved to be effective or safer,
becausetheyhaveunacceptableratesofadverseevents,
such as dehydroepiandrosterone (DHEA)111 (A), fatty
acids82 (A), hydroxychloroquine89,90 (A), azathioprine94
(A), leflunomide97 (A), mycophenolate98 (A) and
anti-TNF agents102 (A). At the discretion of the clinician,
rituximab103–105,112 (A) may be a therapeutic option,
consideringtheevidenceofinflammationandtheimpact
onfunctionalcapacityandqualityoflife.
Q12.Whatisthetreatmentofneurologicalmanifestations
intheperipheralnervoussysteminpatientswithSS?
32. ForthetreatmentofPNSinvolvement,thecombination
ofhigh-doseglucocorticoids(withsubsequenttapering)
and an immunosuppressant (azathioprine,
cyclophos-phamide,mycophenolate)isrecommended60,113,114(C).
33. Patients with mononeuritis multiplex should start
a scheme of intravenous methylprednisolone and
cyclophosphamide113(C).
34. Patients with ataxic polyneuropathy and sensory
ganglioneuronopathy have poorer responses to all
treatments113 (C). Therefore, for these patients, the
association of IVIG to the therapeutic regimen with
glucocorticoids and immunosuppressive drugs is
rec-ommended, in an attempt to achieve a better clinical
response115(C).
35. When no clinical improvement is observed in the
initial treatment, rituximab is recommended116 (C).
Patientswithvasculitisandcryoglobulinemiahavebetter
responsestorituximab116(C).
36. IVIGisatherapeuticoptionforalltypesofPNS
involve-ment,whenpreviousschemesfailed115,117,118(C).
37. Plasmapheresisshouldbereservedforseverecases
refrac-tory to all previous measures, since no studies were
publishedjustifyingitsroutineuse119(C).
Q13.Whatisthetreatmentofneurologicalmanifestations
inthecentralnervoussysteminpatientswithSS?
38. For the treatment of CNS involvement, a combination
ofglucocorticoidsandcyclophosphamideathigh doses
isrecommended113,120–124 (C). In the faceofno clinical
improvement,rituximabisrecommended60,125(C).
39. Subacutefebrileasepticmeningitiscanbetreatedinitially
solely with glucocorticoids, depending on the clinical
condition122(C).
Q14.What isthe treatmentofpulmonary(parenchymal
andlowerairways)manifestationsinpatientswithSS?
40. In the presenceof symptomaticinterstitialpulmonary
disorder,treatmentwithglucocorticoidsplusan
immuno-suppressive agent (azathioprine or cyclophosphamide)
isrecommended126–129 (C).Mycophenolatemofetilisan
option in refractory cases or in those patients with
contraindicationtootherimmunosuppressiveagents130
(C). Rituximab may be considered in refractory cases
ofinterstitialpneumonia,and overtreatmentoffibrotic
changes related to the sequel should be avoided106
(C).
41. Bronchiolitis respiratory tract manifestations may be
mild,justifyingonlytheuseofinhaledand/orsystemic
corticosteroidtherapy131 (C). Immunosuppressivedrugs
shouldbeaccordingtospecialistopinion60(D).
Q15.Whatisthetreatmentofglomerularand
tubulointer-stitialrenalmanifestationsinpatientswithSS?
42. Consideringclinicianopinionhigh-dose glucocorticoids
mightbeindicated,withorwithoutassociationwithother
immunosuppressiveagents132–137(C).
43. InGNs, intravenousmethylprednisolone in association
withcyclophosphamideisrecommended132,133 (C).
moderatecases132(C).Rituximabshouldbeconsideredin
refractorycases106,132,137(C).
Q16.Whataretheeffectivenessandsafetyofdrugtherapy
inthetreatmentofvasculitisinpatientswithSS?
44. Therecommendedtreatmentforvasculitis,regardlessof
the organ involved, is immunosuppression with
high-dose intravenous methylprednisolone for three days,
in combination with immunosuppressants
(azathio-prine, mycophenolate mofetil, or cyclophosphamide);
cyclophosphamide hasbeen the most commonlyused
immunosuppressant138–141 (C). Treatment ofcutaneous
vasculitis maybeinitiallycarried out withan oral
glu-cocorticoid(0.5–1mg/kg/day)orwithintravenous
methyl-prednisolone(dependingontheseverityofthecondition)
plusanimmunosuppressant. Casesrefractorytoinitial
treatmentcanbetreatedwithrituximab142,143(C).
Funding
BrazilianSocietyofRheumatology.
Conflicts
of
interest
Theauthorsdeclarenoconflictsofinterest.
Acknowledgements
Theauthors acknowledgeWalberVieira Pinto (Presidentof
SBR in the biennium 2012-2014) and César Emile Baaklini
(PresidentofSBRinthebiennium2014-2016),forsupporting
thedevelopmentoftheseguidelines.
r
e
f
e
r
e
n
c
e
s
1. Garcia-CarrascoM,Ramos-CasalsM,RosasJ,PallaresL, Calvo-AlenJ,CerveraR,etal.PrimarySjogren’ssyndrome: clinicalandimmunologicdiseasepatternsinacohortof400 patients.Medicine(Baltimore).2002;81:270–80.
2. ValimV,ZandonadeE,PereiraAM,deBritoFilhoOH, SerranoEV,MussoC,etal.PrimarySjogren’ssyndrome prevalenceinamajormetropolitanareainBrazil.RevBras Reumatol.2013;53:24–34.
3. HagaHJ,NaderiY,MorenoAM,PeenE.Astudyofthe prevalenceofsiccasymptomsandsecondarySjogren’s syndromeinpatientswithrheumatoidarthritis,andits associationtodiseaseactivityandtreatmentprofile.IntJ RheumDis.2012;15:284–8.
4. KosrirukvongsP,NgowyutagonP,PusuwanP,KoolvisootA, NilganuwongS.Prevalenceofdryeyesyndromeand Sjogren’ssyndromeinpatientswithrheumatoidarthritis.J MedAssocThailand.2012;95Suppl.4:S61–9.
5. BaldiniC,TavoniA,MerliniG,SebastianiM,BombardieriS. PrimarySjogren’ssyndrome:clinicalandserologicalfeature ofasinglecentre.Reumatismo.2005;57:256–61.
6. SzodorayP,AlexP,JonssonMV,KnowltonN,DozmorovI, NakkenB,etal.DistinctprofilesofSjogren’ssyndrome patientswithectopicsalivaryglandgerminalcenters
revealedbyserumcytokinesandBAFF.ClinImmunol. 2005;117:168–76.
7.RekstenTR,JonssonMV,SzyszkoEA,BrunJG,JonssonR, BrokstadKA.Cytokineandautoantibodyprofilingrelatedto histopathologicalfeaturesinprimarySjogren’ssyndrome. Rheumatology.2009;48:1102–6.
8.RekstenTR,JohnsenSJ,JonssonMV,OmdalR,BrunJG, TheanderE,etal.Geneticassociationstogerminalcentre formationinprimarySjogren’ssyndrome.AnnRheumDis. 2014;73:1253–8.
9.JonssonMV,TheanderE,JonssonR.Predictorsforthe developmentofnon-Hodgkinlymphomainprimary Sjogren’ssyndrome.PresseMed.2012;41Pt2:e511–6. 10.TheanderE,VasaitisL,BaecklundE,NordmarkG,Warfvinge
G,LiedholmR,etal.Lymphoidorganisationinlabialsalivary glandbiopsiesisapossiblepredictorforthedevelopmentof malignantlymphomainprimarySjogren’ssyndrome.Ann RheumDis.2011;70:1363–8.
11.SerorR,BootsmaH,SarauxA,BowmanSJ,TheanderE,Brun JG,etal.Definingdiseaseactivitystatesandclinically meaningfulimprovementinprimarySjogren’ssyndrome withEularSjogren’sSyndromeDiseaseActivityIndex (ESSDAI)andpatient-reportedindexes(ESSPRI).AnnRheum Dis.2014.
12.SerranoEV,ValimV,MiyamotoST,GiovelliRA,PaganottiMA, CadeNV.TransculturaladaptationoftheEularSjogren’s SyndromeDiseaseActivityIndex(ESSDAI)intoBrazilian Portuguese.RevBrasReumatol.2013;53:483–93.
13.StrombeckB,EkdahlC,ManthorpeR,JacobssonLT.Physical capacityinwomenwithprimarySjogren’ssyndrome:a controlledstudy.ArthritisRheum.2003;49:681–8.
14.StrombeckBE,TheanderE,JacobssonLT.Effectsofexercise onaerobiccapacityandfatigueinwomenwithprimary Sjogren’ssyndrome.Rheumatology.2007;46:868–71. 15.vanAssenS,Agmon-LevinN,ElkayamO,CerveraR,Doran
MF,DougadosM,etal.EULARrecommendationsfor vaccinationinadultpatientswithautoimmune inflammatoryrheumaticdiseases.AnnRheumDis. 2011;70:414–22.
16.PasotoSG,RibeiroAC,VianaVS,LeonEP,BuenoC,NetoML, etal.Shortandlong-termeffectsofpandemic
unadjuvantedinfluenzaA(H1N1)pdm09vaccineonclinical manifestationsandautoantibodyprofileinprimary Sjogren’ssyndrome.Vaccine.2013;31:1793–8. 17.MilanovicM,StojanovichL,DjokovicA,KonticM,
GvozdenovicE.Influenzavaccinationinautoimmune rheumaticdiseasepatients.TohokuJExpMed. 2013;229:29–34.
18.KarshJ,PavlidisN,SchiffmanG,MoutsopoulosHM. ImmunizationofpatientswithSjogren’ssyndromewith pneumococcalpolysaccharidevaccine:arandomizedtrial. ArthritisRheum.1980;23:1294–8.
19.MullerK,OxholmP,SorensenOH,ThymannM, Hoier-MadsenM,BendtzenK.AbnormalvitaminD3 metabolisminpatientswithprimarySjogren’ssyndrome. AnnRheumDis.1990;49:682–4.
20.ErtenS,SahinA,AltunogluA,GemciogluE,KocaC. ComparisonofplasmavitaminDlevelsinpatientswith Sjogren’ssyndromeandhealthysubjects.IntJRheumDis. 2014;18:70–5.
21.BaldiniC,DelleSedieA,LucianoN,PepeP,FerroF,Talarico R,etal.VitaminDin“early”primarySjogren’ssyndrome: doesitplayaroleininfluencingdiseasephenotypes? RheumatolInt.2014;34:1159–64.
23.Agmon-LevinN,KivityS,TzioufasAG,LopezHoyosM, RozmanB,EfesI,etal.Lowlevelsofvitamin-Dare
associatedwithneuropathyandlymphomaamongpatients withSjogren’ssyndrome.JAutoimmun.2012;39:234–9. 24.AlikoA,AlushiA,TafajA,IsufiR.Evaluationoftheclinical
efficacyofBioteneOralBalanceinpatientswithsecondary Sjogren’ssyndrome:apilotstudy.RheumatolInt.
2012;32:2877–81.
25.AlpözE,GuneriP,OnderG,CankayaH,KabasakalY,KoseT. TheefficacyofXialineinpatientswithSjogren’ssyndrome: asingle-blind,cross-overstudy.ClinOralInvestig.
2008;12:165–72.
26.FrostPM,ShirlawPJ,ChallacombeSJ,Fernandes-NaglikL, WalterJD,IdeM.Impactofwearinganintra-orallubricating deviceonoralhealthindrymouthpatients.OralDis. 2006;12:57–62.
27.AlvesMB,MottaAC,MessinaWC,MigliariDA.Saliva substituteinxerostomicpatientswithprimarySjogren’s syndrome:asingle-blindtrial.QuintessenceInt. 2004;35:392–6.
28.JohanssonG,AnderssonG,EdwardssonS,BjornAL, ManthorpeR,AttstromR.Effectsofmouthrinseswith linseedextractSalinumwithout/withchlorhexidineonoral conditionsinpatientswithSjogren’ssyndrome.A
double-blindcrossoverinvestigation.Gerodontology. 2001;18:87–94.
29.RhodusNL,BereuterJ.Clinicalevaluationofacommercially availableoralmoisturizerinrelievingsignsandsymptoms ofxerostomiainpostirradiationheadandneckcancer patientsandpatientswithSjogren’ssyndrome.J Otolaryngol.2000;29:28–34.
30.vanderReijdenWA,vanderKwaakH,VissinkA,Veerman EC,AmerongenAV.Treatmentofxerostomiawith polymer-basedsalivasubstitutesinpatientswithSjogren’s syndrome.ArthritisRheum.1996;39:57–63.
31.VischLL,GravenmadeEJ,SchaubRM,VanPuttenWL, VissinkA.Adouble-blindcrossovertrialofCMC-and mucin-containingsalivasubstitutes.IntJOralMaxillofac Surg.1986;15:395–400.
32.FurnessS,WorthingtonHV,BryanG,BirchenoughS, McMillanR.Interventionsforthemanagementofdry mouth:topicaltherapies.CochraneDatabaseSystRev. 2011:CD340089.
33.daSilvaMarquesDN,daMataAD,PattoJM,BarcelosFA,de AlmeidaRatoAmaralJP,deOliveiraMC,etal.Effectsof gustatorystimulantsofsalivarysecretiononsalivarypH andflowinpatientswithSjogren’ssyndrome:arandomized controlledtrial.JOralPatholMed.2011;40:785–92.
34.AragonaP,RaniaL,RoszkowskaAM,SpinellaR,PostorinoE, PuzzoloD,etal.Effectsofaminoacidsenrichedtears substitutesonthecorneaofpatientswithdysfunctional tearsyndrome.ActaOphthalmol(Copenh).2013;91:e437–44. 35.BrignoleF,PisellaPJ,DupasB,BaeyensV,BaudouinC.
Efficacyandsafetyof0.18%sodiumhyaluronateinpatients withmoderatedryeyesyndromeandsuperficialkeratitis. Graefe’sArchClinExpOphthalmol.2005;243:531–8. 36.McDonaldCC,KayeSB,FigueiredoFC,MacintoshG,Lockett
C.Arandomised,crossover,multicentrestudytocompare theperformanceof0.1%(w/v)sodiumhyaluronatewith 1.4%(w/v)polyvinylalcoholinthealleviationofsymptoms associatedwithdryeyesyndrome.Eye.2002;16:601–7. 37.AragonaP,DiStefanoG,FerreriF,SpinellaR,StiloA.Sodium
hyaluronateeyedropsofdifferentosmolarityforthe treatmentofdryeyeinSjogren’ssyndromepatients.BrJ Ophthalmol.2002;86:879–84.
38.AragonaP,PapaV,MicaliA,SantoconoM,MilazzoG.Long termtreatmentwithsodiumhyaluronate-containing
artificialtearsreducesocularsurfacedamageinpatients withdryeye.BrJOphthalmol.2002;86:181–4.
39.CondonPI,McEwenCG,WrightM,MackintoshG,PrescottRJ, McDonaldC.Doubleblind,randomised,placebocontrolled, crossover,multicentrestudytodeterminetheefficacyofa 0.1%(w/v)sodiumhyaluronatesolution(Fermavisc)inthe treatmentofdryeyesyndrome.BrJOphthalmol.
1999;83:1121–4.
40.TodaI,ShinozakiN,TsubotaK.Hydroxypropyl methylcelluloseforthetreatmentofseveredryeye associatedwithSjogren’ssyndrome.Cornea.1996;15:120–8. 41.ValimV,TrevisaniVF,deSousaJM,VilelaVS,BelfortRJr.
Currentapproachtodryeyedisease.ClinRevAllergy Immunol.2014.
42.SchultzC.Safetyandefficacyofcyclosporineinthe treatmentofchronicdryeye.OphthalmolEyeDis. 2014;6:37–42.
43.AlvesM,FonsecaEC,AlvesMF,MalkiLT,ArrudaGV,Reinach PS,etal.Dryeyediseasetreatment:asystematicreviewof publishedtrialsandacriticalappraisaloftherapeutic strategies.OculSurf.2013;11:181–92.
44.ZhouXQ,WeiRL.TopicalcyclosporineAinthetreatmentof dryeye:asystematicreviewandmeta-analysis.Cornea. 2014;33:760–7.
45.SallK,StevensonOD,MundorfTK,ReisBL.Twomulticenter, randomizedstudiesoftheefficacyandsafetyof
cyclosporineophthalmicemulsioninmoderatetosevere dryeyedisease.CsAPhase3StudyGroup.Ophthalmology. 2000;107:631–9.
46.SallKN,CohenSM,ChristensenMT,SteinJM.Anevaluation oftheefficacyofacyclosporine-baseddryeyetherapywhen usedwithmarketedartificialtearsassupportivetherapyin dryeye.EyeContactLens.2006;32:21–6.
47.DeveciH,KobakS.Theefficacyoftopical0.05%cyclosporine AinpatientswithdryeyediseaseassociatedwithSjogren’s syndrome.IntOphthalmol.2014;34:1043–8.
48.DemiryayE,YaylaliV,CetinEN,YildirimC.Effectsoftopical cyclosporineaplusartificialtearsversusartificialtears treatmentonconjunctivalgobletcelldensityin dysfunctionaltearsyndrome.EyeContactLens. 2011;37:312–5.
49.SuMY,PerryHD,BarsamA,PerryAR,DonnenfeldED, WittpennJR,etal.Theeffectofdecreasingthedosageof cyclosporineA0.05%ondryeyediseaseafter1yearof twice-dailytherapy.Cornea.2011;30:1098–104.
50.Baiza-DuranL,Medrano-PalafoxJ,Hernandez-QuintelaE, Lozano-AlcazarJ,Alaniz-delaOJ.Acomparativeclinical trialoftheefficacyoftwodifferentaqueoussolutionsof cyclosporineforthetreatmentofmoderate-to-severedry eyesyndrome.BrJOphthalmol.2010;94:1312–5.
51.KimEC,ChoiJS,JooCK.Acomparisonofvitaminaand cyclosporinea0.05%eyedropsfortreatmentofdryeye syndrome.AmJOphthalmol.2009;147,206–13e3.
52.RobertsCW,CarnigliaPE,BrazzoBG.Comparisonoftopical cyclosporine,punctalocclusion,andacombinationforthe treatmentofdryeye.Cornea.2007;26:805–9.
53.BarberLD,PflugfelderSC,TauberJ,FoulksGN.PhaseIII safetyevaluationofcyclosporine0.1%ophthalmicemulsion administeredtwicedailytodryeyediseasepatientsforup to3years.Ophthalmology.2005;112:1790–4.
54.KunertKS,TisdaleAS,SternME,SmithJA,GipsonIK. Analysisoftopicalcyclosporinetreatmentofpatientswith dryeyesyndrome:effectonconjunctivallymphocytes.Arch Ophthalmol.2000;118:1489–96.
randomizedtrial.TheCyclosporinAPhase2StudyGroup. Ophthalmology.2000;107:967–74.
56.GünduzK,OzdemirO.Topicalcyclosporintreatmentof keratoconjunctivitissiccainsecondarySjogren’ssyndrome. ActaOphthalmol(Copenh).1994;72:438–42.
57.SerraMSDLM,SimonCastellviC,KabbaniO.Nonpreserved topicalsteroidsandlacrimalpunctalocclusionforsevere keratoconjunctivitissicca.ArchSocEspOftalmol. 2000;75:751–6.
58.AragonaP,SpinellaR,RaniaL,PostorinoE,SommarioMS, RoszkowskaAM,etal.Safetyandefficacyof0.1%
clobetasonebutyrateeyedropsinthetreatmentofdryeyein Sjogrensyndrome.EurJOphthalmol.2013;23:368–76. 59.AragonaP,StiloA,FerreriF,MobriciM.Effectsofthetopical
treatmentwithNSAIDsoncornealsensitivityandocular surfaceofSjogren’ssyndromepatients.Eye.2005;19:535–9. 60.Ramos-CasalsM,Brito-ZeronP,Siso-AlmirallA,BoschX,
TzioufasAG.Topicalandsystemicmedicationsforthe treatmentofprimarySjogren’ssyndrome.NatRev Rheumatol.2012;8:399–411.
61.ErvinAM,WojciechowskiR,ScheinO.Punctalocclusionfor dryeyesyndrome.CochraneDatabaseSystRev.
2010:CD750067.
62.FarrellJ,PatelS,GriersonDG,SturrockRD.Aclinical proceduretopredictthevalueoftemporaryocclusion therapyinkeratoconjunctivitissicca.OphthalmicPhysiol Opt.2003;23:1–8.
63.QiuW,LiuZ,AoM,LiX,WangW.Punctalplugsversus artificialtearsfortreatingprimarySjogren’ssyndromewith keratoconjunctivitissicca:acomparativeobservationof theireffectsonvisualfunction.RheumatolInt. 2013;33:2543–8.
64.HolzchuhR,VillaAlbersMB,OsakiTH,IgamiTZ,SantoRM, Kara-JoseN,etal.Two-yearoutcomeofpartiallacrimal punctalocclusioninthemanagementofdryeyerelatedto Sjogren’ssyndrome.CurrEyeRes.2011;36:507–12. 65.MansourK,LeonhardtCJ,KalkWW,BootsmaH,BruinKJ,
BlanksmaLJ,etal.Lacrimalpunctumocclusioninthe treatmentofseverekeratoconjunctivitissiccacausedby Sjogren’ssyndrome:auniocularevaluation.Cornea. 2007;26:147–50.
66.VivinoFB,Al-HashimiI,KhanZ,LeVequeFG,SalisburyPL 3rd,Tran-JohnsonTK,etal.Pilocarpinetabletsforthe treatmentofdrymouthanddryeyesymptomsinpatients withSjogrensyndrome:arandomized,placebo-controlled, fixed-dose,multicentertrial.P92-01StudyGroup.Arch InternMed.1999;159:174–81.
67.PapasAS,SherrerYS,CharneyM,GoldenHE,MedsgerTAJr, WalshBT,etal.Successfultreatmentofdrymouthanddry eyesymptomsinSjogren’ssyndromepatientswithoral pilocarpine:arandomized,placebo-controlled,
dose-adjustmentstudy.JClinRheumatol.2004;10:169–77. 68.WuCH,HsiehSC,LeeKL,LiKJ,LuMC,YuCL.Pilocarpine
hydrochlorideforthetreatmentofxerostomiainpatients withSjogren’ssyndromeinTaiwan–adouble-blind, placebo-controlledtrial.JFormosMedAssoc. 2006;105:796–803.
69.PetroneD,CondemiJJ,FifeR,GluckO,CohenS,DalginP.A double-blind,randomized,placebo-controlledstudyof cevimelineinSjogren’ssyndromepatientswithxerostomia andkeratoconjunctivitissicca.ArthritisRheum.
2002;46:748–54.
70.TsifetakiN,KitsosG,PaschidesCA,AlamanosY,EftaxiasV, VoulgariPV,etal.Oralpilocarpineforthetreatmentof ocularsymptomsinpatientswithSjogren’ssyndrome:a randomised12weekcontrolledstudy.AnnRheumDis. 2003;62:1204–7.
71.LeungKC,McMillanAS,WongMC,LeungWK,MokMY,Lau CS.Theefficacyofcevimelinehydrochlorideinthe treatmentofxerostomiainSjogren’ssyndromeinsouthern Chinesepatients:arandomiseddouble-blind,
placebo-controlledcrossoverstudy.ClinRheumatol. 2008;27:429–36.
72.FifeRS,ChaseWF,DoreRK,WiesenhutterCW,LockhartPB, TindallE,etal.Cevimelineforthetreatmentofxerostomia inpatientswithSjogren’ssyndrome:arandomizedtrial. ArchInternMed.2002;162:1293–300.
73.OnoM,TakamuraE,ShinozakiK,TsumuraT,HamanoT, YagiY,etal.Therapeuticeffectofcevimelineondryeyein patientswithSjogren’ssyndrome:arandomized,
double-blindclinicalstudy.AmJOphthalmol.2004;138:6–17. 74.NoaisehG,BakerJF,VivinoFB.Comparisonofthe
discontinuationratesandside-effectprofilesofpilocarpine andcevimelineforxerostomiainprimarySjogren’s syndrome.ClinExpRheumatol.2014;32:575–7.
75.BrimhallJ,JhaveriMA,YepesJF.Efficacyofcevimelinevs. pilocarpineinthesecretionofsaliva:apilotstudy.SpecCare Dentist.2013;33:123–7.
76.WaltersMT,RubinCE,KeightleySJ,WardCD,CawleyMI.A double-blind,cross-over,studyoforalN-acetylcysteinein Sjogren’ssyndrome.ScandJRheumatolSuppl.
1986;61:253–8.
77.ManthorpeR,HagenPetersenS,PrauseJU.PrimarySjogren’s syndrometreatedwithEfamol/Efavit.Adouble-blind cross-overinvestigation.RheumatolInt.1984;4:165–7. 78.OxholmP,ManthorpeR,PrauseJU,HorrobinD.Patientswith
primarySjogren’ssyndrometreatedfortwomonthswith eveningprimroseoil.ScandJRheumatol.1986;15:103–8. 79.AragonaP,BucoloC,SpinellaR,GiuffridaS,FerreriG.
Systemicomega-6essentialfattyacidtreatmentandpge1 tearcontentinSjogren’ssyndromepatients.Investig OphthalmolVisSci.2005;46:4474–9.
80.PinheiroMNJr,dosSantosPM,dosSantosRC,BarrosJdeN, PassosLF,CardosoNetoJ.Oralflaxseedoil(Linum
usitatissimum)inthetreatmentfordry-eyeSjogren’s syndromepatients.ArqBrasOftalmol.2007;70:649–55. 81.SinghM,StarkPC,PalmerCA,GilbardJP,PapasAS.Effectof
omega-3andvitaminEsupplementationondrymouthin patientswithSjogren’ssyndrome.SpecCareDentist. 2010;30:225–9.
82.TheanderE,HorrobinDF,JacobssonLT,ManthorpeR. Gammalinolenicacidtreatmentoffatigueassociatedwith primarySjogren’ssyndrome.ScandJRheumatol.
2002;31:72–9.
83.TishlerM,YaronI,ShiraziI,YaronM.Hydroxychloroquine treatmentforprimarySjogren’ssyndrome:itseffecton salivaryandseruminflammatorymarkers.AnnRheumDis. 1999;58:253–6.
84.RihlM,UlbrichtK,SchmidtRE,WitteT.Treatmentofsicca symptomswithhydroxychloroquineinpatientswith Sjogren’ssyndrome.Rheumatology.2009;48:796–9. 85.FoxRI,DixonR,GuarrasiV,KrubelS.Treatmentofprimary
Sjogren’ssyndromewithhydroxychloroquine:a retrospective,open-labelstudy.Lupus.1996;5Suppl.1: S31–6.
86.CankayaH,AlpozE,KarabulutG,GuneriP,BoyaciogluH, KabasakalY.Effectsofhydroxychloroquineonsalivaryflow ratesandoralcomplaintsofSjogren’spatients:a
prospectivesamplestudy.OralSurgOralMedOralPathol OralRadiolEndod.2010;110:62–7.
87.YavuzS,AsfurogluE,BicakcigilM,TokerE.
Hydroxychloroquineimprovesdryeyesymptomsofpatients withprimarySjogren’ssyndrome.RheumatolInt.
88.FoxRI,ChanE,BentonL,FongS,FriedlaenderM,HowellFV. TreatmentofprimarySjogren’ssyndromewith
hydroxychloroquine.AmJMed.1988;85:62–7.
89.KruizeAA,HeneRJ,KallenbergCG,vanBijsterveldOP,van derHeideA,KaterL,etal.Hydroxychloroquinetreatment forprimarySjogren’ssyndrome:atwoyeardoubleblind crossovertrial.AnnRheumDis.1993;52:360–4.
90.GottenbergJE,RavaudP,PuechalX,LeGuernV,SibiliaJ, GoebV,etal.Effectsofhydroxychloroquineonsymptomatic improvementinprimarySjogren’ssyndrome:theJOQUER randomizedclinicaltrial.JAMA.2014;312:249–58. 91.FoxPC,DatilesM,AtkinsonJC,MacynskiAA,ScottJ,
FletcherD,etal.Prednisoneandpiroxicamfortreatmentof primarySjogren’ssyndrome.ClinExpRheumatol.1993;11: 149–56.
92.PijpeJ,KalkWW,BootsmaH,SpijkervetFK,KallenbergCG, VissinkA.Progressionofsalivaryglanddysfunctionin patientswithSjogren’ssyndrome.AnnRheumDis. 2007;66:107–12.
93.MiyawakiS,NishiyamaS,MatobaK.Efficacyoflow-dose prednisolonemaintenanceforsalivaproductionand serologicalabnormalitiesinpatientswithprimarySjogren’s syndrome.InternMed.1999;38:938–43.
94.PriceEJ,RigbySP,ClancyU,VenablesPJ.Adoubleblind placebocontrolledtrialofazathioprineinthetreatmentof primarySjogren’ssyndrome.JRheumatol.1998;25:896–9. 95.DrososAA,SkopouliFN,GalanopoulouVK,KitridouRC,
MoutsopoulosHM.Cyclosporinatherapyinpatientswith primarySjogren’ssyndrome:resultsatoneyear.ScandJ RheumatolSuppl.1986;61:246–9.
96.SkopouliFN,JagielloP,TsifetakiN,MoutsopoulosHM. MethotrexateinprimarySjogren’ssyndrome.ClinExp Rheumatol.1996;14:555–8.
97.vanWoerkomJM,KruizeAA,GeenenR,vanRoonEN, GoldschmedingR,VerstappenSM,etal.Safetyandefficacy ofleflunomideinprimarySjogren’ssyndrome:aphaseII pilotstudy.AnnRheumDis.2007;66:1026–32.
98.WillekeP,SchluterB,BeckerH,SchotteH,DomschkeW, GaubitzM.Mycophenolatesodiumtreatmentinpatients withprimarySjogrensyndrome:apilottrial.ArthritisRes Ther.2007;9:R115.
99.MoutsopoulosNM,KatsifisGE,AngelovN,LeakanRA, SankarV,PillemerS,etal.Lackofefficacyofetanerceptin Sjogrensyndromecorrelateswithfailedsuppressionof tumournecrosisfactoralphaandsystemicimmune activation.AnnRheumDis.2008;67:1437–43.
100.MavraganiCP,NiewoldTB,MoutsopoulosNM,PillemerSR, WahlSM,CrowMK.Augmentedinterferon-alphapathway activationinpatientswithSjogren’ssyndrometreatedwith etanercept.ArthritisRheum.2007;56:3995–4004.
101.SankarV,BrennanMT,KokMR,LeakanRA,SmithJA,Manny J,etal.EtanerceptinSjogren’ssyndrome:atwelve-week randomized,double-blind,placebo-controlledpilotclinical trial.ArthritisRheum.2004;50:2240–5.
102.MarietteX,RavaudP,SteinfeldS,BaronG,GoetzJ,Hachulla E,etal.InefficacyofinfliximabinprimarySjogren’s syndrome:resultsoftherandomized,controlledTrialof RemicadeinPrimarySjogren’sSyndrome(TRIPSS).Arthritis Rheum.2004;50:1270–6.
103.DassS,BowmanSJ,VitalEM,IkedaK,PeaseCT,HamburgerJ, etal.ReductionoffatigueinSjogren’ssyndromewith rituximab:resultsofarandomised,double-blind, placebo-controlledpilotstudy.AnnRheumDis. 2008;67:1541–4.
104.MeijerJM,MeinersPM,VissinkA,SpijkervetFK,Abdulahad W,KammingaN,etal.Effectivenessofrituximabtreatment inprimarySjogren’ssyndrome:arandomized,double-blind, placebo-controlledtrial.ArthritisRheum.2010;62:960–8.
105.Devauchelle-PensecV,MarietteX,Jousse-JoulinS,Berthelot JM,PerdrigerA,PuechalX,etal.Treatmentofprimary Sjogren’ssyndromewithrituximab:arandomizedtrial.Ann InternMed.2014;160:233–42.
106.GottenbergJE,CinquettiG,LarrocheC,CombeB,HachullaE, MeyerO,etal.Efficacyofrituximabinsystemic
manifestationsofprimarySjogren’ssyndrome:resultsin78 patientsoftheAutoImmuneandRituximabregistry.Ann RheumDis.2013;72:1026–31.
107.AdlerS,KornerM,ForgerF,HuscherD,CaversaccioMD, VilligerPM.Evaluationofhistologic,serologic,andclinical changesinresponsetoabatacepttreatmentofprimary Sjogren’ssyndrome:apilotstudy.ArthritisCareRes. 2013;65:1862–8.
108.MeinersPM,VissinkA,KroeseFG,SpijkervetFK, Smitt-KammingaNS,AbdulahadWH,etal.Abatacept treatmentreducesdiseaseactivityinearlyprimarySjogren’s syndrome(open-labelproofofconceptASAPstudy).Ann RheumDis.2014;73:1393–6.
109.TsuboiH,MatsumotoI,HagiwaraS,HirotaT,TakahashiH, EbeH,etal.Efficacyandsafetyofabataceptforpatients withSjogren’ssyndromeassociatedwithrheumatoid arthritis:RheumatoidArthritiswithOrenciaTrialtoward Sjogren’ssyndromeEndocrinopathy(ROSE)trial-an open-label,one-year,prospectivestudy-Interimanalysisof 32patientsfor24weeks.ModeRheumatol.2014:1–7. 110.MarietteX,SerorR,QuartuccioL,BaronG,SalvinS,FabrisM,
etal.EfficacyandsafetyofbelimumabinprimarySjogren’s syndrome:resultsoftheBelissopen-labelphaseIIstudy. AnnRheumDis.2013.
111.HartkampA,GeenenR,GodaertGL,BootsmaH,KruizeAA, BijlsmaJW,etal.Effectofdehydroepiandrosterone administrationonfatigue,well-being,andfunctioningin womenwithprimarySjogren’ssyndrome:arandomised controlledtrial.AnnRheumDis.2008;67:91–7.
112.CarubbiF,CiprianiP,MarrelliA,BenedettoP,RuscittiP, BerardicurtiO,etal.Efficacyandsafetyofrituximab treatmentinearlyprimarySjogren’ssyndrome:a prospective,multi-center,follow-upstudy.ArthritisRes Ther.2013;15:R172.
113.DelalandeS,deSezeJ,FauchaisAL,HachullaE,StojkovicT, FerribyD,etal.Neurologicmanifestationsinprimary Sjogren’ssyndrome:astudyof82patients.Medicine (Baltimore).2004;83:280–91.
114.DanieliMG,PettinariL,MorariuR,MonteforteF,LogulloF. Intravenousimmunoglobulinandmycophenolatemofetil forlong-standingsensoryneuronopathyinSjogren’s syndrome.CaseRepImmunol.2012;2012,186320. 115.RistS,SellamJ,HachullaE,SordetC,PuechalX,HatronPY,
etal.Experienceofintravenousimmunoglobulintherapyin neuropathyassociatedwithprimarySjogren’ssyndrome:a nationalmulticentricretrospectivestudy.ArthritisCareRes. 2011;63:1339–44.
116.MekinianA,RavaudP,HatronPY,LarrocheC,LeoneJ, GombertB,etal.EfficacyofrituximabinprimarySjogren’s syndromewithperipheralnervoussysteminvolvement: resultsfromtheAIRregistry.AnnRheumDis.2012;71:84–7. 117.MoriK,IijimaM,KoikeH,HattoriN,TanakaF,WatanabeH,
etal.Thewidespectrumofclinicalmanifestationsin Sjogren’ssyndrome-associatedneuropathy.Brain.2005;128 Pt11:2518–34.
118.MorozumiS,KawagashiraY,IijimaM,KoikeH,HattoriN, KatsunoM,etal.Intravenousimmunoglobulintreatment forpainfulsensoryneuropathyassociatedwithSjogren’s syndrome.JNeurolSci.2009;279:57–61.
120.TobonGJ,PersJO,Devauchelle-PensecV,YouinouP. NeurologicaldisordersinprimarySjogren’ssyndrome. AutoimmuneDis.2012;2012:645967.
121.LafitteC,AmouraZ,CacoubP,Pradat-DiehlP,PicqC, SalachasF,etal.Neurologicalcomplicationsofprimary Sjogren’ssyndrome.JNeurol.2001;248:577–84.
122.RossiR,ValeriaSaddiM.Subacuteasepticmeningitisas neurologicalmanifestationofprimarySjogren’ssyndrome. ClinNeurolNeurosurg.2006;108:688–91.
123.SantosaA,LimAY,VasooS,LauTC,TengGG.Neurosjogren: earlytherapyisassociatedwithsuccessfuloutcomes.JClin Rheumatol.2012;18:389–92.
124.deSezeJ,DelalandeS,FauchaisAL,HachullaE,StojkovicT, FerribyD,etal.MyelopathiessecondarytoSjogren’s syndrome:treatmentwithmonthlyintravenous cyclophosphamideassociatedwithcorticosteroids.J Rheumatol.2006;33:709–11.
125.MekinianA,RavaudP,LarrocheC,HachullaE,GombertB, Blanchard-DelaunayC,etal.Rituximabincentralnervous systemmanifestationsofpatientswithprimarySjogren’s syndrome:resultsfromtheAIRregistry.ClinExp Rheumatol.2012;30:208–12.
126.DeheinzelinD,CapelozziVL,KairallaRA,BarbasFilhoJV, SaldivaPH,deCarvalhoCR.Interstitiallungdiseasein primarySjogren’ssyndrome.Clinical–pathological
evaluationandresponsetotreatment.AmJRespirCritCare Med.1996;154Pt1:794–9.
127.ParambilJG,MyersJL,LindellRM,MattesonEL,RyuJH. InterstitiallungdiseaseinprimarySjogren’ssyndrome. Chest.2006;130:1489–95.
128.ZhangL,MoH,ZhuM,WangL.Effectofcyclophosphamide oncytokinesinpatientswithprimarySjogren’s
syndrome-associatedinterstitiallungdiseaseinSouth China.RheumatolInt.2013;33:1403–7.
129.ShiJH,LiuHR,XuWB,FengRE,ZhangZH,TianXL,etal. PulmonarymanifestationsofSjogren’ssyndrome.RespirInt RevThoracDis.2009;78:377–86.
130.FischerA,BrownKK,DuBoisRM,FrankelSK,CosgroveGP, Fernandez-PerezER,etal.Mycophenolatemofetilimproves lungfunctioninconnectivetissuedisease-associated interstitiallungdisease.JRheumatol.2013;40:640–6. 131.BorieR,SchneiderS,DebrayMP,Adle-BiasssetteH,DanelC,
BergeronA,etal.Severechronicbronchiolitisasthe presentingfeatureofprimarySjogren’ssyndrome.Respir Med.2011;105:130–6.
132.GoulesAV,TatouliIP,MoutsopoulosHM,TzioufasAG. ClinicallysignificantrenalinvolvementinprimarySjogren’s syndrome:clinicalpresentationandoutcome.Arthritis Rheum.2013;65:2945–53.
133.KaufmanI,SchwartzD,CaspiD,ParanD.Sjogren’s syndrome–notjustSicca:renalinvolvementinSjogren’s syndrome.ScandJRheumatol.2008;37:213–8.
134.RenH,WangWM,ChenXN,ZhangW,PanXX,WangXL, etal.Renalinvolvementandfollowupof130patientswith primarySjogren’ssyndrome.JRheumatol.2008;35:278–84. 135.YamamotoS,OkadaY,MoriH,HirataS,SaitoK,InokuchiN,
etal.Successfultreatmentofosteomalaciacausedbyrenal tubularacidosisassociatedwithSjogren’ssyndrome.Mod Rheumatol.2013;23:401–5.
136.YilmazH,KayaM,OzbekMK,SafaYildirimUUI. HypokalemicperiodicparalysisinSjogren’ssyndrome secondarytodistalrenaltubularacidosis.RheumatolInt. 2013;33:1879–82.
137.MaripuriS,GrandeJP,OsbornTG,FervenzaFC,MattesonEL, DonadioJV,etal.RenalinvolvementinprimarySjogren’s syndrome:aclinicopathologicstudy.ClinJAmSocNephrol CJASN.2009;4:1423–31.
138.HasilogluZI,AlbayramS,TasmaliK,ErerB,SelcukH,IslakC. AcaseofprimarySjogren’ssyndromepresentingprimarily withcentralnervoussystemvasculiticinvolvement. RheumatolInt.2012;32:805–7.
139.TsaiTC,ChenCY,LinWT,LeeWJ,ChenHC.Sjogren’s syndromecomplicatedwithIgAnephropathyand leukocytoclasticvasculitis.RenFail.2008;30:755–8. 140.CardosoR,GoncaloM,TellecheaO,MaiaR,BorgesC,Silva
JA,etal.Livedoidvasculopathyandhypercoagulabilityina patientwithprimarySjogren’ssyndrome.IntJDermatol. 2007;46:431–4.
141.GolanTD,KerenD,EliasN,NaschitzJE,ToubiE,Misselevich I,etal.Severereversiblecardiomyopathyassociatedwith systemicvasculitisinprimarySjogren’ssyndrome.Lupus. 1997;6:505–8.
142.GottenbergJE,GuillevinL,LambotteO,CombeB,AllanoreY, CantagrelA,etal.Toleranceandshorttermefficacyof rituximabin43patientswithsystemicautoimmune diseases.AnnRheumDis.2005;64:913–20.