Printed in Brazil - ©2004 Sociedade Brasileira de Química 0103 - 5053 $6.00+0.00
Article
* e-mail: icsfj@iqm.unicamp.br
# This papaer is dedicated to professors Kenneth and Carol Collins
Chromatographic Comparison of Self-Immobilized and Radiation-Immobilized
Poly(methyloctylsiloxane) Stationary Phases on Various Silicas
Tania A. Anazawa, Kenneth E. Collins and Isabel Cristina S. F. Jardim*,#
Instituto de Química, Universidade Estadual de Campinas, CP 6154, 13084–971 Campinas - SP, Brazil
Dois tipos de fase estacionária, auto-imobilizada e imobilizada por radiação, foram preparadas com poli(metiloctilsiloxano) sobre oito suportes de sílica com tamanho e forma de partículas, e tamanho de poros diferentes. As colunas recheadas com as fases estacionárias preparadas através dos dois procedimentos de imobilização apresentaram eficiências, resoluções e fatores de separação similares, mas as colunas recheadas com a fase estacionária auto-imobilizada tiveram percentagens de carga menores e, portanto, fatores de retenção menores. Os resultados evidenciam diferenças estruturais entre as fases.
Self-immobilized and radiation-immobilized stationary phases were prepared with poly(methyloctylsiloxane) on eight silica supports having different particle sizes, particle shapes and pore sizes. Columns prepared by the two immobilization procedures had similar efficiencies, resolutions and separation factors but columns with self-immobilized stationary phases had lower percent loadings and, thus, lower retention factors. Results show structural differences between the phases.
Keywords: silica, radiation immobilization, stationary phase, poly(metyloctylsiloxane)
Introduction
Silica-based reversed-phase high-performance liquid chromatography (RP-HPLC) has become the method of choice for most liquid chromatographic separations. Much of this popularity can be attributed to the silica itself because of the wide variety of available pore diameters, the uniformity of the pores, the high specific surface areas, the high mechanical strength and chemical reactivity for easy functionalization by reactions with the surface silanol groups.1,2 This reactivity, although the key to the success
of silica supports, is also a source of its limitations. Silanol groups that are not removed or covered lead to irreversible adsorption of basic solutes and strong peak asymmetry.3
Organic polymers have been sorbed onto, or produced on, the surfaces of HPLC silica to obtain more complete coverage, hence more efficient shielding of residual silanol
groups.4 The LabCrom group (UNICAMP) has been
developing such stationary phases by coating poly(methyloctylsiloxane) (PMOS) onto chromatographic silica particles,5–7 then immobilizing these phase by
γ -irradiation8–11 and by self-immobilization at ambiente
temperature.12
In the present work we directly compare the physical and chromatographic properties of PMOS phases that have
been immobilized by γ-irradiation or by
self-immobilization upon the surfaces of various HPLC-silica supports.
Experimental
Reagents and materials
Analytical-reagent grade or HPLC-grade solvents were obtained from Merck (Rio de Janeiro, RJ, Brazil) (methanol, dichloromethane, carbon tetrachloride, acetone, benzonitrile, benzene, toluene and naphthalene) and not further purified. Water was distilled and then purified through a Milli-Q system from Millipore.
Poly(methyloctylsiloxane) (PMOS) polymer (average molar mass of 6 200, viscosity 600 – 1000 cSt, 25 °C) was obtained from Hüls America (Pescataway, NJ, USA).
Preparation of stationary phase and immobilization by gamma irradiation
A known quantity of silica (dried at 150 °C for 24 h) was added to a solution of PMOS in dichloromethane to
prepare a PMOS-loaded material, SiO2(PMOS), having an
initial loading of 40% or 50% PMOS. This mixture was slowly agitated at room temperature for three hours and then the solvent was allowed to evaporate, without stirring, at room temperature in the fume hood.
Samples of 5.0 g of prepared stationary phase were sealed in glass ampoules under air. The sealed samples were irradiated to 80 or 120 kGy (irregular silicas)8 or 20
kGy (spherical silicas) of absorbed dose, using an industrial Cobalt-60 source (IBRAS-CBO, Campinas, SP, Brazil).
Physical and chemical characterization
The elemental analyses of the immobilized stationary phases were obtained with a Model CHN–2400 Perkin– Elmer analyzer.
Specific surface areas of the various packings were
determined by the conventional BET method13 using a
Model 2300 Micromeritics Flow Sorb II instrument. Infrared spectroscopy was done with a Perkin-Elmer Model 1600 FT–IR spectrophotometer and thermogra-vimetric analysis with a TA Instruments 2050 TGA.
Solvent extractions were done with a series of three independent extractions (with methanol, benzene and dichloromethane) of six hours each, on each sample, in a Soxhlet extractor using a modification of the method of Sanchez et al..14 After each extraction the solvent was
evaporated from the sample and the remaining mass was determined before initiating the next extraction.
Column packing
Columns (125 mm x 3.4 mm i.d.) were made locally from type 316 stainless tubing whose inner surface was highly polished in our laboratory.15 The columns were slurry
packed using 10% (irregular) or 20% (spherical) slurries (m/ v) of the stationary phase in carbon tetrachloride. A packing pressure of 38 MPa (Haskel Packing Pump) was used, with methanol as propulsion solvent. Columns were conditioned for four hours with mobile phase (methanol:water, 70:30, v/ v) at 0.2 mL min-1 prior to testing.
Chromatographic evaluation
Chromatographic evaluations were performed with a
modular HPLC instrument, equipped with a Waters Model 510 pump, SSI Model 3XL pneumatic injector with a
10 µL loop, a Waters Model 481 spectrophotometric
detector (14 µL cell volume) and a Waters Model 740
integrator.
All measurements were carried out at ambient temperature using methanol-water mobile phase at a
flow-rate of 0.2 mL min-1, near the optimal flow-rate as
determined by a van Deemter plot. The column dead time,
tM, was determined using methanol as an unretained
compound.
Two test mixtures were used in this study: (I) acetone, benzonitrile, benzene, toluene and naphthalene and (II) aniline, o-, m-, p–toluidine , and N,N–dimethylaniline. In
mixture I, a mobile phase of MeOH:H2O 70:30 (v/v) was
used while for mixture II, the mobile phase was MeOH:H2O 55:45 (v/v). Injections were of 10 µL with UV detection at 254 nm.
Chromatographic performance was evaluated by means of the efficiency (plates/m, N/L), retention factor (k), resolution (Rs), separation factor (α) and asymmetry factor (As), manually determined from the chromatograms. The asymmetry factor was calculated at 10% of the peak height.16
Results and Discussion
The infrared spectra of some self-immobilized and γ -immobilized stationary phases (Figure 1) show that the intensity of the signals characteristic of PMOS (2900, 1466 and 1258 cm-1) present a slight increase in intensity
in the immobilized stationary phases due to the larger amount of PMOS in the silicas, as a result of cross-linking of the PMOS – as is also indicated by the results of solvent extraction and % carbon.
Table 1. Physical and chemical characteristics of self-immobilized and γ-immobilized stationary phases
1 2 3 4 5 6 % PMOS % PMOS % PMOS ratio
Stationary phases Particle Silica Silica Silica Silica after packinga after extractionsb (self-immob./g-immob.) (% PMOS in initial shape particle pore specific specific
loading) size size volume surface 7 8 9 10 11 12
(µm) (nm) (mL g-1) area self-immob. γ-immob. self-immob. γ-immob. after Pc after Xd (m2 g-1)
40% PMOS-Davisil irregular 10 15 1.6 237 18.6 26.3 18.5 25.1 0.71 0.74
50% PMOS-Sigma irregular 10 6 1.1 393 28.5 35.0 24.4 29.2 0.81 0.83
50% PMOS-Si-100 irregular 10 10 1.25 290 27.3 39.5 26.9 34.1 0.69 0.79
50% PMOS-Si-60 irregular 10 6 0.9 267 - 35.5 19.9 28.5 - 0.70
50% PMOS-Si-60 irregular 7 6 0.9 305 29.0 34.8 20.2 29.4 0.83 0.69
50% PMOS-Si-60 irregular 5 6 0.9 402 30.3 32.9 23.4 26.9 0.92 0.87
40% PMOS-Spher. spherical 8 8 0.45 149 24.0 24.3 17.0 18.0 0.99 0.94
40% PMOS-Spher. spherical 5 8 0.45 186 21.8 - 17.9 18.8 - 0.95
a % PMOS obtained from the %C of used packing material by dividing the %C by the carbon fraction (0.62) of PMOS; b % PMOS obtained from the %C of non-used (non-packed) packing material following a series of extractions (methanol, benzene, dichloromethane); c after P = after packing; d after X = after extractions.
12 of Table 1 give estimates of the relative quantities of PMOS immobilized by the two immobilization procedures. Thermogravimetric analyses (Figure 2) show good thermal stability of self-immobilized and γ-immobilized stationary phases in the temperature range used for HPLC,
with decomposition of the stationary phase only starting at about 400 °C for all phases except self-immobilization
50% PMOS-Lichrosorb Si–60, 10 µm (~ 370 °C) and
γ-immobilized 40% PMOS-Davisil (~ 290 °C).
Table 2 lists the efficiencies (N/L) obtained from
Table 2. Comparisons of column efficienciesa
Stationary phase dp (µm) N/L (N/L)5 b
Self-immob. γ-immob. Self-immob. γ-immob.
Davisil 10 24 000 26 400 62 400 68 640
Sigma 10 34 400 36 400 68 800 72 800
Si-100 10 33 600 36 400 67 200 72 800
Si-60 10 35 200 43 600 70 400 87 200
Si-60 7 41 200 37 600 57 700 52 640
Si-60 5 50 400 66 400 50 400 66 400
Spherisorb 8 58 400 39 600 93 440 63 400
Spherisorb 5 74 400 70 400 74 400 70 400
mean 68 090 69 280
68 685 ± 595
a efficiencies were obtained from the naphthalene peak of test mixture I; b (N/L)
5 = plates per meter normalized to 5 µm particle size by the factor dp/5. For example: for the case of 10 µm particles, (N/L)5 = (N/L)10 x 10/5.
columns packed with the stationary phases. The efficiencies normalized to 5 µm particles, (N/L)5, show that column efficiencies are, on the average, no better for
γ-immobilized PMOS phases than for self-immobilized
phases. Nevertheless the overall average efficiency of almost 70 000 plates per meter compares well with commercial phases.
Table 3 shows that, for the test solutes of mixture I, the
As, Rs and α values do not differ greatly between the
different silicas or for the two immobilization procedures. The k values, however, show consistent differences for the
two immobilization procedures: the γ-immobilized
stationary phases, with their greater % PMOS values, have consistently higher k, probably due to the thickness of the PMOS coating, so there is a decrease in the velocity of mass transfer and, consequently, an increase in the retention factor (Figure 3). This seems to imply that the self-immobilized stationary phases, with separation efficiencies similar to those of the γ-immobilized phases, can perform separations faster, which could be a decided advantage.
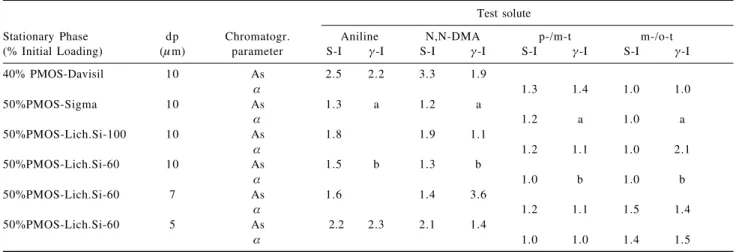
Table 4 shows the results obtained using test mixture II, part of the mixture proposed by Engelhardt and co-workers17, 18 as basic probes, with aniline as a weak base,
N,N-dimethylaniline (N,N-DMA) as a strong base, and the isomeric o-, m- and p-toluidines as probes for silanophilic interactions. Methanol:water (55:45, v/v), without addition of buffer or salt solution, was used as the mobile phase. A column can be considered “good” for the analysis of basic compounds, according to the Engelhardt criteria17,18 if the
isomeric toluidines coelute or have value below 1.3. The findings obtained from self-immobilized stationary phases were discussed in previous work.7
Table 4 reveals that, although the separation factors (α) for the toluidines may be quite similar for both the
self-immobilized and γ-immobilized phases, the asymmetry
factors for N,N-DMA tend to be consistently different, thus pointing again to the possibility that the actual interaction surfaces of the stationary phases which are immobilized in different ways have fundamentally different structures, as
Table 3. Chromatographic parameters obtained using test mixture I
Stationary Phase dp Asa ka R
s
b αb
(% Initial Loading) (µm) Self-immob. γ-immob. Self-immob. γ-immob. Self-immob. γ-immob. Self-immob. γ-immob.
40% PMOS-Davisil 10 1.4 1.3 1.8 2.5 1.8 1.9 1.2 1.2
50% PMOS-Sigma 10 1.1 1.2 3.6 6.6 2.7 2.7 1.2 1.2
50% PMOS-Si-100 10 1.0 1.1 3.2 6.3 2.4 2.6 1.2 1.2
50% PMOS-Si-60 10 1.2 1.2 1.5 6.2 2.0 3.0 1.2 1.2
50% PMOS-Si-60 7 1.3 0.9 3.1 6.4 2.7 2.7 1.2 1.2
50% PMOS-Si-60 5 1.2 0.9 4.8 6.4 3.2 3.6 1.2 1.2
40% PMOS-Spher. 8 1.0 0.7 4.1 6.5 3.4 3.0 1.2 1.2
40% PMOS-Spher. 5 1.3 0.9 2.1 4.7 3.3 4.0 1.2 1.2
a calculated for the naphthalene peak; b calculated for the toluene-naphthalene pair.
Figure 3. Chromatograms of test mixture I: 1 = acetone, 2 = benzonitrile, 3 = benzene, 4 = toluene and 5 = naphthalene, obtained with columns packed with: (A) 40% PMOS-Davisil self-immobilized and γ-immobilized, (B) 50% PMOS-Sigma self-immobilized and γ -immobilized, (C) 50% PMOS- Lichrosorb Si-60, 10 µm, self-immo-bilized and γ-immobilized, (D) 40% PMOS-Spherisorb, 5 µm, self-immobilized and γ-immobilized. Chromatographic conditions: mo-bile phase: methanol:water (70:30, v/v), flow-rate: 0.2 mL min-1, volume of injected sample: 10 µL, detection: UV, 254 nm.
Table 4. Chromatographic parameters obtained using test mixture II
Test solute
Stationary Phase dp Chromatogr. Aniline N,N-DMA p-/m-t m-/o-t
(% Initial Loading) (µm) parameter S-I γ-I S-I γ-I S-I γ-I S-I γ-I
40% PMOS-Davisil 10 As 2.5 2.2 3.3 1.9
α 1.3 1.4 1.0 1.0
50%PMOS-Sigma 10 As 1.3 a 1.2 a
α 1.2 a 1.0 a
50%PMOS-Lich.Si-100 10 As 1.8 1.9 1.1
α 1.2 1.1 1.0 2.1
50%PMOS-Lich.Si-60 10 As 1.5 b 1.3 b
α 1.0 b 1.0 b
50%PMOS-Lich.Si-60 7 As 1.6 1.4 3.6
α 1.2 1.1 1.5 1.4
50%PMOS-Lich.Si-60 5 As 2.2 2.3 2.1 1.4
α 1.0 1.0 1.4 1.5
p-/m-t = p-/m-toluidine pair; m-/o-t = m-/o-toluidine pair; a = result not reproducible; b = problems of absorption of the compounds ; S-I = self-immobilized ; γ-I = γ-immobilized.
Conclusions
Although self-immobilized and γ-immobilized PMOS
phases have similar chromatographic properties, the
γ-immobilized phases show somewhat higher efficiencies
and the self-immobilized phases, having lower carbon contents, can give more rapid separations. Both types of immobilized phase may be used at moderately elevated pH.
Acknowledgements
The authors acknowledge financial support from FAPESP and CNPq and thank IBRAS-CBO (Campinas, SP, Brazil) for performing the irradiations of the stationary phases and Prof. C. H. Collins for helpful discussions and suggestions.
References
1. Majors, R.E.; LC-GC1997, 5, 508.
2. Unger, K.K.; Porous Silica: Its Properties and Use as Support
in Column Liquid Chromatography, Elsevier: Amsterdam,
1986.
3. Nawrocki, J.; J. Chromatogr. A1997, 779, 29. 4. Petro, M.; Berek, D.; Chromatographia1993, 37, 549. 5. Anazawa, T.A.; Jardim, I.C.S.F.; J. Liq. Chromatogr. 1994,
17, 1265.
6. Anazawa, T.A.; Carraro, F.; Collins, K.E.; Jardim, I.C.S.F.; J.
Chromatogr. A 1995, 697, 159.
7. Anazawa, T.A.; Jardim, I.C.S.F; J. Liq. Chromatogr. Relat.
Technol.1998, 21, 645.
8. Jardim, I.C.S.F.; Collins, K.E.; Anazawa, T.A.; J. Chromatogr. A1999, 849, 299.
9. Melo, L.F.C.; Jardim, I.C.S.F.; J. Chromatogr. A1999, 845,
423.
10. Silva, R.B.; Collins, C.H.; J. Chromatogr. A1999, 845, 417. 11. Silva, R. B.; Collins, K.E.; Collins, C.H.; J. Chromatogr. A
2000, 869, 137.
12. Collins, K.E.; Bottoli, C.B.G.; Bachmann, S.; Vigna, C.R.M.; Collins, C. H.; Albert, K.; J. Chromatogr. A2004, in press. 13. Brunauer, S.; Emmett, P.H.; Teller, E.; J. Am. Chem. Soc.
1938, 60, 309.
14. Sanchez, E. F.; Dominguez, J.A.; Munoz , J.E.; Molera, M.J.;
J. Chromatogr. 1984, 299, 151.
15. Collins, K.E.; Franchon, A.C.; Jardim, I.C.S.F.; Radovanovic, E.; Gonçalves, M.C.; LC-GC2000, 18, 106.
16. Snyder, L.R.; Kirkland, J. J.; Glajch, J.L.; Pratical HPLC
Method Development, 2nd ed., Wiley: New York, 1997, ch. 5.
17. Engelhardt, H.; Jungheim, M.; Chromatographia1990, 29,
59.
18. Engelhardt, H.; Löw, H.; Götzzinger, W.; J. Chromatogr.1991,
544, 371.
Received: September 16, 2002 Published on the web: January 19, 2004