REVISTA
BRASILEIRA
DE
ANESTESIOLOGIA
PublicaçãoOficialdaSociedadeBrasileiradeAnestesiologiawww.sba.com.br
SCIENTIFIC
ARTICLE
Dexmedetomidine
preconditioning
protects
against
lipopolysaccharides-induced
injury
in
the
human
alveolar
epithelial
cells
Lei
Zhang
a,b,
Xian-Jin
Zhou
b,c,
Li-Ying
Zhan
a,b,
Xiao-Jing
Wu
a,b,
Wen-Lan
Li
a,b,
Bo
Zhao
a,b,
Qing-Tao
Meng
a,b,∗,
Zhong-Yuan
Xia
a,b,∗aWuhanUniversity,RenminHospital,DepartmentofAnesthesiology,Wuhan,Hubei,China
bWuhanUniversity,RenminHospital,LaboratoryofAnesthesiologyandCriticalCareMedicine,Wuhan,Hubei,China cTongjiUniversity,FirstMaternityandInfantHospital,DepartmentofAnesthesiology,Shanghai,China
Received17March2016;accepted27February2017 Availableonline26June2017
KEYWORDS Dexmedetomidine; Lipopolysaccharides; Preconditioning; Acutelunginjury; Alveolarepithelial cell
Abstract
Backgroundandobjectives: Dexmedetomidine (DEX) has demonstrated the preconditioning
effectandshownprotectiveeffectsagainstorganizeinjury.Inthisstudy,usingA549(human
alveolar epithelial cell) celllines, we investigated whether DEX preconditioning protected
againstacutelunginjury(ALI)invitro.
Methods:A549 were randomly divided into four groups (n=5): control group, DEX group,
lipopolysaccharides(LPS) group,andD-LPS (DEX+LPS)group. Phosphatebuffer saline(PBS)
orDEXwereadministered.After2hpreconditioning,themediumwasrefreshedandthecells
were challengedwith LPSfor 24honthe LPSand D-LPSgroup. Thenthe malondialdehyde
(MDA),superoxidedismutase(SOD), Bcl-2,Bax,caspase-3andthecytochromecintheA549
weretested.Theapoptosiswasalsoevaluatedinthecells.
Results:ComparewithLPSgroup,DEXpreconditioningreducedtheapoptosis(26.43%±1.05%
vs.33.58%±1.16%,p<0.05)intheA549,whichiscorrelatedwithdecreasedMDA(12.84±1.05
vs. 19.16±1.89nmoL.mg−1 protein, p<0.05) and increased SOD activity (30.28±2.38 vs.
20.86±2.19U.mg−1 protein, p<0.05). DEX preconditioning also increased the Bcl-2 level
(0.53±0.03vs.0.32±0.04,p<0.05)anddecreasedthelevelofBax(0.49±0.04vs.0.65±0.04,
p<0.05),caspase-3(0.54±0.04vs.0.76±0.04,p<0.05)andcytochromec.
Conclusion:DEX preconditioning hasa protectiveeffect against ALI in vitro. The potential
mechanismsinvolvedaretheinhibitionofcelldeathandimprovementofantioxidation.
©2017SociedadeBrasileiradeAnestesiologia.PublishedbyElsevierEditoraLtda.Thisisan
openaccessarticleundertheCCBY-NC-NDlicense(
http://creativecommons.org/licenses/by-nc-nd/4.0/).
∗Correspondingauthor.
E-mail:674612814@qq.com(Z.Y.Xia). http://dx.doi.org/10.1016/j.bjane.2017.02.002
PALAVRAS-CHAVE Dexmedetomidina; Lipopolissacarídeos;
Pré-condicionamento; Lesãopulmonar aguda;
Célulasepiteliais alveolares
Pré-condicionamentocomdexmedetomidinaprotegecontralesõesinduzidas porlipopolissacarídeosemcélulasepiteliaisalveolareshumanas
Resumo
Justificativaeobjetivos: Dexmedetomidina(DEX)demonstroupossuirefeitopré-condicionante
e também efeitos protetores contra lesão organizada. Neste estudo, usando células A549
(célulasepiteliaisalveolareshumanas),investigamosseopré-condicionamentocomDEX
pro-porcionariaprotec¸ãocontralesãopulmonaraguda(LPA)invitro.
Métodos: CélulasA549foramaleatoriamentedistribuídasemquatrogrupos(n=5):grupo
con-trole, grupo DEX, grupo lipopolissacarídeos (LPS) e grupo D-LPS (DEX+LPS). Administramos
soluc¸ãodePBS(tampãofosfato-alcalino)ouDEX.Após2hdepré-condicionamento,omeiofoi
renovadoeascélulas desafiadascomLPSpor24hnosgruposLPSeD-LPS.Emseguida,
mal-ondialdeído(MDA),superóxidodismutase(SOD),Bcl-2,Bax,caspase-3ecitocromocemA549
foramtestados.Apoptosetambémfoiavaliadanascélulas.
Resultados: Emcomparac¸ãocomogrupoLPS,opré-condicionamentocomDEXreduziua
apop-tose(26,43%±1,05%vs.33,58%±1,16%,p<0,05)emcélulasA549,oqueestácorrelacionado
comadiminuic¸ãodeMDA(12,84±1,05vs.19,16±1,89nmoL.mg−1deproteína,p<0,05)e
aumentodaatividadedeSOD(30,28±2,38vs.20,86±2,19U.mg−1deproteína,p<0,05).O
pré-condicionamentocomDEXtambémaumentouoníveldeBcl-2(0,53±0,03vs.0,32±0,04,
p<0,05)ediminuiuoníveldeBax(0,49±0,04vs.0,65±0,04,p<0,05),caspase-3(0,54±0,04 vs.0,76±0,04,p<0,05)ecitocromoc.
Conclusão:Opré-condicionamentocomDEXtemefeitoprotetorcontraLPAinvitro.Os
poten-ciaismecanismosenvolvidossãoinibic¸ãodamortecelularemelhoradaantioxidac¸ão.
©2017SociedadeBrasileiradeAnestesiologia.PublicadoporElsevierEditoraLtda.Este ´eum
artigo OpenAccess sobumalicenc¸aCCBY-NC-ND(
http://creativecommons.org/licenses/by-nc-nd/4.0/).
Introduction
Acute Lung Injury (ALI) and its most severe form, Acute RespiratoryDistress Syndrome(ARDS),is arelatively com-mon syndrome in critically ill patients associated with high morbidity and mortality. Whichare characterizedby high-proteinpulmonaryedemaandseverehypoxemic respi-ratory failure and may result from many clinical insults, includingsepsisandpneumonia.1---3Despiteintenseresearch
andan improved understanding of the pathophysiologyof ALI/ARDS,therearenospecificpharmacologicaltreatments ofprovenbenefitforthem.Sothemorbidityandmortality remainssignificantat35%---40%.3---5
Preconditioninghasbeen reportedtoofferaneffective protection against ALI by a previous stimulus.6,7 To date,
it has been extensively studied for its potential to offer a unique opportunity to exert protective effects in clini-cal practice. Today, we know that the protection can be elicitedmoresafely bymanydrugs suchas dexmedetomi-dine(DEX).8,9
DEXhasbeencommonlyusedasasedativeinclinical sett-ings.Recently,investigatorsfoundthatDEXwascapableof mimickingthepreconditioningeffectandshownprotective effects against organize injury caused by lipopolysaccha-rides(LPS).10,11ThatevidenceindicatesthatDEXmayhave
protectiveeffectagainstALI.Here,weusedtheLPS-induced A549 injury model to simulate ALI in vitro, investigat-ingwhetherDEXpreconditioningwouldproduceprotection againstALI. Wehave alsoinvestigatedthe effects of DEX preconditioningoncelldeathandantioxidationfunctionto gainabetterinsightintothemechanism(s).
Materials
and
methods
Cellcultureandtreatments
Human alveolar epithelial cell line A549 was obtained fromtheChinaCenterforTypeCultureCollection(CCTCC, Wuhan University, Wuhan, China). A549 were cultured in RPMI-1640 medium supplemented with 10% fetal bovine serum, 2mmol.L−1 glutamine, 100U.mL−1 penicillin and
100mg.mL−1streptomycin,andmaintainedinahumid
envi-ronmentat37◦Cand5%CO2.
TheA549atthelogphaseofgrowthwereseededintoa96 wellplate,2×103well.Afterovernightculture,thesecells
wererandomlyallocatedtofourdifferenttreatmentgroups: theDEX(JiangsuHengruiMedicineCo.;Ltd.,Jiangsu,China) and D-LPS group received DEX (10g.mL−1); the control
andLPS(Sigma---Aldrich,SanLuis,MO,USA)groupreceived the same volume of phosphate buffer saline (PBS). After 2hexposuretothepharmacologicalagenteachwellplate washedandthecellswerechallengedwithLPS(50g.mL−1)
for24h ontheLPSand D-LPSgroup. Thenthecells were collectedforfurtheranalysis.
Measurementofmalondialdehyde(MDA)formation
andsuperoxidedismutase(SOD)activity
BCAproteinassaykit(BestBioCo.;Shanghai,China) accord-ingtothemanufacturer’sprotocol.
Westernblotting
Cellswerehomogenized and theprotein concentrationof itssupernatant wasdetermined by the BCA method. The supernatantcontaining 50gof protein wasseparatedby SDS---PAGE and transferred to a nitrocellulose membrane. Primary antibodies included caspase-3, Bcl-2, Bax (Santa Cruz Biotechnology, Santa Cruz, CA, USA), and -actin (Abcam, Cambridge, MA, USA). The membrane was then incubated with an HRP-conjugated anti-rabbit secondary antibody(1:20,000;Pierce)for1handtheblotwas devel-opedwitha Supersignal chemiluminescence detectionkit (Pierce).The immunoblotting wasvisualized withaKodak X-ray Processor 102 (EastmanKodak, Rochester, NY, USA) and analyzed with Quantity One-4.2.3 software(Bio-Rad, Hercules,CA).
Cytochromecreleaseassay
Aspreviouslydescribed,12theA549cellswerefixedwith4%
paraformaldehydefor15min,permeabilizedwith0.1% Tri-tonX-100for10min,andblockedusing3%serumdissolved inPBSfor30minatroomtemperature.Thecellswerethen probedwithanti-cytochromecantibody(1:100;SantaCruz Biotechnology)overnightat4◦C.Thecellswerewashedwith
PBStwice and incubated withFITC conjugated secondary antibody(1:200;Biovision,China)for2hinthedarkat37◦C.
Afterwashing,imagesofstainedcellswereobtainedusing afluorescencemicroscope.
Flowcytometricdetectionofapoptosis
Usingan AnnexinV-PE/7-AADApoptosisDetectionKit (Bio-Vision,MA,USA),theapoptoticcellswasevaluatedbyflow cytometryanalysis(FACScan,BDBiosciences,SanJose,CA, USA).13ThedatawereanalyzedusingtheCellQuestsoftware
(BDBiosciences).
Statisticalanalysis
Thedataarereportedasthemean±SEMoffiveindependent experiments.ANOVAandStudent---Newman---Keuls(SNK)test wereperformedtodeterminestatisticalsignificance. Signif-icantdifferenceswereestablishedatp<0.05.
Results
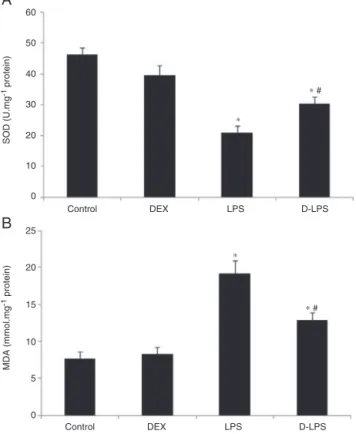
EffectsofDEXonoxidativestressintheA549
Compared with the control group, LPS decreased SOD activity in the A549 (46.34±2.24U.mg−1 protein vs.
20.86±2.19U.mg−1 protein, p<0.05) (Fig. 1A) and
increased the formation of MDA (7.68±0.92nmoL.mg−1
protein vs. 19.16±1.89nmoL.mg−1 protein, p<0.05)
(Fig. 1B). Such an effect was significantly attenuated
A
60
50
40
30
20
25
20
15
10
5
0 10
0
Control DEX
SOD (U.mg
-1
protein)
MDA (mmol.mg
-1 protein)
LPS D-LPS
Control DEX LPS D-LPS
∗ #
∗ #
∗
∗
B
Figure1 (A)SODactivityintheA549.(B)MDAlevelsinthe A549. Control, without injury; DEX, dexmedetomidine; LPS, lipopolysaccharide;D-LPS,DEX+LPS.Valueswerepresentedas mean±SEM,n=5foreachgroup.*p<0.05vs.Controlgroupand
#p<0.05vs.LPSgroup.
by DEX preconditioning (30.28±2.38U.mg−1 protein and
12.84±1.05nmoL.mg−1protein,p<0.05vs.LPSgroup).
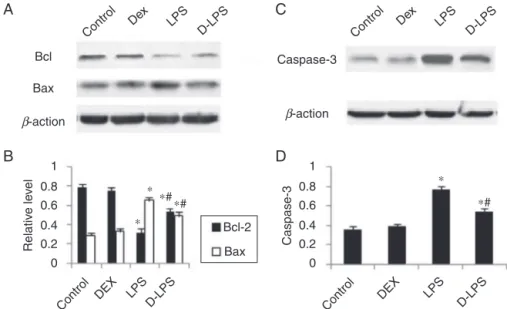
EffectsofDEXontheexpressionofBcl-2,Baxand
caspase-3
Compared with the control group, LPS decreased Bcl-2 level (0.79±0.03 vs. 0.32±0.04, p<0.05) and increased theexpressionofBax(0.29±0.03vs.0.65±0.04,p<0.05) in the A549 (Fig. 2A and B). Such an effect was signifi-cantlyattenuatedbyDEXpreconditioning(0.53±0.03and 0.49±0.04, p<0.05 vs. LPS group). Compared with the control group, LPS increased the expression of caspase-3 (0.36±0.03 vs.0.76±0.04, p<0.05) inthe A549 (Fig.2C andD).SuchaneffectwassignificantlyattenuatedbyDEX preconditioning(0.54±0.04,p<0.05vs.LPSgroup).
EffectsofDEXoncytochromecrelease
A
Bcl Caspase-3
Control
Dex LPS
D-LPS Control
Dex LPS
D-LPS
Bax
Bcl-2 ∗
∗ ∗
∗# ∗#
∗#
Bax
Relativ
e le
vel
Caspase-3
1
0.8 0.6
0.4
0.2 0
1
0.8 0.6
0.4
0.2 0
Control DEX Control DEX
LPS LPS
D-LPS D-LPS
β-action β-action
B
D
C
Figure2 (A)RepresentativepictureofBcl-2andBax.(B)ThelevelofBcl-2andBax.(C)Representativepictureofcaspase-3. (D)Thelevelofcaspase-3.Control,withoutinjury;DEX,dexmedetomidine;LPS,lipopolysaccharide;D-LPS,DEX+LPS.Valueswere presentedasmean±SEM,n=5foreachgroup.*p<0.05vs.Controlgroupand#p<0.05vs.LPSgroup.
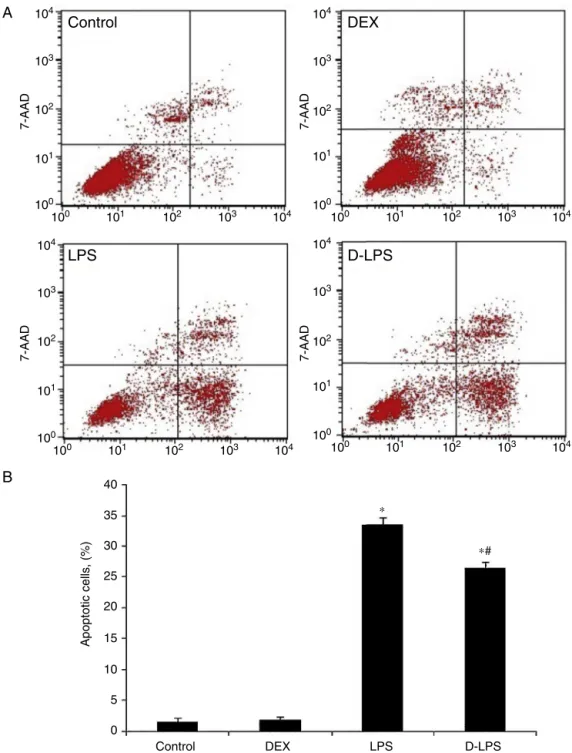
EffectsofDEXonapoptosis
Compared withthe controlgroup, LPSincreased the per-centage of apoptotic cells in the A549 (1.57%±0.52% vs. 33.58%±1.16%, p<0.05) (Fig. 4A and B). Such an effectwassignificantlyattenuatedbyDEXpreconditioning (26.43%±1.05%,p<0.05vs.LPSgroup).
Discussion
This study demonstrates that DEX preconditioning signifi-cantlydecreasedLPS-inducedinjuryintheA549.Themain findingsareasfollows:(1)DEXpreconditioningreducedMDA and increased SOD activity in the A549; (2) DEX precon-ditioningincreasedthe Bcl-2/Baxratioanddecreased the caspase-3andcytochromeclevel;(3)DEXpreconditioning attenuated apoptosisin the A549. This data provides the firstevidencethatDEXpreconditioningattenuatestheA549 injurycausedbyLPS.
Studieshavebeenreportedthatoxidativestressplaysan important roleon LPS-induced injury.14,15 Consistent with
theprevious study,we also found the LPS-induced oxida-tivestressbyincreasedMDAbutdecreasedSODintheA549. MDAisthedegradationproductsoftheoxygen-derivedfree radicalsandlipidperoxidation.Therefore,theincreaseof MDAimpliesimpairmentofthenormalmembranestructure andoxidative tissuedamage.16 Incontrast,SODis thought
tobe an important intracellular antioxidant enzyme with multiplebiologicalfunctions.Withanincreaseinthe antiox-idantenzymeSOD,itcanindicatethecellularcapabilityof scavengingandquenchingfreeradicals.17,18 Previous
stud-iesalsoreportedthatDEXpreventedperoxidationreactions byincreasedSODbut decreasedMDAintissues.19,20 Inthe
experiment, our results obviously demonstrated that DEX preconditioning resulted in an increase of the SOD activ-ityandadecreaseof theMDAin theA549.Thesefindings bothsuggestthatDEXpreconditioningcouldprotectagainst oxidativedamagebyLPS-inducedintheA549,thereby mod-ulatingthecellularinjuryanddysfunction.
Control
Cytochrome c
DAPI
DEX LPS D-LPS
A
B
Control
LPS
D-LPS
DEX
100 101 100
101
7-AAD
7-AAD
7-AAD
7-AAD
102 103 104
100 101 102 103 104
100 101 102 103 104
102 103 104
100 101 102 103 104 100 101
40
∗
∗# 35
30
25
20
15
10
5
0
Control DEX
Apoptotic cells
,
(%)
LPS D-LPS
102 103 104 100
100 101 102 103 104
101 102 103 104
Figure4 (A)Representativepictureofflowcytometer.(B)Thegradeofcellapoptosis.Control,withoutinjury;DEX, dexmedeto-midine;LPS,lipopolysaccharide;D-LPS,DEX+LPS.Valueswerepresentedasmean±SEM,n=5foreachgroup.*p<0.05vs.Control groupand#p<0.05vs.LPSgroup.
Several evidences have indicated that oxidative stress wasinvolvedinunderlyingpathologicalmechanismsof LPS-inducedapoptosisinA549.21,22Assuggestedfromourresults,
we also found that LPS had significantly reducedratio of Bcl-2/Bax associated with the increase of MDA and the decreaseofSODactivityinA549.BothBcl-2andBaxbelong to Bcl-2 family. However, Bcl-2 may be regarded as an importantcellularcomponentthatnotonlyguardsagainst apoptotic cell death but also influences multiple cellular events.Inrecentstudies,Bcl-2wasfoundtoprotectagainst LPS-induced injury in some organs including A549.23,24 In
contrast,Baxexhibitsproapoptoticactions.WhenBaxwas
over expressed, it may form channels or pores allowing for the release of factorssuch ascytochrome cfrom the mitochondriatopropagateapoptosis.25 ThustheBcl-2/Bax
proteinratioislikelycriticalforcellsurvivalafterinjury.24
Inthepresentstudy,ourresultsindicatedthatDEXincreased theexpressionofBcl-2anddecreasedtheexpressionofBax, leadingtoanincreaseoftheBcl-2/Baxratio.
Cytochrome c release and caspase-3 activation is piv-otalpointintheapoptoticcascadeandcanberegulatedby theBcl-2/Baxratio.26 Previousstudiesshowedthat
depressed by Bcl-2. The released cytochrome c and acti-vatedcaspase-3cleavesdownstreamcriticalcellulartargets involved in chromatin condensation, DNA fragmentation, and cytoskeletal destruction, thereby expressing the dra-maticmorphologicalchangesofapoptosis.25---27LPShasbeen
demonstratedtobeoneofthewaysofinducingcytochrome creleaseandcaspase-3activationinA549.28Inthepresent
study,ourdata alsoshows thatcytochromec releaseand caspase-3activationsignificantlyincreasedafterLPS admin-istrationintheA549,consistentwiththepreviousstudies. Furthermore,we observedthat the cytochrome crelease and caspase-3 activation wasdepressedby DEX precondi-tioning.
Apoptosis is a fundamental process of cell death that occursviaactivation ofdistinctsignaling pathways involv-ing down-regulation of Bcl-2/Bax ratio and release of cytochromecandactivationofcaspase-3invitro.28,29
Ulti-mately,cellsundergodestructionandformationofapoptotic bodies.30 LPS have been shown to initiate this apoptotic
cascade in A549.24 Using an in vitro model of ALI, our
data demonstrate significant reduction of apoptosis after DEXpreconditioning,whichcorrelatedwithup-regulationof Bcl-2/Baxratioanddepressionofcaspase-3activationand cytochromecrelease.
However, the descriptive study has some obvious lim-itations that need to be addressed. Which included one sampling timepoint, thebrief periodof observation, and lackofcorrelationwithclinicalmeasurementsofALI. More-over, we haveonly investigatedsingleDEX concentration, whichisverylargetotheclinicalpractice.Comparedwith clinical,alargedoseisusuallyusedinlaboratory.31,32Some
studies evenreporteda heavy dose in experiment.These may be related with different species.31,32 The relation
betweendoseandeffectremainsunclear.Therefore,studies relatedtodose---effectrelationshipneedfurtherexploration inthemodel.
Summary
Our data show that DEX preconditioning can effectively attenuate the LPS-induced injury in A549. The protective effectsmayinvolveareductioninoxidativestressandcell deathinducedbyLPS.Theseexperimentalresultssuggested thatDEXmaybeefficaciousinthetreatmentofLPS-induced ALI.
Conflict
of
interest
Theauthorsdeclarenoconflictsofinterest.
References
1.YangB,HuangW,HanJ,etal.Studyoftheroleofepidermal growthfactoronlungfluidtransportinrabbitswithacutelung injurycausedbyendotoxin.ExpTherMed.2012;4:611---4. 2.VincentJL,SakrY,RanieriVM.Epidemiologyand outcomeof
acuterespiratoryfailurein intensivecare unitpatients. Crit CareMed.2003;31:S296---9.
3.Matthay MA, Zemans RL. The acute respiratory distress syndrome: pathogenesis and treatment. Annu Rev Pathol. 2011;6:147---63.
4.WareLB,MatthayMA.Theacuterespiratorydistresssyndrome. NewEnglJMed.2000;342:1334---49.
5.Rubenfeld GD, Herridge MS. Epidemiology and outcomes of acutelunginjury.Chest.2007;131:554---62.
6.Pang YL, Chen BS, Li SP, et al. The preconditioning pul-monaryprotective effectofvolatile isoflurane inacute lung injuryismediatedbyactivationofendogenousiNOS.JAnesth. 2012;26:822---8.
7.LiQF,ZhuYS,JiangH,etal.Isofluranepreconditioning amelio-ratesendotoxin-inducedacutelunginjuryandmortalityinrats. AnesthAnalg.2009;109:1591---7.
8.Fang B, Li XQ, Bi B, et al. Dexmedetomidine attenuates blood---spinal cord barrier disruption induced by spinal cord ischemia reperfusion injury in rats. Cell Physiol Biochem. 2015;36:373---83.
9.Wang H, Chen H, Wang L, et al. Acute hyperglycemia prevents dexmedetomidine-induced preconditioning against renal ischemia-reperfusion injury. Acta Cir Bras. 2014;29: 812---8.
10.TanF,ChenY,YuanD,etal.Dexmedetomidineprotectsagainst acutekidneyinjurythroughdownregulatinginflammatory reac-tionsinendotoxemiarats.BiomedRep.2015;3:365---70. 11.BagcikE,OzkardeslerS,BoztasN,etal.Effectsof
dexmedeto-midineinconjunctionwithremoteischemicpreconditioningon renalischemia-reperfusioninjuryinrats.RevBrasAnestesiol. 2014;64:382---90.
12.Wang L, Huang H, Fan Y, et al. Effects of downregulation of microRNA-181a on H2O2-induced H9c2 cell apoptosis via themitochondrial apoptotic pathway. Oxid MedCellLongev. 2014;2014:960362.
13.Dong S, QuX, Li W, et al. Thelong non-coding RNA, GAS5, enhances gefitinib-induced cell death in innate EGFR tyro-sinekinaseinhibitor-resistantlungadenocarcinomacellswith wide-typeEGFRviadownregulationoftheIGF-1Rexpression.J HematolOncol.2015;8:43.
14.ZhangXY,CaoJB,ZhangLM,etal.Deferoxamineattenuates lipopolysaccharide-induced neuroinflammation and memory impairmentinmice.JNeuroinflamm.2015;12:20.
15.Cui K, Kou JQ, GuJH,et al. Najanaja atra venom amelio-rates pulmonary fibrosis byinhibitinginflammatory response and oxidative stress. BMC Complement Altern Med. 2014; 14:461.
16.LenazG.Roleofmitochondriainoxidativestressandageing. BiochimBiophysActa.1998;1366:53---67.
17.Xiao J, Rui Q, Guo Y, et al. Prolonged manganese expo-sure induces severe deficits in lifespan, development and reproductionpossiblybyalteringoxidativestressresponsein Caenorhabditiselegans.JEnvironSci.2009;21:842---8. 18.BenovL,Batinic-HaberleI.Amanganeseporphyrinsuppresses
oxidative stress andextends thelifespan of streptozotocin-diabeticrats.FreeRadicalRes.2005;39:81---8.
19.Shou-ShiW, Ting-TingS,Ji-ShunN,etal.Preclinicalefficacy ofdexmedetomidineonspinalcordinjuryprovokedoxidative renaldamage.RenFail.2015;37:1190---7.
20.LiS,YangY,YuC,etal.Dexmedetomidineanalgesiaeffectsin patientsundergoingdentalimplantsurgeryanditsimpacton postoperativeinflammatoryandoxidativestress.OxidMedCell Longev.2015;2015:186736.
21.LinWC,ChenCW,HuangYW,etal.Kallistatinprotectsagainst sepsis-relatedacutelunginjuryviainhibitinginflammationand apoptosis.SciRep.2015;5:12463.
22.KimW,YounH,KangC,etal.Inflammation-induced radiore-sistanceismediatedbyROS-dependentinactivationofprotein phosphatase1 innon-smallcelllungcancercells.Apoptosis. 2015;20:1242---52.
24.ZhaoJ,Li X,ZouM,etal.miR-135ainhibitionprotectsA549 cellsfrom LPS-inducedapoptosis bytargetingBcl-2.Biochem BiophysResCommun.2014;452:951---7.
25.Wolter KG, HsuYT, Smith CL, et al. Movementof Bax from the cytosol to mitochondria during apoptosis. J Cell Biol. 1997;139:1281---92.
26.HaunstetterA,IzumoS.Apoptosis:basicmechanismsand impli-cationsforcardiovasculardisease.CircRes.1998;82:1111---29. 27.Hengartner MO. The biochemistry of apoptosis. Nature.
2000;407:770---6.
28.VeenaVK,PopavathRN,KennedyK,etal.Invitro antiprolif-erative, pro-apoptotic, antimetastatic and anti-inflammatory potentialof2,4-diacteylphloroglucinol(DAPG)byPseudomonas aeruginosastrainFP10.Apoptosis.2015;20:1281---95.
29.Lee US, Ban JO, Yeon ET, et al. Growth inhibitory effect of (E)-2,4-bis(p-hydroxyphenyl)-2-butenal diacetate through induction of apoptotic cell death by increasing DR3 expres-sioninhumanlungcancercells.BiomolTher(Seoul).2012;20: 538---43.
30.vanHeerdeWL, Robert-OffermanS, Dumont E,et al. Mark-ersofapoptosisincardiovasculartissues:focusonAnnexinV. CardiovascRes.2000;45:549---59.
31.ChenSL,ZhouW,HuaFZ,etal.Invitroeffectof dexmedeto-midineontherespiratoryburstofneutrophils.GenetMolRes. 2016:15.