ARTIGOS
Genetic variability of
Puccinia triticina
Eriks. in Brazil
Vânia Bianchin
1; Amarilis Labes Barcellos
2; Erlei Melo Reis
3; Camila Turra
41Enga.-Agra., doutoranda do Programa de Pós-graduação em Agronomia (PPGAgro) da Faculdade de Agronomia e Medicina Veterinária (FAMV) da Universidade de Passo Fundo (UPF). 2Enga.-Agra, Doutora em Fitopatologia, pesquisadora, OR Melhoramento de Sementes Ltda. 3Eng.-Agr., Ph.D. em Fitopatologia, professor do PPGAgro e FAMV da UPF. 4Bióloga, OR Melhoramento de Sementes Ltda.
Autor para correspondência: Erlei Melo Reis (erleireis@ upf.br) Data de chegada: 10/07/2011. Aceito para publicação em: 13/02/2012.
1768
RESUMO
Studies on the genetic variability of Puccinia triticina in inoculum collected in Brazil started in 1941 with Vallega (20). The pioneering work in Brazil dates from 1949 (16) at “Instituto Agronômico do Sul”, Ministry of Agriculture (MA), in Pelotas, Rio Grande do Sul State (RS), and continued after 1975 at Embrapa Wheat in Passo Fundo, RS. In 2002, analyses for the identification of P. triticina
races continued at OR Seed breeding, simultaneously to Embrapa’s program, both in Passo Fundo. The investigators involved in the identification of races in Brazil were Ady Raul da Silva in Pelotas (MA), Eliza Coelho in Pelotas (MA) and in Passo Fundo (Embrapa), Amarilis Labes Barcellos in Pelotas (MA) and in Passo Fundo (Embrapa and OR), Camila Turra in Passo Fundo (OR) and Marcia Chaves in Passo Fundo (Embrapa). From 1979 to 2010 growing season, 59
Bianchin, V.; Barcellos, A.L.; Reis, E.M.; Turra, Camila. Genetic variability of Puccinia triticina Eriks. in Brazil. Summa Phytopathologica, v.38, n.2, p.113-118, 2012.
Palavras-chave adicionais: Ferrugem da folha, Triticumaestivum, raças.
races were determined, according to the differentiation based on the expression of each Lr resistance gene. On average, one to three new races are detected per year. Research has focused on the use of vertical resistance; however, lately some institutes have searched more durable resistance, of the adult-plant type (horizontal, less race-specific). The uninterrupted monitoring of the w h e a t r u s t pathogenic population i n B r a z i l during so many decades allowed the understanding of the evolution and virulence of races. The use of international nomenclature adopted by some programs has allowed the comparison of the fungus variability in Brazil with that in other countries, especially where frontiers are not barriers for spore transportation, confirmed by the occurrence of the same races all over one region.
Os estudos da variabilidade genética de Puccinia triticina em inóculo coletado no Brasil começaram em 1941 por Vallega. O trabalho pioneiro no Brasil teve início em 1949 no Instituto Agronômico do Sul, Ministério da Agricultura (MA) em Pelotas, RSs, depois de 1975 na Embrapa Trigo em Passo Fundo, RS. Em 2002 prosseguiram as análises de identificação de raças na OR Melhoramento de Sementes, paralelamente ao programa da Embrapa, ambos em Passo Fundo. Trabalharam na identificação de raças no Brasil, Ady Raul da Silva em Pelotas (MA), Eliza Coelho em (Pelotas (MA) e em Passo Fundo (Embrapa), Amarilis Labes Barcellos em Pelotas (MA) e em Passo Fundo (Embrapa e OR), Camila Turra em (Passo Fundo (OR) e Márcia Chaves em (Passo Fundo (Embrapa). Até a safra 2010, 59 raças já foram determinadas, considerando-se a partir de 1979, de acordo
Bianchin, V.; Barcellos, A.L.; Reis, E.M.; Turra, Camila. Variabilidade genética de Puccinia triticina Eriks. no Brasil. Summa Phytopathologica, v.38, n.2, p.113-118, 2012.
com a diferenciação pela expressão de cada gene de resistência Lr. Na média surge uma a três raças por ano. A pesquisa tem se concentrado no uso da resistência vertical, porém, ultimamente, algumas instituições têm buscado a resistência mais durável, do tipo planta adulta (horizontal, menos específica a raças). A não interrupção do monitoramento da população patogênica da ferrugem da folha do trigo no Brasil durante tantas décadas possibilitou conhecer a evolução das raças e da virulência. Embora tenha havido esforço de alguns programas O uso da nomenclatura internacional adotado por alguns programas tem permitido a comparação da variabilidade do fungo ocorrida no Brasil com a de outros países e especialmente com aqueles onde as fronteiras não são barreiras para o transporte dos esporos, como confirmado pela ocorrência de mesmas raças em toda a região.
Additional keywords: Leaf rust, Triticum aestivum, races.
ABSTRACT
Wheat leaf rust caused by Puccinia triticina Eriks. is one of the most important diseases of the crop worldwide. Many years ago, Silva et al. (17) considers the adult plant resistance of the Brazilian
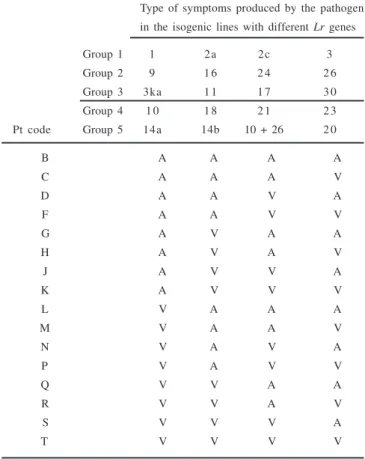
Table 1. Reaction code of 19 genes of a differential cultivar set to
Puccinia triticina (Pt), separated into five groups of four genes
The result of group 1 will lead to the first letter of the race code; group 2, the second letter; and group 3, the third letter. Similarly, for groups 4 and 5, the fourth and fifth letters, respectively.
A = avirulent; V = virulent. Adapted from Long & Kolmer (9).
Type of symptoms produced by the pathogen in the isogenic lines with different Lr genes
Group 1 1 2a 2c 3
Group 2 9 1 6 2 4 2 6
Group 3 3ka 1 1 1 7 3 0
Group 4 1 0 1 8 2 1 2 3
Pt code Group 5 14a 14b 10 + 26 2 0
B A A A A
C A A A V
D A A V A
F A A V V
G A V A A
H A V A V
J A V V A
K A V V V
L V A A A
M V A A V
N V A V A
P V A V V
Q V V A A
R V V A V
S V V V A
T V V V V
Secretariat of Agriculture of Rio Grande do Sul State decided not to study the basic principles, while “IPEAS”, Ministry of Agriculture – (MA) continued the surveys on wheat leaf rust and evaluations at seedling stage, as supplemental information. This guideline resulted in regression and leaf rust became one of the most important wheat diseases.
This is one of the most studied diseases and has worldwide importance. The dynamic nature of the fungus, the annual occurrence of the disease and the cultivation of genotypes with specific resistance favor the emergence of new races. The search for genotypes with durable resistance (adult plant resistance, horizontal resistance or non-specific resistance), which allows the maintenance of resistance effectiveness over the years, has been a major challenge for plant breeding programs.
The pathogen co-evolves to adapt to new resistant genotypes introduced by the breeding programs as the area of new cultivars increases. This co-evolution is noticed by the interaction of pathogen genes with the host genes, demonstrating the fungus capability to adapt.
The change in the pathogen population results in new physiological races, not morphologically but physiologically different and having the ability to infect different wheat cultivars (12).
The aim of this study was to gather the information in the literature and consult wheat leaf rust experts regarding the variability of the fungus Puccinia triticina Eriks. in Brazil. Most data were not published or are difficult to be found.
Methodology for race identification
Initially, a literature survey on the early work on the identification of P. triticina races in Brazil was conducted to recover the races found until 2008. For the identification of races occurring in 2008 and 2009, infected wheat leaves were collected and the races were identified.
Sampling was done in wheat fields over all growing areas of Brazil. The rust-infected leaves were collected and sealed in wax paper bags and brought to the rust laboratory of OR Seed Breeding Ltd., located in Passo Fundo – RS, where they were stored in refrigerator at 5oC.
Inoculum isolation and multiplication were done in living plants due to the condition of the biotrophic parasite. Thus, the cultivars susceptible to all races (Devoid of Lr seedling genes), PG1 and
Morocco, were used. The inoculation was done at seedling stage 11 (First expanded leaf) (17), initially removing plant serosity by moistening the leaves and passing hand fingers. Uredospores were scraped from the leaves and transferred to the plants by using a spatula. Then, inoculated plants were sprayed with water + Tween 20 (Polyoxiethylenesorbitane monolaurate - Synth - four drops per liter of water). The incubation period was 20 to 24 hours in the dark at 20°C and 100% relative humidity. Once the inoculum had multiplied, the spores were collected and stored in gelatin capsules, using suitable collectors coupled to a vacuum pump.
To identify the race, a suspension of uredospores in Soltrol mineral oil was sprayed on a differential set of wheat isogenic lines (Table 1) using a proper sprayer adapted to a compressed air pump. Inoculation was separately done with each individual rust sample.
The races are identified based on the symptoms that each gene Lr
can express (10) and grouped according to the nomenclature system for North America (9) and the Brazilian correspondent (Brazilian nomenclature for race classification) for the response avirulence/
virulence genes, concerning 19 known Lr (Leaf rust) genes that were previously inserted in isogenic lines of Thatcher cultivar: Lr1, Lr2a, Lr2c, Lr3, Lr3ka, Lr9, Lr10, Lr11, Lr14a, Lr14b, Lr16, Lr17, Lr18,
Lr20, Lr21, Lr23, Lr24, Lr26 and Lr30 (Table 1). The following differentials were added to the main series: Lr3bg, Lr19, Lr27+Lr31
in addition to a gene not yet cataloged and important to distinguish the actual predominant race.
To achieve the classification proposed by Long & Kolmer (9), one must consider the scale adapted by Roelfs and Martens (14), which determines whether or not the pathogen is virulent (Figure 1 and Table 2).
The reaction pattern of the virulence set of ineffective Lr genes for resistance, the determination of the North American code and the search in the literature allows learning whether or not the pathogen is a new physiologic race.
Studies on the physiologic specialization of P. triticina in Brazil started in 1941 by Vallega (20). During that period, the races were not named as they are today, using the letter “B” and the corresponding number. They were simply referred to as the corresponding number in identification order, e.g. races 19, 64 and 105 (1).
Figure 1. Scale to assess the reaction of close isogenic seedling lines to
Puccinia triticina (Photo: Singh, R.P.).
Table 2. Description of infection type and symptoms caused by Puccinia triticina in whet leaves
0 Low No uredinia or other macroscopic sign of infectiton 0; Low Few faint flecks
; Low No uredinia, but hypersensitive necrotic or chlorotic flecks present
1 Low Small uredinia often surrounded by necrosis 2 Low Small-to-medium uredinia often
surrounded by chlorosis
Y Low Ordered distribution of variable-sized uredinia, largest at the leaf tip
X Low Random distribution of variable-sized uredinia 3 High Medium-sized uredinia without chlorosis or necrosis 4 High Large uredinia without chlorosis or necrosis
The infection types are often refined by modifying characters as follows
(=) uredinia at the lower size limit for the infection type
(-) uredinia somewhat smaller than normal for the infection type
(+) uredinia somewhat larger than normal for the infection type
(++) uredinia at the upper size limit for the infection type
C more chlorosis than normal for the infection type
N more necrosis than normal for the infection type
Adapted from Roelfs & Martens (1988).
Infection type
Low
/High Symptoms
al. (15) and Cenoz (5).
Races 25 and 26 were determined for the first time in 1961, race 27 in 1962, and race 28 in 1970. Among the races identified between 1958 and 1974, the most important occurring races were 4 and 19. In 1975 and 1976, the pathogen population has changed and race 19 lost its importance (1).
Since 1977, P. triticina races have been identified according to the virulence combination obtained by the reaction of lines carrying the
Lr resistance genes. In 1978, virulence formulae were named with the letter “B” (Brazil) followed by the number in order of identification (1). From races B1 to B9, the differential set was different from that used in the subsequent years; therefore, it was not possible to compare these races with B10 to B58 (Last race with Brazilian nomenclature) (Table 3 and 4). Races identified by the early 80s have information of effective and ineffective Lr genes; however, there are difficulties in determining the old races using the North American current code because not all genes that are considered today were part of the differential set (Table 3).
From 1978 to 1980, races B12, B10, B14 and B11 were most expressive, respectively (1). In 1984 and 1985, the most important race was B25 (2).
From 1990 to 1993, seventeen races were identified. In 1990, the most important race was B32; in 1991, B35; in 1992, B38; and in 1993, B25. In terms of importance during this period, races B25 and B35 can be highlighted (11).
From 1999 to 2002, the predominant races were B40 and B48, corresponding in 2002 to the frequency of 41% and 12%, respectively (6). In 2004, race B55 M(DF)T-M(RT) (Lr virulence: Lr1, Lr3, Lr3ka, Lr10, Lr11, Lr14a, Lr14b, Lr17, Lr20, Lr23, Lr24, Lr26, Lr30) was first identified and, from 2005, predominated, causing damage in all wheat growing areas. In 2007, this race underwent a modification and was named B55 4002S, M(DF)T-M(RT) 4002S (Lr virulence: Lr1, Lr3, Lr3ka, Lr10, Lr11, Lr14a, Lr14b, Lr17, Lr20, Lr23, Lr24, Lr26, Lr30, 4002S, due to the virulence in the line OR 4002, while all genes of the current differential series express the same avirulence/virulence default). Race B55 4002S has dominated the leaf rust population from 2005 to the last still partial 2010 survey (Personal information, data OR Seed breeding).
In 2008 growing season, three new races were identified: TDP-MR, TPT-HT and TDP-HR (Table 4). From the samples handled in the Rust Laboratory of OR – Seed breeding, 102 isolates were obtained, 58% of these were identified as race B55 4002S, 16.5% as B57, 6% as B58, and 19.5% as other races.The predominant race in 2008 was B55 4002S.
The environmental conditions were not favorable to rust epidemics in 2009. During this season, 40 isolates were obtained, of which 75% were race B55 4002S. A new race was identified, probably a variant of race B58 identified in 2005. The new race showed the same susceptibility to wheat line 4002 [unknown gene(s)], thus B58 4002S, while 15% of the isolates were identified as B58 4002S.
Breeding as a control strategy
The best strategy to control wheat leaf rust is the development of resistant cultivars. Vertical resistance has no long-term duration, is easily defeated by the pathogen specialization, is easily obtained and widely used in breeding programs. On the other side, the adult plant resistance is long-lasting but more difficult to achieve and remains a major challenge for breeders.
Table 3. Early physiologic races of Puccinia triticina in Brazil according to the Brazilian nomenclature and identified by the virulence formulae Races Avirulence/virulence formulae
Effective Lr genes Ineffective Lr genes B1 2a, 2c, 2d, 9, 10, 16, 17, 18, 21 1, 3, 3ka, 14a B2 2a, 2c, 2d, 9, 16, 17, 18, 21 1, 3, 3ka, 10, 14a B3 2a, 3, 3ka, 9, 10, 16, 17, 18 1, 2c, 2d, 14a, 21 B4 2a, 3, 3ka, 9, 10, 16, 17, 18, 21 1, 2c, 2d, 14a
B5 2a, 3, 3ka, 9, 16, 17, 18 1, 2c, 2d, 10, 14a, 21
B6 2a, 3, 3ka, 9, 16, 17, 18, 21 1, 2c, 2d, 10, 14a
B7 2a, 3, 9, 16, 17, 18, 21 1, 2c, 2d, 3ka, 10, 14a
B8 3, 3ka, 9, 16 1, 2a, 2c, 2d, 10, 14a, 17, 18, 21
B9 3, 3ka, 9, 16, 21 1, 2a, 2c, 2d, 10, 14a, 17, 18
B10 2a, 3, 3ka, 9, 10, 16, 17, 18, 21 , 23, 24, 26 1, 2c, 2d, 14a, 14b B11 2a, 3, 3ka, 9, 10, 16, 17, 18, 21 , 24, 26 1, 2c, 2d, 14a, 14b, 23 B12 2a, 3, 3ka, 9, 10, 16, 17, 18, 21 , 23, 24, 26 1, 2c, 2d, 10, 14a, 14b B13 2a, 3, 9, 16, 17, 18, 21 , 23 1, 2c, 2d, 3ka, 10, 14a, 14b B14 3, 3ka, 9, 16, 21 , 23, 24, 26 1, 2a, 2c, 2d, 10, 14a, 14b, 17, 18, B15 1, 2a, 3, 3ka, 9, 10, 16,17, 18, 21, 23, 24, 26 2c, 2d, 14a,14b
B16 2a, 2c, 2d, 3, 3ka, 9, 10, 16,17, 18, 21, 24, 26 1, 14a, 14b, 23 B17 3, 3ka, 9, 10, 14a, 16, 21, 23, 24, 26 1, 2a, 2d, 17, 18 B18 2a, 2d, 9, 10, 16,17, 18, 21, 24, 26 1, 3, 3ka, 14a, 14b, 23 B19 1, 2a, 3, 3ka, 9, 10, 16, 17, 18, 21, 24, 26 2d, 14a, 14b, 23 B20 1, 2a, 3, 3ka, 9, 10, 16, 17, 18, 21, 23, 26 2d, 14a, 14b, 24 B21 2a, 2d, 3, 3ka, 9, 16, 17, 18, 21, 24, 26 1, 10, 14a, 14b, 23 B22 2a, 3, 3ka, 9, 16, 17, 18, 21, 24, 26 1, 2d, 10, 14a, 14b, 23 B23 2a, 2d, 3, 9, 16, 17, 18, 21, 24, 26 1, 3ka, 10, 14a, 14b, 23 B24 2a, 3, 3ka, 9, 10, 16, 17, 18, 21 , 23, 24, 26 1, 2d, 14a, 14b
the germplasm of the Southern Cone of South America region. The most common resistance genes in the Brazilian germplasm published in 2002 were Lr10, Lr23, Lr24 and Lr26 (21). For adult plants, the resistance genes Lr13 and Lr34 are present in Brazilian cultivars (21). Adult plant resistance can be effective by two other genes described in Toropi, Trp1 and Trp2 (3), and according to Brammer et al. (4), there is a third unidentified gene in BR35 cultivar.
In Argentina, the effective genes Lr9, Lr10, Lr19, Lr20, Lr24, Lr26, Lr34, Lr37 and Lr47 were identified in 98 recent cultivars, alone or in combinations of two or three (7). In Uruguay, Lr3, Lr10,
Lr14b, Lr16, Lr17a, Lr24, Lr26 were found for seedling resistance and Lr13 and Lr34 for adult plant resistance (8).
There are other genes that express adult plant resistance, some less race-specific than others and consequently lasting longer and shorter. Genes Lr35, Lr37 and Lr22a are race-specific for adult plant resistance. Lr34 and Lr46 are genes for adult plant resistance,
non-race-specific.
Some additive minor genes confer resistance to adult plants and have been used in breeding programs (18). Some examples of materials distributed by CIMMYT with these minor additive genes are Parula (Lr34, Lr46 + one or two minor genes), Chapio (Lr34 + three or four
minor genes), Amadina (four minor genes) among other lines with this type of resistance (19). These materials distributed by CIMMYT are used in crosses with adapted material in breeding programs in South America.
Adopting the methodology of ‘One Backcross’, divulged by Ravi Singh, from CIMMYT, the OR Seed breeding wheat leaf rust program developed lines in one same adapted OR cultivar, each of them with the following resistances: Amadina, Chapio, Frontana, Kukuna, Tukuru and Toropi.
The pyramiding of genes in order to obtain additive effect by them is a viable strategy. In a single cultivar, through breeding techniques, multiple genes are introduced in order to achieve greater efficiency and most enduring resistance to the fungus; however, the disadvantage is to expose many genes at once at risk of pathogen adaptation. Another strategy is the genetic control by means of cultivation of different combinations of genes in different epidemiological zones and the inclusion of gene rotation. However, this practice is difficult to implement as it requires commitment by all people involved in the cultivation (13).
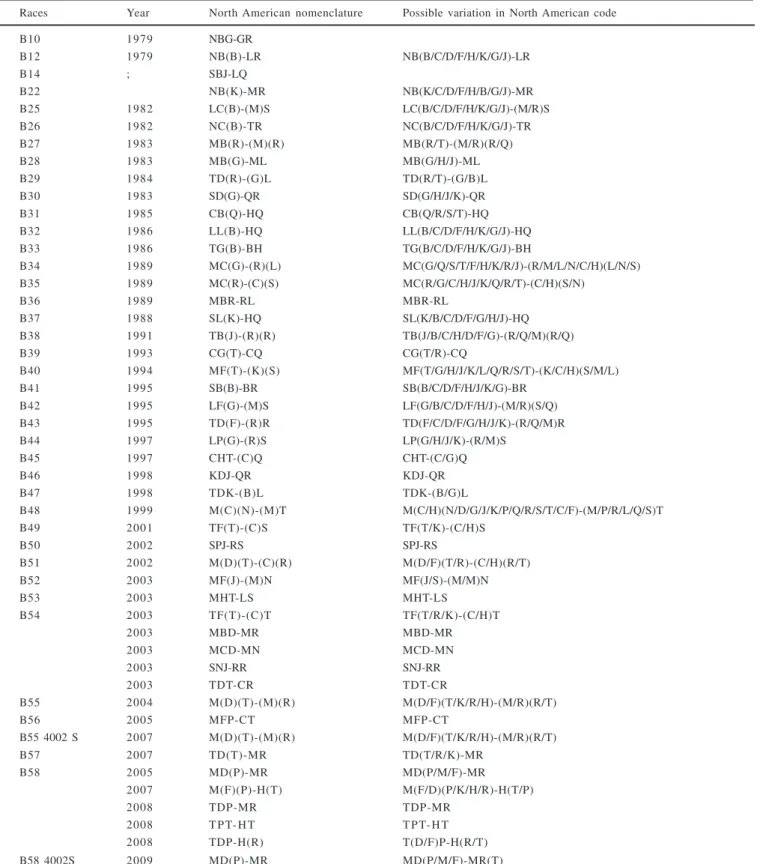
Table 4. Physiologic races of Puccinia triticina (Brazilian nomenclature) identified in Brazil, year of identification, United States code and corresponding genes of possible variations from the differential set
Races Year North American nomenclature Possible variation in North American code
B10 1979 NBG-GR
B12 1979 NB(B)-LR NB(B/C/D/F/H/K/G/J)-LR
B14 ; SBJ-LQ
B22 NB(K)-MR NB(K/C/D/F/H/B/G/J)-MR
B25 1982 LC(B)-(M)S LC(B/C/D/F/H/K/G/J)-(M/R)S
B26 1982 NC(B)-TR NC(B/C/D/F/H/K/G/J)-TR
B27 1983 MB(R)-(M)(R) MB(R/T)-(M/R)(R/Q)
B28 1983 MB(G)-ML MB(G/H/J)-ML
B29 1984 TD(R)-(G)L TD(R/T)-(G/B)L
B30 1983 SD(G)-QR SD(G/H/J/K)-QR
B31 1985 CB(Q)-HQ CB(Q/R/S/T)-HQ
B32 1986 LL(B)-HQ LL(B/C/D/F/H/K/G/J)-HQ
B33 1986 TG(B)-BH TG(B/C/D/F/H/K/G/J)-BH
B34 1989 MC(G)-(R)(L) MC(G/Q/S/T/F/H/K/R/J)-(R/M/L/N/C/H)(L/N/S)
B35 1989 MC(R)-(C)(S) MC(R/G/C/H/J/K/Q/R/T)-(C/H)(S/N)
B36 1989 MBR-RL MBR-RL
B37 1988 SL(K)-HQ SL(K/B/C/D/F/G/H/J)-HQ
B38 1991 TB(J)-(R)(R) TB(J/B/C/H/D/F/G)-(R/Q/M)(R/Q)
B39 1993 CG(T)-CQ CG(T/R)-CQ
B40 1994 MF(T)-(K)(S) MF(T/G/H/J/K/L/Q/R/S/T)-(K/C/H)(S/M/L)
B41 1995 SB(B)-BR SB(B/C/D/F/H/J/K/G)-BR
B42 1995 LF(G)-(M)S LF(G/B/C/D/F/H/J)-(M/R)(S/Q)
B43 1995 TD(F)-(R)R TD(F/C/D/F/G/H/J/K)-(R/Q/M)R
B44 1997 LP(G)-(R)S LP(G/H/J/K)-(R/M)S
B45 1997 CHT-(C)Q CHT-(C/G)Q
B46 1998 KDJ-QR KDJ-QR
B47 1998 TDK-(B)L TDK-(B/G)L
B48 1999 M(C)(N)-(M)T M(C/H)(N/D/G/J/K/P/Q/R/S/T/C/F)-(M/P/R/L/Q/S)T
B49 2001 TF(T)-(C)S TF(T/K)-(C/H)S
B50 2002 SPJ-RS SPJ-RS
B51 2002 M(D)(T)-(C)(R) M(D/F)(T/R)-(C/H)(R/T)
B52 2003 MF(J)-(M)N MF(J/S)-(M/M)N
B53 2003 MHT-LS MHT-LS
B54 2003 TF(T)-(C)T TF(T/R/K)-(C/H)T
2003 MBD-MR MBD-MR
2003 MCD-MN MCD-MN
2003 SNJ-RR SNJ-RR
2003 TDT-CR TDT-CR
B55 2004 M(D)(T)-(M)(R) M(D/F)(T/K/R/H)-(M/R)(R/T)
B56 2005 MFP-CT MFP-CT
B55 4002 S 2007 M(D)(T)-(M)(R) M(D/F)(T/K/R/H)-(M/R)(R/T)
B57 2007 TD(T)-MR TD(T/R/K)-MR
B58 2005 MD(P)-MR MD(P/M/F)-MR
2007 M(F)(P)-H(T) M(F/D)(P/K/H/R)-H(T/P)
2008 TDP-MR TDP-MR
2008 T P T- H T T P T- H T
2008 TDP-H(R) T(D/F)P-H(R/T)
B58 4002S 2009 MD(P)-MR MD(P/M/F)-MR(T)
Data from the literature and Dr. Amarilis Labes Barcellos, personal information. Letters in brackets mean the possible race variations due to response instability of some genes on the basis of environmental conditions.
Vertical resistance has been used by most Brazilian wheat breeding programs which subjected P. triticina to a high selecting pressure, resulting in a vicious cycle of new resistant cultivar – new virulent race.
Due to the variability of P. triticina in Brazil from 1977 (beginning of the use of the differential cultivar set with isolated resistance genes) to 2009, an average of two new races per year are identified.
In 2008, the predominant race was B55 4002S and three new races, TDP-MR, TPT-HT and TDP-HR, were identified.
In 2009, the prevalent race was again B55 4002S and as a new race, MDP MR-4002S, a possible change in race B58 (MDP-MR), was identified.
The use of the international methodology and nomenclature should be pursued for all South American countries with some adaptation.
REFERENCES
01. Barcellos, A.L. As ferrugens do trigo no Brasil. In: Fundação Cargill, Campinas, SP. Trigo no Brasil. Campinas, 1982, v.2, p. 375-419.
02. Barcellos, A.L. Ferrugem da folha do trigo no Brasil em 1984 e 1985 – Ocorrência e virulência. In: Resultados de pesquisa do Centro Nacional de Pesquisa de Trigo apresentados na XIV Reu-nião Nacional de Pesquisa de Trigo – RENAPET, Londrina, 1986. P. 117-134.
03. Barcellos, A.L.; Roelfs A.P.; Moraes-Fernandes, M.I.B. Inheri-tance of adult plant leaf rust resisInheri-tance in the Brazilian wheat cultivar Toropi. Plant Dis, v.84, p. 90-93. 2000.
04. Brammer, S.P.; Moraes-Fernandes, M.I.B.; Barcellos, A.L.; Mila-ch, S.C.K. Genetic analysis of adult-plant resistance to leaf rust in a double haploid wheat (Triticum aestivum L. em Thell.) popu-lation. Genet Mol Biol, v. 27, p. 432-436. 2004.
05. Cenoz, H. Primeira reunión inmunologica de cereales da región sudeste de America Del Sul. Robigo, v.12, p. 14-16, 1961.
06. Chaves, M.S. & BARCELLOS, A.L. Especialização fisiológica de Puccinia triticina no Brasil em 2002. Fitopatologia Brasileira 31:057-062. 2006.
07. Demichellis, M.; Vanzetti, L.; Bainotti, C.; Campos, P. Identifi-cacion de genes Lr presentes em germoplasma argentino de trigo hexaploide (Triticum aestivum L.), mediante tecnicas molecula-res. In: VII Congresso Nacional de Trigo, 2008, Santa Rosa, La Pampa, Argentina. INTA/Universidad Nacional de La Pampa, Santa Rosa. 2008.
08. Germán, S.; Chaves, M.; Campos, P.; Viedma, L.; Madariaga, R. Are rust pathogens under control in the Southern Cone of South America? In: Technical Workshop – Borlaug Global Rust Initiati-ve (BGRI). Obregon, México, 2009. p. 65-73. 2009.
09. Long, D. L. and Kolmer, J. A. A North American System of Nomenclature for Puccinia triticina. Phytopathology 79:525-529, 1989.
10. Mcintosh, R. A.; Wellings, C. R.; Park, R. F. Methodologies in wheat rust diseases. In: Wheat Rusts an atlas of resistance genes. CSIRO, Australia. p. 7-20. 1995.
11. Medeiros, M.C.; Barcellos, A.L. Ferrugem da folha do trigo -ocorrência e distribuição geográfica de raças fisiológicas, no perí-odo de 1990 a 1993, no Brasil. In: XVII Reunião Nacional de Pesquisa de Trigo – RENAPET, Embrapa cnpt. Passo Fundo, 1994 - Resumos. P. 84.
12. Reis, E.M. Doenças do trigo V: Ferrugens. São Paulo, 1991. 20p. 13. Roelfs, A. P.; Singh, R. P. y Saari, E. E. Las royas del trigo: Conceptos y métodos para el manejo de esas enfermedades. Mé-xico, D.F.: CIMMYT. 1992. 81pp.
14. Roelfs, A.P. & Martens, J.W. An international system of nomen-clature for Puccinia graminis f. sp. tritici. Phytopathology, v.78, p. 526-533, 1988.
15. Silva, A.R.; Coelho, E.T.; Silva, A.V. Identificação de raças de ferrugem da folha do trigo no Brasil pelo uso de um novo grupo de variedades. Robigo, v.10, p. 7-12, 1960.
16. Silva, A.R.; Silva, A.V.; Rincon, R.P. Levantamento de raças fisi-ológicas de Puccinia graminis tritici e Puccinia rubigo-vera triti-ci no Brasi. Agros, 8 (l/2): 18-32, 1955.
17. Silva, A.R. et alii. Melhoramento para resistência do trigo às doenças e às pragas. IN: REUNIÃO LATINO AMERICANA DO TRIGO, Porto Alegre, 1974p.56-76
18. Ingh, R.P.; Huerta-Espino, J.; William, M. Resistencia durable a roya de la hoja y roya amarilla del trigo: genetica y mejoramiento en El Cimmyt. In: Kohli, M.M.; Diaz, M.; Castro, M. (eds). Es-trategias y metodologias utilizadas en el mejoramiento de trigo: un enfoque multidisciplinario, 8-11 oct 2001, La Estanzuela, Colonia, Uruguay. CIMMYT-INIA, Montevideo, p. 109-118. 2003.
19. Vallega, J. Especialization fisiologica de Puccinia graminis tritici
en Brasil. Anales del InstitutoFitotecnico de Santa Catalina, 3: 29-36.1941.
20. Zadoks, J.C.; Chang, T.T.; Konzak, C.F. A decimal code for the growth stages of cereals. Weed Research, Oxford, v.14, n.6, p.415-421, 1974.