A Validated HPTLC Method for Simultaneous Quantification of Nebivolol and Hydrochlorothiazide in Bulk and Tablet Formulation
Texto
Imagem
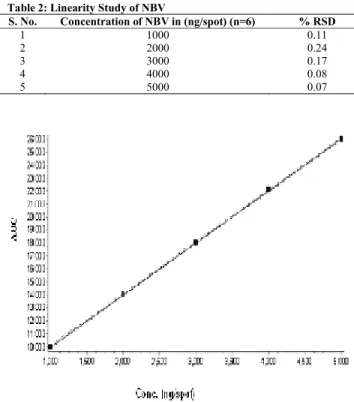
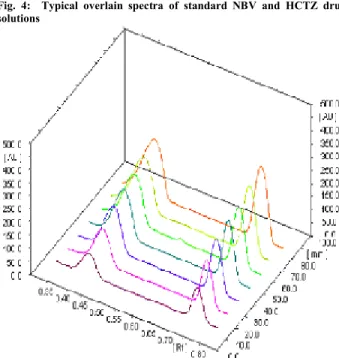
![Table 8: Results of Repeatability Studies S. No Application volume [µl] % RSD NBV [4000 ng/spot] (n=7) % RSD of HCTZ[1600 ng/spot] (n=7) 1 4 0.13 0.58 Specificity](https://thumb-eu.123doks.com/thumbv2/123dok_br/18202246.333633/4.918.96.453.164.217/table-results-repeatability-studies-application-volume-hctz-specificity.webp)
Documentos relacionados
In present study, rapid, validated HPTLC method has been established for the determination of piperine and piperlongumine in methanolic root extract and its commercial
Commercial capsule formulation was successfully analyzed using the developed method and the proposed method is applicable to routine analysis of determination of
The percentage of Levocetrizine in marketed formulation (Levocetrizine tablet) was calculated from the calibration curve of Levocetrizine. %Assay was found to be 98.78%
The following research was done in order to develop a simple, precise and accurate method for analysis of Fluconazole and Ivermectin in bulk and tablet
Several manipulations were performed on the raw overlapping spectral data to enable mixture resolution for example, using different order derivatives [1-11].The aim of the
A new simple high performance thin layer chromatographic method (HPTLC) for simultaneous determination of nabumetone and paracetamol in combined tablet dosage form was developed
A simple, accurate, precise, reproducible, highly sensitive, economic UV spectrophotometric method has been developed for the estimation of metaxalon in bulk and tablet dosage
A simple, sensitive, rapid, robust and reproducible method for the determination of Quetiapine Hemi Fumarate (QHF) in bulk and Pharmaceutical Formulation (Tablets) was developed