and Subsequent Vessel Maturation via Angiopoietin-1
Induce Functional Neovasculature after Ischemia
Di Qin1,2, Teresa Trenkwalder1,3, Seungmin Lee1, Omary Chillo3, Elisabeth Deindl3, Christian Kupatt1,4, Rabea Hinkel1,4*
1Medizinische Klinik und Poliklinik I, Klinikum Großhadern, Munich, Germany,2Department of Senile Disease, China-Japan Union Hospital of Jilin University, Changchun, Jilin, People’s Republic of China,3Walter-Brendel-Centre of Experimental Medicine, LMU Munich, Munich, Germany,4DZHK (German Centre for Cardiovascular Research), partner site Munich Heart Alliance, Munich, Germany
Abstract
Background:We assessed whether Angiopoietin-2 (Ang2), a Tie2 ligand and partial antagonist of Angiopoietin-1 (Ang1), is required for early vessel destabilization during postischemic angiogenesis, when combined with vascular growth factors.
Methods:In vitro, matrigel co-cultures assessed endothelial-cell tube formation and pericyte recruitment after stimulation of VEGF-A, Apelin (APLN), Ang1 with or without Ang2. In a murine hindlimb ischemia model, adeno-associated virus (rAAV, 361012virusparticles) transduction of VEGF-A, APLN and Ang1 with or without Ang2 (continuous or early expression d0-3)
was performed intramuscularly (d-14). Femoral artery ligation was performed at d0, followed by laser doppler perfusion meassurements (LDI) 7 and 14. At d7 (early timepoint) and d14 (late timepoint), histological analysis of capillary/muscle fiber ratio (CMF-R, PECAM-1) and pericyte/capillary ratio (PC-R, NG2) was performed.
Results:In vitro, VEGF-A, APLN and Ang1 induced ring formation, but only APLN and Ang1 recruited pericytes. Ang2 did not affect tube formation by APLN, but reduced pericyte recruitment after APLN or Ang1 overexpression. In vivo, rAAV.VEGF-A did not alter LDI-perfusion at d14, consistent with an impaired PC-R despite a rise in CMF-R. rAAV.APLN improved perfusion at d14, with or without continuous Ang2, increasing CMF-R and PC-R. rAAV.Ang1 improved perfusion at d14, when combined with rAAV.Ang2 (d0-3), accompanied by an increased CMF-R and PC-R.
Conclusion:The combination of early vessel destabilization (Ang2 d0-3) and continuous Ang1 overexpression improves hindlimb perfusion, pointing to the importance of early vessel destabilization and subsequent vessel maturation for enhanced therapeutic neovascularization.
Citation:Qin D, Trenkwalder T, Lee S, Chillo O, Deindl E, et al. (2013) Early Vessel Destabilization Mediated by Angiopoietin-2 and Subsequent Vessel Maturation via Angiopoietin-1 Induce Functional Neovasculature after Ischemia. PLoS ONE 8(4): e61831. doi:10.1371/journal.pone.0061831
Editor:Rudolf Kirchmair, Medical University Innsbruck, Austria
ReceivedAugust 24, 2012;AcceptedMarch 14, 2013;PublishedApril 16, 2013
Copyright:ß2013 Qin et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Funding:This work was in part supported by Deutsche Forschungsgemeinschaft Grant KU 1019/11-2 to CK and a Fo¨FoLe grant to TT. No additional external funding was received for this study. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Competing Interests:The authors have declared that no competing interests exist. * E-mail: rabea.hinkel@med.uni-muenchen.de
Introduction
Molecular evidence demonstrates the feasibility of vessel growth in ischemic muscle tissue (for review cf. [1,2]). If applied to patients suffering from manifest atherosclerosis of the heart or peripheral vessels, this fundamental principle would add to the therapeutic armamentarium of ischemic muscle disease. The surgical or interventional treatment options, though highly efficient and constantly improved, may at times be exhausted leaving a growing number of no option patients without functional revascularization. Earlier trials with neovascularization therapy yielded inconsis-tent results [3] which indicated a necessity to further improve therapeutic agents or combinations thereof in order to translate the biological principle of balanced vascular growth into treatment. Currently, three distinct processes of neovascularization appear as minimal requirements for efficient vascular therapy: capillary growth (angiogenesis) and pericyte recruitment
(matura-tion), followed by conductance vessel growth (arteriogenesis) [2]. In the recent years, microvessel maturation advanced to become an important target of therapeutic neovascularization, the lack of which would impair the efficient perfusion of microvessels and the successful growth of conductance vessels [4–6].
immature capillaries lacking the investment of mural cell are prone to capillary regression and fading of an initial angiogenic response [7].
The Angiopoietin/Tie2 system regulates vascular maturation during development and postnatal angiogenesis. Ang1 binding to the Tie2 receptor has emerged as an essential event in the maintenance of a quiescent vasculature [8]. Ang1 acts by strengthening the contact between endothelial cells as well as endothelial cells and pericytes [9] and by increasing the migration of mural cells towards endothelial cells [10,11]. Of note, Ang1 is also capable of induction of endothelial cell proliferation and migration [12,13], and modulates VEGF-A induced neovascular-ization in vivo [14,15]. Angiopoietin-2, in contrast, competes with Ang1 binding at the level of its receptor Tie2 inducing destabilization and regression of microvessels [16]. Ang2 trans-genic mice reveal an impaired neovascularization after hindlimb ischemia induction [17].
For therapeutic neovascularization, a delicate balance appears desirable: whereas early destabilization may support the initial capillary proliferation process, subsequent maturation is required for the development of functional and lasting microvascular networks. In accordance, resistance to early destabilization may inhibit the sprouting of neovascularization and reduce the angiogenic response. Therefore, controlled early destabilization followed by enforced maturation of neo-vessels may constitute an attractive and novel concept of therapeutic angiogenesis in vivo. In the present study, we tested this hypothesis by evaluating Ang2 in combination therapies of three distinct profiles: In a first series, we assessed the combination of Ang2 and VEGF-A, a growth factor efficiently inducing capillary proliferation, but weakly providing capillary maturation [6,7]. Second, we tested the combination of Ang2 and APLN, a growth factor providing both, capillary proliferation and maturation, leading to stable and non-leaky vessels [18]. Third, we assessed the potential of early Ang2-induced destabilization (d0-3) on top of continuous Ang1-overexpression providing neovessel maturation.
Materials and Methods
Animals
Animal care and all experimental procedures were performed in strict accordance to the German and NIH animal legislation guidelines and were approved by the Bavarian Animal Care and Use Committee (AZ 55.2-1-54-2531-130-8 and 35-12). All animal experiments were conducted at the Walter Brendel Centre of Experimental Medicine.
Plasmids and virus
For cell transient transfection we used plasmid cDNA contain-ing target gene (pcDNA, hVEGF-A, mAPLN, mAng2 and hAng1) controlled by the constitutive viral promoter cytomegalovirus (CMV). The tet-off gene expression system was utilized for mAng2 conditional turn-off by doxycycline (Dox) in the late stage. It contains two critical components: one is regulatory protein TetR and VP16. The latter converts the former to the hybrid protein tetracycline-controlled transactivator (tTA). Another is the re-sponse plasmid expressing the target gene with the minimal CMV promoter (PminCMV), and a tetracycline response element (TRE) upstream of the tTA which activates transcription in the absence of Dox. Production of recombinant adeno-associated viral vector was performed using a triple transfection method as described earlier [19]. In brief, plasmid one serves as a helper plasmid, plasmid two encodes for the AAV rep and cap sequences and the third plasmid encodes for the transgen including the CMV
promoter. rAAV production is performed via U293 cell transfec-tion and caesium gradient purificatransfec-tion of the vectors [20]. Titer of the rAAVs was determined using rt-PCR utilizing the polyA tail of the vector bGH (primers: tctagttgccagccatctgttgt-3 forward, 5’-tgggagtggcaccttcca-3’ reverse). Trans and helper plasmids were kindly provided by James M. Wilson, University of Pennsylvania. rAAV vectors were produced for the transgenes: CMV-LacZ, CMV-hVEGF-A, CMV-mAPLN, CMV-mAng2, CMV-tetoff-mAng2 and CMV-hAng1.
Quantitative real-time PCR analysis
Successful transfections were confirmed by quantitative real-time PCR analysis (BIO-RAD, MyiQ, single color Real-Time PCR Detection System, Cat.No. 170-9740). The AAV vectors were applied to ischemic limb 14 days before artery ligation via i.m. injection. Muscle tissue of the lower limb (left and right gastrogenmius muscle) was obtained at day 7 after femoral artery ligation. qRT-PCR was performed as followed: Total RNA was extracted by using the Trizol Reagent-RNA/DNA/Protein Isolation Reagent (Molecular Research Center, Cat.No. TR-118) and transcribed into cDNA using IQ SYBR,RGREEN Supermix (BIO-RAD, Cat.No. 170-8882). Primers for detection of overex-pression were: VEGF-A forward (59- TTT TAC GCT ATG TGG ATA CGC-39) and reverse (59-AAG AGA CAG CAA CCA GGA TTT-39); Ang2 forward (59-TCG AAT ACG ATG ACT CGG TG -39) and reverse (59-GTT TGT CCC TAT TTC TAT C -39); APLN forward (59-GGC CAT CAC CAG CCA TTC CTT G-39) and reverse (59-GCA GCG TTA GCA GCA GCA TAG-39); Ang1 forward (59- GGT GGT TTG ATG CTT GTG G -39) and reverse (59- GGA TTC TAG TTG TGG TTT GTG-39); S18 forward (59-GACCCATTCGAACGTCGTCCCTATCAA-39) and reverse (59 -GTAATTTGCGCGCTTGCTGCCTTCCTT-39). The baseline and threshold were adjusted according to the manufacturer’s instructions. The relative abundance of transcripts was normalized using the expression level of S18 mRNA. The data of the treated ischemic leg were compared to the sham operated leg and to non-treated control animals (Fig. S1).
In vitro tube formation assay and co-culture
Human microvascular endothelial cells (HMECs) or murine endothelial cells (bEnd.3) were cultured in Dulbecco’s modified Eagle’s medium (DMEM+10% fetal calf serum (FCS) and 1% P/ S); Pericytes (C3H/10T1/2, ATCC) were cultured in the complete growth medium including Eagle’s Basal medium (BME) with 2 mM L-glutamine, 1.5 g/L sodium bicarbonate, Earle’s BSS and 10% FCS. Expression of pericyte specific markers were analyzed via FACS measurements. After washing the pericytes in PBS, cells were incubated with primary antibody against NG-2 (Millipore AB 5320) and PDFG-R (abcam ab91066 rat monoclonal) for 45 min. Incubation with secondary antibody (for NG2 Molecular Probes anti-rabbit Alexa 488 A11008, for PDGF-R anti-rat sekunda¨r: anti-rat-PE Santa Cruz sc-3740) was performed after washing of the cells for 45 min. Isotype controls were only incubated with the secondary antibody (Fig. S2A,B).
Cat.No. 22887). Cells were plated on matrigel in angiogenesism -slides (ibidi, Cat.No. 81501). After 18 h tube formation was investigated and pictures were taken for quantification. The green stained pericytes (DiO, Vybrant, Cat.No. 22886) where added to the tubes in a concentration of 16103 cells/20ml. After 6 h fluorescence pictures for red (endothelial cells) and green (pericytes) were taken and the pericyte per ring ratio was calculated.
Mouse hindlimb ischemia model
In male C57BL/6J mice (n = 7 per group, Charles River, Sulzfeld, Germany) i.m. injection into the whole limb (10 injections 5ml each) was performed with rAAV.hVEGF-A (high dose was 361012 virus particles, low dose was 561011 virus
particles), rAAV.mAPLN, rAAV.hAng1, with or without co-application of rAAV.Ang2 or rAAV.tetoff-mAng2 (361012 virus particles), 14 days before surgery. Hindlimb ischemia model was performed as described previously. [21,22] In brief, the right femoral artery was ligated distally of the profunda fermoral artery, whereas the ligature at the sham operated left femoral artery was only loosely winded around the artery at the same position.
Laser Doppler imaging
Hind-limb perfusion was assessed using laser doppler perfusion imaging (MoorLDI-2; Moor Instruments, Devon, UK) [22]. After general anesthesia of the mice with 1.5% Isoflurane, mice were put in a heating chamber and maintained at 37uC. After an adaption time of 10 min in the heating chamber, measurements were conducted. LDI measurements were carried out before and after surgery on day 0 d, 7 d and 14 d. Results are given as right to left leg ratio including subtraction of background tissue value.
Immunofluorescent histochemical staining
Tissue from the gastrocnemius muscle was harvested at day 7 (early timepoint) and day 14 (late timepoint) after femoral artery ligation. Tissue was embedded in tissuetech, stored at280uC, cut to 5mm slices and endothelial cells were stained with a CD31 goat anti-mouse polyclonal IgG (Santa Cruz, Cat.No. sc1506, dilution 1:200) for primary antibody. A donkey anti-goat IgG-R (Santa Cruz, Cat.No. sc2094, dilution 1:200) was used as secondary antibody. Pericyte staining was performed via NG2 staining (primary antibody of Chondroitin Sulfate Proteoglycan, Millipore, Cat.No. AB5320, dilution 1:200) and secondary antibody of bovine anti-rabbit IgG FITC (Santa Cruz, Cat.No. sc2365, dilution 1:200). Capillary density was analyzed as the ratio of CD31 positive cells per muscle fiber (CMF-R), and the maturation of capillaries was represented by pericyte to capillary ratio (PC-R). From each mouse 5 random pictures from the gastrocnemius muscle were analyzed.
Statistical analysis
All data are presented as mean6SEM and analyzed by SPSS 19.0 software. Comparisons among groups were made by one-way ANOVA testing with Gabriel’s post hoc test. Games-Howell analysis was used for heterogeneity of variance. Differences between groups were considered significant for p-value of,0.05.
Results
Angiopoietin-2 antagonizes pericyte recruitment to growing tubes in vitro
First, we sought to establish an in vitro model of pericyte
recruitment to growing tubes. Therefore, DiD-labeled,
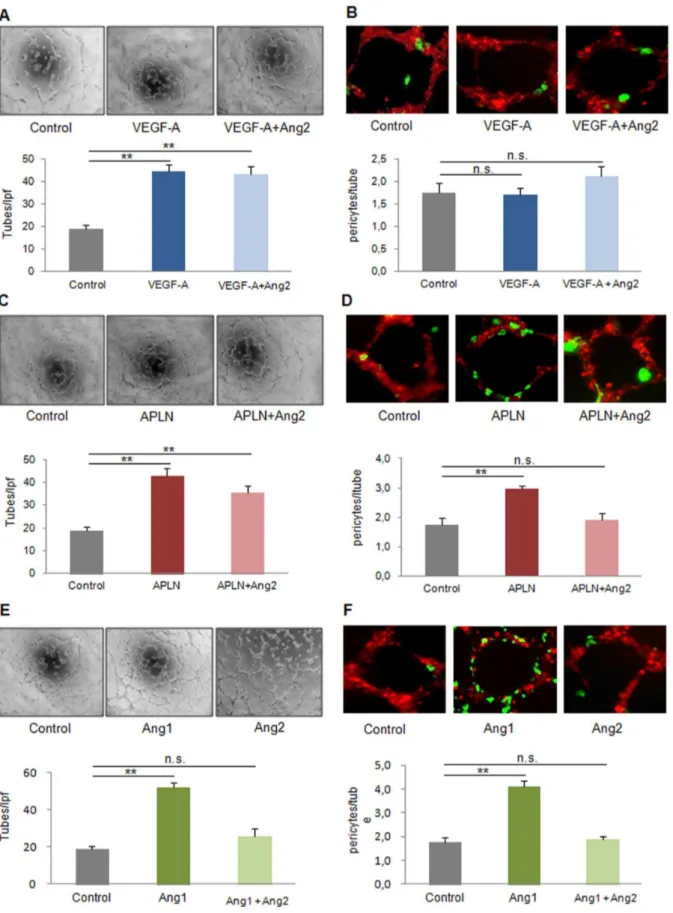
growth-factor-transfected endothelial cells (HMECS or bEnd.3) embedded in matrigel were seeded with DiO-labeled pericytes. Tube analysis revealed that VEGF-A, despite robust tube formation (4563 tubes/lpf vs. 1962 tubes/lpf in control), did not alter pericyte recruitment (1.760.2 pericyte/tube vs. 1.860.2 pericytes/tube in control, Fig. 1A,B, Fig. S3A). In contrast, APLN, known to provide stable, non-leaky vessels in vivo [23], increased both, capillary proliferation (4363 tubes/lpf, Fig. 1C) and pericyte investment (3.060.1 perictes/tube, Fig. 1D, Fig S3B). Ang1 was also capable of inducing tube formation and pericyte recruitment (Fig. 1E,F, Fig. S3C). Ang2, however, which did not alter the already limited pericyte attraction to VEGF-A treated endothelial cells, lowered the number of pericytes attracted to both, the APLN- (1.960.2 pericytes/tube) and the Ang1-transfected cell group (1.960.1 pericytes/tube). In vitro, tube formation capacity of APLN was unaffected by Ang2 (3663 tubes/lpf, Fig. 1C), but suffered in the case of Ang1, when Ang2 was present (Fig 1E).
These in vitro experiments promped us to assess the effect of Ang2 in combination with VEGF-A, APLN and Ang1 in vivo.
Early perfusion recovery of ischemic hindlimbs (d7) does not improve upon rAAV.VEGF-A, but decreases after combining VEGF-A+Angiopoietin-2
Employing a murine hindlimb ischemia model, we investigated the impact of VEGF-A, a well-characterized pro-angiogenic agent with weak vessel maturation potential. The overexpression of target genes (pCMV-hVEGF-A, pCMV-mAng2, pCMV-tetoff-mAng2, pCMV-mAPLN, pCMV-hAng1) as well as the reporter gene (CMV-LacZ), which were applied intramuscular via recom-binant AAV (rAAV) vectors, was analyzed by qRT-PCR and histology (Fig. S1). Analysis of ischemic hindlimb perfusion by LDI indicated that rAAV.VEGF-A-either low dose (561011 virus
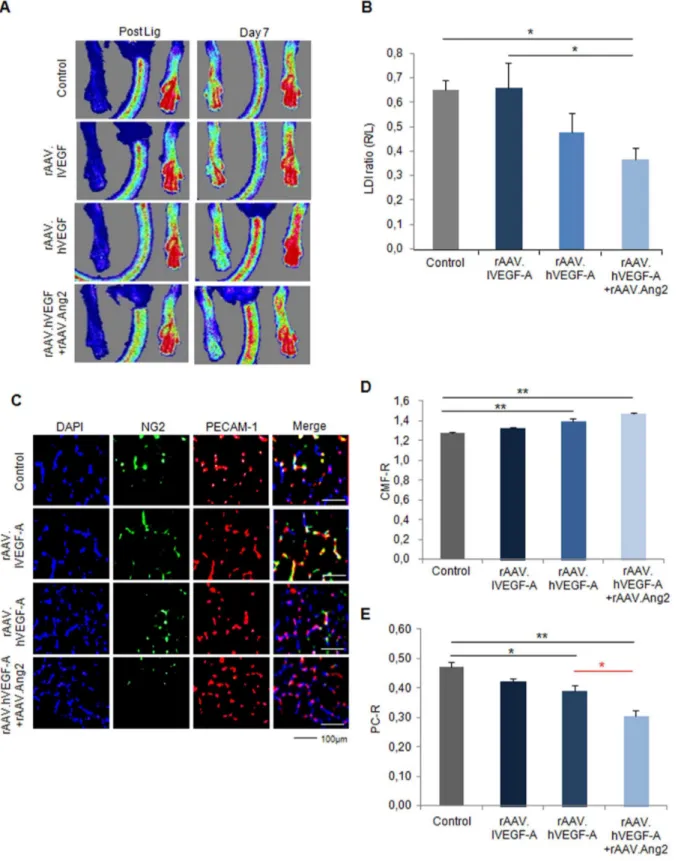
particles, 0.6660.10 LDI ratio (r/l)) or high dose (361012virus particles, 0.4860.08 LDI ratio (r/l))-did not alter the recovery of perfusion (0.6560.04 LDI ratio (r/l) in control, Fig. 2A,B), whereas the combination of high rAAV.VEGF-A and rAAV.Ang2 significantly impaired perfusion recovery at d7 (0.3660.05 LDI ratio (r/l)).
To explore the effect VEGF-A and Ang2 on angiogenesis capillary sprouting in vivo, we analyzed capillary/muscle fiber ratio (CMF-R, PECAM-1 staining) and pericyte/capillary ratio (PC-R, NG2 staining) by fluorescence histology (Fig. 2C-E). Of note, CMF-R was upregulated in the high rAAV.VEGF-A (1.4060.03 CMF-R), but not in the low rAAV.VEGF-A (1.3260.01 CMF-R) compared to control (1.2860.01 CMF-R). In addition, the combination of high rAAV.VEGF-A with Ang2 revealed a significant higher CMF-R (1.4760.01 CMF-R, Fig. 2D) than control group. In contrast, PC-R was decreased in the rAAV.VEGF-A/Ang2 group (0.3160.02 PC-R) compared to control (0.4760.02 PC-R) and low and high rAAV.VEGF-A treatment groups (0.4260.01 PC-R for low and 0.3960.02 PC-R for high VEGF-A, Fig 2E). Taken together, even though hVEGF alone and in combination with Ang2 significantly induces capillary growth, the failure to provide pericyte coverage is associated with a lack of perfusion recovery of the ischemic hindlimb, compared to controls and low VEGF. The combination of hVEGF and Ang2 even further decreased pericyte covarage and thereby showed a trend to a lower perfusion than the hVEGF alone.
Late neovascularization (d14) is not provided by VEGF-A combined with Angiopoietin-2
Figure 1. Capillary growth and maturation profile of VEGF-A, APLN and Ang1 with or without Ang2 in vitro. (A)Capillary-like tube formation was enhanced by VEGF-A alone transfection in HMECs (16104per well ofm-slide angiogenesis plate) on 2D-matrigel.(B)16103Pericytes labeled by DiO (green) are plated after capillary-like tube formation of HMECs with DiD labeling (red). 24 h later, co-cultures reveal a low rate of pericytes attraction by VEGF-A, which was unaffected by Ang2.(C)Apelin (APLN) promoted tube formation with or without Ang2.(D)Pericyte recruitment was enhanced by Apelin, an effect attenuated by Ang2.(E)Ang1 induced tubes formationin the absence of Ang2.(F)However, the tube maturation provided by Ang1 was abolished in the presence of Ang2. (MEAN6SEM, n = 5, ** p,0.01).
Figure 2. The failed therapeutic neovascularization after VEGF-A overexpression in the early stage (7 day) of mouse hindlimb ischemia model.(A) Laser Doppler Imaging (LDI) measured after right femoral artery ligation and at d7. (B) Quantification of LDI (ischemic versus non-ischemic hindlimb) revealed no significant neovascularization by VEGF-A at low (rAAV.lVEGF-A) or high doses (rAAV.hVEGF-A), but deterioration of perfusion in the rAAV.hVEGF-A+Ang2 group compared vehicle group (#P,0.05, vs. vehicle). (C) Fluorescence microscopy images of gastrocnemius muscle sections from control (rAAV.LacZ) or ischemic hind limb co-stained with DAPI (blue, nucleus), anti-NG2 (green, pericyte) antibody and anti-PECAM-1 (red, endothelial cell) antibody at d 7 after ligation. (D) Quantitative evaluation of capillary/muscle fibre ratio (CMF-R) demonstrated the ability of high VEGF-A with or without Ang2 to induce capillary growth in vivo. (E) Pericyte coverage was unaffected by lVEGF-A, but reduced in hVEGF-Agroup compared to vehicle group and even further reduced through the co-application of rAAV.Ang2. (MEAN6SEM, n = 7, * p,0.05, ** p,0.01).
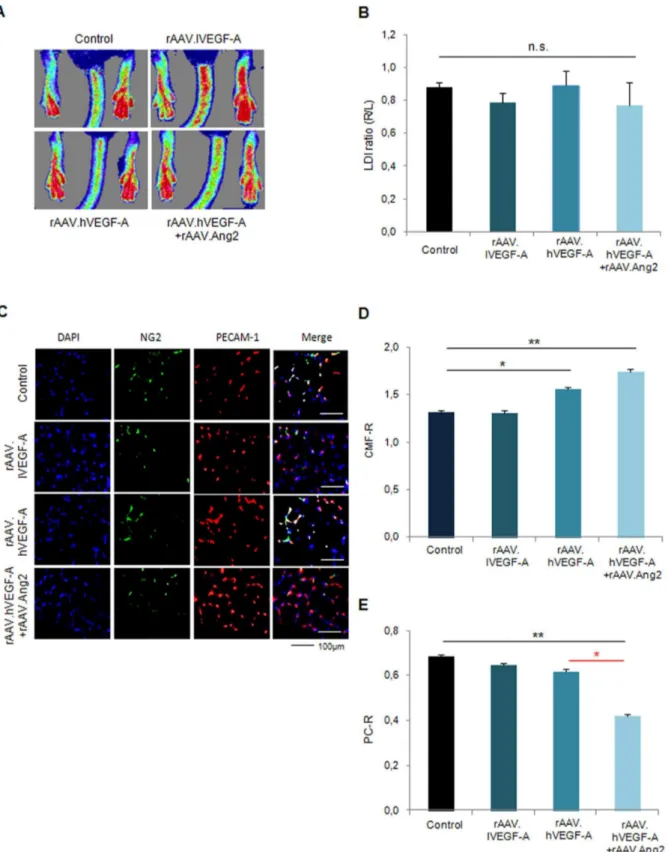
late gain of perfusion (d14). As indicated in Fig. 3A,B, whereas no significant gain of perfusion was achieved by rAAV.hVEGF-A only, the combination of VEGF-A and continuous Ang2 delivery, which impaired perfusion recovery at d7 (Fig. 2), did not impair perfusion at d14 compared to controls (0.8860.03 LDI ratio (r/l) in control vs 0.7760.14 LDI ratio (r/l) in hVEGF-A+Ang2). Histologically, the higher CMF-R in the hVEGF-A+Ang2 group (1.7460.04 CMF-R vs. 1.3160.02 CMF-R in controls, Fig. 3C,D) was offset by a significantly decreased PC-R (0.4260.01), compared to control (0.6960.02 PC-R) and VEGF-A muscle specimen (0.6260.02 PC-R in hVEGF-A, Fig. 3C,E).
Early Apelin-induced neovascularization is antagonized by permanent, but not temporary Angiopoietin-2 overexpression
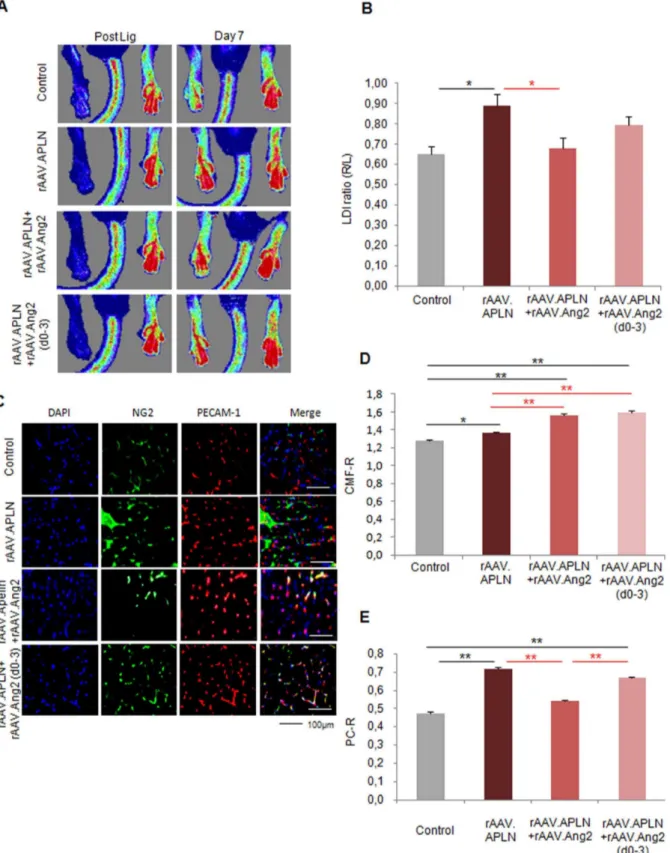
Since VEGF-A did not provide a gain of perfusion in the ischemic hindlimb, we investigated the potential of apelin (APLN), a vascular growth factor inducing capillary sprouting and maturation, to improve perfusion after ischemia induction. Indeed, rAAV.APLN sufficed to significantly increase perfusion at d7 (0.8960.06 LDI ratio (r/l), Fig. 4A, B). This effect was completely abolished by co-application of rAAV.Ang2 (0.6860.05 LDI ratio (r/l)), in fact reducing the perfusion at d7 to control level. Histological analysis revealed that the gain of CMF-R induced by APLN (1.3460.01) was even increased by rAAV.Ang2 coapplication (1.5660.03) Fig. 4C,D). However, the PC-R, which was significantly increased upon apelin stimulation (0.7260.01), was reduced to control level after rAAV.APLN/Ang2 co-application (0.5460.01 PC-R, Fig. 4E). Thus, the improved early perfusion (d7) after APLN overexpression critically depended on pericyte recruitment. Preventing pericyte recruitment by Ang2-cotreatment sufficed to block a gain of perfusion upon apelin, despite an even improved capillary growth.
Next, we investigated whether temporary Angiopoietin-2 expression would improve the efficacy of APLN. Since we found that APLN induces vessel maturation in vitro, we compared both, either continuous or early vessel destabilization by Ang2. The latter was achieved by Doxycycline-inducible (Tet-off) Ang2, which allowed to switch off Ang2 expression significantly (Fig S1D). Using Doxycycline from d3 on, we limited Ang2 expression to d0-3 and investigated whether early Ang2 overexpression may boost APLN-induced vessel growth. Of note, we found a strong trend towards improvement of perfusion (0.8060.04 LDI ratio (r/ l), p = 0.08) (Fig. 4A,B) in the rAAV.APLN+Ang2 (d0-3) group, which co-incided with improved CMF-R (1.5960.02) compared to rAAV.APLN only and improved PC-R (0.6760.01), compared to rAAV.APLN+Ang2 (Fig. 4C–E). However, although early Ang2 overexpression favorably altered capillary growth and maturation indices at d7, it did not suffice to improve early hindlimb perfusion provided by APLN.
Late neovascularization is provided by Apelin with or without Angiopoietin-2
In contrast, rAAV.APLN provided a significantly increased perfusion at d14 (1.0460.02 vs. 0.8860.03 LDI ratio (r/l)), an effect still present during continuous co-expression of APLN and Ang2 (1.1060.12 LDI ratio (r/l)), but not after APLN+Ang2 (d0-3) transduction, which provided Ang2 only in the first 3 days of postischemic vessel growth (Fig. 5A,B). The capillary/muscle fiber ratio increased in both, the rAAV.APLN and the rAAV.APL-N+Ang2 groups and after rAAV.Apln+Ang2 (d0-3) transduction (Fig. 5C,D). The pericyte/capillary ratio, which was provided by APLN alone at d14, was significantly reduced in the APLN+
Ang2-treated animals (Fig. 5C,E), whereas temporary overexpression of Ang2 showed a non-significant trend towards reduction of pericyte coverage. Thus, although APLN+Ang2 was efficient in improving capillarization at d14, a lack of mature vessels was associated with a lack of further improvement of perfusion beyond the level provided by APLN alone. When Ang2 overexpression is limited to the d0-3 period and switched off by Doxycycline application thereafter, Apelin fails to further increase perfusion above control level, and did not provide capillary growth or pericyte investment (Fig. 5A–E).
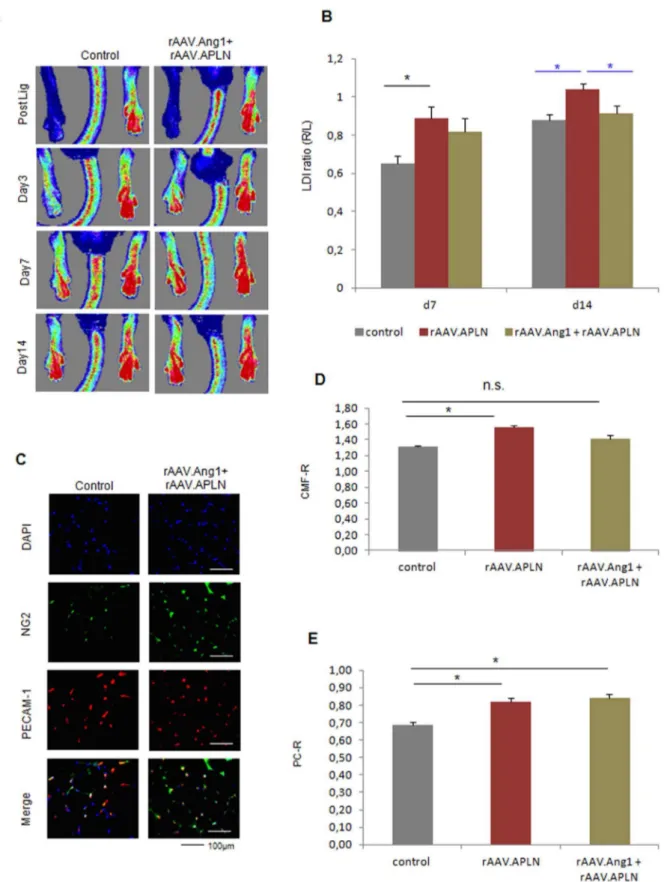
rAAV.Angiopoietin1 requires temporary Angiopoietin2 expression for functional early vessel growth
Next, we assessed the potential of Angiopoietin-1, an antagonist of Ang2 and a powerful vessel maturation factor. Of note, neither Ang1 nor Ang2 induced significant increase of perfusion alone (0.7860.06 LDI ratio rAAV.Ang1 and 0.7260.06 LDI ration rAAV.Ang2, Fig. 6A,B). However, a combination of Ang1 and temporary overexpression of Ang2 (d0-3) (Fig. S1E) provided a significant gain of perfusion (Fig. 6A,B) at d7, along with a high capillary/muscle fiber ratio and a high pericyte/capillary ratio (Fig. 6C–E). rAAV.Ang1 and rAAV.Ang2 alone did not improve capillary growth (PECAM-1 positive cells, Fig. 6C,D), whereas pericyte coverage of the capillaries was enhanced after Ang1 overexpression and unaltered after Ang2 application (Fig. 6C,E).
Late neovascularization is provided by Angiopoietin-1 combined with early Angiopoietin-2 treatment (d0-3)
Next, we assessed the late-timepoint efficacy of sequential Tie2-activation by Ang2 (d0-3) and continuous rAAV.Ang1 overex-pression. In contrast to rAAV.Ang1 or rAAV.Ang2 alone, which failed to significantly exceed the control groups perfusion at d14 (Fig. 7A,B), we could demonstrate a profound sustained gain of perfusion by combining Ang1 with early Ang2 overexpression (Fig. 7A,B). This effect was accompanied by an improved capillary/muscle fiber ratio (CMF-R) in the Ang1+Ang2 (d0-3) group (1.6160.08 CMF-R, Fig. 7C,D) as well as an increase in pericyte coverage (0.8060.03 PC-R, Fig. 7C,E). Neither rAA-V.Ang1 nor rAAV.Ang2 provided increased CMF-R (1.3160.02 CMF-R in control vs. 1.4460.04 CMF-R (Ang1) or 1.4160.13 CMF-R (Ang2)). Of note, whereas Ang1 increased PC-R, Ang2 decreased pericyte investment (0.6460.04, Fig. 7C,E). Thus, an early vessel destabilization regimen, e.g. provided by Ang2 (d0-3), expanded the vascular growth response of rAAV.Ang1, which itself was capable of enhancing capillary maturation, but not capillary growth over time.
Combination of Ang1 and Apelin does not provide enhanced neovascularization
Figure 3. rAAV.VEGF-A did not provide enhanced neovascularization at d14. (A, B)Laser Doppler images and quantification of LDI of low and high VEGF-A groups displayed no difference to controls, similar to the combination of high VEGF-A with Ang2.(C)Fluorescence microscopy images of gastrocnemius muscle for capillaries (PECAM-1, red), pericytes (NG2, green) and nuclei (DAPI, blue) for the different groups. (D) Quantification of CMF-R displayed increased capillary growth for high VEGF-A with or without Ang2, whereas(E)PC-R revealed a diminished vessel maturation only for high VEGF-A combined with Ang2. (MEAN6SEM, n = 7,* p,0.05, ** p,0.01).
Figure 4. Apelin did not provide early neovascularization with Ang2. (A, B)LDI of blood reperfusion induced by APLN is enhanced without continuous Ang2 overexpression (rAAV.Ang2), where as the combination of APLN and Ang2 displayed no significant alteration. APLN combined with early Ang2 overexpression (rAAV.Apln+Ang2(d0-3)) induced a trend towards higher perfusion (p = 0.08).(C–E)PECAM-1 positive capillaries were slightly increased by rAAV.APLN, whereas the combination with continuous rAAV.Ang2 or rAAV.Ang2(d0-3) clearly enhaced capillary formation compared to APLN alone. Capillary maturation, indicated by pericyte coverage(E)was enhanced by rAAV.APLN with or without rAAV.Ang2(d0-3), whereas continous Ang2 reduced PC-R. (MEAN6SEM, n = 7,* p,0.05, ** p,0.01).
Discussion
In this study, we elucidated the vital importance of the balance between capillary proliferation and stabilization in adult
postis-chemic neovascularization. By virtue of continuously or tempo-rarily active rAAV vectors, we assessed the role of early or continuous pericyte detachment via Angiopoietin-2. First, we found that VEGF-A failed to improve perfusion in a mouse
hindlimb ischemia model, a finding further impaired by Ang2 a second vessel destabilization factor (Fig. 1, 2). Second, we found that APLN, itself a weaker capillary proliferating agent than
high-VEGF-A, improved perfusion, associated with a high pericyte coverage (Fig. 1, 3). Addition of Ang2 to APLN, which enhanced capillary density in the ischemic hindlimb, delayed improvement
Figure 6. Ang1 combined with early Ang2 (d0-3) induces enhanced neovascularization at d7. (A, B)LDI analysis revealed no alteration of perfusion at d7 by either Ang1 or Ang2 (continuous overexpression), but a significant increase in the Ang1+Ang2(d0-3) group.(C–D)Analysis of
capillaries revealed an elevated CMF-R only in the Ang1 and Ang2(d0-3) group, whereas the capillarization was unaltered if Ang 1 and Ang2 were overxpressed alone.(C,E)Ang1 alone as well as Ang1+Ang2(d0-3) was capable of enhancing pericytes coverage compared to control, Ang2 alone
of perfusion to the late time point (d14, Fig. 4,5). Combining APLN and early Ang2 (d0-3) did not improve perfusion in the same manner. (Fig. 4, 5)
Third, we observed that modulation of the Tie2 receptor by early Ang2 overexpression in addition to continuous Ang1
expression significantly increased hindlimb perfusion, associated with an increased capillary growth and maturation (Fig. 6, 7). Neither Ang1 nor Ang2 overexpression alone was able to accomplish this effect. Finally, the combination two vessel stabilizing agents (Ang1 and APLN) abolished therapeutic
Figure 7. Ang1 combined with early Ang2 (d0-3) enhanced neovascularization at d14. (A,B)Ang1 improved hindlimb perfusion only, if combined with Ang2 (d0-3), a condition which also elevated(C,D)capillary/muscle fiber ratio (CMF-R) and(C,E)pericyte/capillary ratio (PC-R) to a significant higher level. (MEAN6SEM, n = 7,* p,0.05, ** p,0.01).
Figure 8. Apelin did not enhance neovascularization when combined with Ang1. (A, B)rAAV.APLN combined with rAAV. Ang1 was not capable of elevating hindlimb perfusion at d7 or d14 achieved by APLN alone.(C–E)The combination of APLN with Ang1 failed to increase capillary growth at d14, but provided a capillary maturation index (PR-R). (MEAN6SEM, n = 7,* p,0.05, ** p,0.01).
neovascularization achieved by APLN alone, but provided a very high maturation level, i.e. pericyte coverage (Fig. 8).
These results demonstrate that modulation of the delicate balance of vessel growth and maturation during postischemic neovascularization is a complex task. We first established that prevention of vessel maturation by adding Ang2 to VEGF-A-treatment is potentially harmful, most likely because VEGF-A itself is a powerful vessel destabilizing agent [5]. Here, further destabilization by Ang2 impaired early perfusion (d7), and no improvement above control levels at the later timepoint (d14), although VEGF-A+Ang2 treatment enhanced sprouting of vessels at early and late timepoints (Fig. 2, 5). These results fit the earlier observation in a genetic model of inducible endothelial Ang2 overexpression at a high dose, which suppressed recovery of neovascularization in the murine hindlimb ischemia model over 21 d [17]. Potentially, lower doses of Ang2 would be more desirable, since Felcht et al. recently observed a dual mode of action of Ang2 [24], acting pro-angiogenic on Tie2-low expressing endothelial cells, as opposed to the destabilizing activity of Ang2 on Tie2-expression endothelial cells [16]. On the other hand, it has repetitively been demonstrated that VEGF-A would act more efficiently in postnatal neovascularization, when coupled to a vessel stabilization agent such as Ang1 [15] or PDGF-B [6].
This strategy, a combination of a growth and maturation factor with a vessel destabilizing agent, was pursued by using either APLN or Ang1 in combination with Ang2. The neovasculatory potential of apelin was recently investigated, and a distinct antagonism to the VEGF-A induced hyperpermeability was revealed [23]. Of note, plasmid transfer of VEGF-A helped to raise the number of vessels induced by apelin, and provided an additional gain of perfusion compared to apelin alone. Consis-tently, in our study the limited capability of APLN to form new vessels, coupled with a high maturation potential (Fig. 4, 5), was improved by Ang2. However, this combination did not provide a further improvement of perfusion of the ischemic hindlimb, if used continuously, most likely due to the pericyte detachment induced by Ang2 (Fig. 5E). It is puzzling that the combination of APLN with early Ang2 overexpression did not further increase perfusion at the later timepoint (Fig. 5). Although the functions conceptu-alized before (early destabilization by Ang2 followed by enhanced maturation by APLN) can be demonstrated in vitro (Fig. 1) and in vivo (Fig. 3, 7), the efficacy of this approach is lacking in a clearcut and statistical significant manner. Obviously, this combination becomes dependent on the Ang2 effect, the subtraction of which by switching off the inducible vector compromises other compo-nents of the vessel growth process which cannot be compensated by APLN furtheron.
In order to rule out unforeseen effects of Ang2 on the maturation principle such as APLN, we speculated that combining early vessel destabilization via Ang2 with continuous Ang1 overexpression might provide a more balanced vessel growth than combination of Ang2 with other vasoactive agents. Since neither Ang1 nor Ang2 alone had an effect on perfusion recovery, we were surprised to note that the short Ang2-overexpression (d0-3), which did not increase the perfusion achieved in the APLN series (Fig. 4, 5), was highly efficient in raising the perfusion. Of note, several studies have reported a boost of the vascular growth response by late Ang1 addition [25]. Thus, remodeling the physiologic sequence of early Ang2 induction and subsequent Ang1 increase found in postischemic myocardial tissue [26] may serve the cause of early vessel destabilization and subsequent pericyte recruitment. The continuous Ang1 increase seems instrumental for vascular integrity and prevention of vessel regression, as observed in development [27] and in tumor vasculature [28].
The pro-angiogenic effects seen with the combination of a vessel destabilization (Ang2) and a subsequent vessel maturation (APLN or Ang1, Fig 5, 7) display a perfusion rate which is above 1, pointing to a supra-physiological perfusion. An improved perfusion index.1 after hindlimb ischemia in mice was already shown by Abe and colleagues. They demonstrated a blood flow recovery rate of 1.4– 1.6 (LDI ratio R/L) at 14 days after repeated adrenomedullin pcDNA injection in a model of hindlimb ischemia. [29] In addition, values of LDI ratio right to left of 1 can already be achieved in control mice, depending on their age and genetical background. [30] [31] Furthermore, the operation technique (ligation vs. excision of the femoral artery) clearly influences the flow reduction and recovery capacity in this hindlimb ischemia model in mice. [30] [32] Taken together, these data confirm that a perfusion index.1 can be achieved, but might be dependent on the longterm or repeated overexpression of the right transgene. In addition, the mouse model of femoral artery ligation has high recovery capacity; therefore results should be viewed as a first evidence for a therapeutic treatment. Furthermore it allows some insights into signaling cascades and pathophysiological correlations. These concepts need to be reassessed in additional (more stable) animal models before translated to a clinical situation.
In summary, we showed that the concept of early vessel destabilization and late maturation is valid in providing enhanced postischemic hindlimb perfusion. We demonstrated that combin-ing two destabilizcombin-ing agents (VEGF-A and Ang2) at times deteriorates perfusion, whereas the combination of two growth and maturation factors (Ang1 and APLN) can even prohibit the efficacy of one (APLN) when applied alone. However, the choice of vascular agents is delicate: in our model, only the sequence of early Ang2 overexpression combined with continuous Ang1 overexpression provided enhanced perfusion, even superior to APLN, due to a induction of mature microvessels. However, this concept might add to the armamentarium of therapeutic approaches of chronic ischemic muscle disease.
Supporting Information
Figure S1 Efficacy of rAAV mediated growth factor transduction. Intramuscular injection of rAAV.VEGF-A (A), rAAV.Ang2 (B) and rAAV.APLN (C) into the ischemic limb displayed a significant increase of mRNA levels compared to the non-injected sham operated leg as well as to controls (displayed as DDCT normalized to S18, day 7 post ligation).(D) Analysis of Ang1 levels revealed higherDDCT levels at day 3 post ligation as well as on day 7, when Doxycycline was applied.(E)rAAV.Ang2 (day0-3) transduction showed enhanced Ang2 levels at day 3 (no Doxycycline) and a clear reduction of Ang2 at day 7 when the vector was shut off by Doxycycline. (F) I.m. injection of rAAV.LacZ revealed a clear blue staining for ß-Galaktosidase in the treated leg compared to control. (MEAN 6 SEM, n = 3, ** p,0.05).
(TIF)
Figure S2 In vitro expression analysis. Pericytes (C3H/ 10T1/2) obtain from ATCC, express pericyte markers as NG2(A) and PDGF-R (B) in FACS analysis. To asure that the co-transfection of two transgenes within one approach does not alter the expression level, RT-PCR analysis were performed. (C,D) APLN as well as Ang2 display the same expression level if transfected alone or in combination forDDCT of APLN or Ang2. (E;F) Analysis of cells transfected with VEGF alon or in combination with Ang2 revieled similar expression levels for DDCT of VEGF or Ang2 in both groups.
Figure S3 In vitro pericyte recruitment. (A) 16103
Pericytes labeled by DiO (green) are plated after capillary-like tube formation of bEnd.3 cells (murine endothelial cells) with DiD labeling (red). 24 h later, co-cultures reveal a low rate of pericytes attraction by VEGF-A, which was unaffected by Ang2. (B) Pericyte recruitment to the murine endothelial cells was enhanced by APLN, an effect attenuated by Ang2.(C)However, the tube maturation of the bEnd.3 cells provided by Ang1 was abolished in the presence of Ang2. (MEAN6SEM, n = 5, ** p,0.01). (TIF)
Acknowledgments
We would like to thank Andre´ W. Bra¨ndli for the Apelin plasmid and Cuong Kieu and Elisabeth Raatz for excellent technical assistance.
Author Contributions
Conceived and designed the experiments: RH CK. Performed the experiments: DQ TT SL OC. Analyzed the data: DQ ED RH CK. Contributed reagents/materials/analysis tools: ED. Wrote the paper: CK RH.
References
1. Yla-Herttuala S, Rissanen TT, Vajanto I, Hartikainen J (2007) Vascular endothelial growth factors: biology and current status of clinical applications in cardiovascular medicine. J Am Coll Cardiol 49: 1015–1026.
2. Hinkel R, Trenkwalder T, Kupatt C (2011) Gene therapy for ischemic heart disease. Expert Opin Biol Ther 11: 723–737. 10.1517/14712598.2011.570749 [doi].
3. Karvinen H, Yla-Herttuala S (2010) New aspects in vascular gene therapy. Curr Opin Pharmacol 10: 208–211. S1471-4892(10)00005-6 [pii];10.1016/ j.coph.2010.01.004 [doi].
4. Jain RK (2003) Molecular regulation of vessel maturation. Nat Med 9: 685–693. 5. Greenberg JI, Shields DJ, Barillas SG, Acevedo LM, Murphy E, et al. (2008) A role for VEGF as a negative regulator of pericyte function and vessel maturation. Nature 456: 809–813.
6. Kupatt C, Hinkel R, Pfosser A, El-Aouni C, Wuchrer A, et al. (2010) Cotransfection of Vascular Endothelial Growth Factor-A and Platelet-Derived Growth Factor-B Via Recombinant Adeno-Associated Virus Resolves Chronic Ischemic Malperfusion: Role of Vessel Maturation. J Am Coll Cardiol 56: 414– 422.
7. Dor Y, Djonov V, Abramovitch R, Itin A, Fishman GI, et al. (2002) Conditional switching of VEGF provides new insights into adult neovascularization and pro-angiogenic therapy. EMBO J 21: 1939–1947.
8. Thomas M, Augustin H (2009) The role of the Angiopoietins in vascular morphogenesis. Angiogenesis 12: 125–137.
9. Saharinen P, Eklund L, Miettinen J, Wirkkala R, Anisimov A, et al. (2008) Angiopoietins assemble distinct Tie2 signalling complexes in endothelial cell-cell and cell-matrix contacts. Nat Cell Biol 10: 527–537.
10. Kobayashi H, DeBusk LM, Babichev YO, Dumont DJ, Lin PC (2006) Hepatocyte growth factor mediates angiopoietin-induced smooth muscle cell recruitment. Blood 108: 1260–1266.
11. Zhu WH, Han J, Nicosia RF (2003) Requisite Role of p38 MAPK in Mural Cell Recruitment during Angiogenesis in the Rat Aorta Model. Journal of Vascular Research 40: 140–148.
12. Koblizek TI, Weiss C, Yancopoulos GD, Deutsch U, Risau W (1998) Angiopoietin-1 induces sprouting angiogenesis in vitro. Current Biology 8: 529–532.
13. Abdel-Malak NA, Srikant CB, Kristof AS, Magder SA, Di Battista JA, et al. (2008) Angiopoietin-1 promotes endothelial cell proliferation and migration through AP-1GC¸ oˆdependent autocrine production of interleukin-8. Blood 111: 4145–4154.
14. Asahara T, Chen D, Takahashi T, Fujikawa K, Kearney M, et al. (1998) Tie2 receptor ligands, angiopoietin-1 and angiopoietin-2, modulate VEGF-induced postnatal neovascularization. Circ Res 83: 233–240.
15. Arsic N, Zentilin L, Zacchigna S, Santoro D, Stanta G, et al. (2003) Induction of functional neovascularization by combined VEGF and angiopoietin-1 gene transfer using AAV vectors. Molecular Therapy 7: 450–459.
16. Scharpfenecker M, Fiedler U, Reiss Y, Augustin HG (2005) The Tie-2 ligand Angiopoietin-2 destabilizes quiescent endothelium through an internal autocrine loop mechanism. J Cell Sci 118: 771–780.
17. Reiss Y, Droste J, Heil M, Tribulova S, Schmidt MHH, et al. (2007) Angiopoietin-2 Impairs Revascularization After Limb Ischemia. Circ Res CIRCRESAHA.
18. Kidoya H, Takakura N (2012) Biology of the apelin-APJ axis in vascular formation. Journal of Biochemistry 152: 125–131.
19. Raake PW, Hinkel R, Muller S, Delker S, Kreuzpointner R, et al. (2008) Cardio-specific long-term gene expression in a porcine model after selective pressure-regulated retroinfusion of adeno-associated viral (AAV) vectors. Gene Ther 15: 12–17.
20. Gao GP, Alvira MR, Wang L, Calcedo R, Johnston J, et al. (2002) Novel adeno-associated viruses from rhesus monkeys as vectors for human gene therapy. Proc Natl Acad Sci U S A 99: 11854–11859. 10.1073/pnas.182412299 [doi];182412299 [pii].
21. Pagel JI, Ziegelhoeffer T, Heil M, Fischer S, Fernandez B, et al. (2012) Role of early growth response 1 in arteriogenesis: impact on vascular cell proliferation and leukocyte recruitment in vivo. Thromb Haemost 107: 562–574. 11-07-0490 [pii];10.1160/TH11-07-0490 [doi].
22. Limbourg A, Korff T, Napp LC, Schaper W, Drexler H, et al. (2009) Evaluation of postnatal arteriogenesis and angiogenesis in a mouse model of hind-limb ischemia. Nat Protoc 4: 1737–1746. nprot.2009.185 [pii];10.1038/ nprot.2009.185 [doi].
23. Kidoya H, Naito H, Takakura N (2010) Apelin induces enlarged and nonleaky blood vessels for functional recovery from ischemia. Blood 115: 3166–3174. 24. Felcht M, Luck R, Schering A, Seidel P, Srivastava K, et al. (2012)
Angiopoietin-2 differentially regulates angiogenesis through TIEAngiopoietin-2 and integrin signaling. J Clin Invest 122: 1991–2005.
25. Smith AH, Kuliszewski MA, Liao C, Rudenko D, Stewart DJ, et al. (2012) Sustained improvement in perfusion and flow reserve after temporally separated delivery of vascular endothelial growth factor and angiopoietin-1 plasmid deoxyribonucleic acid. J Am Coll Cardiol 59: 1320–1328.
26. Sandhu R, Teichert-Kuliszewska K, Nag S, Proteau G, Robb MJ, et al. (2004) Reciprocal regulation of angiopoietin-1 and angiopoietin-2 following myocardial infarction in the rat. Cardiovasc Res 64: 115–124.
27. Holash J, Wiegand SJ, Yancopoulos GD (1999) New model of tumor angiogenesis: dynamic balance between vessel regression and growth mediated by angiopoietins and VEGF. Oncogene 18: 5356–5362. 10.1038/ sj.onc.1203035 [doi].
28. Fagiani E, Lorentz P, Kopfstein L, Christofori G (2011) Angiopoietin-1 and -2 Exert Antagonistic Functions in Tumor Angiogenesis, yet Both Induce Lymphangiogenesis. Cancer Research 71: 5717–5727.
29. Abe M, Sata M, Nishimatsu H, Nagata D, Suzuki E, et al. (2003) Adrenomedullin augments collateral development in response to acute ischemia. Biochem Biophys Res Commun 306: 10–15. S0006291X03009033 [pii]. 30. Scholz D, Ziegelhoeffer T, Helisch A, Wagner S, Friedrich C, et al. (2002)
Contribution of arteriogenesis and angiogenesis to postocclusive hindlimb perfusion in mice. J Mol Cell Cardiol 34: 775–787. S0022282802920134 [pii]. 31. Helisch A, Wagner S, Khan N, Drinane M, Wolfram S, et al. (2006) Impact of mouse strain differences in innate hindlimb collateral vasculature. Arterioscler Thromb Vasc Biol 26: 520–526. 01.ATV.0000202677.55012.a0 [pii];10.1161/ 01.ATV.0000202677.55012.a0 [doi].