RevistaBrasileiradeFarmacognosia26(2016)701–704
w ww . e l s e v i e r . c o m / l o c a t e / b j p
Original
Article
Cucumol
A:
a
cytotoxic
triterpenoid
from
Cucumis
melo
seeds
Sabrin
Ibrahim
a,b,∗,
Rwaida
Al
Haidari
a,
Gamal
Mohamed
c,d,
Ehab
Elkhayat
d,
Mohamed
Moustafa
eaDepartmentofPharmacognosyandPharmaceuticalChemistry,CollegeofPharmacy,TaibahUniversity,AlMadinahAlMunawwarah,SaudiArabia
bDepartmentofPharmacognosy,FacultyofPharmacy,AssiutUniversity,Assiut,Egypt
cDepartmentofNaturalProductsandAlternativeMedicine,FacultyofPharmacy,KingAbdulazizUniversity,Jeddah,SaudiArabia
dDepartmentofPharmacognosy,FacultyofPharmacy,Al-AzharUniversity,AssiutBranch,Assiut,Egypt
eDepartmentofPharmaceuticalChemistry,FacultyofPharmacy,KingAbdulazizUniversity,Jeddah,SaudiArabia
a
r
t
i
c
l
e
i
n
f
o
Articlehistory:
Received31January2016 Accepted2March2016 Availableonline21May2016
Keywords:
Cucumismelo
Cucurbitaceae Triterpenoid CucumolA Cytotoxicactivity
a
b
s
t
r
a
c
t
PhytochemicalinvestigationoftheMeOHextractofCucumismeloL.var.reticulates,Cucurbitaceae,seeds ledtotheisolationofanewtriterpenoid:cucumolA(27-hydroxytaraxerol-3-ol),alongwiththree knowncompounds:␣-spinasterolandD:B-friedoolean-5-ene-3--ol.Theirstructureswereestablished byextensive1D(1H,13C,andDEPT)and2D(1H–1HCOSY,HMQC,andHMBC)NMR,aswellasIRand
HRESIMSspectralanalyses.Compound3displayedcytotoxicactivityagainstL5178YandHelacancer celllineswithED50of1.30and5.40g/ml,respectivelycomparedtopaclitaxel(0.07and0.92g/ml,
respectively).
©2016SociedadeBrasileiradeFarmacognosia.PublishedbyElsevierEditoraLtda.Thisisanopen accessarticleundertheCCBY-NC-NDlicense(http://creativecommons.org/licenses/by-nc-nd/4.0/).
Introduction
CucumismeloL.(cantaloupe)belongingtoCucurbitaceaefamily, iscultivatedintemperate,subtropical,andtropicalregions
world-wide(Yasaretal.,2006;Ibrahim,2010).Itsfruitsareconsumedin
thesummerperiodbecausethepulpofthefruitisvery refresh-ing,highnutritional,andsweetwithapleasantaroma,whichmay beusedasanappetizer,adessertorassalad(Meloetal.,2000;
Baghaeietal.,2008).InChinesefolkmedicine,theseedsofC.melo
areusedasdigestive,febrifuge,antitussive,demulcent,and ver-mifuge(Dukeand Ayensu,1985;De Marino et al.,2009).Their extractcanbeused asananti-diabetic andis usefulinchronic eczema(LalandLata,1980;TeotiaandRamakrishna,1984).Seed kernelis commonlyusedinrenal disorderssuchaskidneyand bladderstones,ulcersin theurinarytractand stomach,painful andburningmicturation,jaundice,vitiligo,ascites,suppressionof urine,chronicfevers,inflammationoftheliverand kidney,and ingeneraldebilityintheIndiantraditionalmedicine(Nayarand
Singh,1998;Baitar,2003;Gilletal.,2011;Milindand Kulwant,
2011; Ibrahim, 2014; Ullah et al., 2015).The fruit’s pulp
pos-sessesdiureticandanthelminticproperties(Ullahetal.,2015).Its
∗ Correspondingauthor.
E-mail:sribrahim@taibahu.edu.sa(S.Ibrahim).
lotionisemployedforacuteandchroniceczema.Rootsareemetic agents.Thefruitsareusedasafirstaidtreatmentforburnsand abrasions.Peduncle isusedtomanageanasarcaandindigestion
(MilindandKulwant,2011).C.melorevealedawiderangeof
bio-logicalactivitiessuchasantioxidant,analgesic,anti-inflammatory, andantimicrobial(Vouldoukisetal.,2004;MariodandMatthaus,
2008; Gillet al., 2011; Ibrahim, 2014). Previous phytochemical
studiesonC.meloL.var.reticulatesseedsresultedintheisolation ofchromone derivatives,triterpene,andsterols(Ibrahim,2010;
Ibrahim, 2014,Ibrahim andMohamed, 2015a,b).In thepresent
work,investigationoftheMeOHextractofC.meloL.var. reticu-latesseedsaffordedanewtriterpenoid:cucumolA(27-hydroxy taraxerol-3-o1) (3), along with ␣-spinasterol (1) and D:B-friedoolean-5-ene-3--ol(2).Thenewcompoundwasevaluated foritscytotoxicactivityagainstL5178Y,PC12,andHelacancercell lines.
Materialsandmethods
Generalexperimentalprocedures
Meltingpoint wascarriedout usingan Electrothermal9100 DigitalMeltingPointapparatus(ElectrothermalEngineeringLtd, Essex,England).OpticalrotationwasrecordedonaPerkin-Elmer
http://dx.doi.org/10.1016/j.bjp.2016.03.012
702 S.Ibrahimetal./RevistaBrasileiradeFarmacognosia26(2016)701–704
Model341LCPolarimeter(Perkin-Elmer,Waltham,MA,USA).EIMS wasrecordedonJEOLJMS-SX/SX102Amassspectrometer(Joel, Peabody,MA, USA).HRESIMSspectrum wasrecordedona LTQ Orbitrap(ThermoFinnigan,Bremen,Germany).1Dand2DNMR spectrawererecorded ona BrukerDRX400 NMR spectrometer usingstandardBrukersoftwareandC5D5NandCDCl3assolvents,
withTMS as the internal reference (Bruker, Rheinstetten, Ger-many).Columnchromatographicseparationswereperformedon silicagel60 (0.04–0.063mm,Merck, Darmstadt,Germany).TLC wasperformedon precoated TLCplates withsilica gel 60 F254
(layerthickness0.2mm,Merck,Darmstadt,Germany).The chro-matogramsweredevelopedusingthefollowingsolventsystems: hexane:EtOAc(95:5,S1)andhexane:EtOAc(90:10,S2).The
com-pounds were detected by spraying with p-anisaldehyde/H2SO4
reagentandheatingat110◦Cfor1–2min.
Plantmaterial
SeedsofCucumismeloL.var.reticulates,Curcubitaceae,were obtainedfrom thecultivated plants El-GalaaVillage, Samalout,
Table1
NMRspectraldataofcompound3(C5D5N,400and100MHz).
No. ıH[mult.,
J(Hz)]
ıC(mult.) HMBC
1 1.51m 1.47m
37.7(CH2) –
2 2.05m 1.83m
28.0(CH2) 3
3 3.42dd(9.2, 6.6)
78.1(CH) 5,24
4 – 39.2(C) –
5 0.81dd(9.6, 1.3)
55.9(CH) 1,4,6,24
6 1.53m 1.34m
19.1(CH2) –
7 1.92m 1.89m
41.6(CH2) 14
8 – 39.4(C) –
9 1.42m 49.5(CH) –
10 – 38.7(C) –
11 1.64m 1.41m
17.8(CH2) –
12 1.31m 1.27m
33.2(CH2) –
13 – 41.1(C) –
14 – 158.6(C) –
15 5.62dd(10.6, 4.3)
116.8(CH) 8,13,17
16 2.68dd(13.8, 10.6) 1.86dd(13.8, 4.3)
31.7(CH2) 14,15,17,18,
27,15
17 – 41.1(C) –
18 0.70dd(10.8, 4.8)
45.5(CH) 14,27
19 1.50m 1.03m
36.8(CH2) –
20 – 38.7(C)
21 – 28.4(CH2) –
22 1.62m 1.46m
33.8(CH2) –
23 1.21s 28.6(CH3) 3,4,5,24
24 1.05s 16.4(CH3) 3,4,5,23,25
25 0.92s 15.6(CH3) 1,5,9,24
26 1.08s 26.2(CH3) 7,9,14
27 3.59d(11.8) 3.44d(11.8)
64.5(CH2) 12,14,16,18,
16 28 1.02s 33.7(CH3) 18,19,21
29 0.98s 30.1(CH3) 19,21,30
30 1.09s 21.9(CH3) 21,22,29
OH 5.96brs – –
OH 5.75brs – –
Minia,Egypt.Theplantmaterialwasidentifiedandauthenticated (voucherspecimen2014-5)byProf.Dr.MohamedA.Farghali, Pro-fessor ofHorticulture (Vegetable Crops), Faculty of Agriculture, AssiutUniversity.
Extractionandisolation
Fruitswerecutandseedswereremovedfromstringypiths.The seedswererubbedbyhandandwashedquicklywithtapwater, then transferredto a colanderand dried at roomtemperature. Driedseeds(200g) were trituratedin a ball milland screened throughameshof0.5mmdiameter.Thetrituratedseeds(175g) werepackedin a Soxhletapparatusand defattedusing hexane (3×1l), then extracted with MeOH severaltimes (4×1l). The
MeOHextract wasevaporatedand concentrated underreduced pressuretoaffordadarkbrownresidue(9.3g).Thelatterwas sub-jectedtoVLC(vacuumliquidchromatography)usinghexane:EtOAc andEtOAc:MeOHgradientstoaffordfivefractions:CA-ItoCA-V; CA-I(2.6g,hexane:EtOAc75:25),CA-II(1.3g,hexane:EtOAc50:50), CA-III(2.1g,hexane:EtOAc25:75),CA-IV(0.7g,EtOAc100%),and CA-V(1.2g,MeOH100%).FractionCA-I(2.6g) wassubjectedto silicagelcolumnchromatography(120g×50×2cm)using
hex-ane:EtOAcgradienttoaffordfoursubfractionsCA-IA:CA-ID.Silica gelcolumnchromatography(80g×50×2cm)ofsubfraction
CA-IB (0.55g) using hexane:EtOAcas an eluent gave compound 1 (15mg,whitecrystallineneedles).SubfractionCA-IC(0.67g)was chromatographedoversilicagelcolumn(90g×50×2cm)using
S.Ibrahimetal./RevistaBrasileiradeFarmacognosia26(2016)701–704 703
Spectraldata
Cucumol A (3): White needles (7.5mg); mp 203–204◦C; [˛]D22+36.1(C=1.0,CHCl3);UV(MeOH)max(logε):258(4.25) nm;IR(KBr)max:3435,2982,1610,1070cm−1;NMRdata(C5D5N, 400MHzand100MHz),seeTable1:HRESIMSm/z443.38840(calcd forC30H51O2[M+H(proton)]+,443.38836).
Cytotoxicityassay
Thecytotoxicactivityofcompound3wasexaminedtowards mouselymphoma(L5178Y),ratbrain(PC12),andhumancervix (Hela) cancer cell lines using MTT assay as described earlier
(Mohamed, 2014; Mohamed et al., 2013).Exponentially
grow-ingcellswereharvested, counted,anddilutedappropriately.Of the cell suspension, 50l containing 3750 cells were pipetted into 96-well microtiter plates. Subsequently, 50l of a solu-tion of the tested sample was added to each well. The test plateswereincubatedat37◦Cwith5%CO2 for72h.Asolution of3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazoliumbromide (MTT)waspreparedat5mg/mlinphosphatebufferedsaline(PBS; 1.5mMKH2PO4,6.5mMNa2HPO4,137mMNaCl,2.7mMKCl;pH
7.4)and fromthis solution, 20lwas pipetted intoeach well. The yellowMTT penetrates the healthy living cells and in the presenceof mitochondrial dehydrogenases,MTT istransformed toitsblueformazancomplex.Afteranincubation periodof4h at37◦Cinahumidifiedincubatorwith5%CO
2,themediumwas
centrifuged(15min,20◦C,210
×g)with200lDMSO,thecells werelysedtoliberatetheformedformazanproduct.Cellviability wasevaluatedbymeasurementoftheabsorbance520nmusing a scanning microtiter-well spectrophotometer. Compound con-centrationsthatproduce50%cellgrowthinhibition(ED50)were
calculated fromcurves constructedby plotting cellsurvival (%) versusdrugconcentration(g/ml).Allexperimentswerecarried outintriplicatesandrepeatedthreetimes.Asnegativecontrols, mediawith0.1%(v/v)EtOHwereincludedinallexperiments.The paclitaxelwasusedasapositivecontrol(Ibrahimetal.,2015c).
Resultsanddiscussion
Compound3wasobtainedaswhiteneedles.Itgaveapositive Liebermann-Burchard’s test, indicating its triterpenoidalnature
(Ibrahimetal.,2012;Sayedetal.,2007;Reinhold,1935).Its
HRES-IMSspectrumshowedaquasi-molecularionpeakatm/z443.3884 [M+H]+,suggestingamolecularformulaC
30H50O2,whichimplied
sixdegreesofunsaturation.TheIRspectrumshowedabsorption bandsat3435and1070cm−1characteristicforthepresenceofa hydroxylgroup(SilversteinandWebster,1998;AlMusayeibetal., 2014).Thiswasconfirmedbytheappearanceof two exchange-able protons signals at ıH 5.96(1H, brs) and 5.75 (1H, brs) in 1HNMRspectrum.13CNMRandDEPTspectraof3revealedthe
presenceofresonancesforthirtycarbons:sevenmethyls,eleven methylenesoneofthemisoxygen-bondedatıC64.5(C-27),five
methines,andsevenquaternarycarbons.The1Hand13CNMR
spec-traexhibitedsevenmethylgroupssignalsatıH1.21(H-23)/ıC28.6
(C-23),1.05(H-24)/16.4(C-24),0.92(H-25)/15.6(C-25),1.08 (H-26)/26.2(C-26),1.02(H-28)/33.7(C-28),0.98(H3-29)/30.1(C-29),
and1.09(H-30)/21.9(C-30),suggestingapentacyclictriterpenoidal natureof3(MahatoandKundu,1994;Laphookhieoetal.,2004;Al
Muqarrabunetal.,2014).Moreover,signalsfortri-substituted
dou-blebondatıH5.62(dd,J=10.6,4.3Hz,H-15)/ıC116.8(C-15)and
158.6(C-14)wereobserved.ItspositionatC14–C15wasestablished
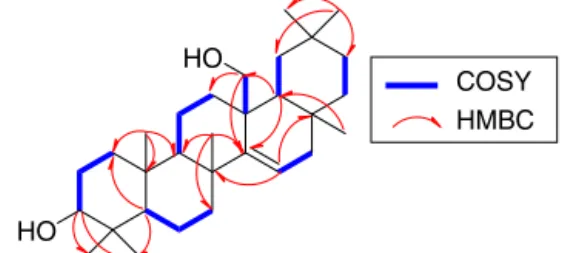
bythe3JHMBCcrosspeaksofH-7,H-16,andH-18toC-14and
H-15toC-8,C-13,andC-17.TheZgeometryofthedoublebondwas assignedbasedonthecouplingconstantvalueJ15,16ax=10.6and
HO
HO
COSY
HMBC
Fig.1.Somekey1H–1HCOSYandHMBCcorrelationsofcompound3.
J15,16eq4.3Hz(Ibrahim,2014).The1HNMRspectrumrevealed
sig-nalsforanoxymethineatıH3.42(1H,dd,J=9.2,6.6Hz,H-3)and
oxymethyleneatıH3.59and3.44(eachd,J=11.8Hz,H-27),which
correlatedwiththecarbonsignalsresonatingatıC78.1(C-3)and
64.5(C-27),respectivelyinHMQCspectrum.TheHMBCcrosspeaks ofH-2,H-23,andH-24toC-3,H-16andH-18toC-27,andH-27 toC-12,C-14,andC-18establishedthepositionsofthe oxyme-thineandoxyemthylene moietiesatC-3andC-27,respectively. ThreemethineprotonsignalsatıH0.81(dd,J=9.6,1.3Hz,H-5),
1.42(m,H-9),and0.70 (dd,J=10.8,4.8Hz, H-18),which corre-latedwiththecarbonsignalsresonatingatıC55.9(C-5),49.5(C-9),
and45.5(C-18),respectivelyinHMQCspectrumwereobserved. Theirassignmentwasestablishedbasedontheobserved correla-tioninthe1H–1HCOSYandHMBCspectra(Fig.1).Onthebasis
oftheabovespectraevidenceandbycomparisonwithliterature
(MahatoandKundu,1994;Laphookhieoetal.,2004),thestructure
ofcompound3wasestablishedas27-hydroxytaraxerol-3ˇ-o1and namedcucumolA.
Theknowncompoundswereidentifiedbyanalysisofthe spec-troscopicdata(1H,13CNMR,COSY,andHMQC)andcomparison
oftheirdatawiththoseintheliteraturetobe:␣-spinasterol(2)
(Ibrahim, 2014)and D:B-friedoolean-5-ene-3--ol(3) (Ibrahim,
2014).
Thecytotoxiceffectofcompound3wastestedtowardsL5178Y, PC12,andHelacancercelllines.Compound3wasfoundto dis-playcytotoxicactivitytowardsL5178YandHelacancercelllines withED50valuesof1.30and5.40g/ml,respectivelycomparedto
paclitaxel(0.07and0.92g/ml,respectively).While,itwasinactive againstPC12cancercellline.
Conclusion
A new triterpenoid: cucumol A (3) and three known com-poundswereisolatedfromC.meloseedsafforded.Theirstructures wereestablishedbydifferentspectroscopicanalyses.Compound3
showedcytotoxicactivitytowardsL5178YandHelacells.
Ethicaldisclosures
Protectionofhumanandanimalsubjects. Theauthorsdeclare
thatnoexperimentswereperformedonhumansoranimalsfor thisstudy.
Confidentialityofdata. Theauthorsdeclarethatnopatientdata appearinthisarticle.
Righttoprivacyandinformedconsent.Theauthorsdeclarethat nopatientdataappearinthisarticle.
Authors’contribution
spectro-704 S.Ibrahimetal./RevistaBrasileiradeFarmacognosia26(2016)701–704
scopicdata,andwritingthemanuscript.SRMIandESEhaverevised andapprovedthesubmission.
Conflictsofinterest
Theauthorsdeclarenoconflictsofinterest.
Acknowledgments
WewouldliketoexpressourdeepthankstoDr.VolkerBrecht foracquiringNMRandMSspectroscopicdata.Wearegratefulto Prof.Dr.W.E.G.Müller(InstitutefürPhysiologischeChemie,Dues bergweg6,D-55099Mainz,Germany)forcarryingoutcytotoxicity assay.
References
AlMuqarrabun,L.M.R.,Ahmat,N.,Aris,S.R.S.,Norizan,N.,Shamsulrijal,N.,Yusof, F.Z.M.,Suratman,M.N.,Yusof,M.I.M.,Salim,F.,2014.Anewtriterpenoidfrom Sapiumbaccatum(Euphorbiaceae).Nat.Prod.Res.28,1003–1009.
AlMusayeib,N.M.,Mothana,R.A.,Ibrahim,S.R.M.,ElGamal,A.A.,Al-Massarani,S.M., 2014.KlodoroneAandklodorolA:newtriterpenesfromKleiniaodora.Nat.Prod. Res.28,1142–1146.
Baghaei,H.,Shahidi,F.,Varidi,M.J.,Mahallati,M.N.,2008.Orange-cantaloupeseed beverage:nutritivevalue,effectofstoragetimeandconditiononchemical, sensoryandmicrobialproperties.WorldAppl.Sci.J.3,753–758.
Baitar,I.E.,2003.AljamaiulMufradat-ulAdviaWalAghzia.CCRUM,NewDelhi,pp. 248.
DeMarino,S.,Festa,C.,Zollo,F.,Iorizzi,M.,2009.PhenolicglycosidesfromCucumis melovar.inodorusseeds.Phytochem.Lett.2,130–133.
Duke,J.A.,Ayensu,E.S.,1985.MedicinalPlantsofChina,ISBN0-9177256-20-4.
Gill,N.S.,Bajwa,J.,Dhiman,K.,Sharma,P.,Sood,S.,Sharma,P.D.,Singh,B.,Bali,M., 2011.TherapeuticpotentialoftraditionallyconsumedCucumismeloseeds.Asian J.PlantSci.10,86–91.
Ibrahim,S.R.M.,2010.New2-(2-phenylethyl)chromonederivativesfromtheseeds ofCucumismeloLvar.reticulates.Nat.Prod.Commun.5,403–407.
Ibrahim,S.R.M.,Mohamed,G.A.,2015a.CucuminS,anewphenylethylchromone fromCucumismelovar.reticulatusseeds.Rev.Bras.Farmacogn.25,462–464.
Ibrahim, S.R.M., Mohamed, G.A., 2015b. Natural occurring 2-(2-phenylethyl)chromones, structure elucidation and biological activities. Nat.Prod.Res.29,1489–1520.
Ibrahim,S.R.M.,2014.NewchromoneandtriglyceridefromCucumismeloseeds.Nat. Prod.Commun.9,205–208.
Ibrahim,S.R.M.,Mohamed,G.A.,Zayed,M.F.,Sayed,H.M.,2015c.IngeninesAand B,twonewalkaloidsfromtheIndonesianspongeAcanthostrongylophoraingens. DrugRes.65,361–365.
Ibrahim,S.R.M.,Mohamed,G.A.,Shaala,L.A.,Banuls,L.M.Y.,VanGoietsenoven,G., Kiss,R.,Youssef,D.T.A.,2012.Newursane-typetriterpenesfromtherootbark ofCalotropisprocera.Phytochem.Lett.5,490–495.
Lal,S.D.,Lata,K.,1980.PlantsusedbyBhatcommunityforregulatingfertility.Econ. Bot.34,273–275.
Laphookhieo,S.,Karalai, C.,Ponglimanont,C.,2004. Newsesquiterpenoidand triterpenoidsfromthefruitsofRhizophoramucronata.Chem.Pharm.Bull.52, 883–885.
Mahato,S.B.,Kundu,A.P.,1994.13CNMRspectraofpentacyclictriterpenoidsA compilationandsomesalientfeatures.Phytochemistry37,1517–1575.
Mariod,A.,Matthaus,B.,2008.Investigationsonfattyacids,tocopherols,sterols, phenolicprofilesandoxidativestabilityofCucumismelovar.agrestisoil.J.Food Lipids15,56–67.
Melo,M.L.S.,Narain,N.,Bora,P.S.,2000.Characterisationofsomenutritional con-stituentsofmelon(CucumismelohybridAF-522)seeds.FoodChem.68,411–414.
Milind,P.,Kulwant,S.,2011.Muskmeloniseat-mustmelon.Int.Res.J.Pharm.2, 52–57.
Mohamed,G.A.,2014.NewcytotoxiccycloartanetriterpenefromCassiaitalicaaerial parts.Nat.Prod.Res.28,976–983.
Mohamed,G.A.,Ibrahim,S.R.M.,Ross,S.A.,2013.Newceramidesandisoflavonefrom theEgyptianIrisgermanicaL.rhizomes.Phytochem.Lett.6,340–344.
Nayar,N.M.,Singh,R.,1998.In:Nayar,N.M.,More,T.A.(Eds.),Taxonomy, Distri-butionandEthnobotanicalUsesinCucurbits.SciencePublishers,Inc.,USA,pp. 1–18.
Reinhold,J.G.,1935.Liebermann-Burchardreactionvelocities ofsterols:I. Dif-ferencesbetweenfreeandestercholesterolappliedtothedeterminationof cholesterolesters,II.Atestforthepresenceofcoprostenolinplasma.Am.J. Med.Sci.189,302.
Sayed,H.M.,Mohamed,M.H.,Farag,S.F.,Mohamed,G.A.,Proksch,P.,2007.Anew steroidglycosideandfurochromonesfromCyperusrotundusL.Nat.Prod.Res. 21,343–350.
Silverstein,R.M.,Webster,F.X.,1998.SpectrometricIdentificationofOrganic Com-pounds,6thed.NewYork,JohnWiley.
Teotia,M.S.,Ramakrishna,P.,1984.Chemistryandtechnologyofmelonseeds.J. FoodSci.Technol.21,332–340.
Ullah,N.,Khan,S.,Khan,A.,Ahmad,W.,Shah,Y.,Ahmad,L.,Ullah,I.,2015.A prospec-tivepharmacologicalreviewofmedicinalherbs,CucumismeloandBerberis vulgaris,commonlyusedinthetreatmentofrenaldiseasesinPakistan.Acta PoloniaePharm.DrugRes.72,651–654.
Vouldoukis,I.,Lacan,D.,Kamate,C.,Coste,P.,Calenda,A.,Mazier,D.,Conti,M., Dugas,B.,2004.Antioxidantandanti-inflammatorypropertiesofCucumismelo LC.extractrichinsuperoxidedismutaseactivity.J.Ethnopharmacol.94,67–75.